85c05401aaf5048c313c2d151c247dfe.ppt
- Количество слайдов: 74
 Air Pollution Chapter 15
Air Pollution Chapter 15
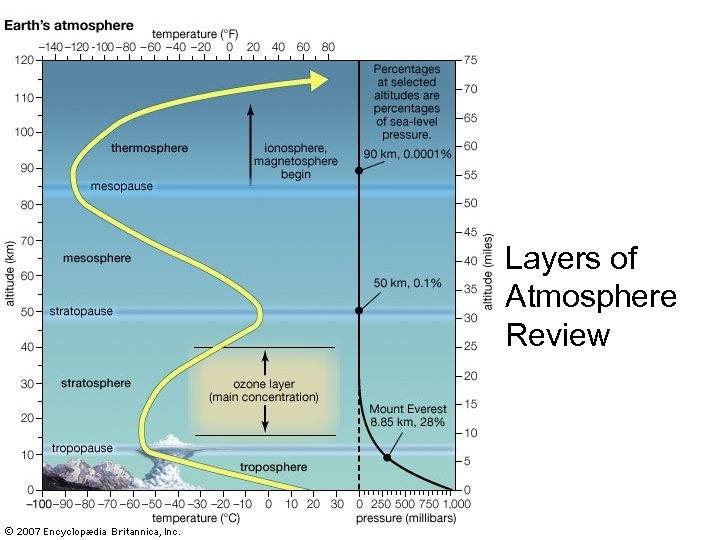 Layers of Atmosphere Review
Layers of Atmosphere Review
 Structure of the Atmosphere • Troposphere: – All weather confined here – Temp. decreases as altitude increases – 78% N 2 & 22% O 2 – Ozone (O 3) in troposphere = bad!! • Stratosphere: – Temp increases with altitude (temp inversion) – UV absorbing O 3 (ozone) = good!!! • UVA, UVB and UVC • sunburn, skin/eye cancer, cataracts • Keeps O 2 in troposphere
Structure of the Atmosphere • Troposphere: – All weather confined here – Temp. decreases as altitude increases – 78% N 2 & 22% O 2 – Ozone (O 3) in troposphere = bad!! • Stratosphere: – Temp increases with altitude (temp inversion) – UV absorbing O 3 (ozone) = good!!! • UVA, UVB and UVC • sunburn, skin/eye cancer, cataracts • Keeps O 2 in troposphere
 Air Pollution • Introduction of chemical, particulate matter, or microorganisms into atmosphere @ concentrations high enough to harm plants, animals, & alter ecosystems. – Refers to pollutants in troposphere (aka “Ground Level Pollution” • Most polluted areas: Asia (outdoor) & Asia, Africa, & SA (indoor) – Natural (fires & volcanoes) or anthropogenic (cars & factories) – Global system: atmosphere envelops the whole globe • Acid rain in W. Coast of US from Asia
Air Pollution • Introduction of chemical, particulate matter, or microorganisms into atmosphere @ concentrations high enough to harm plants, animals, & alter ecosystems. – Refers to pollutants in troposphere (aka “Ground Level Pollution” • Most polluted areas: Asia (outdoor) & Asia, Africa, & SA (indoor) – Natural (fires & volcanoes) or anthropogenic (cars & factories) – Global system: atmosphere envelops the whole globe • Acid rain in W. Coast of US from Asia
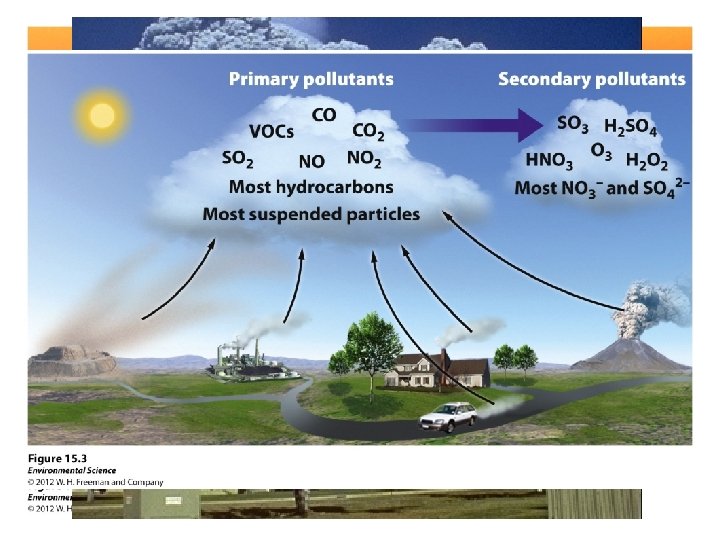
 Primary & Secondary Pollution • Primary: pollutants that come directly out of the smokestack, exhaust pipe, or natural emission source. – Ex: CO, CO 2, SO 2, NOX, suspended particulate matter & VOC • Secondary: primary pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. – Ex: Ozone (O 3), SO 42 -, NO 3– Reduce the primary pollutant to reduce the secondary pollutant.
Primary & Secondary Pollution • Primary: pollutants that come directly out of the smokestack, exhaust pipe, or natural emission source. – Ex: CO, CO 2, SO 2, NOX, suspended particulate matter & VOC • Secondary: primary pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. – Ex: Ozone (O 3), SO 42 -, NO 3– Reduce the primary pollutant to reduce the secondary pollutant.
 Major Air Pollutants • Sulfur dioxide (SO 2) or (SOx): – 1/3 SO 2 occurs naturally through the sulfur cycle. – 2/3 from human sources • Combustion of sulfur fuels (oil, coal, gasoline) – Respiratory Irritant, harms plant tissue, forms sulfuric acid – Secondary Pollution: • SO 2 + O 2 SO 3 • SO 3 + H 2 O H 2 SO 4 – Acid deposition – harms terrestrial and aquatic life
Major Air Pollutants • Sulfur dioxide (SO 2) or (SOx): – 1/3 SO 2 occurs naturally through the sulfur cycle. – 2/3 from human sources • Combustion of sulfur fuels (oil, coal, gasoline) – Respiratory Irritant, harms plant tissue, forms sulfuric acid – Secondary Pollution: • SO 2 + O 2 SO 3 • SO 3 + H 2 O H 2 SO 4 – Acid deposition – harms terrestrial and aquatic life
 Ø Nitrogen oxides (NOX) : l N 2 + O 2 2 NO l 2 NO + O 2 2 NO 2 • Combustion of fossil fuels, biomass • NO can also form from lightning and certain soil bacteria. l Respiratory irritant, precursor to ozone and nitric acid, NO 3 - (over fertilization of terrestrial and aquatic ecosystems) l Secondary: • 3 NO 2 + H 20 2 HNO 3 + NO • NO 2 +UV NO + O l O+O 2 O 3
Ø Nitrogen oxides (NOX) : l N 2 + O 2 2 NO l 2 NO + O 2 2 NO 2 • Combustion of fossil fuels, biomass • NO can also form from lightning and certain soil bacteria. l Respiratory irritant, precursor to ozone and nitric acid, NO 3 - (over fertilization of terrestrial and aquatic ecosystems) l Secondary: • 3 NO 2 + H 20 2 HNO 3 + NO • NO 2 +UV NO + O l O+O 2 O 3
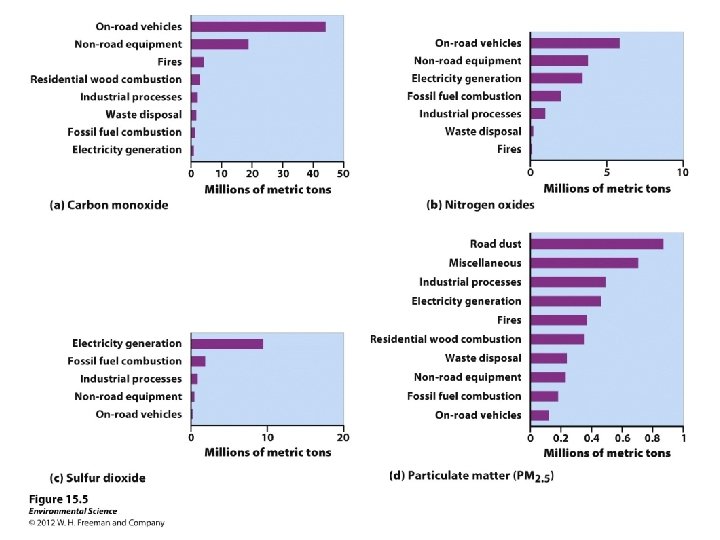
 • Carbon oxides: – Carbon monoxide (CO) • toxic gas (suffocation) • during the incomplete combustion of carbon compounds • cigarette smoke – Carbon dioxide (CO 2) • 93% natural carbon cycle • 7% of CO 2 from human activities (mostly burning fossil fuels). • greenhouse gas – climate effects • Acidification of aquatic systems • not considered a pollutant by government standards
