a83906298b9545ee99d91ba18f1ef9cc.ppt
- Количество слайдов: 71
 Alameda County Influenza Update Rosilyn Ryals, M. D. Division of Communicable Disease Control & Prevention Alameda County Public Health Department November 2005
Alameda County Influenza Update Rosilyn Ryals, M. D. Division of Communicable Disease Control & Prevention Alameda County Public Health Department November 2005
 Outline Influenza n The Pandemic Threat n
Outline Influenza n The Pandemic Threat n
 What is Influenza? Contagious, acute, febrile, respiratory illness caused by viruses n Epidemics of influenza occur during winter months in temperate regions, like the U. S. n Every year in the United states: n » 5 to 20% of the population get flu » >200, 000 people are hospitalized from flu complications » Approximately 36, 000 people die from flu (primarily high risk persons)
What is Influenza? Contagious, acute, febrile, respiratory illness caused by viruses n Epidemics of influenza occur during winter months in temperate regions, like the U. S. n Every year in the United states: n » 5 to 20% of the population get flu » >200, 000 people are hospitalized from flu complications » Approximately 36, 000 people die from flu (primarily high risk persons)
 High Risk Population n n n Adults >65 years Children 6 -23 months Persons aged 2 -64 yrs. with chronic lung, heart or metabolic disorders: - heart disease (ever diagnosed) - asthma (taking medication) - diabetes (ever diagnosed) Persons with hemoglobinopathies or immunosuppression Children and adolescents on long-term aspirin therapy Women pregnant during influenza season
High Risk Population n n n Adults >65 years Children 6 -23 months Persons aged 2 -64 yrs. with chronic lung, heart or metabolic disorders: - heart disease (ever diagnosed) - asthma (taking medication) - diabetes (ever diagnosed) Persons with hemoglobinopathies or immunosuppression Children and adolescents on long-term aspirin therapy Women pregnant during influenza season
 High Risk Population (continued) Adults and children who have any condition that can compromise respiratory function. n Residents of nursing homes and other chronic-care facilities Plus those in close contact with high risk persons: n Household members and out-of-home care givers of infants under the age of 6 mos. n Healthcare workers who provide direct, hands-on care to patients - ambulatory health care services - hospitals - nursing and residential care facilities n
High Risk Population (continued) Adults and children who have any condition that can compromise respiratory function. n Residents of nursing homes and other chronic-care facilities Plus those in close contact with high risk persons: n Household members and out-of-home care givers of infants under the age of 6 mos. n Healthcare workers who provide direct, hands-on care to patients - ambulatory health care services - hospitals - nursing and residential care facilities n
 High Risk Group Impact on Alameda County has a population of about 1. 5 million people n The high risk groups identified comprise approximately 1/3 of the county population n
High Risk Group Impact on Alameda County has a population of about 1. 5 million people n The high risk groups identified comprise approximately 1/3 of the county population n
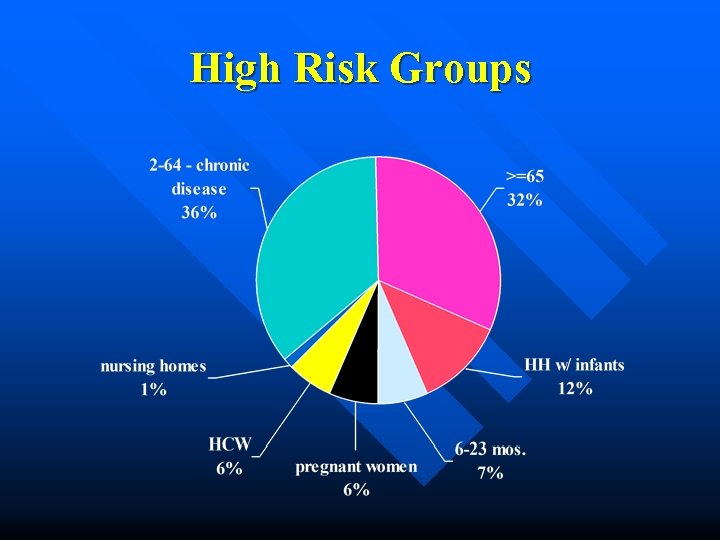 High Risk Groups
High Risk Groups
 Symptoms of Influenza Fever n Headache n Malaise n Cough n Sore Throat n Runny or stuffy nose n Myalgia n GI symptoms (nausea, vomiting, and diarrhea) – primarily in children n
Symptoms of Influenza Fever n Headache n Malaise n Cough n Sore Throat n Runny or stuffy nose n Myalgia n GI symptoms (nausea, vomiting, and diarrhea) – primarily in children n
 Influenza-Course Incubation: 1 -4 days, averagely 2 days n Adults can be infectious from the day before symptoms until about 5 days after onset n Children may be infectious > 10 days n Severely immunocompromised persons may shed virus for weeks or months n Uncomplicated influenza illness typically resolves in 3 -7 days n
Influenza-Course Incubation: 1 -4 days, averagely 2 days n Adults can be infectious from the day before symptoms until about 5 days after onset n Children may be infectious > 10 days n Severely immunocompromised persons may shed virus for weeks or months n Uncomplicated influenza illness typically resolves in 3 -7 days n
 Influenza- Complications Pneumonia – usually secondary bacterial n Dehydration n Exacerbation of chronic conditions n Sinus and ear infections (children) n Febrile seizures in children n
Influenza- Complications Pneumonia – usually secondary bacterial n Dehydration n Exacerbation of chronic conditions n Sinus and ear infections (children) n Febrile seizures in children n
