2f55478c4c2da5212143b6ef4f17c717.ppt
- Количество слайдов: 65
 Advances in VL and PKDL Case Detection, Management and Prevention Dr. Dinesh Mondal, MD, Ph. D Senior Scientist, icddr, b and Project Director, Kala. CORE
Advances in VL and PKDL Case Detection, Management and Prevention Dr. Dinesh Mondal, MD, Ph. D Senior Scientist, icddr, b and Project Director, Kala. CORE
 Plan for today’s discussion ØOverview of Leishmaniasis and visceral leishmanisis (VL) ØRecapitulation of life cycle of Leishmania donovani (LD) ØHistorical milestone ØCorner stone for intervention and prevention ØResearch activities in Bangladesh ØEpidemiology and risk factor ØVL and PKDL case detection and referral ØVL and PKDL diagnosis ØVL and PKDL treatment and follow up ØPrevention: ØEarly case detection ØVector control ØVaccine
Plan for today’s discussion ØOverview of Leishmaniasis and visceral leishmanisis (VL) ØRecapitulation of life cycle of Leishmania donovani (LD) ØHistorical milestone ØCorner stone for intervention and prevention ØResearch activities in Bangladesh ØEpidemiology and risk factor ØVL and PKDL case detection and referral ØVL and PKDL diagnosis ØVL and PKDL treatment and follow up ØPrevention: ØEarly case detection ØVector control ØVaccine