• Carbon oxides: – Carbon monoxide (CO) • toxic gas (suffocation) • during the incomplete combustion of carbon compounds • cigarette smoke – Carbon dioxide (CO 2) • 93% natural carbon cycle • 7% of CO 2 from human activities (mostly burning fossil fuels). • greenhouse gas – climate effects • Acidification of aquatic systems • not considered a pollutant by government standards
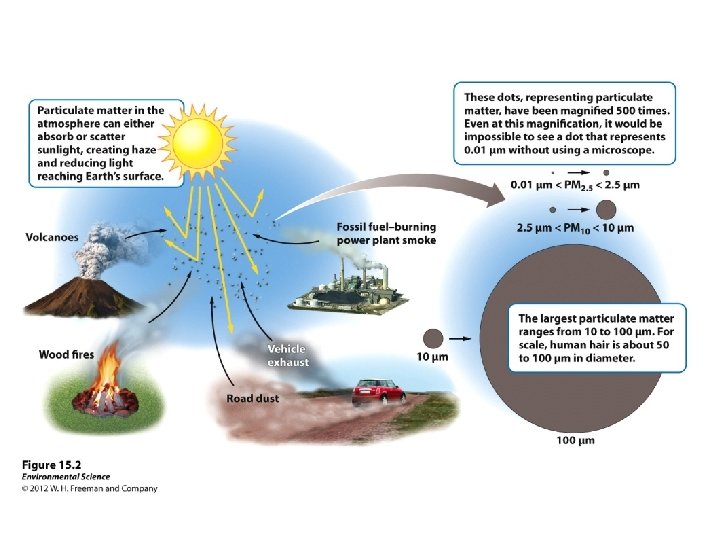
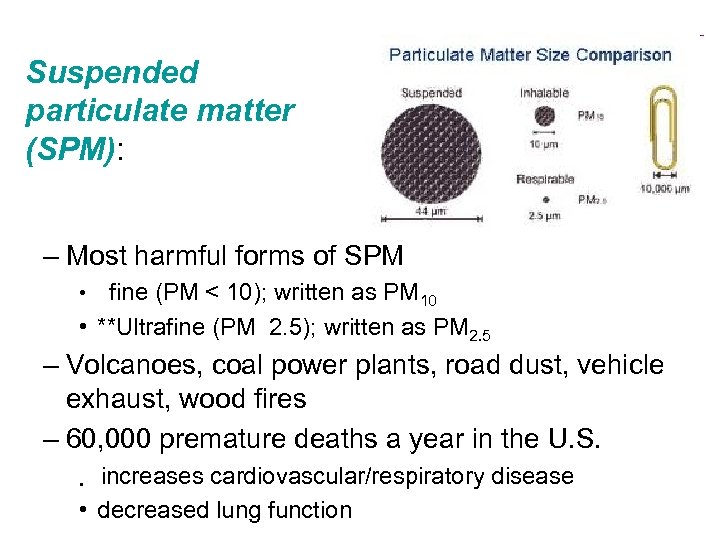 Suspended particulate matter (SPM): – Most harmful forms of SPM fine (PM < 10); written as PM 10 • **Ultrafine (PM 2. 5); written as PM 2. 5 • – Volcanoes, coal power plants, road dust, vehicle exhaust, wood fires – 60, 000 premature deaths a year in the U. S. increases cardiovascular/respiratory disease • decreased lung function •
Suspended particulate matter (SPM): – Most harmful forms of SPM fine (PM < 10); written as PM 10 • **Ultrafine (PM 2. 5); written as PM 2. 5 • – Volcanoes, coal power plants, road dust, vehicle exhaust, wood fires – 60, 000 premature deaths a year in the U. S. increases cardiovascular/respiratory disease • decreased lung function •
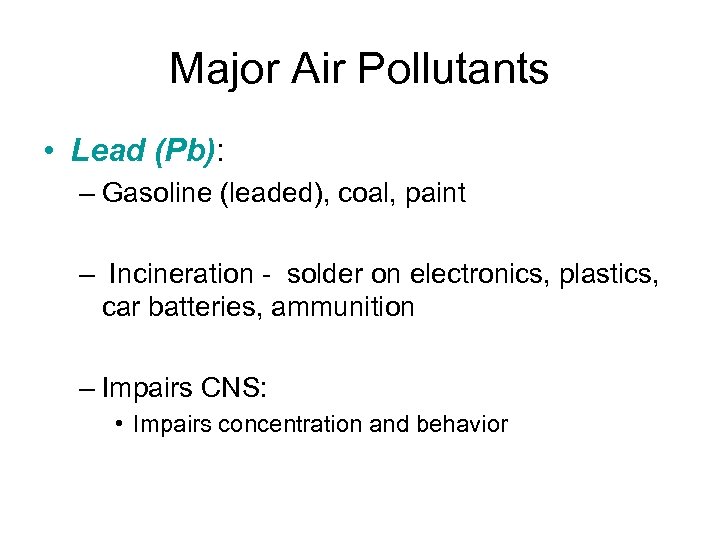 Major Air Pollutants • Lead (Pb): – Gasoline (leaded), coal, paint – Incineration - solder on electronics, plastics, car batteries, ammunition – Impairs CNS: • Impairs concentration and behavior
Major Air Pollutants • Lead (Pb): – Gasoline (leaded), coal, paint – Incineration - solder on electronics, plastics, car batteries, ammunition – Impairs CNS: • Impairs concentration and behavior
 Major Air Pollutants Ø Ozone (O 3): l Increased NOx increases ozone in tropo l Decreased ozone in stratosphere = UVB and C in troposphere l Is a highly reactive gas that is a major component of photochemical smog. l It can • Cause and aggravate respiratory illness. • Can aggravate heart disease. • Damage plants, rubber in tires, fabrics, and paints.
Major Air Pollutants Ø Ozone (O 3): l Increased NOx increases ozone in tropo l Decreased ozone in stratosphere = UVB and C in troposphere l Is a highly reactive gas that is a major component of photochemical smog. l It can • Cause and aggravate respiratory illness. • Can aggravate heart disease. • Damage plants, rubber in tires, fabrics, and paints.
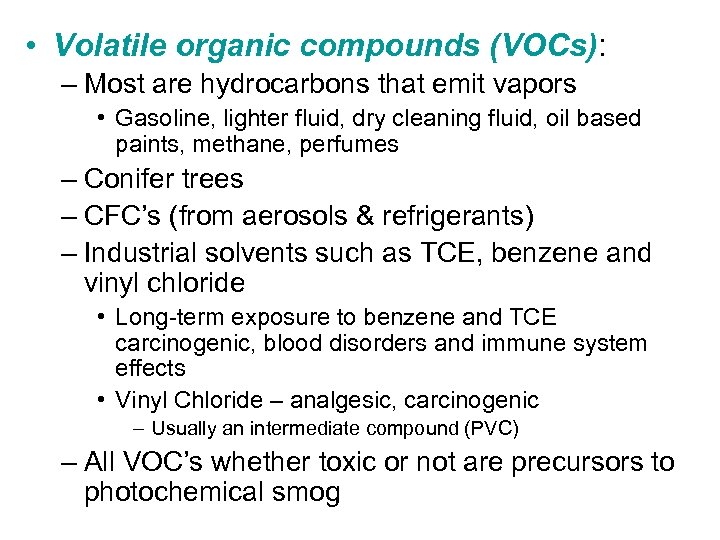 • Volatile organic compounds (VOCs): – Most are hydrocarbons that emit vapors • Gasoline, lighter fluid, dry cleaning fluid, oil based paints, methane, perfumes – Conifer trees – CFC’s (from aerosols & refrigerants) – Industrial solvents such as TCE, benzene and vinyl chloride • Long-term exposure to benzene and TCE carcinogenic, blood disorders and immune system effects • Vinyl Chloride – analgesic, carcinogenic – Usually an intermediate compound (PVC) – All VOC’s whether toxic or not are precursors to photochemical smog
• Volatile organic compounds (VOCs): – Most are hydrocarbons that emit vapors • Gasoline, lighter fluid, dry cleaning fluid, oil based paints, methane, perfumes – Conifer trees – CFC’s (from aerosols & refrigerants) – Industrial solvents such as TCE, benzene and vinyl chloride • Long-term exposure to benzene and TCE carcinogenic, blood disorders and immune system effects • Vinyl Chloride – analgesic, carcinogenic – Usually an intermediate compound (PVC) – All VOC’s whether toxic or not are precursors to photochemical smog
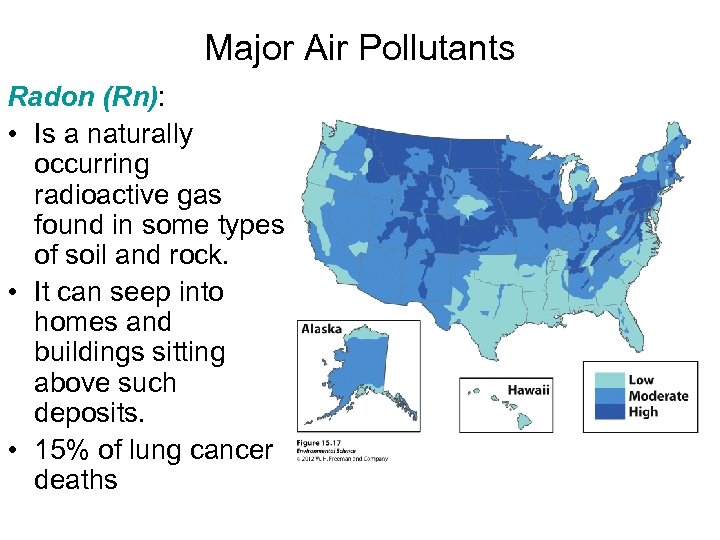 Major Air Pollutants Radon (Rn): • Is a naturally occurring radioactive gas found in some types of soil and rock. • It can seep into homes and buildings sitting above such deposits. • 15% of lung cancer deaths
Major Air Pollutants Radon (Rn): • Is a naturally occurring radioactive gas found in some types of soil and rock. • It can seep into homes and buildings sitting above such deposits. • 15% of lung cancer deaths
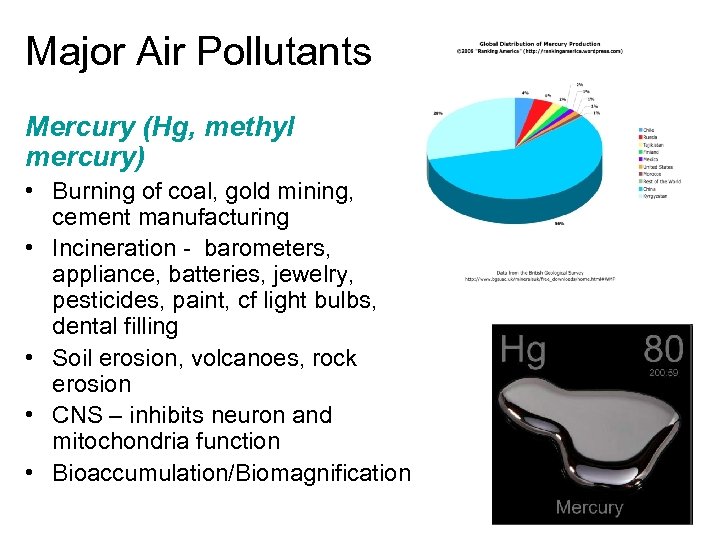 Major Air Pollutants Mercury (Hg, methyl mercury) • Burning of coal, gold mining, cement manufacturing • Incineration - barometers, appliance, batteries, jewelry, pesticides, paint, cf light bulbs, dental filling • Soil erosion, volcanoes, rock erosion • CNS – inhibits neuron and mitochondria function • Bioaccumulation/Biomagnification
Major Air Pollutants Mercury (Hg, methyl mercury) • Burning of coal, gold mining, cement manufacturing • Incineration - barometers, appliance, batteries, jewelry, pesticides, paint, cf light bulbs, dental filling • Soil erosion, volcanoes, rock erosion • CNS – inhibits neuron and mitochondria function • Bioaccumulation/Biomagnification
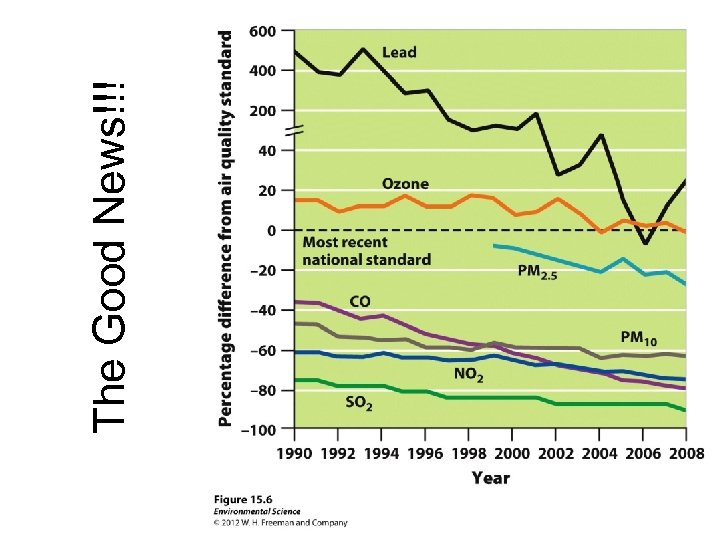 The Good News!!!
