c253096e5a0e9d17a47f7ecef8dc582b.ppt
- Количество слайдов: 80
 Air Pollution Chapter 19
Air Pollution Chapter 19
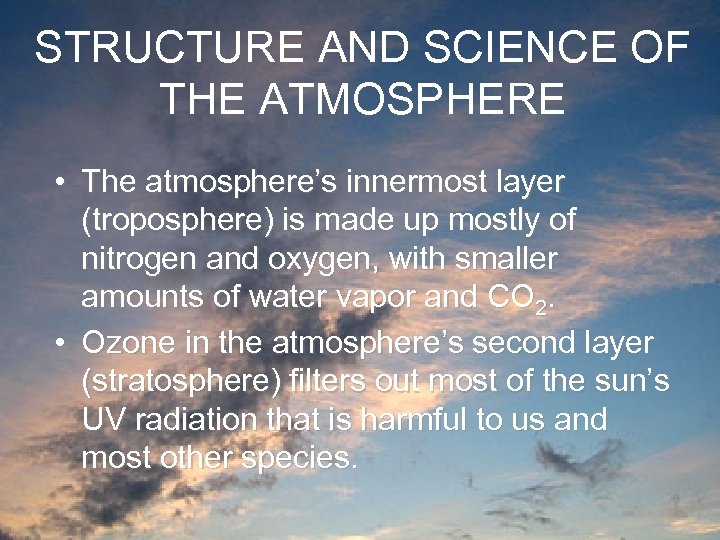 STRUCTURE AND SCIENCE OF THE ATMOSPHERE • The atmosphere’s innermost layer (troposphere) is made up mostly of nitrogen and oxygen, with smaller amounts of water vapor and CO 2. • Ozone in the atmosphere’s second layer (stratosphere) filters out most of the sun’s UV radiation that is harmful to us and most other species.
STRUCTURE AND SCIENCE OF THE ATMOSPHERE • The atmosphere’s innermost layer (troposphere) is made up mostly of nitrogen and oxygen, with smaller amounts of water vapor and CO 2. • Ozone in the atmosphere’s second layer (stratosphere) filters out most of the sun’s UV radiation that is harmful to us and most other species.
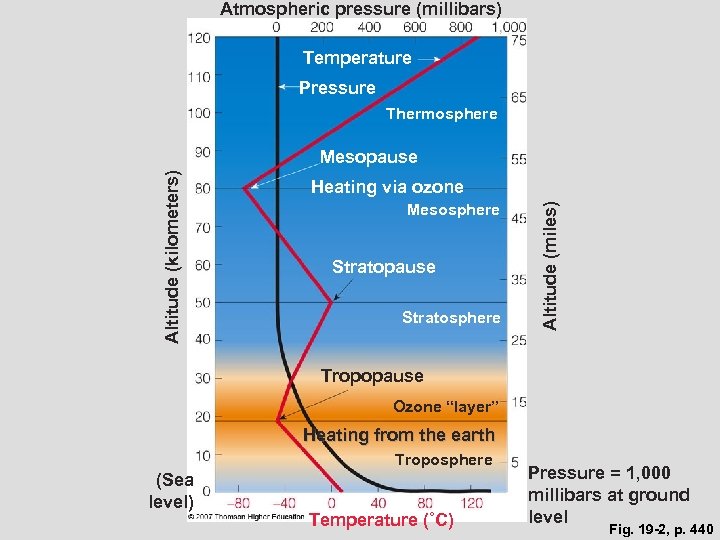 Atmospheric pressure (millibars) Temperature Pressure Thermosphere Heating via ozone Mesosphere Stratopause Stratosphere Altitude (miles) Altitude (kilometers) Mesopause Tropopause Ozone “layer” Heating from the earth Troposphere (Sea level) Temperature (˚C) Pressure = 1, 000 millibars at ground level Fig. 19 -2, p. 440
Atmospheric pressure (millibars) Temperature Pressure Thermosphere Heating via ozone Mesosphere Stratopause Stratosphere Altitude (miles) Altitude (kilometers) Mesopause Tropopause Ozone “layer” Heating from the earth Troposphere (Sea level) Temperature (˚C) Pressure = 1, 000 millibars at ground level Fig. 19 -2, p. 440
 Pollution • The term “Smog” (smoke and fog) was first used in 1905 to describe sulfur dioxide emission • Smog is a kind of air pollution; the word "smog" is a blend of smoke and fog. Classic smog results from large amounts of coal burning in an area and is caused by a mixture of smoke and sulfur dioxide.
Pollution • The term “Smog” (smoke and fog) was first used in 1905 to describe sulfur dioxide emission • Smog is a kind of air pollution; the word "smog" is a blend of smoke and fog. Classic smog results from large amounts of coal burning in an area and is caused by a mixture of smoke and sulfur dioxide.
 Clean Air Act • Originally signed 1963 – Individual states controlled standards • 1970 – Uniform Standards by Federal Govt. – Criteria Pollutants • Primary – Released directly into the air • Secondary – Formed from chemical reactions of the primary pollutants
Clean Air Act • Originally signed 1963 – Individual states controlled standards • 1970 – Uniform Standards by Federal Govt. – Criteria Pollutants • Primary – Released directly into the air • Secondary – Formed from chemical reactions of the primary pollutants
 Clean Air Act • 1990 version – Acid rain, urban smog, toxic air pollutants, ozone depletion, marketing pollution rights, VOC’s (Volatile Organic Compounds) • 1997 version – Reduced ambient ozone levels – Cost $15 billion/year -> save 15, 000 lives – Reduce bronchitis cases by 60, 000 per year – Reduce hospital respiratory admission 9000/year
Clean Air Act • 1990 version – Acid rain, urban smog, toxic air pollutants, ozone depletion, marketing pollution rights, VOC’s (Volatile Organic Compounds) • 1997 version – Reduced ambient ozone levels – Cost $15 billion/year -> save 15, 000 lives – Reduce bronchitis cases by 60, 000 per year – Reduce hospital respiratory admission 9000/year
 Air Pollution • Presence of chemicals in atmosphere that affects climate and living organisms. 2 TYPES: 1. Outdoor pollution 2. Indoor pollution Air pollution in China
Air Pollution • Presence of chemicals in atmosphere that affects climate and living organisms. 2 TYPES: 1. Outdoor pollution 2. Indoor pollution Air pollution in China
 Categories of Pollutants • Primary pollutants- emitted directly into troposphere. - ex: CO, soot, SO 2 • Secondary pollutants- formed from primary pollutants combining with air. - ex: sulfuric acid, sulfur trioxide
Categories of Pollutants • Primary pollutants- emitted directly into troposphere. - ex: CO, soot, SO 2 • Secondary pollutants- formed from primary pollutants combining with air. - ex: sulfuric acid, sulfur trioxide
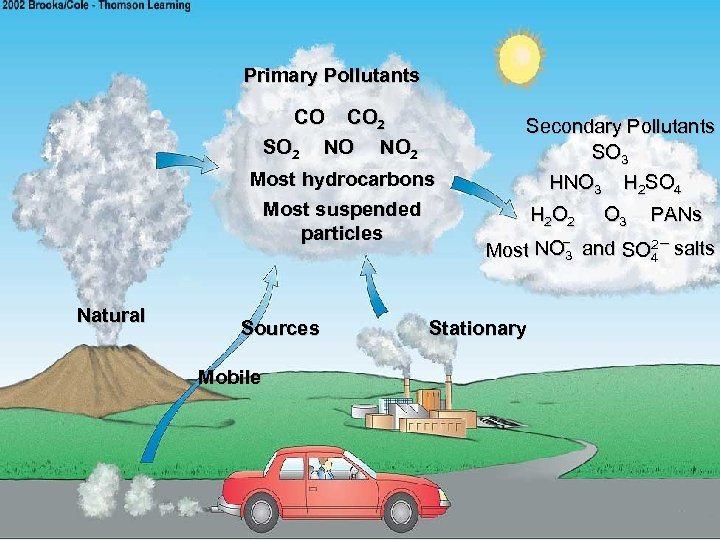 Primary Pollutants CO CO 2 SO 2 NO NO 2 Most hydrocarbons Most suspended particles Natural Sources Mobile Secondary Pollutants SO 3 HNO 3 H 2 SO 4 H 2 O 3 PANs – Most NO 3 and SO 2 – salts 4 Stationary
Primary Pollutants CO CO 2 SO 2 NO NO 2 Most hydrocarbons Most suspended particles Natural Sources Mobile Secondary Pollutants SO 3 HNO 3 H 2 SO 4 H 2 O 3 PANs – Most NO 3 and SO 2 – salts 4 Stationary
 Human Impact on Atmosphere Production of fertilizer and burning of fossil fuels: § Adds CO and O 3 to troposphere § Produces acid rain § Releases NO, NO 2, N 2 O, and NH 3 into troposphere § Releases SO 2 into troposphere § Releases toxic heavy metals (lead and arsenic) into troposphere Air pollution in Brazil
Human Impact on Atmosphere Production of fertilizer and burning of fossil fuels: § Adds CO and O 3 to troposphere § Produces acid rain § Releases NO, NO 2, N 2 O, and NH 3 into troposphere § Releases SO 2 into troposphere § Releases toxic heavy metals (lead and arsenic) into troposphere Air pollution in Brazil
 Criteria Air Pollutants • EPA uses these "criteria pollutants" as indicators of air quality 1. 2. 3. 4. 5. 6. 7. • Nitrogen Oxides: NO 2, N 2 O, NO Ozone: ground level O 3 Carbon oxides: CO, CO 2 Metals and halogens: Pb, Hg, As, CFCs Suspended Particulate Matter: dust, ash, soot, smoke, pollen, spores, asbestos fibers Sulfur oxides: SO 2, SO 3 Volatile Organic Compounds (VOCs): methane, benzene, toluene, formaldehyde EPA established concentrations levels on above substances because they contribute the largest volume of air-quality degradation and are the most serious threat to human health
Criteria Air Pollutants • EPA uses these "criteria pollutants" as indicators of air quality 1. 2. 3. 4. 5. 6. 7. • Nitrogen Oxides: NO 2, N 2 O, NO Ozone: ground level O 3 Carbon oxides: CO, CO 2 Metals and halogens: Pb, Hg, As, CFCs Suspended Particulate Matter: dust, ash, soot, smoke, pollen, spores, asbestos fibers Sulfur oxides: SO 2, SO 3 Volatile Organic Compounds (VOCs): methane, benzene, toluene, formaldehyde EPA established concentrations levels on above substances because they contribute the largest volume of air-quality degradation and are the most serious threat to human health
 Nitrogen Oxides (NOx) • Properties: Nitrogen oxides (NOx) forms when nitrogen and oxygen gas in air react at the highcombustion temperatures in automobile engines and coal-burning plants. NOx can also form from lightning and certain soil bacteria. • Effects: acid rain, lung and heart problems, decreased visibility (yellow haze), suppresses plant growth • Class: Nitrogen oxides (NOx) – NO reacts with air to form NO 2. – NO 2 reacts with water vapor in the air to form nitric acid (HNO 3) and nitrate salts (NO -), which are components of acid deposition.