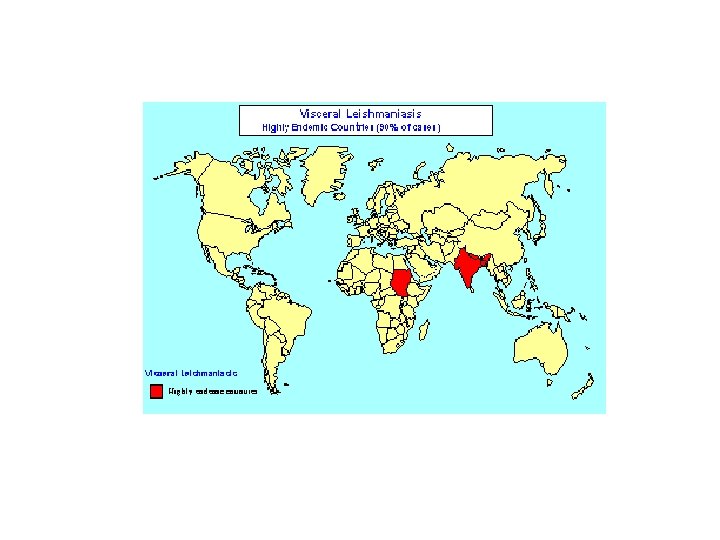
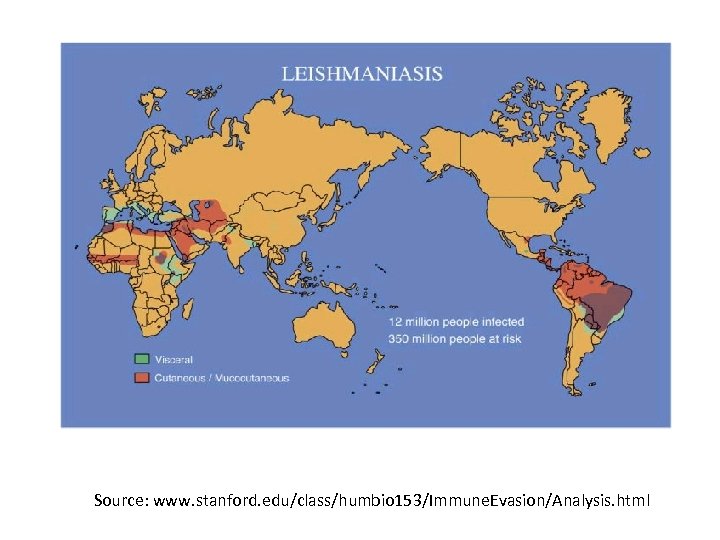 Source: www. stanford. edu/class/humbio 153/Immune. Evasion/Analysis. html
Source: www. stanford. edu/class/humbio 153/Immune. Evasion/Analysis. html
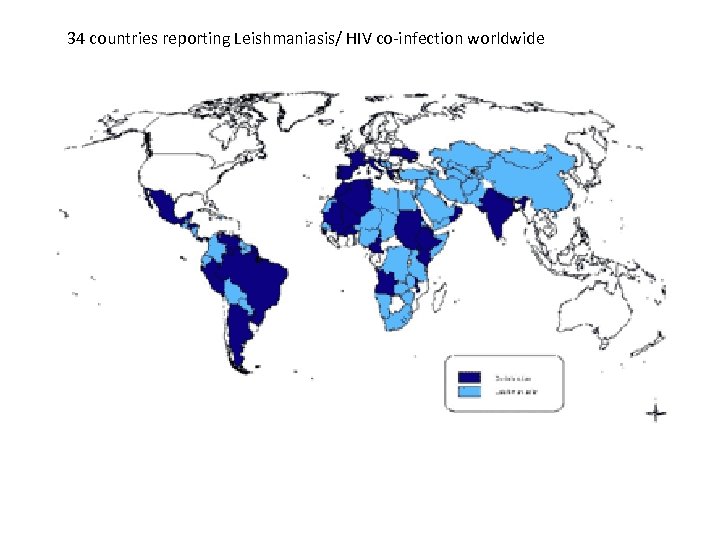 34 countries reporting Leishmaniasis/ HIV co-infection worldwide
34 countries reporting Leishmaniasis/ HIV co-infection worldwide
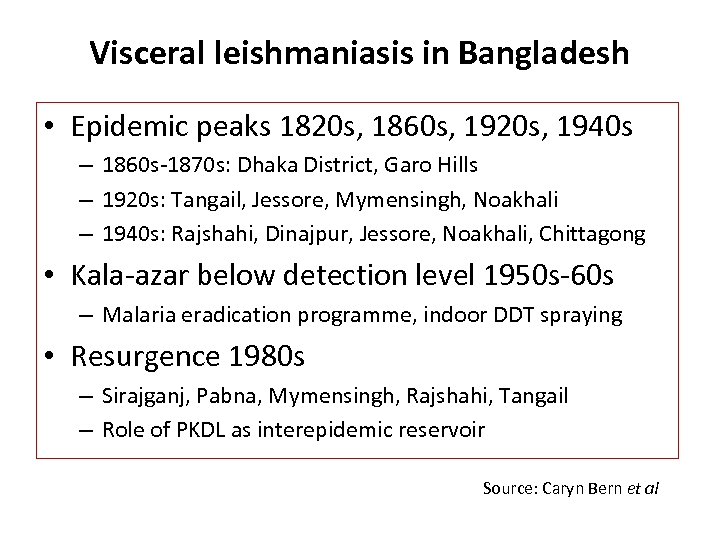 Visceral leishmaniasis in Bangladesh • Epidemic peaks 1820 s, 1860 s, 1920 s, 1940 s – 1860 s-1870 s: Dhaka District, Garo Hills – 1920 s: Tangail, Jessore, Mymensingh, Noakhali – 1940 s: Rajshahi, Dinajpur, Jessore, Noakhali, Chittagong • Kala-azar below detection level 1950 s-60 s – Malaria eradication programme, indoor DDT spraying • Resurgence 1980 s – Sirajganj, Pabna, Mymensingh, Rajshahi, Tangail – Role of PKDL as interepidemic reservoir Source: Caryn Bern et al
Visceral leishmaniasis in Bangladesh • Epidemic peaks 1820 s, 1860 s, 1920 s, 1940 s – 1860 s-1870 s: Dhaka District, Garo Hills – 1920 s: Tangail, Jessore, Mymensingh, Noakhali – 1940 s: Rajshahi, Dinajpur, Jessore, Noakhali, Chittagong • Kala-azar below detection level 1950 s-60 s – Malaria eradication programme, indoor DDT spraying • Resurgence 1980 s – Sirajganj, Pabna, Mymensingh, Rajshahi, Tangail – Role of PKDL as interepidemic reservoir Source: Caryn Bern et al