The Good News!!!
 URBAN OUTDOOR AIR POLLUTION • Photochemical Smog • Temperature Inversions • Acid Deposition LONDON, mostly automobiles
URBAN OUTDOOR AIR POLLUTION • Photochemical Smog • Temperature Inversions • Acid Deposition LONDON, mostly automobiles
 Photochemical Smog • Photochemical oxidants: – Includes O 3 – Pollutants formed when NOX & SOX react with sunlight • Smog: – O 3 reacts with VOC & NOX to more dangerous photochemical oxidants – SOX & NOX reacts with photochemical oxidants to form some particulates – Mixture of oxidants & particulate matter is referred to as smog.
Photochemical Smog • Photochemical oxidants: – Includes O 3 – Pollutants formed when NOX & SOX react with sunlight • Smog: – O 3 reacts with VOC & NOX to more dangerous photochemical oxidants – SOX & NOX reacts with photochemical oxidants to form some particulates – Mixture of oxidants & particulate matter is referred to as smog.
 Smog Categories • Los Angeles-type smog: – Dominated by oxidants & O 3 – AKA “brown smog” • London-type smog: – Sulfurous smog or gray smog – Dominated by SO 2 & sulfate compounds • Atmospheric Brown Cloud: – Derived from fossil fuel combustion & burning biomass – Found mostly is Asia
Smog Categories • Los Angeles-type smog: – Dominated by oxidants & O 3 – AKA “brown smog” • London-type smog: – Sulfurous smog or gray smog – Dominated by SO 2 & sulfate compounds • Atmospheric Brown Cloud: – Derived from fossil fuel combustion & burning biomass – Found mostly is Asia

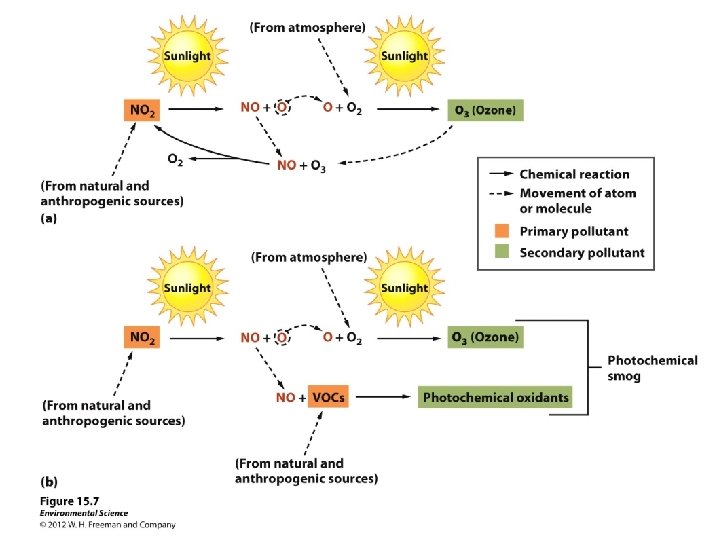
 Sunlight plus Cars Equals Photochemical Smog • Photochemical smog – air pollutants formed by the reaction of nitrogen oxides and volatile organic hydrocarbons under the influence of sunlight. Warm temps expedite chemical reactions.
Sunlight plus Cars Equals Photochemical Smog • Photochemical smog – air pollutants formed by the reaction of nitrogen oxides and volatile organic hydrocarbons under the influence of sunlight. Warm temps expedite chemical reactions.
 Case Study: South Asia’s Massive Brown Cloud • Coal Burning Countries – China and India – Pollution stretches over much of southeastern Asia. – Reduced photosynthesis and crop interference. – Fine particles and droplets in the cloud appear to be changing regional climates (including rainfall). • May have contributed to floods in 2002 and 2005 which killed thousands of people.
Case Study: South Asia’s Massive Brown Cloud • Coal Burning Countries – China and India – Pollution stretches over much of southeastern Asia. – Reduced photosynthesis and crop interference. – Fine particles and droplets in the cloud appear to be changing regional climates (including rainfall). • May have contributed to floods in 2002 and 2005 which killed thousands of people.
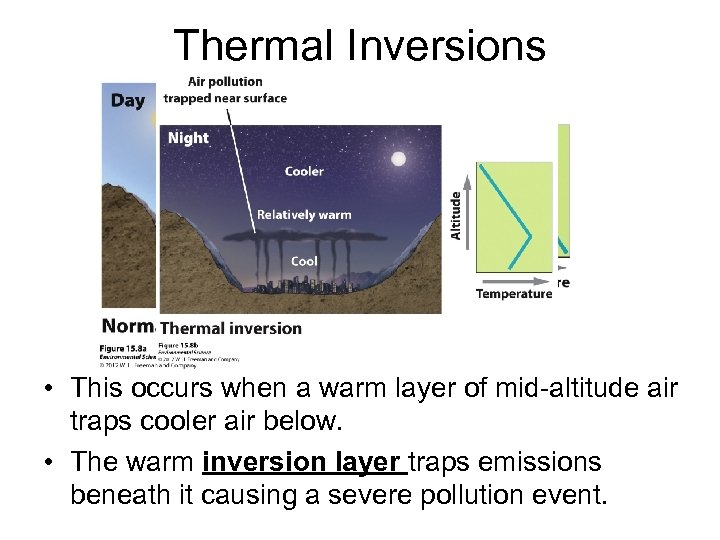 Thermal Inversions • This occurs when a warm layer of mid-altitude air traps cooler air below. • The warm inversion layer traps emissions beneath it causing a severe pollution event.
Thermal Inversions • This occurs when a warm layer of mid-altitude air traps cooler air below. • The warm inversion layer traps emissions beneath it causing a severe pollution event.
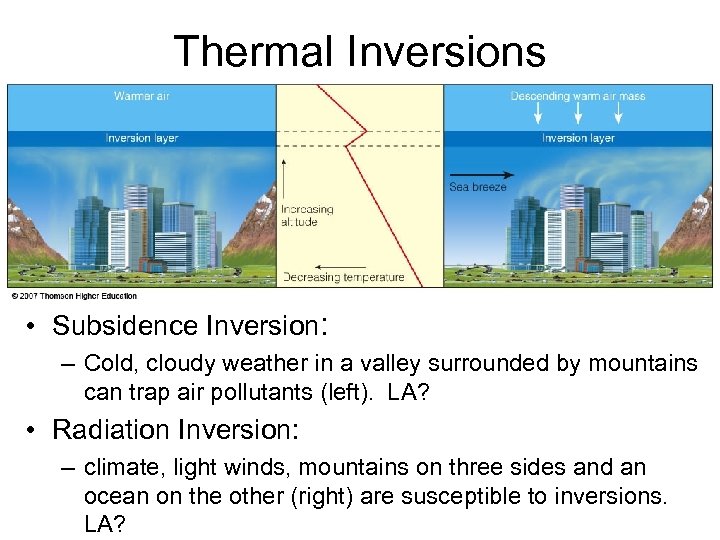 Thermal Inversions • Subsidence Inversion: – Cold, cloudy weather in a valley surrounded by mountains can trap air pollutants (left). LA? • Radiation Inversion: – climate, light winds, mountains on three sides and an ocean on the other (right) are susceptible to inversions. LA?
Thermal Inversions • Subsidence Inversion: – Cold, cloudy weather in a valley surrounded by mountains can trap air pollutants (left). LA? • Radiation Inversion: – climate, light winds, mountains on three sides and an ocean on the other (right) are susceptible to inversions. LA?
 Factors Influencing Levels of Outdoor Air Pollution • Outdoor air pollution can be reduced by: – settling out, precipitation, sea spray, winds, and chemical reactions. • Outdoor air pollution can be increased by: – urban buildings (slow wind dispersal of pollutants), mountains (promote temperature inversions), and high temperatures (promote photochemical reactions).