Nitrogen Oxides (NOx) • Properties: Nitrogen oxides (NOx) forms when nitrogen and oxygen gas in air react at the highcombustion temperatures in automobile engines and coal-burning plants. NOx can also form from lightning and certain soil bacteria. • Effects: acid rain, lung and heart problems, decreased visibility (yellow haze), suppresses plant growth • Class: Nitrogen oxides (NOx) – NO reacts with air to form NO 2. – NO 2 reacts with water vapor in the air to form nitric acid (HNO 3) and nitrate salts (NO -), which are components of acid deposition.
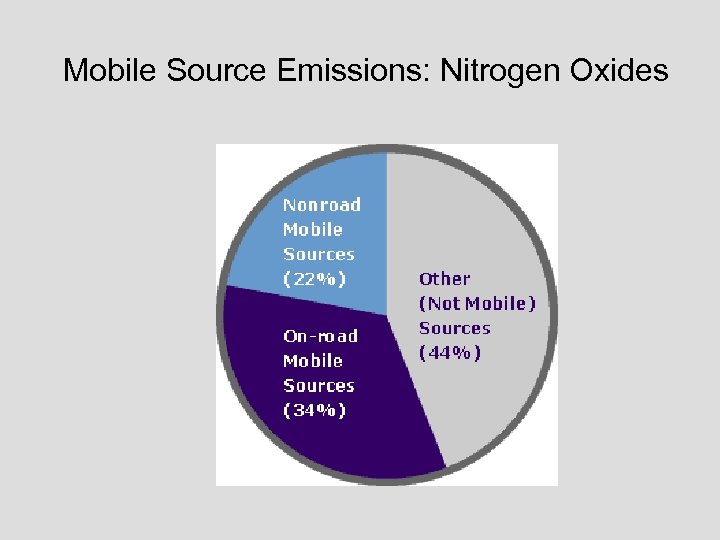 Mobile Source Emissions: Nitrogen Oxides
Mobile Source Emissions: Nitrogen Oxides
 Ozone (O 3) • Properties: colorless, unpleasant odor, major part of photochemical smog • Effects: lung irritant, damages plants • Sources: Created by sunlight acting on NOx and VOC , photocopiers, cars, industry, gas vapors, chemical solvents, incomplete fuel combustion products • Class: photochemical oxidants
Ozone (O 3) • Properties: colorless, unpleasant odor, major part of photochemical smog • Effects: lung irritant, damages plants • Sources: Created by sunlight acting on NOx and VOC , photocopiers, cars, industry, gas vapors, chemical solvents, incomplete fuel combustion products • Class: photochemical oxidants
 Ozone (O 3) • Is a highly reactive gas that is a major component of photochemical smog. • 10, 000 to 15, 000 people in US admitted to hospitals each year due to ozone-related illness • Children more susceptible – Airways narrower – More time spent outdoors • It can – Cause and aggravate respiratory illness. – Can aggravate heart disease. – Damage plants, rubber in tires, fabrics, and paints.
Ozone (O 3) • Is a highly reactive gas that is a major component of photochemical smog. • 10, 000 to 15, 000 people in US admitted to hospitals each year due to ozone-related illness • Children more susceptible – Airways narrower – More time spent outdoors • It can – Cause and aggravate respiratory illness. – Can aggravate heart disease. – Damage plants, rubber in tires, fabrics, and paints.
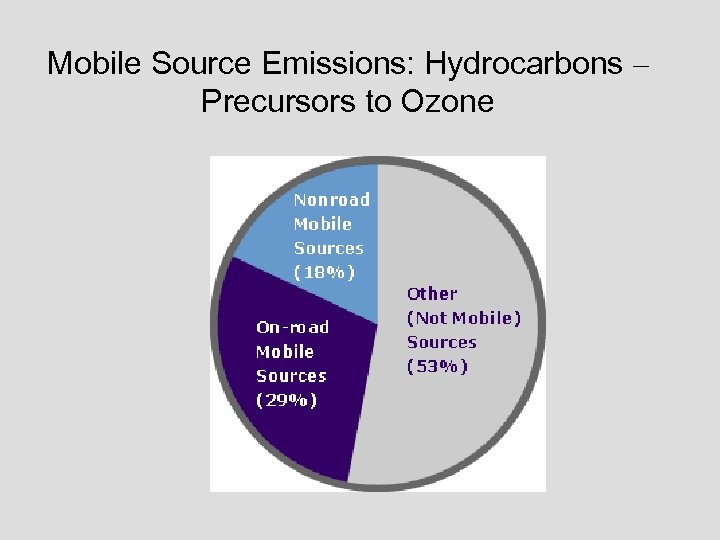 Mobile Source Emissions: Hydrocarbons – Precursors to Ozone
Mobile Source Emissions: Hydrocarbons – Precursors to Ozone
 Carbon Monoxide (CO) • Properties: colorless, odorless, heavier than air • Effects: binds tighter to hemoglobin than O 2, so body can’t remove it; person asphyxiates or suffers diminished mental functions and vision • Sources: incomplete combustion of fossil fuels 60 95% from auto exhaust (can also occur inside homes from incomplete combustion in gas furnaces) • Class: carbon oxides (CO 2, CO) • 5. 5 billion tons enter atmosphere/year
Carbon Monoxide (CO) • Properties: colorless, odorless, heavier than air • Effects: binds tighter to hemoglobin than O 2, so body can’t remove it; person asphyxiates or suffers diminished mental functions and vision • Sources: incomplete combustion of fossil fuels 60 95% from auto exhaust (can also occur inside homes from incomplete combustion in gas furnaces) • Class: carbon oxides (CO 2, CO) • 5. 5 billion tons enter atmosphere/year
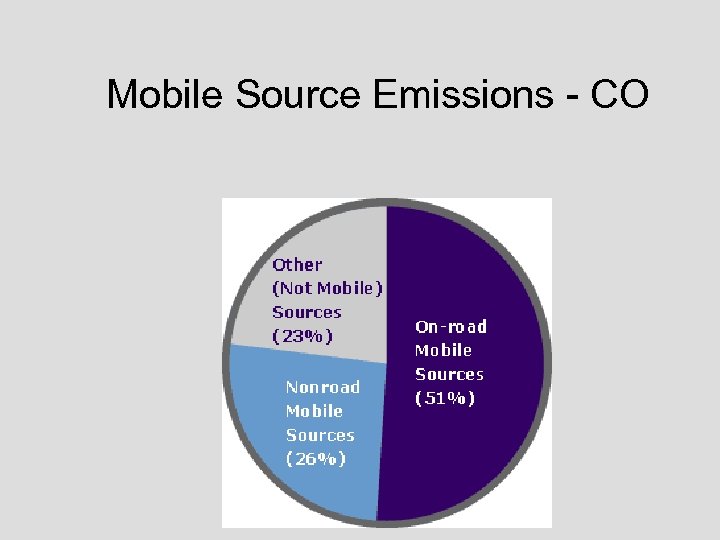 Mobile Source Emissions - CO
Mobile Source Emissions - CO
 Lead (Pb) • Properties: grayish metal • Effects: accumulates in tissue; affects kidneys, liver and nervous system (children most susceptible); mental retardation; possible carcinogen • Sources: paint, smelters (metal refineries), batteries • Class: toxic or heavy metals
Lead (Pb) • Properties: grayish metal • Effects: accumulates in tissue; affects kidneys, liver and nervous system (children most susceptible); mental retardation; possible carcinogen • Sources: paint, smelters (metal refineries), batteries • Class: toxic or heavy metals
 Suspended Particulate Matter (SPM) • Properties: Consists of a variety of solid particles and liquid droplets small and light enough to remain suspended in the air. The most harmful forms of SPM are fine particles (PM-10, with an average diameter < 10 micrometers) and ultrafine particles (PM-2. 5). • Effects: lung damage, carcinogenic, asthma • Sources: burning coal or diesel, volcanoes, factories, unpaved roads, plowing, lint, pollen, spores, burning fields – According to the EPA, SPM is responsible for about 60, 000 premature deaths a year in the U. S.
Suspended Particulate Matter (SPM) • Properties: Consists of a variety of solid particles and liquid droplets small and light enough to remain suspended in the air. The most harmful forms of SPM are fine particles (PM-10, with an average diameter < 10 micrometers) and ultrafine particles (PM-2. 5). • Effects: lung damage, carcinogenic, asthma • Sources: burning coal or diesel, volcanoes, factories, unpaved roads, plowing, lint, pollen, spores, burning fields – According to the EPA, SPM is responsible for about 60, 000 premature deaths a year in the U. S.