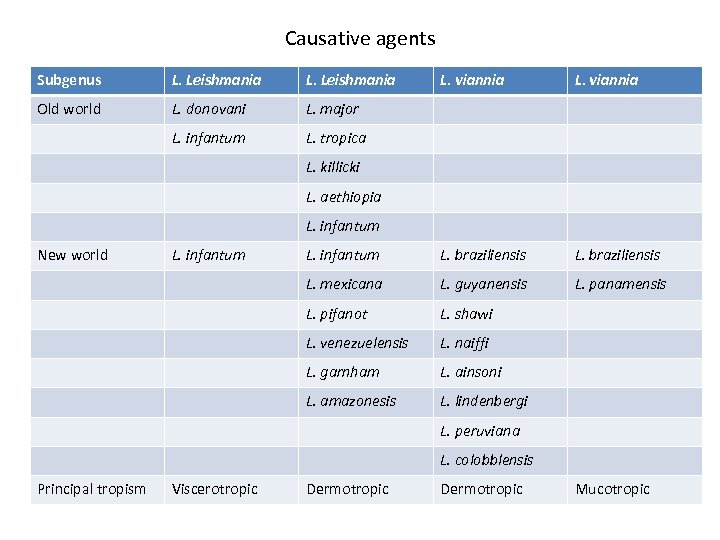 Causative agents Subgenus L. Leishmania L. viannia Old world L. donovani L. major L. infantum L. tropica L. infantum L. braziliensis L. mexicana L. guyanensis L. panamensis L. pifanot L. shawi L. venezuelensis L. naiffi L. garnham L. ainsoni L. amazonesis L. lindenbergi L. killicki L. aethiopia L. infantum New world L. infantum L. peruviana L. colobblensis Principal tropism Viscerotropic Dermotropic Mucotropic
Causative agents Subgenus L. Leishmania L. viannia Old world L. donovani L. major L. infantum L. tropica L. infantum L. braziliensis L. mexicana L. guyanensis L. panamensis L. pifanot L. shawi L. venezuelensis L. naiffi L. garnham L. ainsoni L. amazonesis L. lindenbergi L. killicki L. aethiopia L. infantum New world L. infantum L. peruviana L. colobblensis Principal tropism Viscerotropic Dermotropic Mucotropic
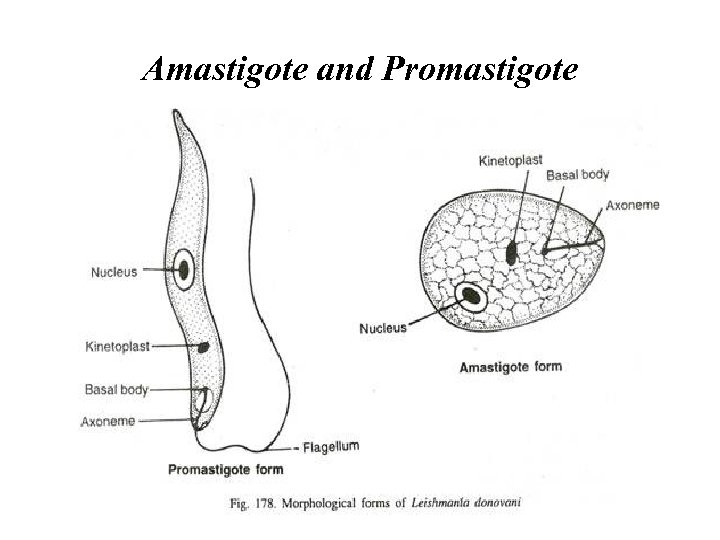 Amastigote and Promastigote
Amastigote and Promastigote
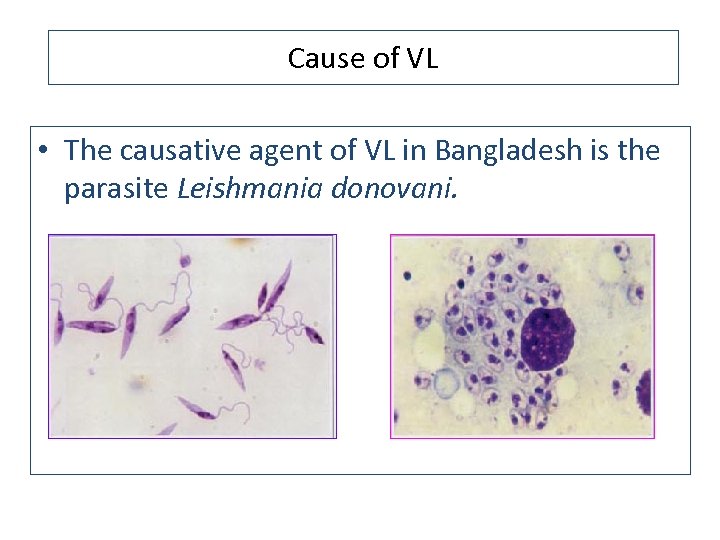 Cause of VL • The causative agent of VL in Bangladesh is the parasite Leishmania donovani.
