46aaf9cf0c44e3fa4ea6791d6fc48f9b.ppt
- Количество слайдов: 31

Advisory Committee for Pharmaceutical Science October 25 -26, 2005 OPS Update Helen N. Winkle Director, Office of Pharmaceutical Science

Outline • • • CDER objectives and goals Move to White Oak Current management structure in OPS Reorganizations in OPS Important Initiatives for OPS – Pharmaceutical CGMP for the 21 st Century • CMC Review • Field and Review Interaction – Drug Safety Initiative – Critical Path Initiative – Follow-on Proteins • Importance of this meeting • Agenda for meeting and what hope to accomplish 2

CDER State of the Center – Dr. Galson’s Vision for CDER’s Future • CDER’s leadership – International scientific leader in drug development and innovative regulatory science – Many active, robust, productive scientific partnerships with outside groups – Regulatory programs are consistent, efficient and transparent 3

Vision • CDER’s Organization – Quality Systems throughout our organization – Improve communication with the public and health -care community about the risks and benefits of pharmaceutical products – Electronic vs. paper environment for submission, review, post-marketing surveillance – Respect and tolerance for differences of opinion – More mechanisms for involving stakeholders in peer review – High degree of professionalism in resolving 4 disputes

Supporting the Vision • Enhance the ability of the Center to respond to challenges of Critical Path (CP) initiatives and improve regulatory and drug development science – Locus needed for ownership of CP activities • Reflect the commitment of CDER to sustain a multidisciplinary, cross-Center approach to drug safety – Placement in organization must reflect level of commitment – Need focus and consistency and improvement in communication about drug risks and benefits – Need focus for cross-center policy development 5

White Oak 6

White Oak Offers Opportunity • Allows for flexibility to reorganize • Brings OPS together in one location • Provides opportunity to work closely with all review groups • Potential for better interaction when rest of CDER moves 7

Management Structure • Deputy Directors – Ajaz Hussain – Keith Webber • Associate Directors – Nakissa Sadrieh – Jon Clark • Office Directors – Office of Generic Drugs – Gary Buehler – Office of New Drug Quality Assessment – Moheb Nasr – Office of Biotechnology – Steve Kozlowski, Acting – Office of Testing and Research – Cindy Buhse, Acting 8

Reorganizations • Have had several reorganizations in OPS during the past few months – OGD – new chemistry division – ONDQA • Change in organizational structure • New division • Other reorganizations in Center that affect OPS 9

Pharmaceutical CGMP for the 21 st Century • What is current status? – Set direction for modernization of pharmaceutical regulation – Continue to focus on pharmaceutical quality issues through Council on Pharmaceutical Quality – Still implementing various recommendations 10
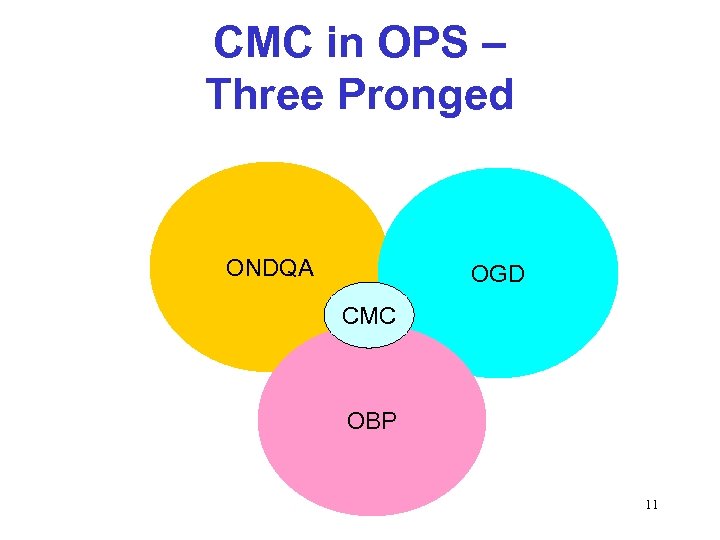
CMC in OPS – Three Pronged ONDQA OGD CMC OBP 11