Factors Influencing Levels of Outdoor Air Pollution • Outdoor air pollution can be reduced by: – settling out, precipitation, sea spray, winds, and chemical reactions. • Outdoor air pollution can be increased by: – urban buildings (slow wind dispersal of pollutants), mountains (promote temperature inversions), and high temperatures (promote photochemical reactions).
 ACID DEPOSITION • Formation: – NOX & SO 2 are transformed by water & O 2 into secondary pollutants of nitric acid & sulfuric acid. – Reactions occur over a number of days & pollutants can travel 1, 000 km – Washed out of air by precipitation & deposited on vegetation, soil, or water
ACID DEPOSITION • Formation: – NOX & SO 2 are transformed by water & O 2 into secondary pollutants of nitric acid & sulfuric acid. – Reactions occur over a number of days & pollutants can travel 1, 000 km – Washed out of air by precipitation & deposited on vegetation, soil, or water
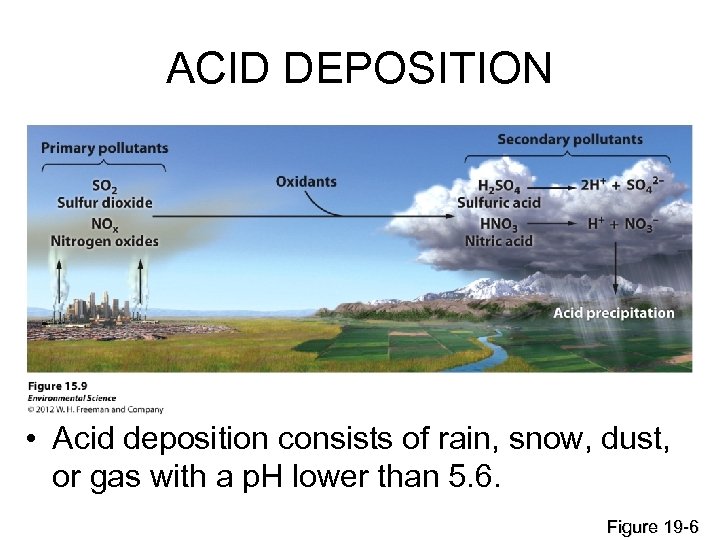 ACID DEPOSITION • Acid deposition consists of rain, snow, dust, or gas with a p. H lower than 5. 6. Figure 19 -6
ACID DEPOSITION • Acid deposition consists of rain, snow, dust, or gas with a p. H lower than 5. 6. Figure 19 -6
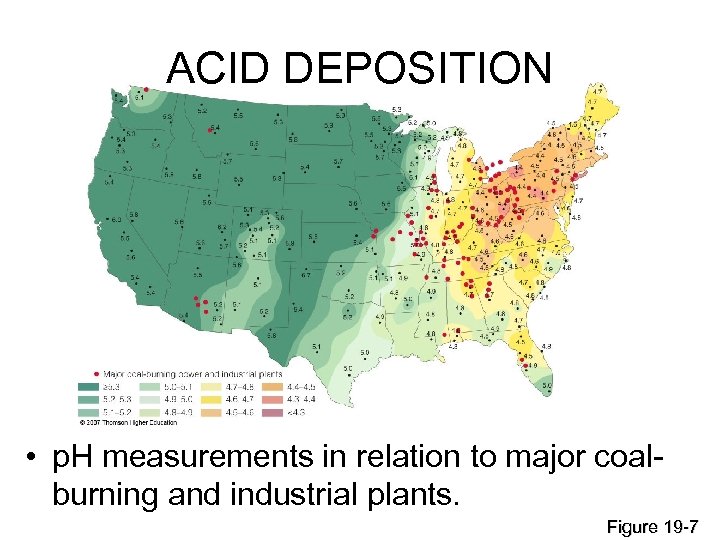 ACID DEPOSITION • p. H measurements in relation to major coalburning and industrial plants. Figure 19 -7
ACID DEPOSITION • p. H measurements in relation to major coalburning and industrial plants. Figure 19 -7
 ACID DEPOSITION • Acid deposition contributes to chronic respiratory disease and can leach toxic metals (such as lead and mercury) from soils and rocks into acidic lakes used as sources for drinking water.
ACID DEPOSITION • Acid deposition contributes to chronic respiratory disease and can leach toxic metals (such as lead and mercury) from soils and rocks into acidic lakes used as sources for drinking water.
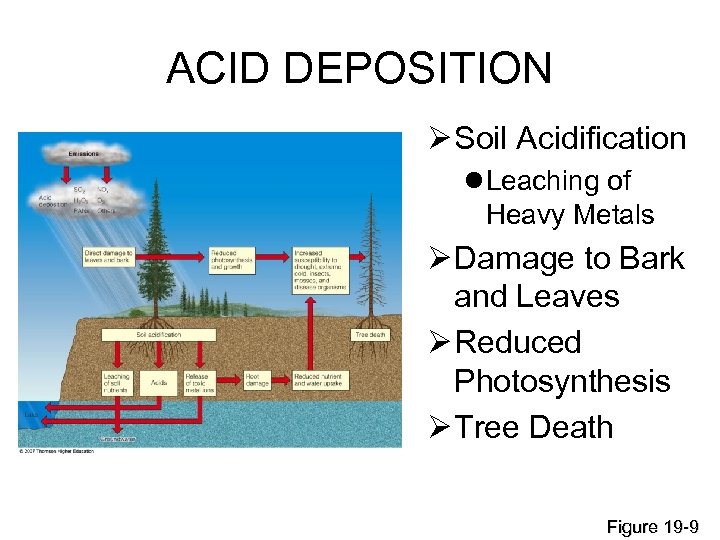 ACID DEPOSITION Ø Soil Acidification l Leaching of Heavy Metals Ø Damage to Bark and Leaves Ø Reduced Photosynthesis Ø Tree Death Figure 19 -9
ACID DEPOSITION Ø Soil Acidification l Leaching of Heavy Metals Ø Damage to Bark and Leaves Ø Reduced Photosynthesis Ø Tree Death Figure 19 -9
 Solutions Acid Deposition Prevention Reduce air pollution by improving energy efficiency Cleanup Add lime to neutralize acidified lakes Reduce coal use Increase natural gas use Increase use of renewable energy resources Add phosphate fertilizer to neutralize acidified lakes Burn low-sulfur coal Remove SO 2 particulates & NOx from smokestack gases Remove NOx from motor vehicular exhaust Tax emissions of SO 2 Fig. 19 -10, p. 452
Solutions Acid Deposition Prevention Reduce air pollution by improving energy efficiency Cleanup Add lime to neutralize acidified lakes Reduce coal use Increase natural gas use Increase use of renewable energy resources Add phosphate fertilizer to neutralize acidified lakes Burn low-sulfur coal Remove SO 2 particulates & NOx from smokestack gases Remove NOx from motor vehicular exhaust Tax emissions of SO 2 Fig. 19 -10, p. 452
 PREVENTING AND REDUCING AIR POLLUTION • The Clean Air Acts in the United States have greatly reduced outdoor air pollution from six major pollutants: – Carbon monoxide – Nitrogen oxides – Sulfur dioxides – Lead – Ozone – Suspended particulate matter (less than PM-10) • Decrease by 41% while GDP raised by 64%
PREVENTING AND REDUCING AIR POLLUTION • The Clean Air Acts in the United States have greatly reduced outdoor air pollution from six major pollutants: – Carbon monoxide – Nitrogen oxides – Sulfur dioxides – Lead – Ozone – Suspended particulate matter (less than PM-10) • Decrease by 41% while GDP raised by 64%
 PREVENTING AND REDUCING AIR POLLUTION Ø Environmental scientists point out several deficiencies in the Clean Air Act: l The U. S. continues to rely on cleanup rather than prevention. l The U. S. Congress has failed to increase fuelefficiency standards for automobiles. l Regulation of emissions from motorcycles and two-cycle engines remains inadequate. l There is little or no regulation of air pollution from oceangoing ships in American ports.
PREVENTING AND REDUCING AIR POLLUTION Ø Environmental scientists point out several deficiencies in the Clean Air Act: l The U. S. continues to rely on cleanup rather than prevention. l The U. S. Congress has failed to increase fuelefficiency standards for automobiles. l Regulation of emissions from motorcycles and two-cycle engines remains inadequate. l There is little or no regulation of air pollution from oceangoing ships in American ports.
 PREVENTING AND REDUCING AIR POLLUTION – Airports are exempt from many air pollution regulations. – The Act does not regulate the greenhouse gas CO 2. – The Act has failed to deal seriously with indoor air pollution. – There is a need for better enforcement of the Clean Air Act.
PREVENTING AND REDUCING AIR POLLUTION – Airports are exempt from many air pollution regulations. – The Act does not regulate the greenhouse gas CO 2. – The Act has failed to deal seriously with indoor air pollution. – There is a need for better enforcement of the Clean Air Act.
 Using the Marketplace to Reduce Outdoor Air Pollution Ø To help reduce SO 2 emissions, the Clean Air Act authorized and emission trading (cap-and -trade) program. l Enables the 110 most polluting power plants to buy and sell SO 2 pollution rights. l Between 1990 -2002, the emission trading system reduced emissions. l In 2002, the EPA reported the cap-and-trade system produced less emission reductions than were projected.
Using the Marketplace to Reduce Outdoor Air Pollution Ø To help reduce SO 2 emissions, the Clean Air Act authorized and emission trading (cap-and -trade) program. l Enables the 110 most polluting power plants to buy and sell SO 2 pollution rights. l Between 1990 -2002, the emission trading system reduced emissions. l In 2002, the EPA reported the cap-and-trade system produced less emission reductions than were projected.