Cause of VL • The causative agent of VL in Bangladesh is the parasite Leishmania donovani.
 Sandfly
Sandfly
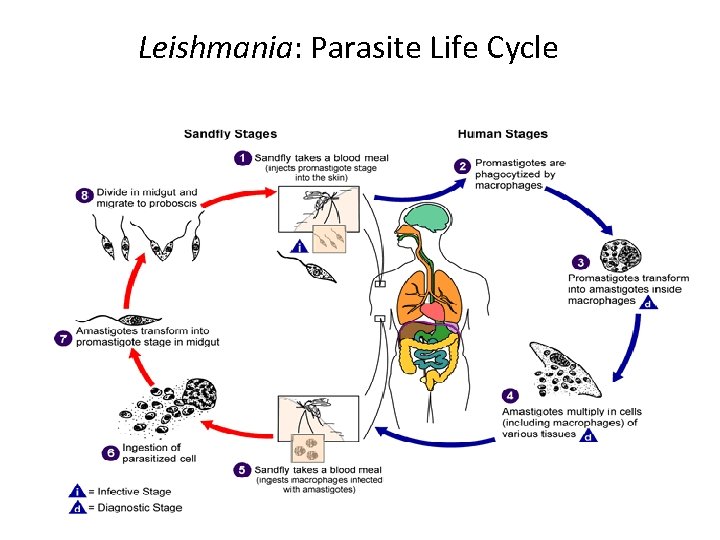 Leishmania: Parasite Life Cycle
Leishmania: Parasite Life Cycle
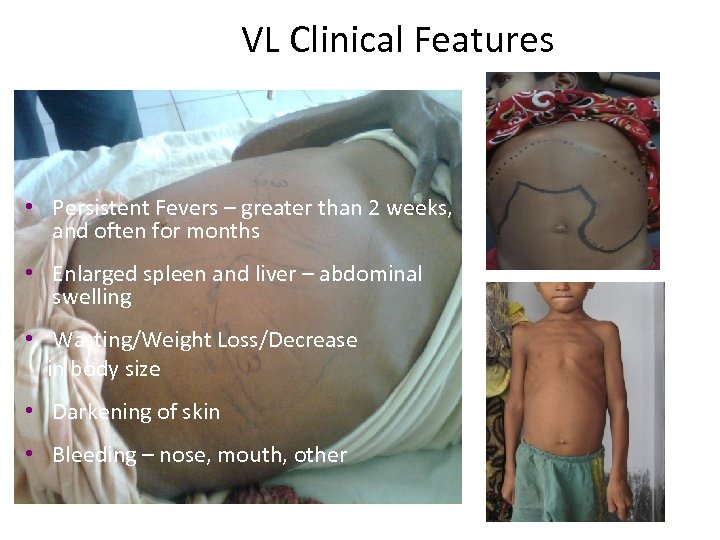 VL Clinical Features • Persistent Fevers – greater than 2 weeks, and often for months • Enlarged spleen and liver – abdominal swelling • Wasting/Weight Loss/Decrease in body size • Darkening of skin • Bleeding – nose, mouth, other
VL Clinical Features • Persistent Fevers – greater than 2 weeks, and often for months • Enlarged spleen and liver – abdominal swelling • Wasting/Weight Loss/Decrease in body size • Darkening of skin • Bleeding – nose, mouth, other
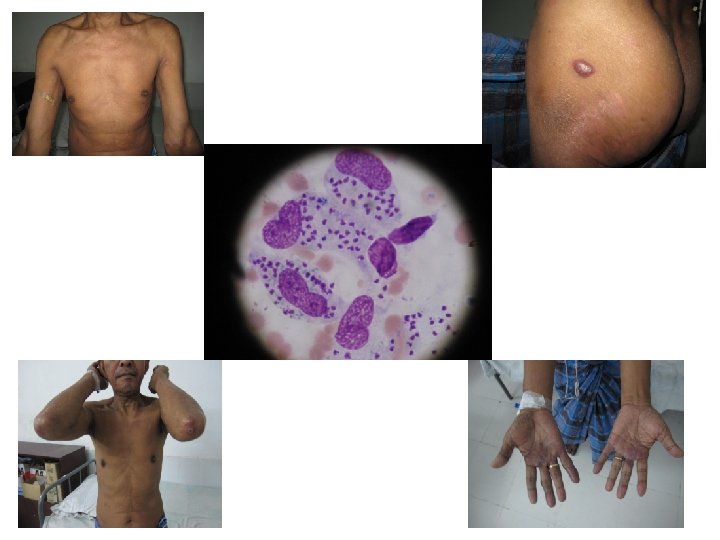
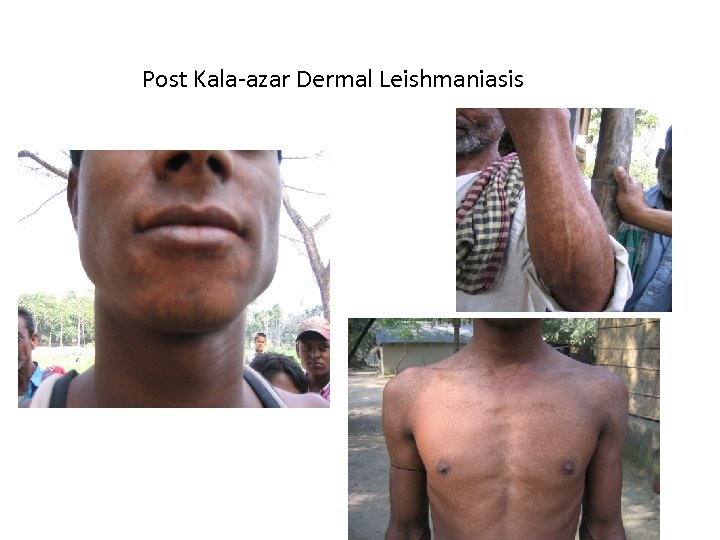 Post Kala-azar Dermal Leishmaniasis
Post Kala-azar Dermal Leishmaniasis
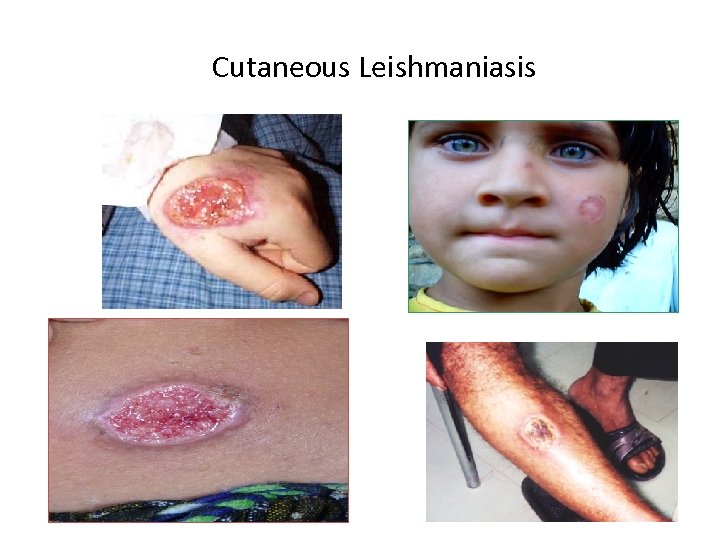 Cutaneous Leishmaniasis
Cutaneous Leishmaniasis
 Historical Milestones • 1824 – First Disease Description • 1903 – Discovery of causative agent by W. B. Leishman and C. Donovan • 1904 – The parasite was cultured in vitro by Rogers and morphology, cultural characteristics and protozological features of the parasite