Changing the CMC Review Processes • Office of New Drug Quality Assessment • Question-based review • Integration of biotech products into new paradigm • All come together as one 12
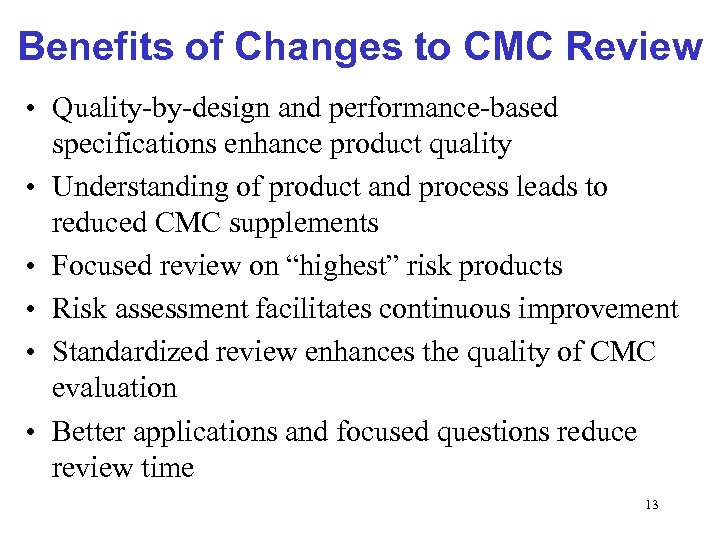
Benefits of Changes to CMC Review • Quality-by-design and performance-based specifications enhance product quality • Understanding of product and process leads to reduced CMC supplements • Focused review on “highest” risk products • Risk assessment facilitates continuous improvement • Standardized review enhances the quality of CMC evaluation • Better applications and focused questions reduce review time 13

CMC Workshop • • Held October 5, 6, and 7 Over 600 in attendance Focused on new paradigm for CMC review Discussed quality-by-design (Qb. D), design space, pharmaceutical development data (PD), continuous improvement (CI) and quality overall summary (QOS) • Set stage for moving forward with the new processes 14

Agreements Reached at Workshop • Support concept of Qb. D built into PD • PD to illustrate product/process understanding to serve as the basis of science and risk-based assessment • Regulatory flexibility predicated on scientific knowledge and process understanding and is welcomed by industry and regulators • Concept of regulatory agreement supported • Improved, streamlined, frequent communications required - We must partner!! • Specifications tied to clinical safety/efficacy • Guidances and training will need to be different 15

Challenges Realized as Result of Workshop • Lack of adequate scientific understanding of products and processes both by FDA & industry • Implementation – “devil is in the details” • Setting specifications • Legacy products • Culture change • Industry buy-in for new processes (in many cases industry resistant to make changes) • Global harmonization 16
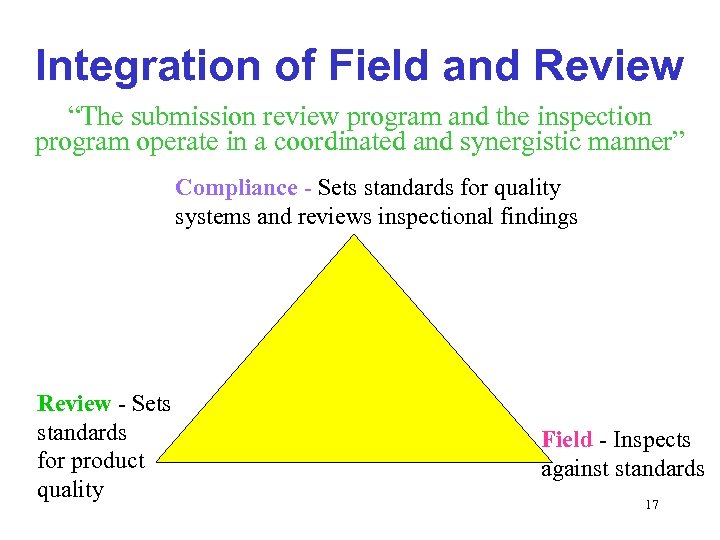
Integration of Field and Review “The submission review program and the inspection program operate in a coordinated and synergistic manner” Compliance - Sets standards for quality systems and reviews inspectional findings Review - Sets standards for product quality Field - Inspects against standards 17

Roles and Responsibilities • Review side (lead) – Scientific assessment of product and process design – Evaluate product quality in light of established FDA standards (e. g. , impurities, stability, etc. ) – Set and maintain product quality standards 18

Roles and Responsibilities • Field (lead) – Evaluate implementation of process design – Evaluate quality system – Implement enforcement actions – Set certain compliance policies 19

Roles and Responsibilities • Compliance Offices (lead) – Establish and maintain quality system standards for c. GMPs – Establish and maintain risk management system for inspections – Establish and maintain compliance policies and standards 20