 Solutions: Reducing Outdoor Air Pollution • There are ways to prevent and control air pollution from coal-burning facilities. – Electrostatic precipitator: are used to attract negatively charged particles in a smokestack into a collector. – Wet scrubber: fine mists of water vapor trap particulates and convert them to a sludge that is collected and disposed of usually in a landfill. – Baghouse Filter: Air passes through fabrics that can remove almost 100% of PM emissions
Solutions: Reducing Outdoor Air Pollution • There are ways to prevent and control air pollution from coal-burning facilities. – Electrostatic precipitator: are used to attract negatively charged particles in a smokestack into a collector. – Wet scrubber: fine mists of water vapor trap particulates and convert them to a sludge that is collected and disposed of usually in a landfill. – Baghouse Filter: Air passes through fabrics that can remove almost 100% of PM emissions
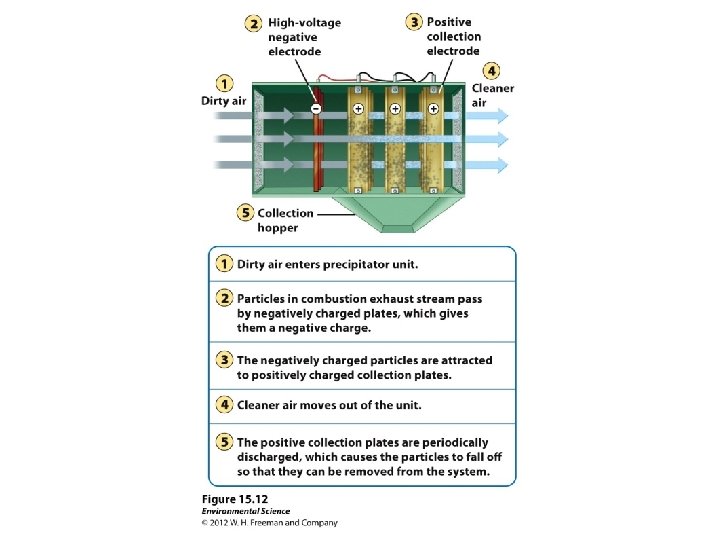
 Electrostatic Precipitator Ø Uses electrical charge to attract particles Ø Can remove 99% of particulate matter Ø Does not remove hazardous ultrafine particles. Ø Produces toxic dust that must be safely disposed of. Ø Uses large amounts of electricity
Electrostatic Precipitator Ø Uses electrical charge to attract particles Ø Can remove 99% of particulate matter Ø Does not remove hazardous ultrafine particles. Ø Produces toxic dust that must be safely disposed of. Ø Uses large amounts of electricity
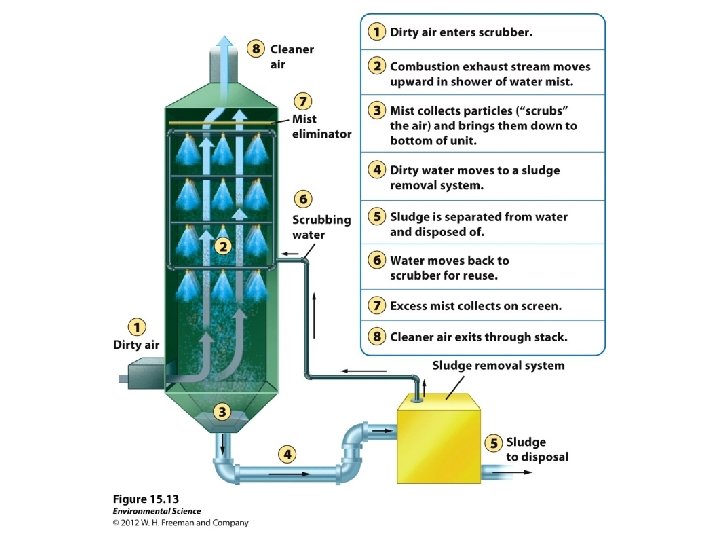
 The Scrubber Ø AKA the “Wet Scrubber” Ø Can remove 98% of SO 2 and particulate matter. Ø Not very effective in removing hazardous fine and ultrafine particles. Ø Uses water and electricity to catch particulate matter and SO 2
The Scrubber Ø AKA the “Wet Scrubber” Ø Can remove 98% of SO 2 and particulate matter. Ø Not very effective in removing hazardous fine and ultrafine particles. Ø Uses water and electricity to catch particulate matter and SO 2
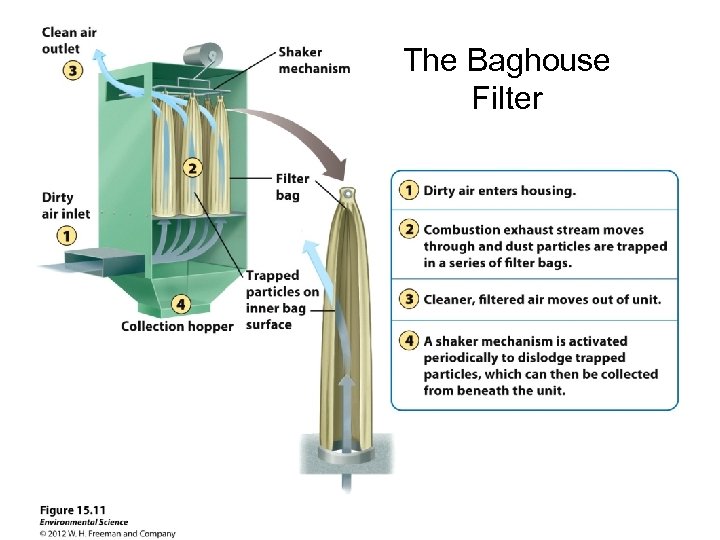 The Baghouse Filter
The Baghouse Filter
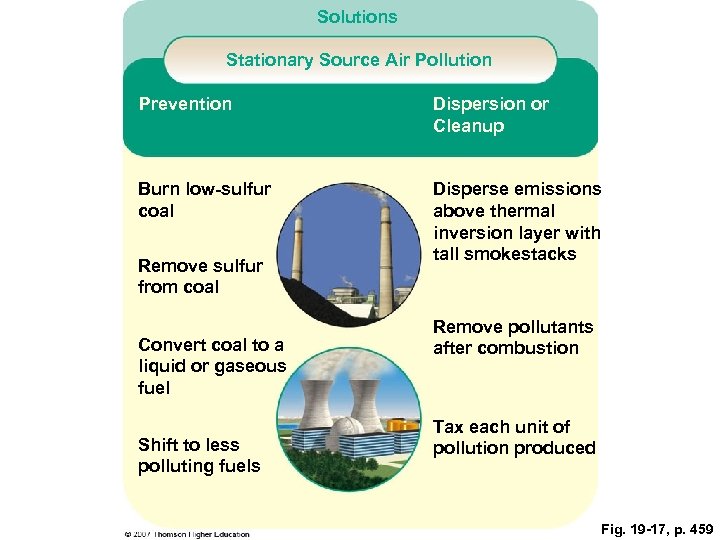 Solutions Stationary Source Air Pollution Prevention Dispersion or Cleanup Burn low-sulfur coal Disperse emissions above thermal inversion layer with tall smokestacks Remove sulfur from coal Convert coal to a liquid or gaseous fuel Shift to less polluting fuels Remove pollutants after combustion Tax each unit of pollution produced Fig. 19 -17, p. 459
Solutions Stationary Source Air Pollution Prevention Dispersion or Cleanup Burn low-sulfur coal Disperse emissions above thermal inversion layer with tall smokestacks Remove sulfur from coal Convert coal to a liquid or gaseous fuel Shift to less polluting fuels Remove pollutants after combustion Tax each unit of pollution produced Fig. 19 -17, p. 459
 Solutions: Reducing Outdoor Air Pollution • In 2003, fourteen states and a number of U. S. cities – sued the EPA to block new rules that would allow older coal-burning power plants to modernize without having to install the most advanced air pollution controls.
Solutions: Reducing Outdoor Air Pollution • In 2003, fourteen states and a number of U. S. cities – sued the EPA to block new rules that would allow older coal-burning power plants to modernize without having to install the most advanced air pollution controls.