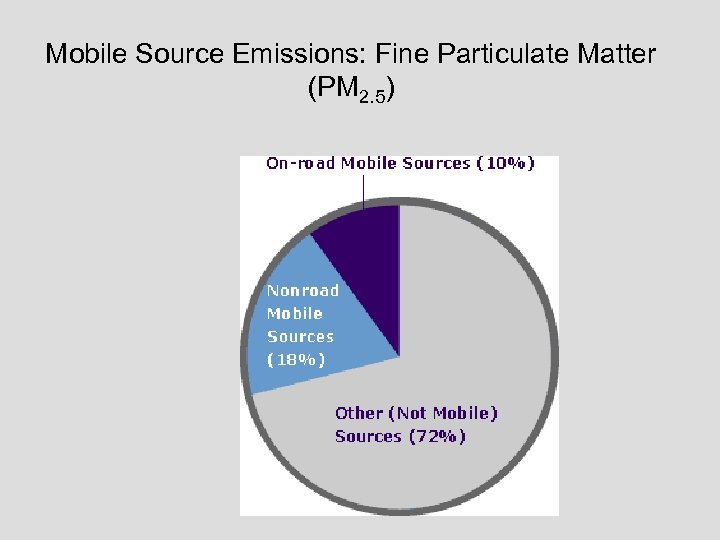 Mobile Source Emissions: Fine Particulate Matter (PM 2. 5)
Mobile Source Emissions: Fine Particulate Matter (PM 2. 5)
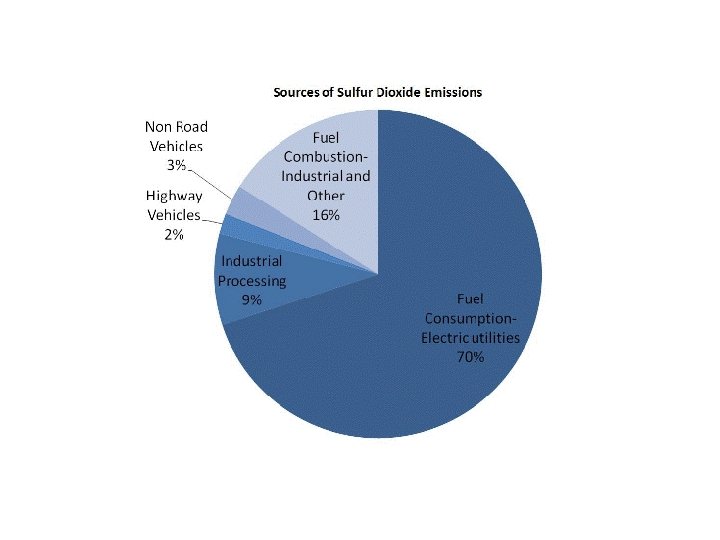
 Sulfur Dioxide (SO 2) and Sulfuric Acid • Properties: colorless gas with irritating odor. S combines with O 2 to form SO 2 • Effects: produces acid rain (H 2 SO 4), breathing difficulties, eutrophication due to sulfate formation; growth abnormalities in lichen and moss are indicators • Sources: burning high sulfur coal or oil, industrial processes contributes 2/3 of existing sulfur dioxide • Class: sulfur oxides
Sulfur Dioxide (SO 2) and Sulfuric Acid • Properties: colorless gas with irritating odor. S combines with O 2 to form SO 2 • Effects: produces acid rain (H 2 SO 4), breathing difficulties, eutrophication due to sulfate formation; growth abnormalities in lichen and moss are indicators • Sources: burning high sulfur coal or oil, industrial processes contributes 2/3 of existing sulfur dioxide • Class: sulfur oxides
 VOCs (Volatile Organic Compounds) • Properties: organic compounds (hydrocarbons) that evaporate easily, usually aromatic. Most are hydrocarbons emitted by the leaves of many plants and methane. • Effects: eye and respiratory irritants; carcinogenic; liver, CNS, or kidney damage; damages plants; lowered visibility due to brown haze; global warming • Sources: vehicles (largest source), evaporation of solvents or fossil fuels, aerosols, paint thinners, dry cleaning • Other VOCs include industrial solvents such as trichlorethylene (TCE), benzene, and vinyl chloride. – Long-term exposure to benzene can cause cancer, blood disorders, and immune system damage.
VOCs (Volatile Organic Compounds) • Properties: organic compounds (hydrocarbons) that evaporate easily, usually aromatic. Most are hydrocarbons emitted by the leaves of many plants and methane. • Effects: eye and respiratory irritants; carcinogenic; liver, CNS, or kidney damage; damages plants; lowered visibility due to brown haze; global warming • Sources: vehicles (largest source), evaporation of solvents or fossil fuels, aerosols, paint thinners, dry cleaning • Other VOCs include industrial solvents such as trichlorethylene (TCE), benzene, and vinyl chloride. – Long-term exposure to benzene can cause cancer, blood disorders, and immune system damage.
 CO 2 as a pollutant? • The Environmental Protection Agency in April, 2009 formally declared carbon dioxide and five other heat-trapping gases to be pollutants that endanger public health and welfare • New ruling from EPA limits CO 2 emissions from new power plants to 1000 lbs/m. Wh.
CO 2 as a pollutant? • The Environmental Protection Agency in April, 2009 formally declared carbon dioxide and five other heat-trapping gases to be pollutants that endanger public health and welfare • New ruling from EPA limits CO 2 emissions from new power plants to 1000 lbs/m. Wh.
 Formation of Pollutants Factors impacting include: • Local climate (inversions, air pressure, temperature, humidity) • Topography (hills and mountains) • Population density • Amount of industry • Fuels used by population and industry for heating, manufacturing, transportation, power • Weather: rain, snow, wind • Economics
Formation of Pollutants Factors impacting include: • Local climate (inversions, air pressure, temperature, humidity) • Topography (hills and mountains) • Population density • Amount of industry • Fuels used by population and industry for heating, manufacturing, transportation, power • Weather: rain, snow, wind • Economics
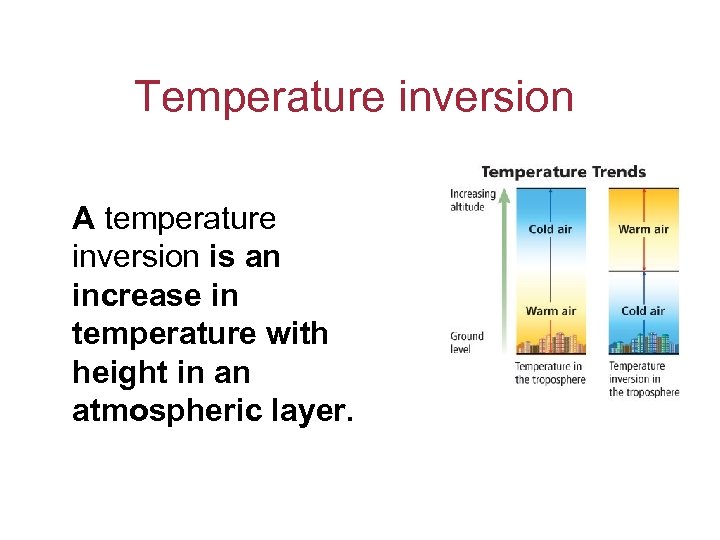 Section 11. 2 Properties of the Atmosphere Temperature inversion A temperature inversion is an increase in temperature with height in an atmospheric layer.
Section 11. 2 Properties of the Atmosphere Temperature inversion A temperature inversion is an increase in temperature with height in an atmospheric layer.
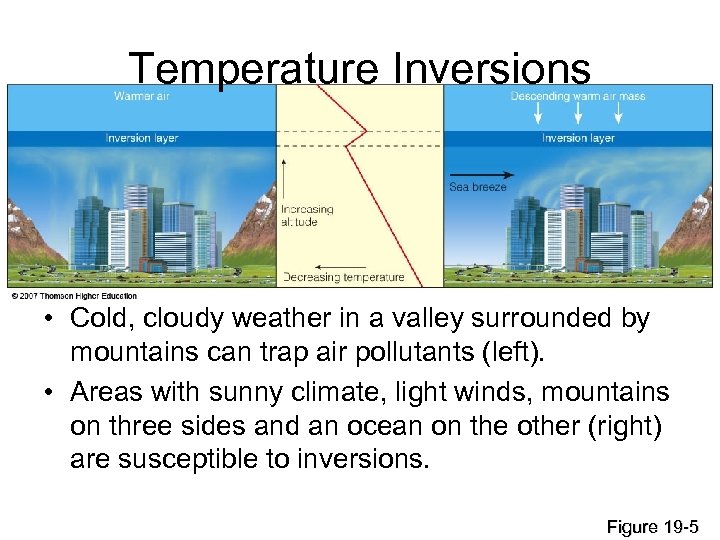 Temperature Inversions • Cold, cloudy weather in a valley surrounded by mountains can trap air pollutants (left). • Areas with sunny climate, light winds, mountains on three sides and an ocean on the other (right) are susceptible to inversions. Figure 19 -5
Temperature Inversions • Cold, cloudy weather in a valley surrounded by mountains can trap air pollutants (left). • Areas with sunny climate, light winds, mountains on three sides and an ocean on the other (right) are susceptible to inversions. Figure 19 -5
 Industrial Smog • Industrial smog is a mixture of sulfur dioxide, droplets of sulfuric acid, and a variety of suspended solid particles emitted mostly by burning coal. – In most developed countries where coal and heavy oil is burned, industrial smog is not a problem due to reasonably good pollution control or with tall smokestacks that transfer the pollutant to rural areas. • We see this brownish haze on sunny, warm, and dry days.
Industrial Smog • Industrial smog is a mixture of sulfur dioxide, droplets of sulfuric acid, and a variety of suspended solid particles emitted mostly by burning coal. – In most developed countries where coal and heavy oil is burned, industrial smog is not a problem due to reasonably good pollution control or with tall smokestacks that transfer the pollutant to rural areas. • We see this brownish haze on sunny, warm, and dry days.
 Sunlight plus Cars Equals Photochemical Smog • Photochemical smog is a mixture of air pollutants formed by the reaction of nitrogen oxides and volatile organic hydrocarbons under the influence of sunlight.