 Biology of Influenza n n n Influenza viruses belong to the family Orthomyxoviridae There are 3 distinct types of influenza: A, B and C Influenza A and B are the two types that cause epidemic human disease Influenza A is further categorized into subtypes on the basis of two surface antigens: hemagglutinin (H) and neuraminidase (N) Type A Influenza has 16 different Hs and 9 different Ns Human disease with Influenza A has historically been caused by three subtypes of H (H 1, H 2, and H 3) and two subtypes of N (N 1 and N 2)
Biology of Influenza n n n Influenza viruses belong to the family Orthomyxoviridae There are 3 distinct types of influenza: A, B and C Influenza A and B are the two types that cause epidemic human disease Influenza A is further categorized into subtypes on the basis of two surface antigens: hemagglutinin (H) and neuraminidase (N) Type A Influenza has 16 different Hs and 9 different Ns Human disease with Influenza A has historically been caused by three subtypes of H (H 1, H 2, and H 3) and two subtypes of N (N 1 and N 2)
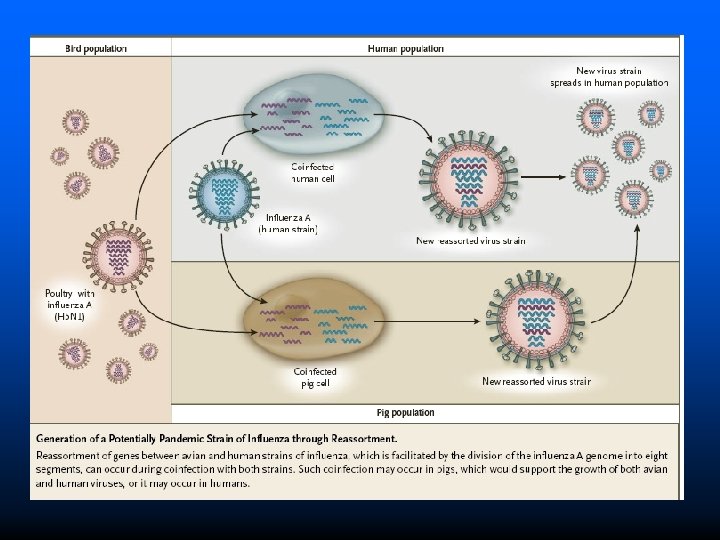
 Biology of Influenza (continued) n n n All known subtypes of influenza A can be found in birds Influenza B viruses are not categorized into subtypes. Standard nomenclature for influenza viruses includes: (1) Influenza type, (2) Place of initial isolation, (3) Strain designation, and (4) Year of isolation, and (5) H and N subtypes for Influenza A. For example, influenza A isolated in a California patient in 2004 would be written as: A/California/7/2004 (H 3 N 2)
Biology of Influenza (continued) n n n All known subtypes of influenza A can be found in birds Influenza B viruses are not categorized into subtypes. Standard nomenclature for influenza viruses includes: (1) Influenza type, (2) Place of initial isolation, (3) Strain designation, and (4) Year of isolation, and (5) H and N subtypes for Influenza A. For example, influenza A isolated in a California patient in 2004 would be written as: A/California/7/2004 (H 3 N 2)
 Biology of Influenza (continued) n Influenza A viruses are the most worrisome of all the well-established infectious diseases: » Mutate rapidly » In addition to humans, they infect pigs, horses, sea mammals, and birds » They have a large number of subtypes maintained in aquatic birds, providing a perpetual source of viruses and a huge pool of genetic diversity
Biology of Influenza (continued) n Influenza A viruses are the most worrisome of all the well-established infectious diseases: » Mutate rapidly » In addition to humans, they infect pigs, horses, sea mammals, and birds » They have a large number of subtypes maintained in aquatic birds, providing a perpetual source of viruses and a huge pool of genetic diversity
 Biology of Influenza (continued) n Influenza A viruses are described as “sloppy, capricious, and promiscuous” because: – They lack a proof-reading mechanism to detect and correct small errors that occur when the viruses copy themselves. – This allows for constant stepwise changes in their genetic makeup termed antigenic drift – Though small, these slight variations keep populations susceptible to infection (This explains need for a new vaccine for each winter season)
Biology of Influenza (continued) n Influenza A viruses are described as “sloppy, capricious, and promiscuous” because: – They lack a proof-reading mechanism to detect and correct small errors that occur when the viruses copy themselves. – This allows for constant stepwise changes in their genetic makeup termed antigenic drift – Though small, these slight variations keep populations susceptible to infection (This explains need for a new vaccine for each winter season)
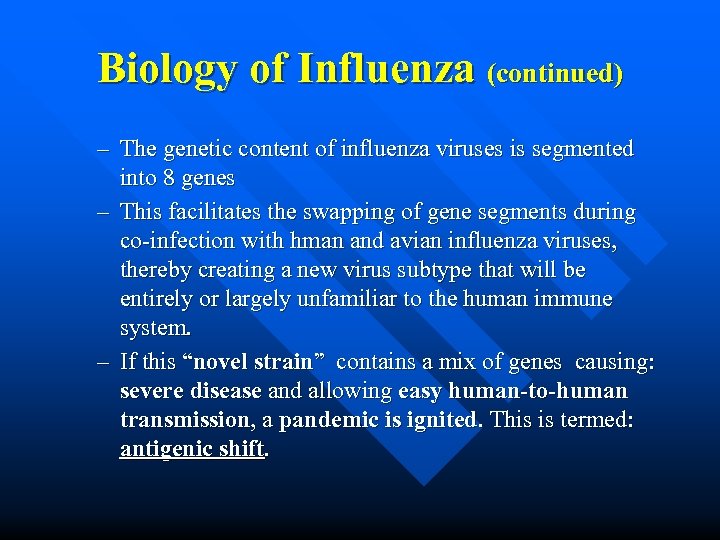 Biology of Influenza (continued) – The genetic content of influenza viruses is segmented into 8 genes – This facilitates the swapping of gene segments during co-infection with hman and avian influenza viruses, thereby creating a new virus subtype that will be entirely or largely unfamiliar to the human immune system. – If this “novel strain” contains a mix of genes causing: severe disease and allowing easy human-to-human transmission, a pandemic is ignited. This is termed: antigenic shift.
Biology of Influenza (continued) – The genetic content of influenza viruses is segmented into 8 genes – This facilitates the swapping of gene segments during co-infection with hman and avian influenza viruses, thereby creating a new virus subtype that will be entirely or largely unfamiliar to the human immune system. – If this “novel strain” contains a mix of genes causing: severe disease and allowing easy human-to-human transmission, a pandemic is ignited. This is termed: antigenic shift.