Drugs Safety and How it Relates to Product Quality • Many issues related to drug safety are caused by product quality problems (e. g. , alcohol-induced dose dumping) • Safety is an important aspect when focusing on product risk • CMC specifications linked to safety and efficacy 21

Drug Safety – How Focused in Center • Drugs safer than they have ever been • FDA as organization learns well from experience with large number of products • What we’ve heard: – More info – address gap between FDA and others – Improve internal processes to manage safety issues – Involve outside experts more • Secretary Leavitt recently announced a new drug safety initiative – Promote a culture of openness and 22 – Enhanced oversight within the FDA

Drug Safety Initiative • New drug safety information initiative – Proposed Drug Watch Program – Patient Information Sheets – Healthcare Professional Sheets • Drug Safety Oversight Board – Focus on product quality 23
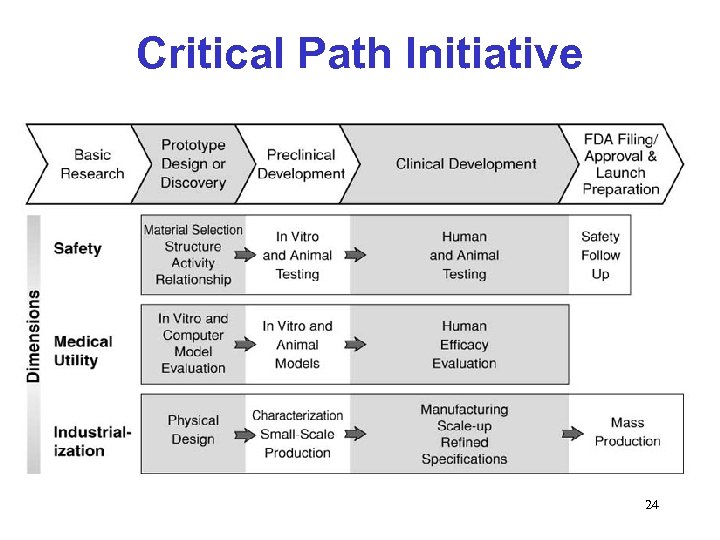
Critical Path Initiative 24

FDA’s Role in Critical Path • FDA has a significant role in enhancing product development and manufacturing – We are involved in review during product development - they see the successes, failures, and missed opportunities – We have no preconceived thoughts on how products need to be developed and manufactured - we are not a competitor, but instead can serve a crucial convening and coordinating role for consensus development between industry, academia and government – We set the standards that need to be met • Critical Path research can make a difference in how we regulate CMC 25

Examples of Critical Path Projects Focused on CMC • Proposed CRADAs to collaborate with industry and academia to better understand manufacturing science and new technologies and their application • Determining how PAT can be applied in product manufacturing to improve efficiencies • Gathering information on determining critical product and process parameters and quality attributes - CMC pilot • Other 26

Follow-on Proteins • Moving toward follow-on proteins, but there a lot of issues to address – Terminology – Legal – Science – Administrative/process • We need to be more open in our thinking and not have preconceived ideas as to how follow-ons should be regulated • Process will evolve – as we learn more – and we will incorporate into regulatory processes 27

Follow-on Proteins (cont. ) • We need to incorporate thinking from the new paradigm – looking at early on in developing regulatory framework • Finalizing guidances to help lay the path for moving forward • It will take all of us working together to make this happen successfully 28

Importance of this Meeting • Step closer to understanding quality-by-design – especially as it relates to dissolution • Showcasing the progress that has been made in changes to CMC review • Introducing several new topics • Saying farewell to Dr. Ajaz Hussain 29

Advisory Committee Meeting Topics • Quality-by-Design and control of drug dissolution – Continuation of discussion from May – Several presentations from outside organizations – FDA presentations on tactical plan • Update from PTIT working group – Fact finding group on dose content uniformity for inhalation products • Alcohol-induced dose dumping – Awareness topic – Basis of concern and our current thinking on regulatory 30 approach

Advisory Committee Meeting Topics • Transitional changes in CMC review – Presentations by all three OPS CMC programs – Implementation of risk-based approach • Laboratory research - developing “peer review” program – How best to handle scientific “peer review” for all laboratory research • State of US pharmaceutical science and engineering education – Important as we move toward new paradigm 31
46aaf9cf0c44e3fa4ea6791d6fc48f9b.ppt