Sunlight plus Cars Equals Photochemical Smog • Photochemical smog is a mixture of air pollutants formed by the reaction of nitrogen oxides and volatile organic hydrocarbons under the influence of sunlight.
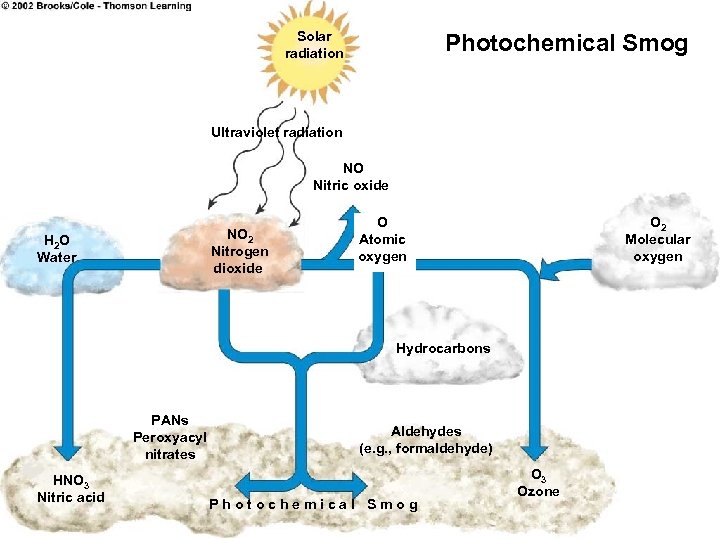 Solar radiation Photochemical Smog Ultraviolet radiation NO Nitric oxide NO 2 Nitrogen dioxide H 2 O Water O Atomic oxygen O 2 Molecular oxygen Hydrocarbons PANs Peroxyacyl nitrates HNO 3 Nitric acid Aldehydes (e. g. , formaldehyde) Photochemical Smog O 3 Ozone
Solar radiation Photochemical Smog Ultraviolet radiation NO Nitric oxide NO 2 Nitrogen dioxide H 2 O Water O Atomic oxygen O 2 Molecular oxygen Hydrocarbons PANs Peroxyacyl nitrates HNO 3 Nitric acid Aldehydes (e. g. , formaldehyde) Photochemical Smog O 3 Ozone
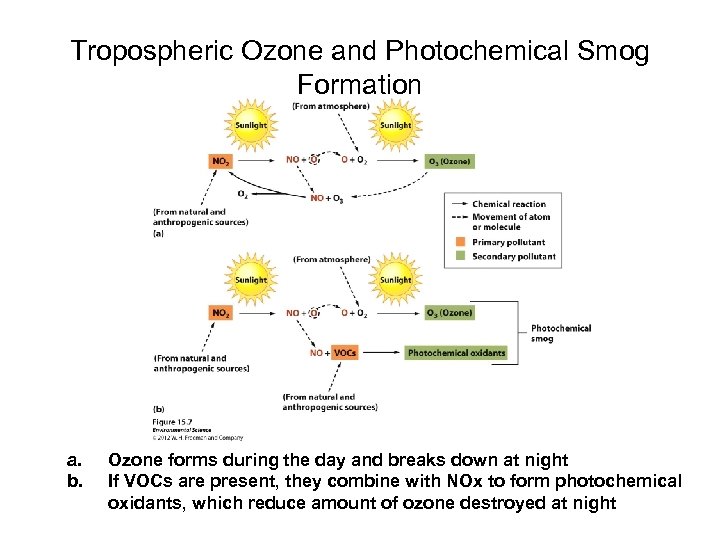 Tropospheric Ozone and Photochemical Smog Formation a. b. Ozone forms during the day and breaks down at night If VOCs are present, they combine with NOx to form photochemical oxidants, which reduce amount of ozone destroyed at night
Tropospheric Ozone and Photochemical Smog Formation a. b. Ozone forms during the day and breaks down at night If VOCs are present, they combine with NOx to form photochemical oxidants, which reduce amount of ozone destroyed at night
 Photochemical Smog Denver Los Angeles
Photochemical Smog Denver Los Angeles
 Smog effects are worse in summer Justin Lampley, 2008 Stone Mountain, GA Mady Scolnick, October 2008
Smog effects are worse in summer Justin Lampley, 2008 Stone Mountain, GA Mady Scolnick, October 2008
 Factors Influencing Levels of Outdoor Air Pollution • Outdoor air pollution can be reduced by: – settling out, precipitation, sea spray, winds, and chemical reactions. • Outdoor air pollution can be increased by: – urban buildings (slow wind dispersal of pollutants), mountains (promote temperature inversions), and high temperatures (promote photochemical reactions).
Factors Influencing Levels of Outdoor Air Pollution • Outdoor air pollution can be reduced by: – settling out, precipitation, sea spray, winds, and chemical reactions. • Outdoor air pollution can be increased by: – urban buildings (slow wind dispersal of pollutants), mountains (promote temperature inversions), and high temperatures (promote photochemical reactions).
 Acid Deposition/Acid Rain • Deposition is the deposition of wet acidic solutions or dry acidic particles from the air • Results from the burning of coal. • SO 2 and NO 2 released via smoke stacks. • Smoke stacks reduce local air pollution, but increase regional pollution downwind. • Industrial areas are bathed in acidic solutions (fog, light precipitation) with a p. H ranging from 23.
Acid Deposition/Acid Rain • Deposition is the deposition of wet acidic solutions or dry acidic particles from the air • Results from the burning of coal. • SO 2 and NO 2 released via smoke stacks. • Smoke stacks reduce local air pollution, but increase regional pollution downwind. • Industrial areas are bathed in acidic solutions (fog, light precipitation) with a p. H ranging from 23.
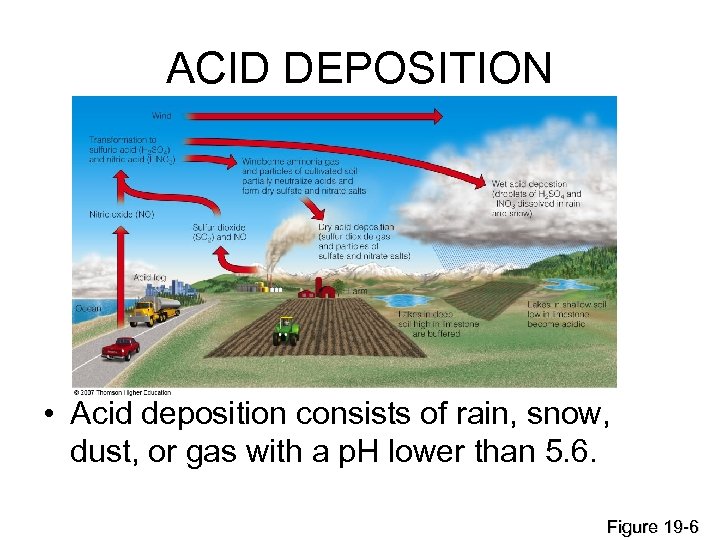 ACID DEPOSITION • Acid deposition consists of rain, snow, dust, or gas with a p. H lower than 5. 6. Figure 19 -6
ACID DEPOSITION • Acid deposition consists of rain, snow, dust, or gas with a p. H lower than 5. 6. Figure 19 -6
 ACID DEPOSITION • Sulfur dioxides, nitrogen oxides, and particulates can react in the atmosphere to produce acidic chemicals that can travel long distances before returning to the earth’s surface. – Tall smokestacks reduce local air pollution but can increase regional air pollution.
ACID DEPOSITION • Sulfur dioxides, nitrogen oxides, and particulates can react in the atmosphere to produce acidic chemicals that can travel long distances before returning to the earth’s surface. – Tall smokestacks reduce local air pollution but can increase regional air pollution.
 Acid Rain • It can damage statues, buildings, metals, and car finishes. • It can damage tree foliage. The areas hardest hit by acid deposition are mountaintop forests, which tend to have thin soils without much buffering capacity. • A combination of acid deposition and other air pollutants can make trees more susceptible to stresses such as cold temperatures, diseases, insects, drought, and fungi.
Acid Rain • It can damage statues, buildings, metals, and car finishes. • It can damage tree foliage. The areas hardest hit by acid deposition are mountaintop forests, which tend to have thin soils without much buffering capacity. • A combination of acid deposition and other air pollutants can make trees more susceptible to stresses such as cold temperatures, diseases, insects, drought, and fungi.
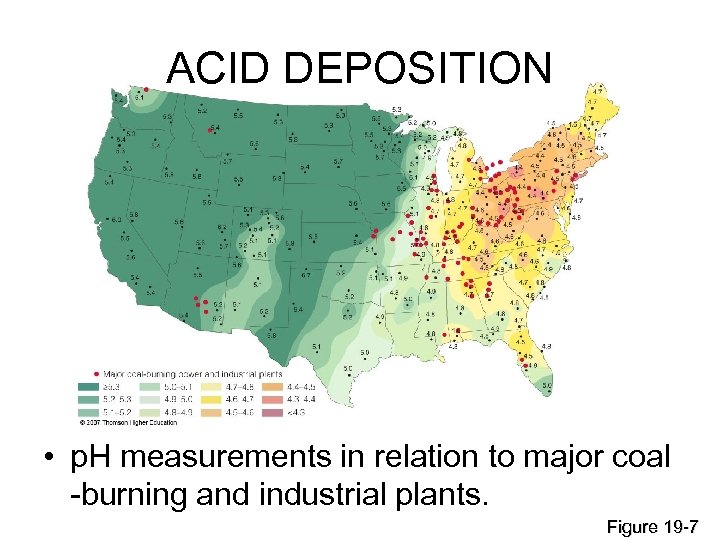 ACID DEPOSITION • p. H measurements in relation to major coal -burning and industrial plants. Figure 19 -7
ACID DEPOSITION • p. H measurements in relation to major coal -burning and industrial plants. Figure 19 -7
 ACID DEPOSITION • Acid deposition contributes to chronic respiratory disease and can leach toxic metals (such as lead and mercury) from soils and rocks into acidic lakes used as sources for drinking water.