 Biology of Influenza (continued) Usually a single strain of influenza virus prevails during an epidemic n Occasionally, two different strains within a single subtype (e. g. A/Victoria/3/75 (H 3 N 2)or A/Texas/1/77 (H 3 N 2) or two different influenza A subtypes (H 1 N 1 and H 3 N 2) may circulate simultaneously. n
Biology of Influenza (continued) Usually a single strain of influenza virus prevails during an epidemic n Occasionally, two different strains within a single subtype (e. g. A/Victoria/3/75 (H 3 N 2)or A/Texas/1/77 (H 3 N 2) or two different influenza A subtypes (H 1 N 1 and H 3 N 2) may circulate simultaneously. n
 Influenza. Transmission Primarily transmitted person-to-person by large virus-laden droplets (as generated by cough or sneezing within 3 feet of susceptible person) n Direct or indirect contact with virus-laden respiratory secretions followed by touching the eyes, nose or mouth of a susceptible person n
Influenza. Transmission Primarily transmitted person-to-person by large virus-laden droplets (as generated by cough or sneezing within 3 feet of susceptible person) n Direct or indirect contact with virus-laden respiratory secretions followed by touching the eyes, nose or mouth of a susceptible person n
 Laboratory Diagnosis n It is difficult to diagnose based on clinical symptoms alone. Similar symptoms can be caused by other illnesses, e. g. Mycoplasma peumoniae n Adenovirus n Respiratory Syncytial Virus n Rhinovirus n Parainfluenza Viruses n Legionella n
Laboratory Diagnosis n It is difficult to diagnose based on clinical symptoms alone. Similar symptoms can be caused by other illnesses, e. g. Mycoplasma peumoniae n Adenovirus n Respiratory Syncytial Virus n Rhinovirus n Parainfluenza Viruses n Legionella n
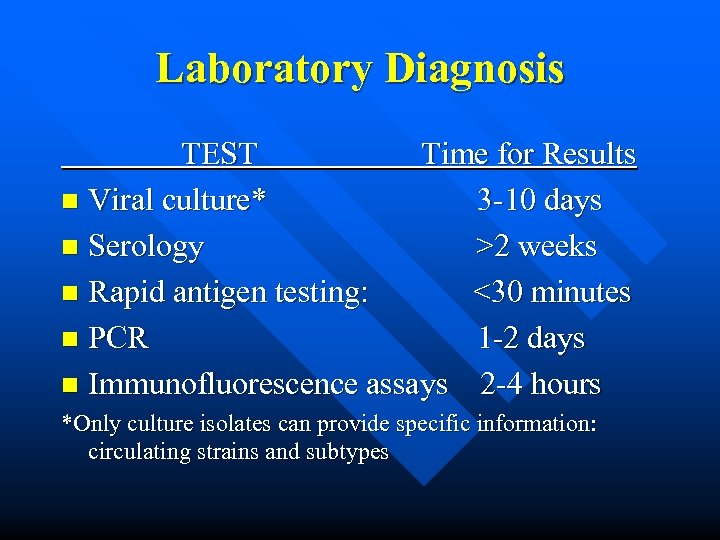 Laboratory Diagnosis TEST Time for Results n Viral culture* 3 -10 days n Serology >2 weeks n Rapid antigen testing: <30 minutes n PCR 1 -2 days n Immunofluorescence assays 2 -4 hours *Only culture isolates can provide specific information: circulating strains and subtypes
Laboratory Diagnosis TEST Time for Results n Viral culture* 3 -10 days n Serology >2 weeks n Rapid antigen testing: <30 minutes n PCR 1 -2 days n Immunofluorescence assays 2 -4 hours *Only culture isolates can provide specific information: circulating strains and subtypes
 Prevention of Influenza Inter-pandemic Period VACCINATION
Prevention of Influenza Inter-pandemic Period VACCINATION
 Inactivated Influenza Vaccine n Influenza vaccine can be given to people 6 months of age and older. It is recommended for people who are at risk of serious influenza or its complications, and for people who can spread influenza to those at high risk (including all household members)
Inactivated Influenza Vaccine n Influenza vaccine can be given to people 6 months of age and older. It is recommended for people who are at risk of serious influenza or its complications, and for people who can spread influenza to those at high risk (including all household members)
 Live Attenuated Influenza Vaccine Live, attenuated influenza vaccine (L. A. I. V. ) was licensed in 2003. L. A. I. V. contains live but attenuated (weakened) influenza virus. It is sprayed into the nostrils rather than injected into the muscle. It is recommended for healthy children and adults from 5 through 49 years of age, who are not pregnant. Recipients may shed virus.
Live Attenuated Influenza Vaccine Live, attenuated influenza vaccine (L. A. I. V. ) was licensed in 2003. L. A. I. V. contains live but attenuated (weakened) influenza virus. It is sprayed into the nostrils rather than injected into the muscle. It is recommended for healthy children and adults from 5 through 49 years of age, who are not pregnant. Recipients may shed virus.
 Influenza Vaccination n Influenza viruses are constantly changing. Therefore, influenza vaccines are updated every year, and annual vaccination is recommended. For most people influenza vaccine prevents serious illness caused by the influenza virus. It will not prevent “influenza-like” illnesses caused by other viruses. It takes about 2 weeks for protection to develop after vaccination, and protection can last up to a year.
Influenza Vaccination n Influenza viruses are constantly changing. Therefore, influenza vaccines are updated every year, and annual vaccination is recommended. For most people influenza vaccine prevents serious illness caused by the influenza virus. It will not prevent “influenza-like” illnesses caused by other viruses. It takes about 2 weeks for protection to develop after vaccination, and protection can last up to a year.