was described. • 1921 – Naiper developed serological test (Aldehyde test) for diagnosis of VL • 1940 – Swaminath proved that P. argentipes was the vector of VL • 1915 – 1939 Development of Anti-leishmanial Drugs
Historical Milestones • 1824 – First Disease Description • 1903 – Discovery of causative agent by W. B. Leishman and C. Donovan • 1904 – The parasite was cultured in vitro by Rogers and morphology, cultural characteristics and protozological features of the parasite was described. • 1921 – Naiper developed serological test (Aldehyde test) for diagnosis of VL • 1940 – Swaminath proved that P. argentipes was the vector of VL • 1915 – 1939 Development of Anti-leishmanial Drugs
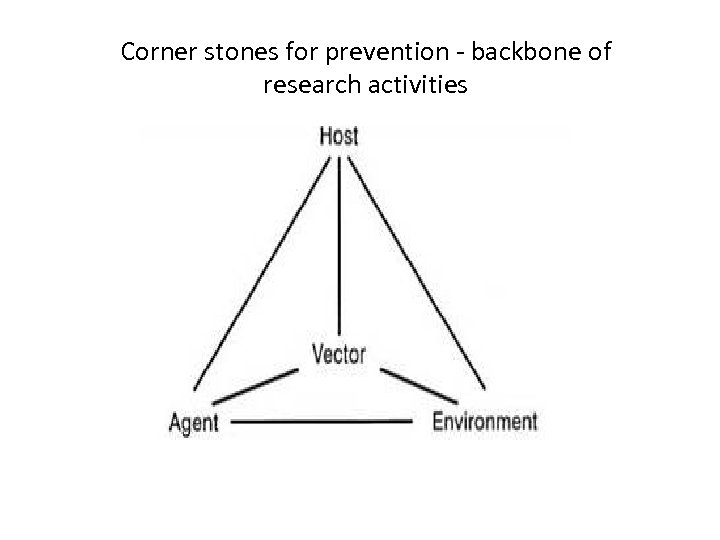 Corner stones for prevention - backbone of research activities
Corner stones for prevention - backbone of research activities
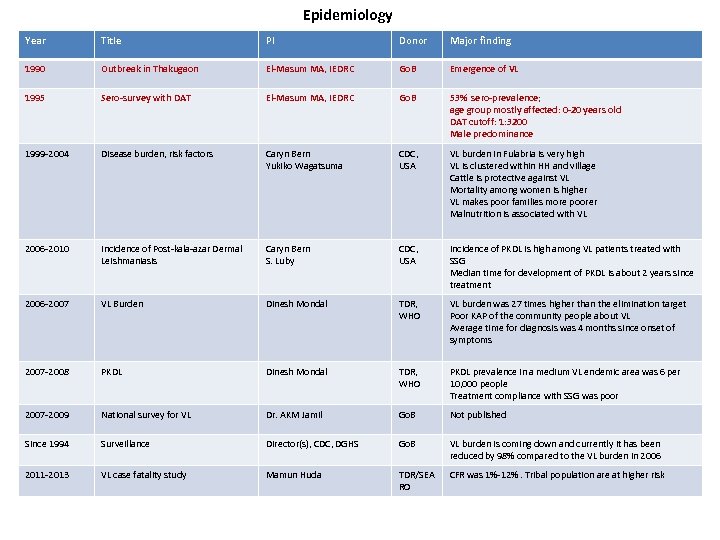 Epidemiology Year Title PI Donor Major finding 1990 Outbreak in Thakugaon El-Masum MA, IEDRC Go. B Emergence of VL 1995 Sero-survey with DAT El-Masum MA, IEDRC Go. B 53% sero-prevalence; age group mostly affected: 0 -20 years old DAT cutoff: 1: 3200 Male predominance 1999 -2004 Disease burden, risk factors Caryn Bern Yukiko