ACID DEPOSITION • Acid deposition contributes to chronic respiratory disease and can leach toxic metals (such as lead and mercury) from soils and rocks into acidic lakes used as sources for drinking water.
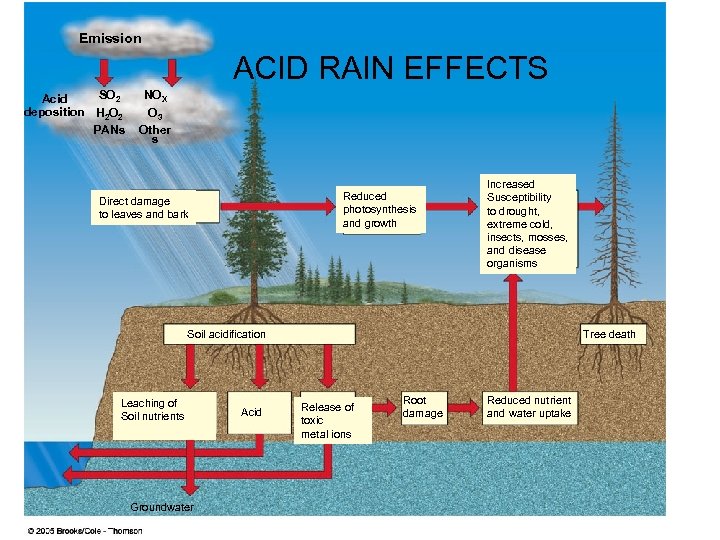 Emission ACID RAIN EFFECTS SO 2 Acid deposition H 2 O 2 PANs NOX O 3 Other s Reduced photosynthesis and growth Direct damage to leaves and bark Increased Susceptibility to drought, extreme cold, insects, mosses, and disease organisms Soil acidification Leaching of Soil nutrients Groundwater Acid Tree death Release of toxic metal ions Root damage Reduced nutrient and water uptake
Emission ACID RAIN EFFECTS SO 2 Acid deposition H 2 O 2 PANs NOX O 3 Other s Reduced photosynthesis and growth Direct damage to leaves and bark Increased Susceptibility to drought, extreme cold, insects, mosses, and disease organisms Soil acidification Leaching of Soil nutrients Groundwater Acid Tree death Release of toxic metal ions Root damage Reduced nutrient and water uptake
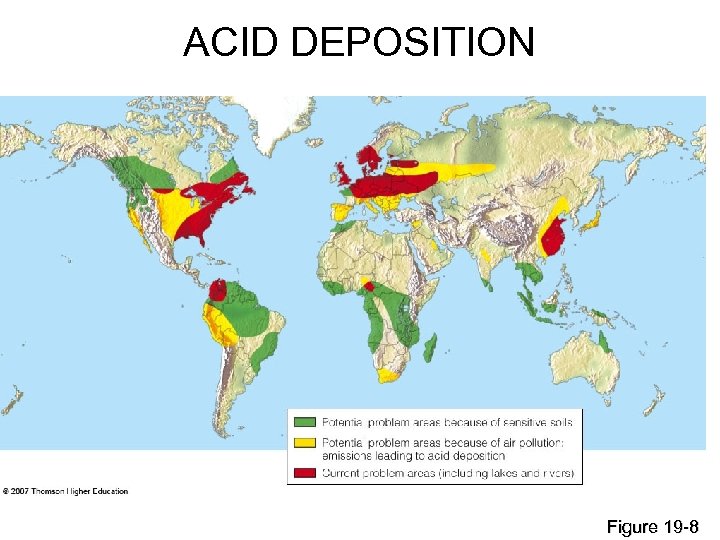 ACID DEPOSITION Figure 19 -8
ACID DEPOSITION Figure 19 -8
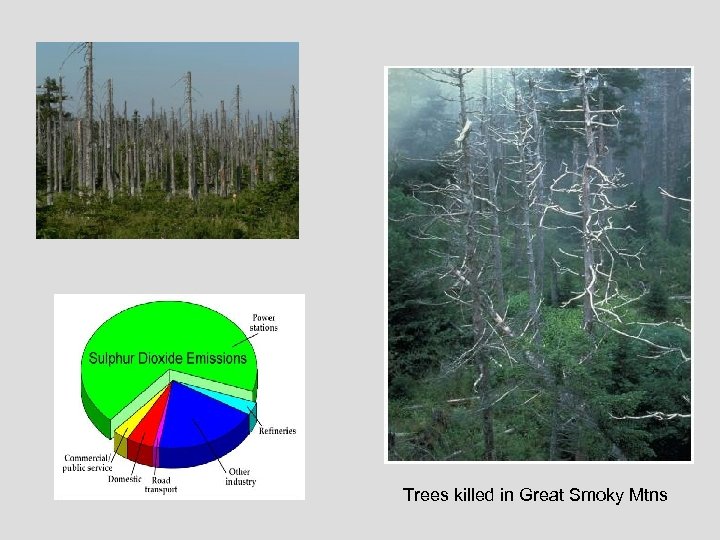 Trees killed in Great Smoky Mtns
Trees killed in Great Smoky Mtns
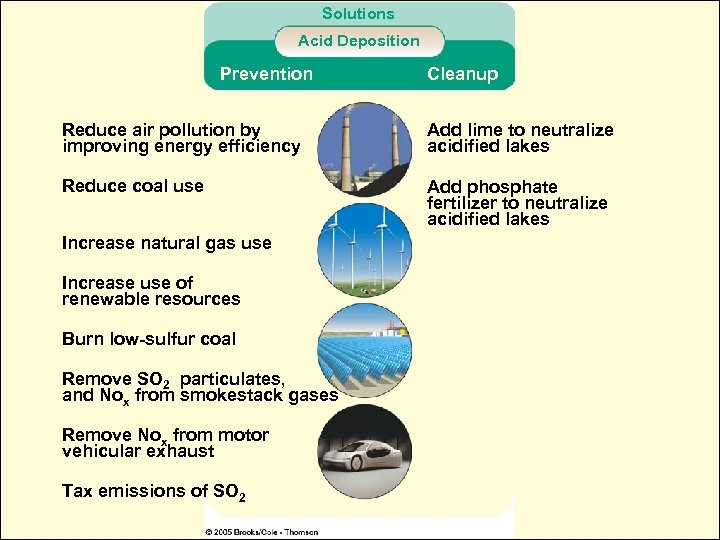 Solutions Acid Deposition Prevention Cleanup Reduce air pollution by improving energy efficiency Add lime to neutralize acidified lakes Reduce coal use Add phosphate fertilizer to neutralize acidified lakes Increase natural gas use Increase use of renewable resources Burn low-sulfur coal Remove SO 2 particulates, and Nox from smokestack gases Remove Nox from motor vehicular exhaust Tax emissions of SO 2
Solutions Acid Deposition Prevention Cleanup Reduce air pollution by improving energy efficiency Add lime to neutralize acidified lakes Reduce coal use Add phosphate fertilizer to neutralize acidified lakes Increase natural gas use Increase use of renewable resources Burn low-sulfur coal Remove SO 2 particulates, and Nox from smokestack gases Remove Nox from motor vehicular exhaust Tax emissions of SO 2
 Outdoor Pollution REDUCTION FACTORS 1. Precipitation (rain/snow) 2. Salty sea spray from oceans 3. Winds INCREASING FACTORS 1. Urban Buildings 2. Mountains and hills 3. High temperatures 4. Grasshopper Effectlong distance travel of pollutants- from country to country via wind
Outdoor Pollution REDUCTION FACTORS 1. Precipitation (rain/snow) 2. Salty sea spray from oceans 3. Winds INCREASING FACTORS 1. Urban Buildings 2. Mountains and hills 3. High temperatures 4. Grasshopper Effectlong distance travel of pollutants- from country to country via wind
 Solutions: Reducing Outdoor Air Pollution • There a number of ways to prevent and control air pollution from coal-burning facilities. – Electrostatic precipitator: are used to attract negatively charged particles in a smokestack into a collector. – Wet scrubber: fine mists of water vapor trap particulates and convert them to a sludge that is collected and disposed of usually in a landfill.
Solutions: Reducing Outdoor Air Pollution • There a number of ways to prevent and control air pollution from coal-burning facilities. – Electrostatic precipitator: are used to attract negatively charged particles in a smokestack into a collector. – Wet scrubber: fine mists of water vapor trap particulates and convert them to a sludge that is collected and disposed of usually in a landfill.
 Electrostatic Precipitator • Can remove 99% of particulate matter • Does not remove hazardous ultrafine particles. • Produces toxic dust that must be safely disposed of. • Uses large amounts of electricity Figure 19 -18
Electrostatic Precipitator • Can remove 99% of particulate matter • Does not remove hazardous ultrafine particles. • Produces toxic dust that must be safely disposed of. • Uses large amounts of electricity Figure 19 -18
 Wet Scrubber • Can remove 98% of SO 2 and particulate matter. • Not very effective in removing hazardous fine and ultrafine particles. Figure 19 -18
Wet Scrubber • Can remove 98% of SO 2 and particulate matter. • Not very effective in removing hazardous fine and ultrafine particles. Figure 19 -18
 Air Pollution Control • Sulfur removal (flue gas scrubbers) – Device that removes sulfur dioxide (SO 2) and nitric oxide (NO) from exhaust gas in industrial processes. There are two types • Wet: use a chemical solvent—lime, limestone, sodium alkali, or diluted sulfuric acid—to remove the SO 2 formed during chemical reactions. • Dry: a lime/limestone mixture or ammonia is sprayed into the flue gases.
Air Pollution Control • Sulfur removal (flue gas scrubbers) – Device that removes sulfur dioxide (SO 2) and nitric oxide (NO) from exhaust gas in industrial processes. There are two types • Wet: use a chemical solvent—lime, limestone, sodium alkali, or diluted sulfuric acid—to remove the SO 2 formed during chemical reactions. • Dry: a lime/limestone mixture or ammonia is sprayed into the flue gases.