 2005 -2006 Influenza Vaccine • Inactivated Influenza Vaccine A/New Caledonia/20/99 (H 1 N 1) A/New York/55/2004 (H 3 N 2) B/Jiangsu/10/2003 • Live attenuated Influenza Vaccine (LAIV) A/New Caledonia/20/99 (H 1 N 1) A/California/7/2004 (H 3 N 2) B/Jiangstu/10/2003
2005 -2006 Influenza Vaccine • Inactivated Influenza Vaccine A/New Caledonia/20/99 (H 1 N 1) A/New York/55/2004 (H 3 N 2) B/Jiangsu/10/2003 • Live attenuated Influenza Vaccine (LAIV) A/New Caledonia/20/99 (H 1 N 1) A/California/7/2004 (H 3 N 2) B/Jiangstu/10/2003
 Pneumococcal Vaccination There are two licensed vaccines: (1) Pneumococcal Conjugate Vaccine, and (2) Pneumococcal Polysaccharide Vaccine. n Recommendations for Pneumococcal Vaccine include population at high risk for influenza and its complications. n
Pneumococcal Vaccination There are two licensed vaccines: (1) Pneumococcal Conjugate Vaccine, and (2) Pneumococcal Polysaccharide Vaccine. n Recommendations for Pneumococcal Vaccine include population at high risk for influenza and its complications. n
 Prevention of Influenza Antivirals
Prevention of Influenza Antivirals
 Antiviral therapy and prophylaxis Adamantine Derivatives: (1) Amantadine (2) Rimantadine Neuraminidase Inhibitors: (1) Zanamivir (2) Oseltamivir (Tamiflu)
Antiviral therapy and prophylaxis Adamantine Derivatives: (1) Amantadine (2) Rimantadine Neuraminidase Inhibitors: (1) Zanamivir (2) Oseltamivir (Tamiflu)
 Antivirals for Treatment Any person with life-threatening influenzarelated illness n Any person at high-risk for serious complications of influenza and who is within the first 2 days of illness onset n
Antivirals for Treatment Any person with life-threatening influenzarelated illness n Any person at high-risk for serious complications of influenza and who is within the first 2 days of illness onset n
 Antivirals for Prophylaxis All persons who live or work in institutions caring for people at high risk of complications from influenza should be given antiviral medication in the event of an institutional outbreak n Persons at high risk of serious influenza complications should be given antivirals if they are likely to be exposed to others infected with influenza. n
Antivirals for Prophylaxis All persons who live or work in institutions caring for people at high risk of complications from influenza should be given antiviral medication in the event of an institutional outbreak n Persons at high risk of serious influenza complications should be given antivirals if they are likely to be exposed to others infected with influenza. n
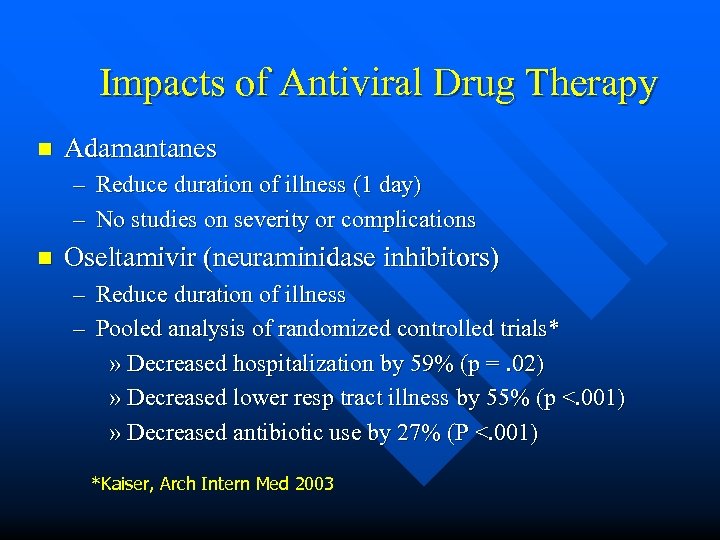 Impacts of Antiviral Drug Therapy n Adamantanes – Reduce duration of illness (1 day) – No studies on severity or complications n Oseltamivir (neuraminidase inhibitors) – Reduce duration of illness – Pooled analysis of randomized controlled trials* » Decreased hospitalization by 59% (p =. 02) » Decreased lower resp tract illness by 55% (p <. 001) » Decreased antibiotic use by 27% (P <. 001) *Kaiser, Arch Intern Med 2003
Impacts of Antiviral Drug Therapy n Adamantanes – Reduce duration of illness (1 day) – No studies on severity or complications n Oseltamivir (neuraminidase inhibitors) – Reduce duration of illness – Pooled analysis of randomized controlled trials* » Decreased hospitalization by 59% (p =. 02) » Decreased lower resp tract illness by 55% (p <. 001) » Decreased antibiotic use by 27% (P <. 001) *Kaiser, Arch Intern Med 2003
 Prevention of Influenza Infection Control
Prevention of Influenza Infection Control
 Infection Control n n Encourage annual Influenza vaccine for Health Care workers Use of Standard Precautions: (1) Handwashing (2) Gloves (3) Mask, Eye Protection, Face Shield: when patient care activities are likely to generate splashes or sprays (4) Gown: protect skin and prevent soiling of clothing in patient care activities that are likely to generate splashes or sprays (5) Patient-Care Equipment (6) Environmental Control (7) Linen (8) Occupational Health and Bloodborne Pathogens (9) Patient Placement: private room or cohorting
Infection Control n n Encourage annual Influenza vaccine for Health Care workers Use of Standard Precautions: (1) Handwashing (2) Gloves (3) Mask, Eye Protection, Face Shield: when patient care activities are likely to generate splashes or sprays (4) Gown: protect skin and prevent soiling of clothing in patient care activities that are likely to generate splashes or sprays (5) Patient-Care Equipment (6) Environmental Control (7) Linen (8) Occupational Health and Bloodborne Pathogens (9) Patient Placement: private room or cohorting
 Infection Control n Droplet Precautions: (1) Patient Placement: Private room; cohort; or maintain at least 3 feet spatial separation (2) Mask: as per Standard precautions and when working within 3 feet of an infected patient (3) Patient transport: limit to essential purposes only; minimize dispersal of droplets by masking patient, if possible.
Infection Control n Droplet Precautions: (1) Patient Placement: Private room; cohort; or maintain at least 3 feet spatial separation (2) Mask: as per Standard precautions and when working within 3 feet of an infected patient (3) Patient transport: limit to essential purposes only; minimize dispersal of droplets by masking patient, if possible.
 Infection Control n Contact Precautions: (1) Patient Placement (2) Gloves and handwashing (3) Gown (4) Patient transport (5) Patient-Care Equipment
Infection Control n Contact Precautions: (1) Patient Placement (2) Gloves and handwashing (3) Gown (4) Patient transport (5) Patient-Care Equipment
 Infection Control n Airborne Precautions (smaller particles than respiratory droplets); may result from procedures like endotracheal intubation, suctioning, nebulizer treatment, or bronchoscopy. These procedures can result in dissemination of airborne droplets over long distances; requires use of special airhandling and ventillation.
Infection Control n Airborne Precautions (smaller particles than respiratory droplets); may result from procedures like endotracheal intubation, suctioning, nebulizer treatment, or bronchoscopy. These procedures can result in dissemination of airborne droplets over long distances; requires use of special airhandling and ventillation.