 Stratospheric Ozone • Ultra-violet Radiation: – UV-A, UV-B, UV-C in order of increasing energy • UV-A – contributes to and possibly initiates skin cancer • UV-B & UV-C can cause significant damage to tissue and DNA of a living organism – However. . . • Layer of O 3 & O 2 in stratosphere absorbs 99% of all incoming UV-B & UV-C radiation
Stratospheric Ozone • Ultra-violet Radiation: – UV-A, UV-B, UV-C in order of increasing energy • UV-A – contributes to and possibly initiates skin cancer • UV-B & UV-C can cause significant damage to tissue and DNA of a living organism – However. . . • Layer of O 3 & O 2 in stratosphere absorbs 99% of all incoming UV-B & UV-C radiation

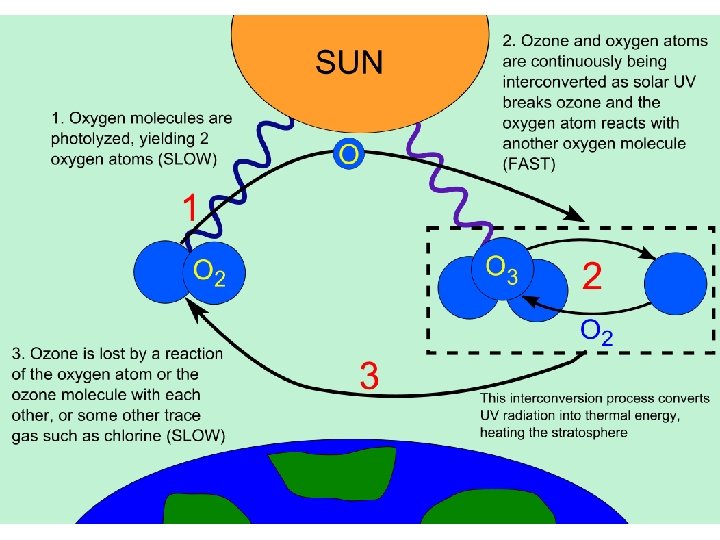
 Stratospheric Ozone • Forms & breaks down naturally in a closed –loop cycle. • Formation of stratospheric ozone: – O 2 + UV-C ® 2 O – O 2 + O ® O 3 – In the presence of UV radiation, oxygen is converted into ozone • Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C & UV-B radiation – O 3 + UV-B or UV-C ® O 2 + O – This maintains a steady-state conc. of O 3
Stratospheric Ozone • Forms & breaks down naturally in a closed –loop cycle. • Formation of stratospheric ozone: – O 2 + UV-C ® 2 O – O 2 + O ® O 3 – In the presence of UV radiation, oxygen is converted into ozone • Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C & UV-B radiation – O 3 + UV-B or UV-C ® O 2 + O – This maintains a steady-state conc. of O 3
 Anthropogenic Contributions to Ozone Destruction • Certain chemicals are catalyst to breaking down ozone. . The most important is chlorine atoms. • CFC’s (chlorofluorocarbons): – Used in AC, refrigerators, aerosols, & injecting air into foam products – When reaches stratosphere, UV radiation breaks bond connecting Cl to CFC molecule
Anthropogenic Contributions to Ozone Destruction • Certain chemicals are catalyst to breaking down ozone. . The most important is chlorine atoms. • CFC’s (chlorofluorocarbons): – Used in AC, refrigerators, aerosols, & injecting air into foam products – When reaches stratosphere, UV radiation breaks bond connecting Cl to CFC molecule
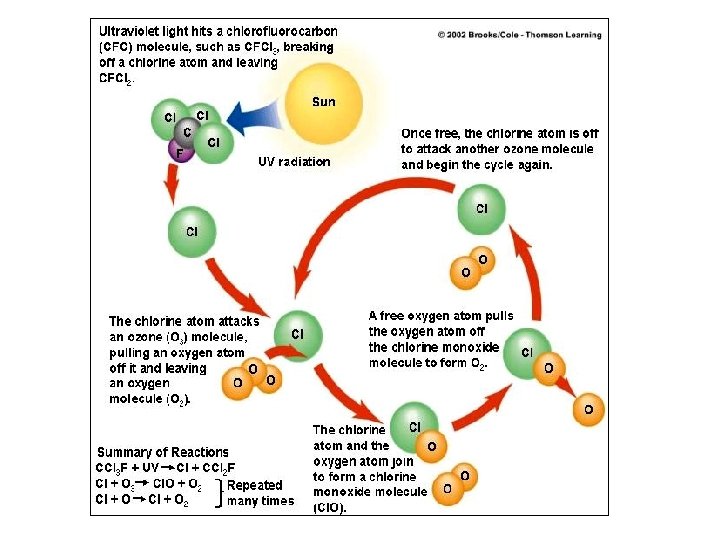
 CFC’s & Ozone Destruction • Free chlorine atom reacts with ozone: – O 3 + Cl ® Cl. O + O 2 (destroying it) • Next a free oxygen pulls the oxygen atom from the Cl. O, freeing the Cl. The free chlorine atom is ready to break down more ozone. – Cl. O + O ® Cl + O 2 – One Cl atom can breakdown as many as 100, 000 ozone molecules
CFC’s & Ozone Destruction • Free chlorine atom reacts with ozone: – O 3 + Cl ® Cl. O + O 2 (destroying it) • Next a free oxygen pulls the oxygen atom from the Cl. O, freeing the Cl. The free chlorine atom is ready to break down more ozone. – Cl. O + O ® Cl + O 2 – One Cl atom can breakdown as many as 100, 000 ozone molecules
 Ozone Depleting Chemicals Ø Chlorofluorocarbons (CFCs) Ø Halons Ø Methyl bromide Ø Carbon tetrachloride Ø Methyl chloroform Ø Hydrogen chloride Ø Sources of CFCs
Ozone Depleting Chemicals Ø Chlorofluorocarbons (CFCs) Ø Halons Ø Methyl bromide Ø Carbon tetrachloride Ø Methyl chloroform Ø Hydrogen chloride Ø Sources of CFCs
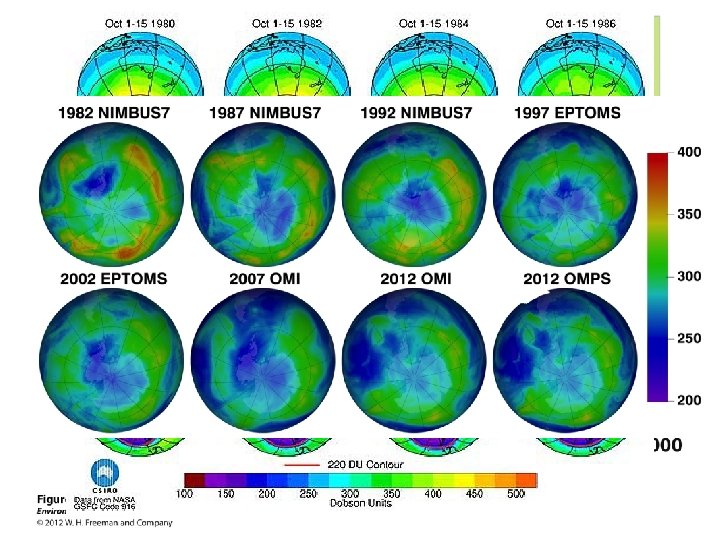
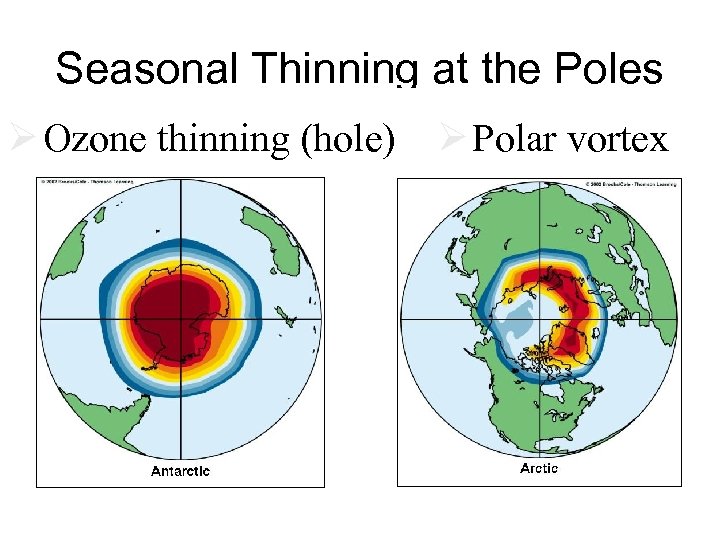 Seasonal Thinning at the Poles Ø Ozone thinning (hole) Ø Polar vortex Fig. 18 -30 p. 475
Seasonal Thinning at the Poles Ø Ozone thinning (hole) Ø Polar vortex Fig. 18 -30 p. 475
 Reasons for Concern Ø Increased incidence and severity of sunburn Ø Increase in eye cataracts Ø Increased incidence of skin cancer Ø Immune system suppression Ø Increase in acid deposition Ø Lower crop yields and decline in productivity Ø Increase in tropospheric ozone
Reasons for Concern Ø Increased incidence and severity of sunburn Ø Increase in eye cataracts Ø Increased incidence of skin cancer Ø Immune system suppression Ø Increase in acid deposition Ø Lower crop yields and decline in productivity Ø Increase in tropospheric ozone
 • http: //www. casadefrias. com/2011/12/myresponse-to-dear-16 -year-old-me-skin. html
• http: //www. casadefrias. com/2011/12/myresponse-to-dear-16 -year-old-me-skin. html
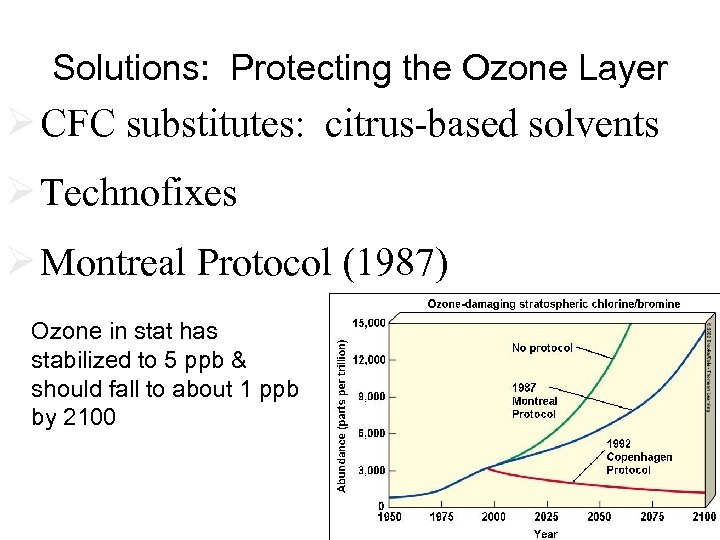 Solutions: Protecting the Ozone Layer Ø CFC substitutes: citrus-based solvents Ø Technofixes Ø Montreal Protocol (1987) Ozone in stat has stabilized to 5 ppb & should fall to about 1 ppb by 2100 Fig. 18 -33 p. 479
Solutions: Protecting the Ozone Layer Ø CFC substitutes: citrus-based solvents Ø Technofixes Ø Montreal Protocol (1987) Ozone in stat has stabilized to 5 ppb & should fall to about 1 ppb by 2100 Fig. 18 -33 p. 479
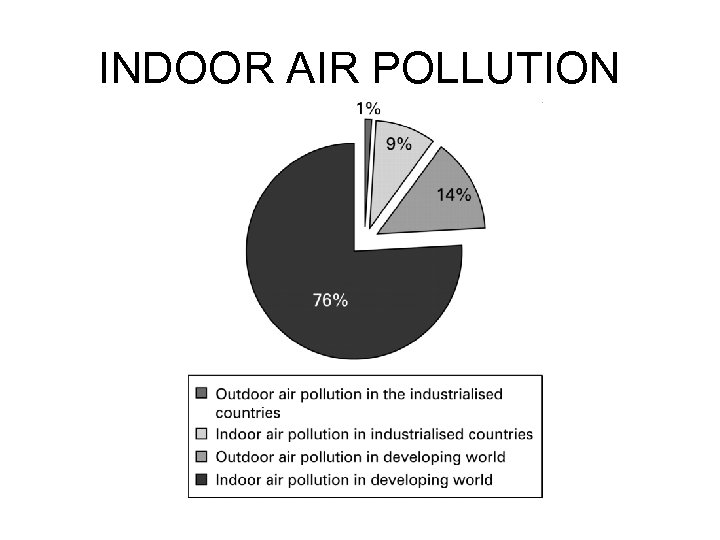 INDOOR AIR POLLUTION
INDOOR AIR POLLUTION
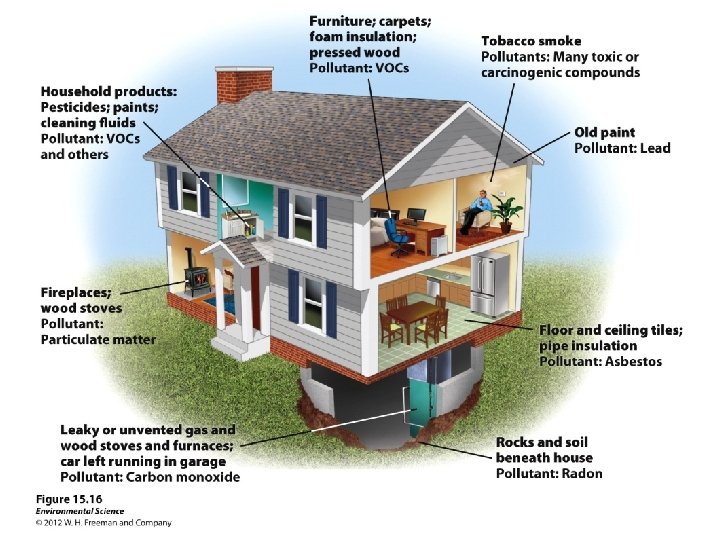
 INDOOR AIR POLLUTION Ø Indoor air pollution usually is a greater threat to human health than outdoor air pollution. Ø According to the EPA, the four most dangerous indoor air pollutants in developed countries are: l Tobacco smoke. l Formaldehyde. l Radioactive radon-222 gas. l Very small fine and ultrafine particles.