 Solutions: Reducing Outdoor Air Pollution • There a number of ways to prevent and control air pollution from motor vehicles. – Because of the Clean Air Act, a new car today in the U. S. emits 75% less pollution than did pre-1970 cars. – There is an increase in motor vehicle use in developing countries and many have no pollution control devices and burn leaded gasoline.
Solutions: Reducing Outdoor Air Pollution • There a number of ways to prevent and control air pollution from motor vehicles. – Because of the Clean Air Act, a new car today in the U. S. emits 75% less pollution than did pre-1970 cars. – There is an increase in motor vehicle use in developing countries and many have no pollution control devices and burn leaded gasoline.
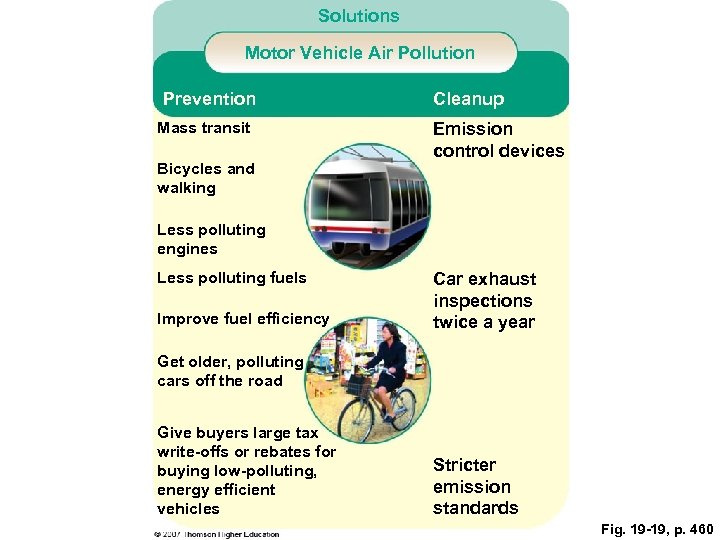 Solutions Motor Vehicle Air Pollution Prevention Mass transit Bicycles and walking Cleanup Emission control devices Less polluting engines Less polluting fuels Improve fuel efficiency Car exhaust inspections twice a year Get older, polluting cars off the road Give buyers large tax write-offs or rebates for buying low-polluting, energy efficient vehicles Stricter emission standards Fig. 19 -19, p. 460
Solutions Motor Vehicle Air Pollution Prevention Mass transit Bicycles and walking Cleanup Emission control devices Less polluting engines Less polluting fuels Improve fuel efficiency Car exhaust inspections twice a year Get older, polluting cars off the road Give buyers large tax write-offs or rebates for buying low-polluting, energy efficient vehicles Stricter emission standards Fig. 19 -19, p. 460
 INDOOR AIR POLLUTION • Indoor air pollution usually is a greater threat to human health than outdoor air pollution. • According to the EPA, the four most dangerous indoor air pollutants in developed countries are: – Tobacco smoke. – Formaldehyde. – Radioactive radon-222 gas. – Very small fine and ultrafine particles.
INDOOR AIR POLLUTION • Indoor air pollution usually is a greater threat to human health than outdoor air pollution. • According to the EPA, the four most dangerous indoor air pollutants in developed countries are: – Tobacco smoke. – Formaldehyde. – Radioactive radon-222 gas. – Very small fine and ultrafine particles.
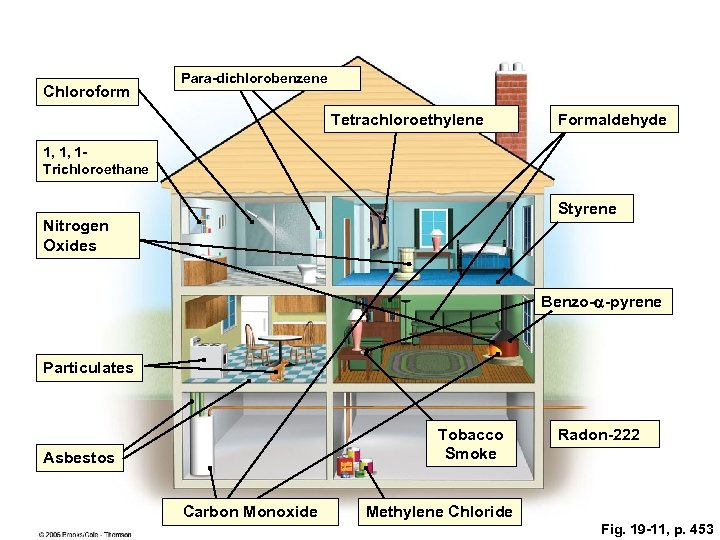 Chloroform Para-dichlorobenzene Tetrachloroethylene Formaldehyde 1, 1, 1 Trichloroethane Styrene Nitrogen Oxides Benzo-a-pyrene Particulates Tobacco Smoke Asbestos Carbon Monoxide Radon-222 Methylene Chloride Fig. 19 -11, p. 453
Chloroform Para-dichlorobenzene Tetrachloroethylene Formaldehyde 1, 1, 1 Trichloroethane Styrene Nitrogen Oxides Benzo-a-pyrene Particulates Tobacco Smoke Asbestos Carbon Monoxide Radon-222 Methylene Chloride Fig. 19 -11, p. 453
 Why is indoor air quality important? • 70 to 90% of time spent indoors, mostly at home • Many significant pollution sources in the home (e. g. gas cookers, paints and glues) • Especially important for susceptible groups – e. g. the sick, old and very young
Why is indoor air quality important? • 70 to 90% of time spent indoors, mostly at home • Many significant pollution sources in the home (e. g. gas cookers, paints and glues) • Especially important for susceptible groups – e. g. the sick, old and very young
 Sources of Indoor Air Pollutants • • • Building materials Furniture Furnishings and fabrics Glues Cleaning products Other consumer products Combustion appliances (cookers and heaters) Open fires Tobacco smoking Cooking House dust mites, bacteria and molds Outdoor air
Sources of Indoor Air Pollutants • • • Building materials Furniture Furnishings and fabrics Glues Cleaning products Other consumer products Combustion appliances (cookers and heaters) Open fires Tobacco smoking Cooking House dust mites, bacteria and molds Outdoor air
 Important Indoor Air Pollutants • • • Nitrogen dioxide Carbon monoxide Formaldehyde Volatile Organic Compounds (VOCs) House dust mites (and other allergens, e. g. from pets) Environmental tobacco smoke Fine particles Chlorinated organic compounds (e. g. pesticides) Asbestos and man-made mineral fibres Radon
Important Indoor Air Pollutants • • • Nitrogen dioxide Carbon monoxide Formaldehyde Volatile Organic Compounds (VOCs) House dust mites (and other allergens, e. g. from pets) Environmental tobacco smoke Fine particles Chlorinated organic compounds (e. g. pesticides) Asbestos and man-made mineral fibres Radon
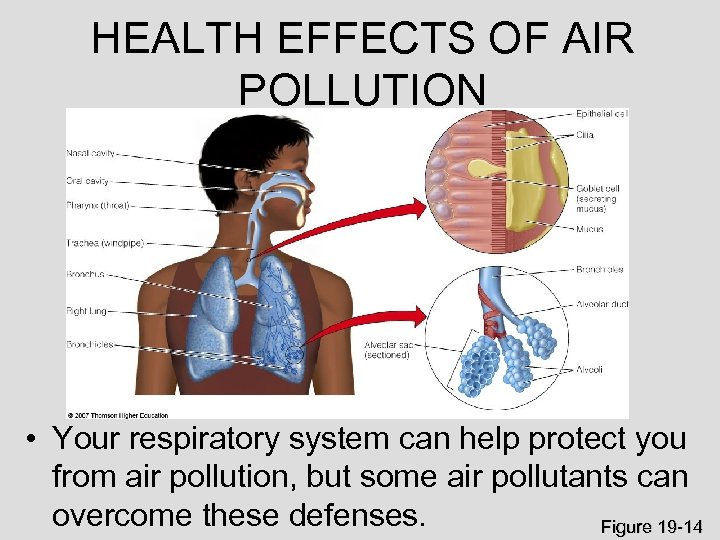 HEALTH EFFECTS OF AIR POLLUTION • Your respiratory system can help protect you from air pollution, but some air pollutants can overcome these defenses. Figure 19 -14
HEALTH EFFECTS OF AIR POLLUTION • Your respiratory system can help protect you from air pollution, but some air pollutants can overcome these defenses. Figure 19 -14
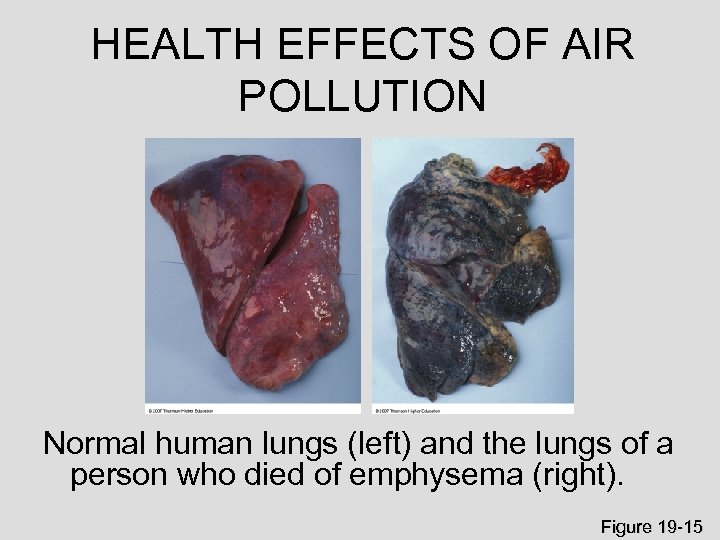 HEALTH EFFECTS OF AIR POLLUTION Normal human lungs (left) and the lungs of a person who died of emphysema (right). Figure 19 -15
HEALTH EFFECTS OF AIR POLLUTION Normal human lungs (left) and the lungs of a person who died of emphysema (right). Figure 19 -15
 Air Pollution is a Big Killer • Each year, air pollution prematurely kills about 3 million people, mostly from indoor air pollution in developing countries. – In the U. S. , the EPA estimates that annual deaths related to indoor and outdoor air pollution range from 150, 000 to 350, 000. – According to the EPA, each year more than 125, 000 Americans get cancer from breathing diesel fumes.