 Infection Control n Airborne Precautions (continued): (1) Patient placement: negative air pressure with 6 -12 air exchanges per hour, and appropriate discharge of air outdoors; keep door closed; can also cohort if private room not available. (2) Respiratory protection: wear an N 95 respirator when entering room (3) Limit movement and transport of patient to essential purposes If transport or movement is necessary, use mask for patient, if possible.
Infection Control n Airborne Precautions (continued): (1) Patient placement: negative air pressure with 6 -12 air exchanges per hour, and appropriate discharge of air outdoors; keep door closed; can also cohort if private room not available. (2) Respiratory protection: wear an N 95 respirator when entering room (3) Limit movement and transport of patient to essential purposes If transport or movement is necessary, use mask for patient, if possible.
 Infection Control In Doctor’s Offices and Clinics, in addition to standard precautions and annual influenza immunization of health care workers: (1) (2) Encourage patients with respiratory and other symptoms consistent with influenza to call in advance of coming in. Encourage the use of masks by symptomatic patients or the use of tissues to cover coughs/sneezes
Infection Control In Doctor’s Offices and Clinics, in addition to standard precautions and annual influenza immunization of health care workers: (1) (2) Encourage patients with respiratory and other symptoms consistent with influenza to call in advance of coming in. Encourage the use of masks by symptomatic patients or the use of tissues to cover coughs/sneezes
 Goals of Influenza Surveillance in the U. S. » Find out when and where influenza activity is occurring » Determine what type of influenza viruses are circulating » Detect changes in the influenza viruses » Track influenza-related illness » Measure the impact influenza is having on deaths in the U. S.
Goals of Influenza Surveillance in the U. S. » Find out when and where influenza activity is occurring » Determine what type of influenza viruses are circulating » Detect changes in the influenza viruses » Track influenza-related illness » Measure the impact influenza is having on deaths in the U. S.
 U. S. Influenza Surveillance System (1) 75 WHO and 50 NREVSS Collaborating Labs throughout U. S. report: # specimens tested, # positive for influenza A or B (2) U. S. Influenza Sentinel Providers Network: 1000 providers around the country report number of persons seen, and number with influenza-like illness by age group (3) 122 Cities Mortality Reporting systems report # of pneumonia or influenza deaths (4) State and Territorial Epidemiologists report the level of influenza activity in the state (5) Influenza-associated pediatric mortality report labconfirmed influenza deaths in children <18 years old
U. S. Influenza Surveillance System (1) 75 WHO and 50 NREVSS Collaborating Labs throughout U. S. report: # specimens tested, # positive for influenza A or B (2) U. S. Influenza Sentinel Providers Network: 1000 providers around the country report number of persons seen, and number with influenza-like illness by age group (3) 122 Cities Mortality Reporting systems report # of pneumonia or influenza deaths (4) State and Territorial Epidemiologists report the level of influenza activity in the state (5) Influenza-associated pediatric mortality report labconfirmed influenza deaths in children <18 years old
 U. S. Influenza Surveillance System (continued) (6) Emerging Infections Program conducts surveillance for lab-confirmed influenza-related hospitalizations in persons less than 18 years of age in 57 counties throughout U. S. (covering 10 states) (7) New Vaccine Surveillance Network provides populationbased estimates of lab-confirmed influenza hospitalization rates for children <5 years who live in 3 U. S. counties(Ohio, Tennessee, and New York)
U. S. Influenza Surveillance System (continued) (6) Emerging Infections Program conducts surveillance for lab-confirmed influenza-related hospitalizations in persons less than 18 years of age in 57 counties throughout U. S. (covering 10 states) (7) New Vaccine Surveillance Network provides populationbased estimates of lab-confirmed influenza hospitalization rates for children <5 years who live in 3 U. S. counties(Ohio, Tennessee, and New York)
 Pandemic Influenza: A Harbinger of Things to Come
Pandemic Influenza: A Harbinger of Things to Come
 Pandemic Influenza Worldwide outbreak of a novel strain n Associated with high morbidity, excess mortality, and social and economic disruption n First recorded pandemic that fits influenza profile occurred in 1580 n
Pandemic Influenza Worldwide outbreak of a novel strain n Associated with high morbidity, excess mortality, and social and economic disruption n First recorded pandemic that fits influenza profile occurred in 1580 n

 Pandemic Influenza in the 20 th Century n 1918 -19 Spanish Flu (H 1 N 1) » 20 -50 million deaths worldwide » >500, 000 U. S. deaths n 1957 -58 Asian Flu (H 2 N 2) » 70, 000 U. S. deaths n 1968 -69 Hong Kong Flu (H 3 N 2) » 50, 000 U. S. deaths
Pandemic Influenza in the 20 th Century n 1918 -19 Spanish Flu (H 1 N 1) » 20 -50 million deaths worldwide » >500, 000 U. S. deaths n 1957 -58 Asian Flu (H 2 N 2) » 70, 000 U. S. deaths n 1968 -69 Hong Kong Flu (H 3 N 2) » 50, 000 U. S. deaths
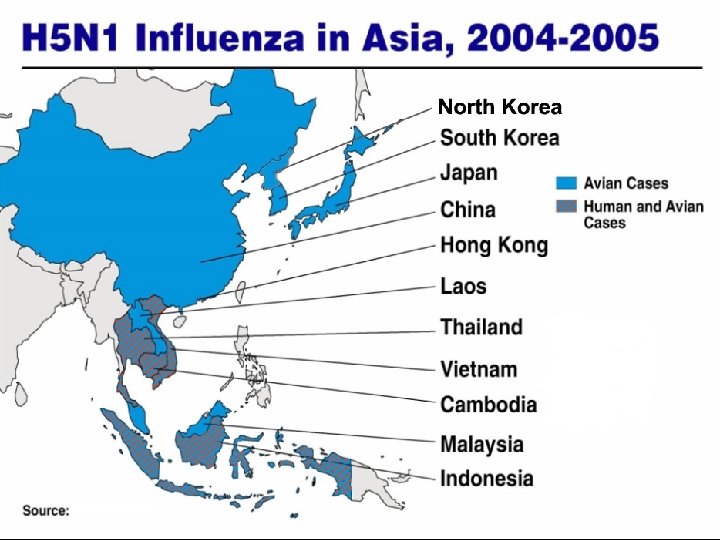
 Avian H 5 N 1 in Asia n Continuing presence in Asia since 1996 – Documented direct avian to human transmission, Hong Kong, 1997 n Enzootic and epizootic of unprecedented size and complexity – 9 countries with ongoing outbreaks (most recently in Malaysia) n Ongoing human cases with high case fatality, mostly in healthy children and young adults n Ongoing evolution of the virus’ antigenic, genetic and functional properties n No sustained human to human transmission to date
Avian H 5 N 1 in Asia n Continuing presence in Asia since 1996 – Documented direct avian to human transmission, Hong Kong, 1997 n Enzootic and epizootic of unprecedented size and complexity – 9 countries with ongoing outbreaks (most recently in Malaysia) n Ongoing human cases with high case fatality, mostly in healthy children and young adults n Ongoing evolution of the virus’ antigenic, genetic and functional properties n No sustained human to human transmission to date
 Why are We Concerned? n Increasing countries/areas with avian influenza – Uncertainties on progress of control n Ongoing human infection with avian H 5 N 1 – Limited implementation of protective measures n Co-Circulating human influenza viruses – Risk of genetic reassortment leading to pandemic strain n n Majority of human population would have no immunity H 5 N 1 resembles the 1918 -19 pandemic influenza in that healthy young persons are affected and a deadly feature is a primary viral pneumonia.