INDOOR AIR POLLUTION Ø Indoor air pollution usually is a greater threat to human health than outdoor air pollution. Ø According to the EPA, the four most dangerous indoor air pollutants in developed countries are: l Tobacco smoke. l Formaldehyde. l Radioactive radon-222 gas. l Very small fine and ultrafine particles.
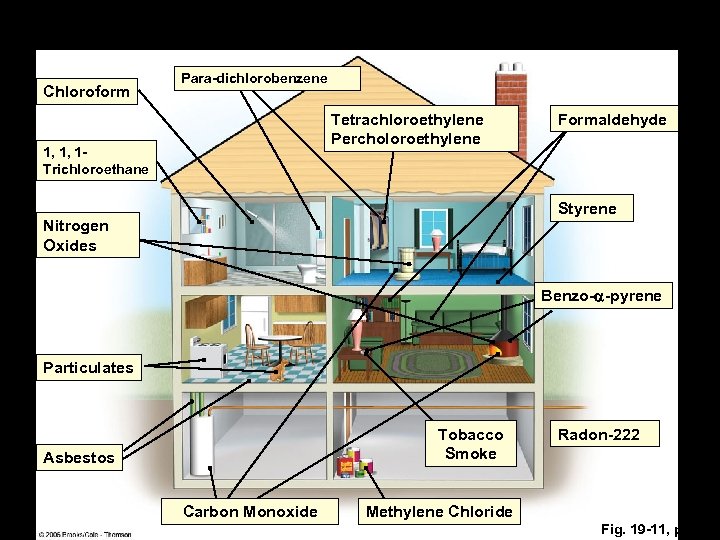 Chloroform Para-dichlorobenzene Tetrachloroethylene Percholoroethylene 1, 1, 1 Trichloroethane Formaldehyde Styrene Nitrogen Oxides Benzo-a-pyrene Particulates Tobacco Smoke Asbestos Carbon Monoxide Radon-222 Methylene Chloride Fig. 19 -11, p. 453
Chloroform Para-dichlorobenzene Tetrachloroethylene Percholoroethylene 1, 1, 1 Trichloroethane Formaldehyde Styrene Nitrogen Oxides Benzo-a-pyrene Particulates Tobacco Smoke Asbestos Carbon Monoxide Radon-222 Methylene Chloride Fig. 19 -11, p. 453
 INDOOR AIR POLLUTION • Household dust mites that feed on human skin and dust, live in materials such as bedding and furniture fabrics. – Can cause asthma attacks and allergic reactions in some people. Figure 19 -12
INDOOR AIR POLLUTION • Household dust mites that feed on human skin and dust, live in materials such as bedding and furniture fabrics. – Can cause asthma attacks and allergic reactions in some people. Figure 19 -12
 INDOOR AIR POLLUTION DEVELOPING NATIONS Ø ~ 3 billion people cook and heat their homes using open fires and leaky stoves burning biomass (wood, animal dung and crop waste) and coal. Ø 2 million people die prematurely from illness attributable to indoor air pollution from household solid fuel use. Ø 50% of pneumonia deaths among children under five are due to particulate matter inhaled from indoor air pollution. Ø >1 million people a year die from chronic obstructive respiratory disease (COPD) that develop due to exposure to such indoor air pollution. l women and men exposed to heavy indoor smoke are 2 -3 times more likely to develop COPD.
INDOOR AIR POLLUTION DEVELOPING NATIONS Ø ~ 3 billion people cook and heat their homes using open fires and leaky stoves burning biomass (wood, animal dung and crop waste) and coal. Ø 2 million people die prematurely from illness attributable to indoor air pollution from household solid fuel use. Ø 50% of pneumonia deaths among children under five are due to particulate matter inhaled from indoor air pollution. Ø >1 million people a year die from chronic obstructive respiratory disease (COPD) that develop due to exposure to such indoor air pollution. l women and men exposed to heavy indoor smoke are 2 -3 times more likely to develop COPD.
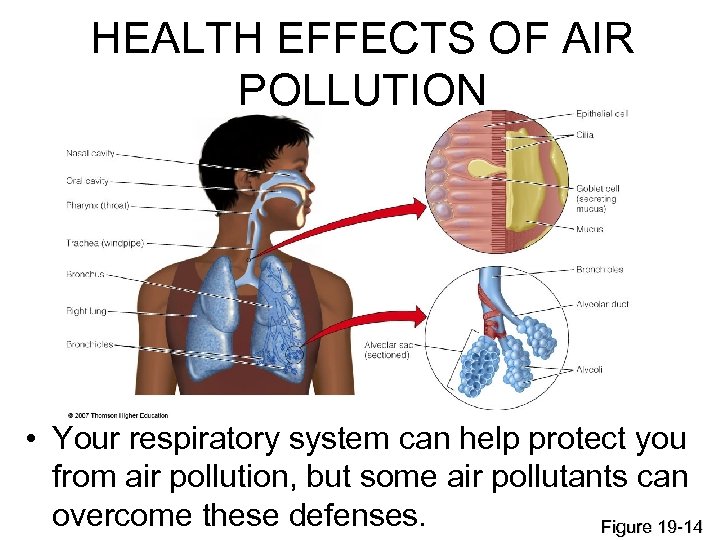 HEALTH EFFECTS OF AIR POLLUTION • Your respiratory system can help protect you from air pollution, but some air pollutants can overcome these defenses. Figure 19 -14
HEALTH EFFECTS OF AIR POLLUTION • Your respiratory system can help protect you from air pollution, but some air pollutants can overcome these defenses. Figure 19 -14
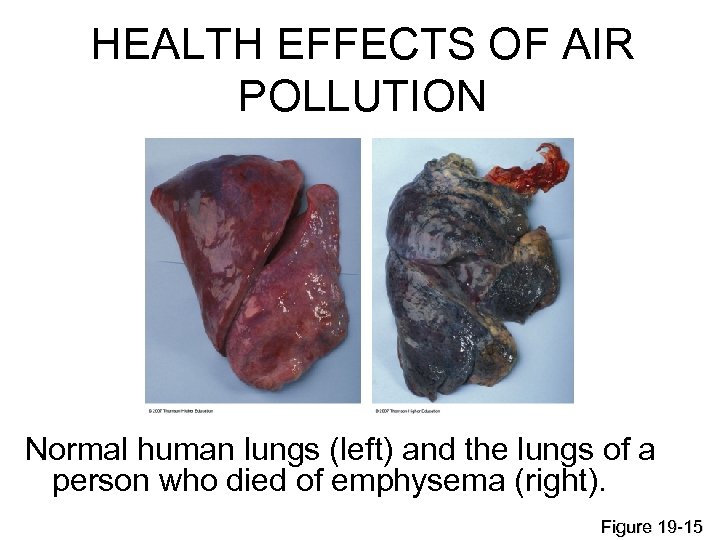 HEALTH EFFECTS OF AIR POLLUTION Normal human lungs (left) and the lungs of a person who died of emphysema (right). Figure 19 -15
HEALTH EFFECTS OF AIR POLLUTION Normal human lungs (left) and the lungs of a person who died of emphysema (right). Figure 19 -15
 Air Pollution is a Big Killer • Each year, air pollution prematurely kills about 3 million people, mostly from indoor air pollution in developing countries. – In the U. S. , the EPA estimates that annual deaths related to indoor and outdoor air pollution range from 150, 000 to 350, 000. – According to the EPA, each year more than 125, 000 Americans get cancer from breathing diesel fumes.
Air Pollution is a Big Killer • Each year, air pollution prematurely kills about 3 million people, mostly from indoor air pollution in developing countries. – In the U. S. , the EPA estimates that annual deaths related to indoor and outdoor air pollution range from 150, 000 to 350, 000. – According to the EPA, each year more than 125, 000 Americans get cancer from breathing diesel fumes.