Air Pollution is a Big Killer • Each year, air pollution prematurely kills about 3 million people, mostly from indoor air pollution in developing countries. – In the U. S. , the EPA estimates that annual deaths related to indoor and outdoor air pollution range from 150, 000 to 350, 000. – According to the EPA, each year more than 125, 000 Americans get cancer from breathing diesel fumes.
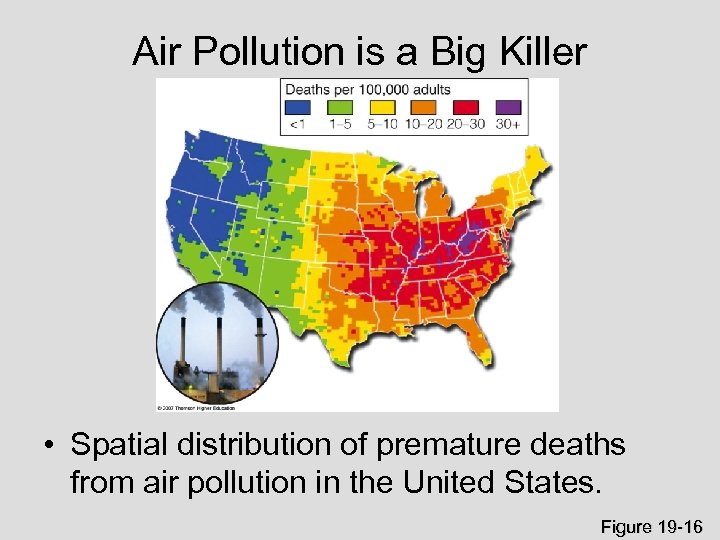 Air Pollution is a Big Killer • Spatial distribution of premature deaths from air pollution in the United States. Figure 19 -16
Air Pollution is a Big Killer • Spatial distribution of premature deaths from air pollution in the United States. Figure 19 -16
 PREVENTING AND REDUCING AIR POLLUTION • Environmental scientists point out several deficiencies in the Clean Air Act: – The U. S. continues to rely on cleanup rather than prevention. – The U. S. Congress has failed to increase fuelefficiency standards for automobiles. – Regulation of emissions from motorcycles and two-cycle engines remains inadequate. – There is little or no regulation of air pollution from oceangoing ships in American ports.
PREVENTING AND REDUCING AIR POLLUTION • Environmental scientists point out several deficiencies in the Clean Air Act: – The U. S. continues to rely on cleanup rather than prevention. – The U. S. Congress has failed to increase fuelefficiency standards for automobiles. – Regulation of emissions from motorcycles and two-cycle engines remains inadequate. – There is little or no regulation of air pollution from oceangoing ships in American ports.
 PREVENTING AND REDUCING AIR POLLUTION – Airports are exempt from many air pollution regulations. – The Act has failed to deal seriously with indoor air pollution. – There is a need for better enforcement of the Clean Air Act.
PREVENTING AND REDUCING AIR POLLUTION – Airports are exempt from many air pollution regulations. – The Act has failed to deal seriously with indoor air pollution. – There is a need for better enforcement of the Clean Air Act.
 Using the Marketplace to Reduce Outdoor Air Pollution • To help reduce SO 2 emissions, the Clean Air Act authorized and emission trading (cap-and-trade) program. – Enables the 110 most polluting power plants to buy and sell SO 2 pollution rights. – Between 1990 -2002, the emission trading system reduced emissions. – In 2002, the EPA reported the cap-and-trade system produced less emission reductions than were projected.
Using the Marketplace to Reduce Outdoor Air Pollution • To help reduce SO 2 emissions, the Clean Air Act authorized and emission trading (cap-and-trade) program. – Enables the 110 most polluting power plants to buy and sell SO 2 pollution rights. – Between 1990 -2002, the emission trading system reduced emissions. – In 2002, the EPA reported the cap-and-trade system produced less emission reductions than were projected.
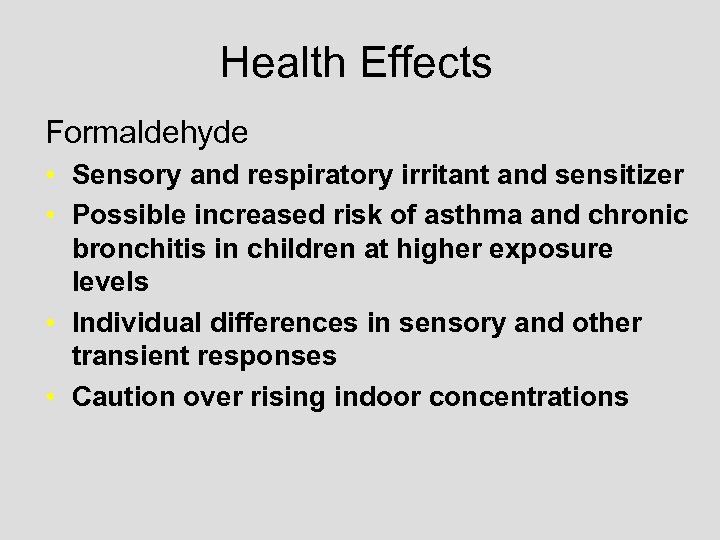 Health Effects Formaldehyde • Sensory and respiratory irritant and sensitizer • Possible increased risk of asthma and chronic bronchitis in children at higher exposure levels • Individual differences in sensory and other transient responses • Caution over rising indoor concentrations
Health Effects Formaldehyde • Sensory and respiratory irritant and sensitizer • Possible increased risk of asthma and chronic bronchitis in children at higher exposure levels • Individual differences in sensory and other transient responses • Caution over rising indoor concentrations
 Health Effects House dust mites • House dust mites produce Der p 1 allergen, a potent sensitizer • Good evidence of increased risk of sensitization with increasing allergen exposure, but this does not necessarily lead to asthma • Small reductions in exposure will not necessarily lead to reduced incidence and/or symptoms • Indoor humidity is important
Health Effects House dust mites • House dust mites produce Der p 1 allergen, a potent sensitizer • Good evidence of increased risk of sensitization with increasing allergen exposure, but this does not necessarily lead to asthma • Small reductions in exposure will not necessarily lead to reduced incidence and/or symptoms • Indoor humidity is important
 Health Effects Fungi and bacteria • Dampness and mold-growth linked to self-reported respiratory conditions, but little convincing evidence for association between measured airborne fungi and respiratory disease • Insufficient data to relate exposure to (nonpathogenic) bacteria to health effects in the indoor environment
Health Effects Fungi and bacteria • Dampness and mold-growth linked to self-reported respiratory conditions, but little convincing evidence for association between measured airborne fungi and respiratory disease • Insufficient data to relate exposure to (nonpathogenic) bacteria to health effects in the indoor environment
 Health Effects Environmental tobacco smoke (ETS) • Sudden infant death syndrome • Lower respiratory tract illness • Middle ear disease • Asthma 12+ million children exposed to secondhand smoke in homes
Health Effects Environmental tobacco smoke (ETS) • Sudden infant death syndrome • Lower respiratory tract illness • Middle ear disease • Asthma 12+ million children exposed to secondhand smoke in homes
 Radon • 55% of our exposure to radiation comes from radon. • colorless, tasteless, odorless gas • formed from the decay of uranium- 238 • found in nearly all soils • levels vary
Radon • 55% of our exposure to radiation comes from radon. • colorless, tasteless, odorless gas • formed from the decay of uranium- 238 • found in nearly all soils • levels vary
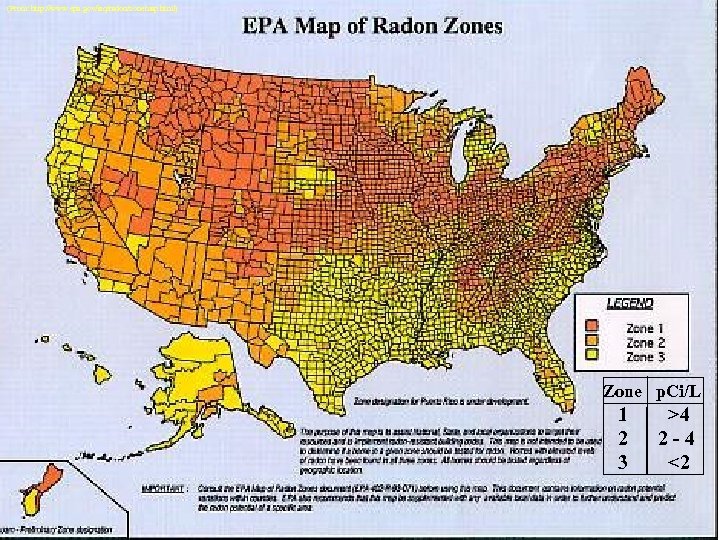 (From: http: //www. epa. gov/iaq/radon/zonemap. html) Zone p. Ci/L 1 2 3 >4 2 -4 <2
(From: http: //www. epa. gov/iaq/radon/zonemap. html) Zone p. Ci/L 1 2 3 >4 2 -4 <2
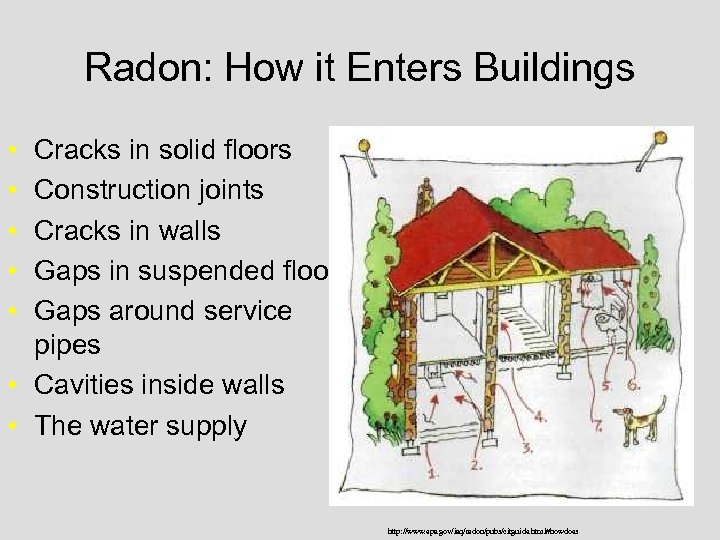 Radon: How it Enters Buildings • • • Cracks in solid floors Construction joints Cracks in walls Gaps in suspended floors Gaps around service pipes • Cavities inside walls • The water supply http: //www. epa. gov/iaq/radon/pubs/citguide. html#howdoes
Radon: How it Enters Buildings • • • Cracks in solid floors Construction joints Cracks in walls Gaps in suspended floors Gaps around service pipes • Cavities inside walls • The water supply http: //www. epa. gov/iaq/radon/pubs/citguide. html#howdoes
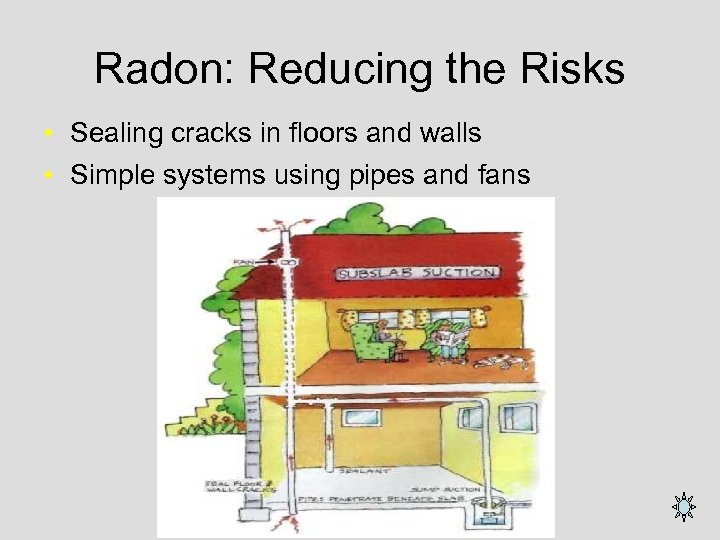 Radon: Reducing the Risks • Sealing cracks in floors and walls • Simple systems using pipes and fans
Radon: Reducing the Risks • Sealing cracks in floors and walls • Simple systems using pipes and fans