Why are We Concerned? n Increasing countries/areas with avian influenza – Uncertainties on progress of control n Ongoing human infection with avian H 5 N 1 – Limited implementation of protective measures n Co-Circulating human influenza viruses – Risk of genetic reassortment leading to pandemic strain n n Majority of human population would have no immunity H 5 N 1 resembles the 1918 -19 pandemic influenza in that healthy young persons are affected and a deadly feature is a primary viral pneumonia.
 Human Infections n H 5 N 1 - severe – – n 1997 Hong Kong: 18 cases; 6 deaths 2003 Hong Kong: 2 cases; 1 death 2004 Vietnam and Thailand: 40 cases; 29 deaths 2005 Hunan (China): 3 cases, 2 deaths H 9 N 2 - mild – 1999 Hong Kong: 2 cases (mild) – 2003 Hong Kong: 1 case (mild)
Human Infections n H 5 N 1 - severe – – n 1997 Hong Kong: 18 cases; 6 deaths 2003 Hong Kong: 2 cases; 1 death 2004 Vietnam and Thailand: 40 cases; 29 deaths 2005 Hunan (China): 3 cases, 2 deaths H 9 N 2 - mild – 1999 Hong Kong: 2 cases (mild) – 2003 Hong Kong: 1 case (mild)
 17 Human Cases 12 Deaths 94 Human Cases 42 Deaths 4 Human Cases 4 Deaths 4 Human case 3 Deaths CIDRAP, 8/2005
17 Human Cases 12 Deaths 94 Human Cases 42 Deaths 4 Human Cases 4 Deaths 4 Human case 3 Deaths CIDRAP, 8/2005
 Influenza H 5 N 1: expanded host range? n n Domestic poultry Wild birds – infected – reservoir n n n Humans Swine (China) Cats? (Netherlands) The natural hosts of the influenza A virus
Influenza H 5 N 1: expanded host range? n n Domestic poultry Wild birds – infected – reservoir n n n Humans Swine (China) Cats? (Netherlands) The natural hosts of the influenza A virus
 People, Pigs and Poultry in China 1968 2004 People 790 million 1. 3 billion Pigs 5. 2 million 508 million 12. 3 million 13 billion Poultry
People, Pigs and Poultry in China 1968 2004 People 790 million 1. 3 billion Pigs 5. 2 million 508 million 12. 3 million 13 billion Poultry
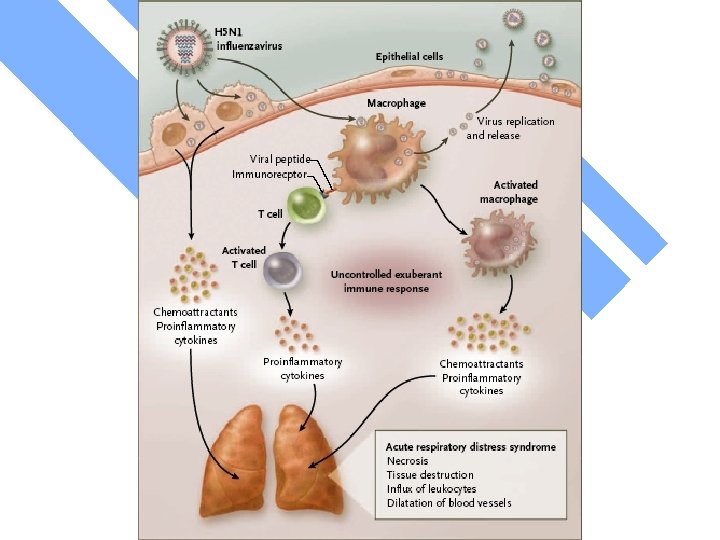
 Understanding Pandemic Influenza • Recent studies in mice using genetically engineered influenza strains similar to the 1918 H 1 N 1 pandemic strain suggest that macrophage activities with high levels of cytokine production maybe a factor in the lung and other organ damage (cytokine storm). Kobasa et al; Nature 2004; 431: 703 • The clinical picture and epidemiology, as well as current studies of H 5 N 1 cases in SE Asia suggest a similar “cytokine storm” phenomena. Peiris et al; Lancet 2004; 363: 617
Understanding Pandemic Influenza • Recent studies in mice using genetically engineered influenza strains similar to the 1918 H 1 N 1 pandemic strain suggest that macrophage activities with high levels of cytokine production maybe a factor in the lung and other organ damage (cytokine storm). Kobasa et al; Nature 2004; 431: 703 • The clinical picture and epidemiology, as well as current studies of H 5 N 1 cases in SE Asia suggest a similar “cytokine storm” phenomena. Peiris et al; Lancet 2004; 363: 617
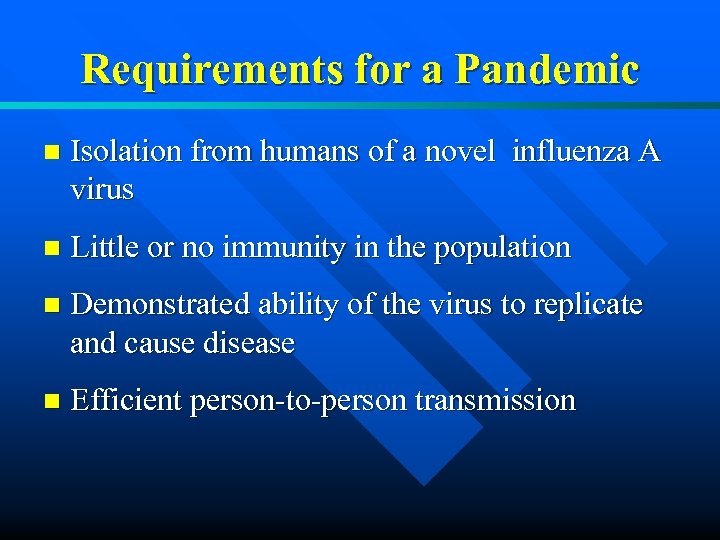 Requirements for a Pandemic n Isolation from humans of a novel influenza A virus n Little or no immunity in the population n Demonstrated ability of the virus to replicate and cause disease n Efficient person-to-person transmission
Requirements for a Pandemic n Isolation from humans of a novel influenza A virus n Little or no immunity in the population n Demonstrated ability of the virus to replicate and cause disease n Efficient person-to-person transmission
 H 5 N 1 All prerequisites met for start of pandemic except efficient human-to-human transmission n More pathogenic than most avian strains – Progressively more pathogenic in poultry – Larger number of animal species affected Ducks are asymptomatic but excrete virus, so sustainable reservoir n Pigs shown to be infected in China n Human cases concentrated in previously healthy children and young adults n