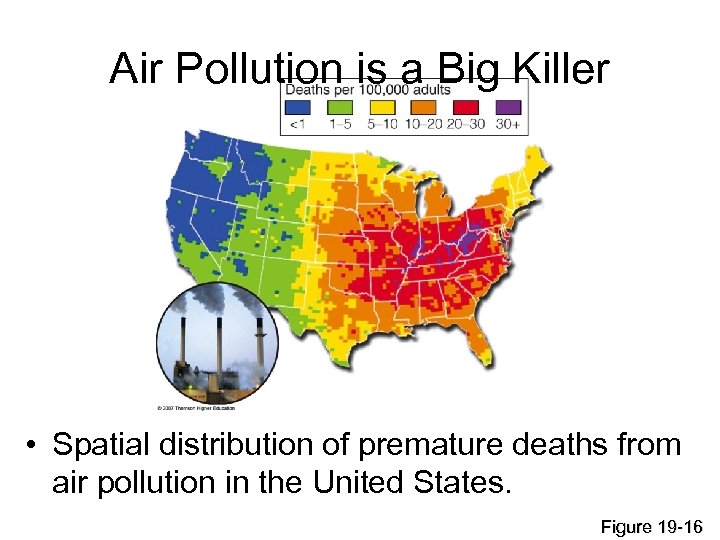 Air Pollution is a Big Killer • Spatial distribution of premature deaths from air pollution in the United States. Figure 19 -16
Air Pollution is a Big Killer • Spatial distribution of premature deaths from air pollution in the United States. Figure 19 -16
 Solutions: Reducing Outdoor Air Pollution • There a of ways to prevent and control air pollution from motor vehicles. – Because of the Clean Air Act, a new car today in the U. S. emits 75% less pollution than did pre 1970 cars. – There is and increase in motor vehicle use in developing countries and many have no pollution control devices and burn leaded gasoline.
Solutions: Reducing Outdoor Air Pollution • There a of ways to prevent and control air pollution from motor vehicles. – Because of the Clean Air Act, a new car today in the U. S. emits 75% less pollution than did pre 1970 cars. – There is and increase in motor vehicle use in developing countries and many have no pollution control devices and burn leaded gasoline.
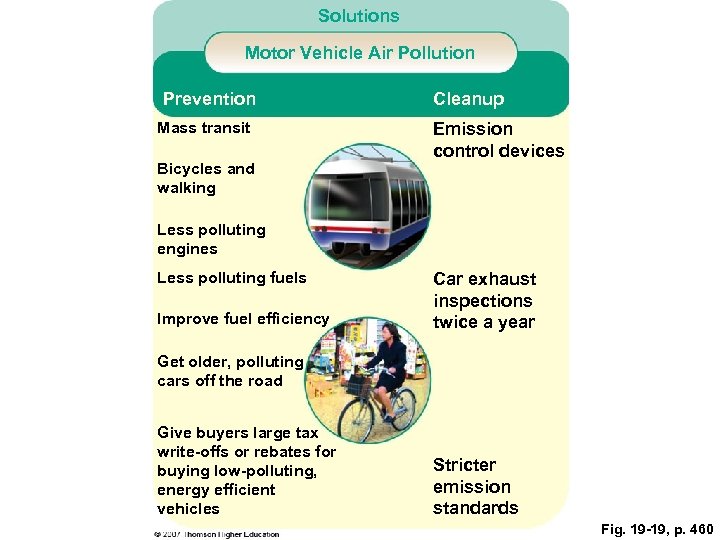 Solutions Motor Vehicle Air Pollution Prevention Mass transit Bicycles and walking Cleanup Emission control devices Less polluting engines Less polluting fuels Improve fuel efficiency Car exhaust inspections twice a year Get older, polluting cars off the road Give buyers large tax write-offs or rebates for buying low-polluting, energy efficient vehicles Stricter emission standards Fig. 19 -19, p. 460
Solutions Motor Vehicle Air Pollution Prevention Mass transit Bicycles and walking Cleanup Emission control devices Less polluting engines Less polluting fuels Improve fuel efficiency Car exhaust inspections twice a year Get older, polluting cars off the road Give buyers large tax write-offs or rebates for buying low-polluting, energy efficient vehicles Stricter emission standards Fig. 19 -19, p. 460
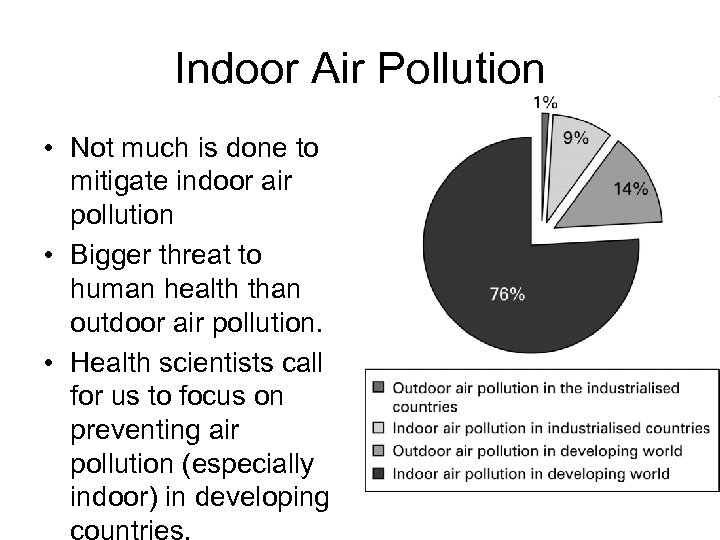 Indoor Air Pollution • Not much is done to mitigate indoor air pollution • Bigger threat to human health than outdoor air pollution. • Health scientists call for us to focus on preventing air pollution (especially indoor) in developing countries.
Indoor Air Pollution • Not much is done to mitigate indoor air pollution • Bigger threat to human health than outdoor air pollution. • Health scientists call for us to focus on preventing air pollution (especially indoor) in developing countries.
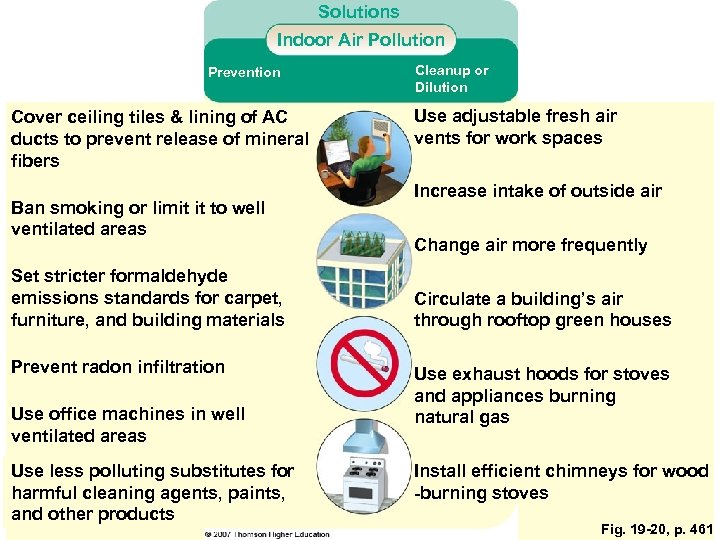 Solutions Indoor Air Pollution Prevention Cover ceiling tiles & lining of AC ducts to prevent release of mineral fibers Ban smoking or limit it to well ventilated areas Set stricter formaldehyde emissions standards for carpet, furniture, and building materials Prevent radon infiltration Use office machines in well ventilated areas Use less polluting substitutes for harmful cleaning agents, paints, and other products Cleanup or Dilution Use adjustable fresh air vents for work spaces Increase intake of outside air Change air more frequently Circulate a building’s air through rooftop green houses Use exhaust hoods for stoves and appliances burning natural gas Install efficient chimneys for wood -burning stoves Fig. 19 -20, p. 461
Solutions Indoor Air Pollution Prevention Cover ceiling tiles & lining of AC ducts to prevent release of mineral fibers Ban smoking or limit it to well ventilated areas Set stricter formaldehyde emissions standards for carpet, furniture, and building materials Prevent radon infiltration Use office machines in well ventilated areas Use less polluting substitutes for harmful cleaning agents, paints, and other products Cleanup or Dilution Use adjustable fresh air vents for work spaces Increase intake of outside air Change air more frequently Circulate a building’s air through rooftop green houses Use exhaust hoods for stoves and appliances burning natural gas Install efficient chimneys for wood -burning stoves Fig. 19 -20, p. 461
 What Can You Do? Indoor Air Pollution • Test for radon and formaldehyde inside your home and take corrective measures as needed. • Do not buy furniture and other products containing formaldehyde. • Remove your shoes before entering your house to reduce inputs of dust, lead, and pesticides. • Test your house or workplace for asbestos fiber levels and for any crumbling asbestos materials if it was built before 1980. • Don't live in a pre-1980 house without having its indoor air tested for asbestos and lead. • Do not store gasoline, solvents, or other volatile hazardous chemicals inside a home or attached garage. • If you smoke, do it outside or in a closed room vented to the outside. • Make sure that wood-burning stoves, fireplaces, and keroseneand gas-burning heaters are properly installed, vented, and maintained. • Install carbon monoxide detectors in all sleeping areas. Fig. 19 -21, p. 461
What Can You Do? Indoor Air Pollution • Test for radon and formaldehyde inside your home and take corrective measures as needed. • Do not buy furniture and other products containing formaldehyde. • Remove your shoes before entering your house to reduce inputs of dust, lead, and pesticides. • Test your house or workplace for asbestos fiber levels and for any crumbling asbestos materials if it was built before 1980. • Don't live in a pre-1980 house without having its indoor air tested for asbestos and lead. • Do not store gasoline, solvents, or other volatile hazardous chemicals inside a home or attached garage. • If you smoke, do it outside or in a closed room vented to the outside. • Make sure that wood-burning stoves, fireplaces, and keroseneand gas-burning heaters are properly installed, vented, and maintained. • Install carbon monoxide detectors in all sleeping areas. Fig. 19 -21, p. 461