 Sick Building Syndrome (SBS) • Used to describe situations in which building occupants experience acute health and comfort effects that appear to be linked to time spent in a building, but no specific illness or cause can be identified. • Problems may result when a building is operated a manner that is inconsistent with its original design. Sometimes indoor air problems are a result of poor building design or occupant activities.
Sick Building Syndrome (SBS) • Used to describe situations in which building occupants experience acute health and comfort effects that appear to be linked to time spent in a building, but no specific illness or cause can be identified. • Problems may result when a building is operated a manner that is inconsistent with its original design. Sometimes indoor air problems are a result of poor building design or occupant activities.
 Indicators of SBS include: • Building occupants complain of symptoms associated with acute discomfort, e. g. , headache; eye, nose, or throat irritation; dry cough; dry or itchy skin; dizziness and nausea; difficulty in concentrating; fatigue; and sensitivity to odors. • The cause of the symptoms is not known. • Most of the complainants report relief soon after leaving the building. • EPA Headquarters is located in a sick building EPA Headquarters, Washington, DC
Indicators of SBS include: • Building occupants complain of symptoms associated with acute discomfort, e. g. , headache; eye, nose, or throat irritation; dry cough; dry or itchy skin; dizziness and nausea; difficulty in concentrating; fatigue; and sensitivity to odors. • The cause of the symptoms is not known. • Most of the complainants report relief soon after leaving the building. • EPA Headquarters is located in a sick building EPA Headquarters, Washington, DC
 Causes of Sick Building Syndrome • Inadequate ventilation • Chemical contaminants from indoor sources • Chemical contaminants from outdoor sources • Biological contaminants
Causes of Sick Building Syndrome • Inadequate ventilation • Chemical contaminants from indoor sources • Chemical contaminants from outdoor sources • Biological contaminants
 Solutions to Sick Building Syndrome • Pollutant source removal or modification • Increasing ventilation rates • Air cleaning • Education and communication
Solutions to Sick Building Syndrome • Pollutant source removal or modification • Increasing ventilation rates • Air cleaning • Education and communication
 Solutions Indoor Air Pollution Prevention Cleanup Cover ceiling tiles and lining of AC ducts to prevent release of mineral fibers Use adjustable fresh air vents for work spaces Ban smoking or limit it to wellventilated areas Increase intake of outside air Set stricter formaldehyde emissions standards for carpet, furniture, and building materials Change air more frequently Circulate building’s air through rooftop greenhouses Prevent radon infiltration Use office machines in wellventilated areas Use exhaust hoods for stoves and appliances burning natural gas Use less polluting substitutes for harmful cleaning agents, paints, and other products Install efficient chimneys for wood-burning stoves
Solutions Indoor Air Pollution Prevention Cleanup Cover ceiling tiles and lining of AC ducts to prevent release of mineral fibers Use adjustable fresh air vents for work spaces Ban smoking or limit it to wellventilated areas Increase intake of outside air Set stricter formaldehyde emissions standards for carpet, furniture, and building materials Change air more frequently Circulate building’s air through rooftop greenhouses Prevent radon infiltration Use office machines in wellventilated areas Use exhaust hoods for stoves and appliances burning natural gas Use less polluting substitutes for harmful cleaning agents, paints, and other products Install efficient chimneys for wood-burning stoves
 What Can You Do? Indoor Air Pollution • Test for radon and formaldehyde inside your home and take corrective measures as needed. • Do not buy furniture and other products containing formaldehyde. • Remove your shoes before entering your house to reduce inputs of dust, lead, and pesticides. • Test your house or workplace for asbestos fiber levels and for any crumbling asbestos materials if it was built before 1980. • Don't live in a pre-1980 house without having its indoor air tested for asbestos and lead. • Do not store gasoline, solvents, or other volatile hazardous chemicals inside a home or attached garage. • If you smoke, do it outside or in a closed room vented to the outside. • Make sure that wood-burning stoves, fireplaces, and kerosene- and gas-burning heaters are properly installed, vented, and maintained. • Install carbon monoxide detectors in all sleeping areas.
What Can You Do? Indoor Air Pollution • Test for radon and formaldehyde inside your home and take corrective measures as needed. • Do not buy furniture and other products containing formaldehyde. • Remove your shoes before entering your house to reduce inputs of dust, lead, and pesticides. • Test your house or workplace for asbestos fiber levels and for any crumbling asbestos materials if it was built before 1980. • Don't live in a pre-1980 house without having its indoor air tested for asbestos and lead. • Do not store gasoline, solvents, or other volatile hazardous chemicals inside a home or attached garage. • If you smoke, do it outside or in a closed room vented to the outside. • Make sure that wood-burning stoves, fireplaces, and kerosene- and gas-burning heaters are properly installed, vented, and maintained. • Install carbon monoxide detectors in all sleeping areas.
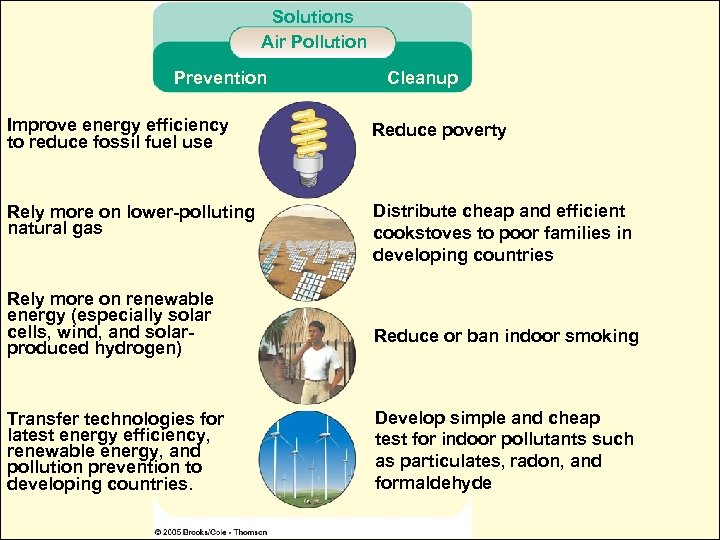 Solutions Air Pollution Prevention Cleanup Improve energy efficiency to reduce fossil fuel use Reduce poverty Rely more on lower-polluting natural gas Distribute cheap and efficient cookstoves to poor families in developing countries Rely more on renewable energy (especially solar cells, wind, and solarproduced hydrogen) Transfer technologies for latest energy efficiency, renewable energy, and pollution prevention to developing countries. Reduce or ban indoor smoking Develop simple and cheap test for indoor pollutants such as particulates, radon, and formaldehyde
Solutions Air Pollution Prevention Cleanup Improve energy efficiency to reduce fossil fuel use Reduce poverty Rely more on lower-polluting natural gas Distribute cheap and efficient cookstoves to poor families in developing countries Rely more on renewable energy (especially solar cells, wind, and solarproduced hydrogen) Transfer technologies for latest energy efficiency, renewable energy, and pollution prevention to developing countries. Reduce or ban indoor smoking Develop simple and cheap test for indoor pollutants such as particulates, radon, and formaldehyde