H 5 N 1 All prerequisites met for start of pandemic except efficient human-to-human transmission n More pathogenic than most avian strains – Progressively more pathogenic in poultry – Larger number of animal species affected Ducks are asymptomatic but excrete virus, so sustainable reservoir n Pigs shown to be infected in China n Human cases concentrated in previously healthy children and young adults n
 National Level Command Control for Pandemic Influenza Lead Departments: Medical Response: DHHS Veterinary Response: Dept. Agriculture International Activities: State Department Overall domestic incident management and federal coordination is through the Department of Homeland Security.
National Level Command Control for Pandemic Influenza Lead Departments: Medical Response: DHHS Veterinary Response: Dept. Agriculture International Activities: State Department Overall domestic incident management and federal coordination is through the Department of Homeland Security.
 Criteria for success of initial control Rapid identification of initial cluster n Rapid case detection, isolation, and treatment n Rapid prophylaxis of targeted population n – Sufficient drug available – No antiviral resistance School and work closures n Population cooperation with strategies After 4 -5 weeks, it will be impossible to contain n
Criteria for success of initial control Rapid identification of initial cluster n Rapid case detection, isolation, and treatment n Rapid prophylaxis of targeted population n – Sufficient drug available – No antiviral resistance School and work closures n Population cooperation with strategies After 4 -5 weeks, it will be impossible to contain n
 Containing an Initial Outbreak of Novel Influenza – Can this be done? n Hong Kong accomplished this in 1997 n 2004 H 5 N 1 situation much more challenging n Large areas affected in a large number of countries n Slow and incomplete reporting of H 5 N 1 findings n Poor public health infrastructure n Complex political and economic situations n International action required: support for antivirals
Containing an Initial Outbreak of Novel Influenza – Can this be done? n Hong Kong accomplished this in 1997 n 2004 H 5 N 1 situation much more challenging n Large areas affected in a large number of countries n Slow and incomplete reporting of H 5 N 1 findings n Poor public health infrastructure n Complex political and economic situations n International action required: support for antivirals
 WHO phase of Pandemic Alert Phase 1: Inter-pandemic phase: Low risk of human cases Phase 2: New virus in animals, no human cases: Higher risk of human cases Phase 3: Pandemic Alert, New Virus causes human cases: No or very limited human-to-human transmission Phase 4: Pandemic Alert, New virus causes human cases: Evidence of increased human-to-human transmission Phase 5: Pandemic Alert, New virus causes human cases: Evidence of significant human-to-human transmission Phase 6: PANDEMIC: Efficient and sustained human-to-human transmission According to WHO, the world is presently in phase 3.
WHO phase of Pandemic Alert Phase 1: Inter-pandemic phase: Low risk of human cases Phase 2: New virus in animals, no human cases: Higher risk of human cases Phase 3: Pandemic Alert, New Virus causes human cases: No or very limited human-to-human transmission Phase 4: Pandemic Alert, New virus causes human cases: Evidence of increased human-to-human transmission Phase 5: Pandemic Alert, New virus causes human cases: Evidence of significant human-to-human transmission Phase 6: PANDEMIC: Efficient and sustained human-to-human transmission According to WHO, the world is presently in phase 3.
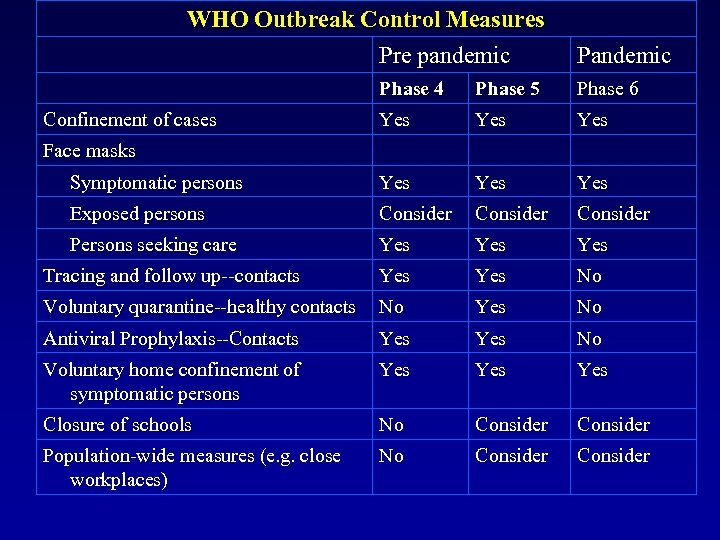 WHO Outbreak Control Measures Pre pandemic Phase 4 Phase 5 Phase 6 Yes Yes Symptomatic persons Yes Yes Exposed persons Consider Persons seeking care Yes Yes Tracing and follow up--contacts Yes No Voluntary quarantine--healthy contacts No Yes No Antiviral Prophylaxis--Contacts Yes No Voluntary home confinement of symptomatic persons Yes Yes Closure of schools No Consider Population-wide measures (e. g. close workplaces) No Consider Confinement of cases Face masks
WHO Outbreak Control Measures Pre pandemic Phase 4 Phase 5 Phase 6 Yes Yes Symptomatic persons Yes Yes Exposed persons Consider Persons seeking care Yes Yes Tracing and follow up--contacts Yes No Voluntary quarantine--healthy contacts No Yes No Antiviral Prophylaxis--Contacts Yes No Voluntary home confinement of symptomatic persons Yes Yes Closure of schools No Consider Population-wide measures (e. g. close workplaces) No Consider Confinement of cases Face masks
 U. S. , State, and Local Control Measures n Currently under development
U. S. , State, and Local Control Measures n Currently under development
 Problems Anticipated in Surge Capacity n Beds – Emergency regulations, increase in beds in existing facilities, alternative facilities, tents, home care n Personnel – Medical Reserve Corps; citizens volunteers; scope of practice changes n n n Equipment/Infection Control Supplies Vaccine Medications
Problems Anticipated in Surge Capacity n Beds – Emergency regulations, increase in beds in existing facilities, alternative facilities, tents, home care n Personnel – Medical Reserve Corps; citizens volunteers; scope of practice changes n n n Equipment/Infection Control Supplies Vaccine Medications
 Vaccine Crisis • Current “standard” vaccine reflects 1950’s technology – grown in chicken eggs – takes 6 months or more to produce – use of “reverse genetics” to develop prototype vaccine virus • Need an immediate and comprehensive international program to develop a cell culture system for vaccine production with surge capacity
Vaccine Crisis • Current “standard” vaccine reflects 1950’s technology – grown in chicken eggs – takes 6 months or more to produce – use of “reverse genetics” to develop prototype vaccine virus • Need an immediate and comprehensive international program to develop a cell culture system for vaccine production with surge capacity
 Influenza Vaccine Crisis • Current annual international capacity for influenza vaccine production using egg culture is approximately 300 million trivalent doses (900 million monovalent) • Almost all of the world’s influenza vaccine is produced in nine countries (12% of the world’s population) • Production capacity will NOT increase significantly in the next several years • New and more timely methods for production are desperately needed and
Influenza Vaccine Crisis • Current annual international capacity for influenza vaccine production using egg culture is approximately 300 million trivalent doses (900 million monovalent) • Almost all of the world’s influenza vaccine is produced in nine countries (12% of the world’s population) • Production capacity will NOT increase significantly in the next several years • New and more timely methods for production are desperately needed and
 Prototype Vaccine n n n Hungary has developed and tested a H 5 N 1 vaccine on 150 persons Claims it is effective “beyond doubt” Set to be approved by European Medicines Agency Sold at $5 -6 per dose Ready to begin production LIMITATION: In that vaccine must be developed based upon pandemic strain
Prototype Vaccine n n n Hungary has developed and tested a H 5 N 1 vaccine on 150 persons Claims it is effective “beyond doubt” Set to be approved by European Medicines Agency Sold at $5 -6 per dose Ready to begin production LIMITATION: In that vaccine must be developed based upon pandemic strain
 Advisory Committee Recommendation for Vaccine n Federal government should purchase all influenza vaccine during a pandemic H 5 N 1 Vaccine for U. S. Stockpile Sanofi Pasteur $100 million for H 5 N 1 vaccine n Chiron 10, 000 doses for testing n HHS plans to buy vaccine for 20 million n Med. Immune developing multiple subtypes n
Advisory Committee Recommendation for Vaccine n Federal government should purchase all influenza vaccine during a pandemic H 5 N 1 Vaccine for U. S. Stockpile Sanofi Pasteur $100 million for H 5 N 1 vaccine n Chiron 10, 000 doses for testing n HHS plans to buy vaccine for 20 million n Med. Immune developing multiple subtypes n
 Antiviral Stockpile (oseltamivir) Recommedations of Advisory Group n 40 million courses minimum – 133 million courses to treat all infected and prophylaxis HCWs and patients at highest risk of infection Status of Antivirals for U. S. Stockpile n n n Currently 2 million courses of Tamiflu Orders for 6 million courses Plans for 20 million courses Glaxo. Smith. Kline $2. 8 million for 84, 300 treatment courses of zanamivir (Relenza) Request for funds to cover up to 50% of population
Antiviral Stockpile (oseltamivir) Recommedations of Advisory Group n 40 million courses minimum – 133 million courses to treat all infected and prophylaxis HCWs and patients at highest risk of infection Status of Antivirals for U. S. Stockpile n n n Currently 2 million courses of Tamiflu Orders for 6 million courses Plans for 20 million courses Glaxo. Smith. Kline $2. 8 million for 84, 300 treatment courses of zanamivir (Relenza) Request for funds to cover up to 50% of population
 Antivirals – Not A Panacea n n n n Global production capacity limited; high cost Ability to use antivirals to limit spread depends on rapid case detection and contact tracing Need to start treatment early Effectiveness on serious illnesses and mortality unknown Prophylaxis may require ongoing use for 6 weeks or longer Antiviral resistance and side effects may limit use Tamiflu produced outside of U. S.
Antivirals – Not A Panacea n n n n Global production capacity limited; high cost Ability to use antivirals to limit spread depends on rapid case detection and contact tracing Need to start treatment early Effectiveness on serious illnesses and mortality unknown Prophylaxis may require ongoing use for 6 weeks or longer Antiviral resistance and side effects may limit use Tamiflu produced outside of U. S.
 Ten Things to Know About Pandemic Influenza 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Pandemic Influenza is different from Avian Influenza pandemics are recurring events The world may be on the brink of another pandemic All countries will be affected Widespread illness will occur Medical supplies will be inadequate Large numbers of deaths will occur Economic and social disruption will be great Every country must be prepared WHO will alert the world when the pandemic threat increases
Ten Things to Know About Pandemic Influenza 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Pandemic Influenza is different from Avian Influenza pandemics are recurring events The world may be on the brink of another pandemic All countries will be affected Widespread illness will occur Medical supplies will be inadequate Large numbers of deaths will occur Economic and social disruption will be great Every country must be prepared WHO will alert the world when the pandemic threat increases
 For the first time mankind is watching a potential pandemic unfolding. World Health Organization
For the first time mankind is watching a potential pandemic unfolding. World Health Organization