Wagatsuma CDC, USA VL burden in Fulabria is very high VL is clustered within HH and village Cattle is protective against VL Mortality among women is higher VL makes poor families more poorer Malnutrition is associated with VL 2006 -2010 Incidence of Post-kala-azar Dermal Leishmaniasis Caryn Bern S. Luby CDC, USA Incidence of PKDL is high among VL patients treated with SSG Median time for development of PKDL is about 2 years since treatment 2006 -2007 VL Burden Dinesh Mondal TDR, WHO VL burden was 27 times higher than the elimination target Poor KAP of the community people about VL Average time for diagnosis was 4 months since onset of symptoms 2007 -2008 PKDL Dinesh Mondal TDR, WHO PKDL prevalence in a medium VL endemic area was 6 per 10, 000 people Treatment compliance with SSG was poor 2007 -2009 National survey for VL Dr. AKM Jamil Go. B Not published Since 1994 Surveillance Director(s), CDC, DGHS Go. B VL burden is coming down and currently it has been reduced by 98% compared to the VL burden in 2006 2011 -2013 VL case fatality study Mamun Huda TDR/SEA RO CFR was 1%-12%. Tribal population are at higher risk
Epidemiology Year Title PI Donor Major finding 1990 Outbreak in Thakugaon El-Masum MA, IEDRC Go. B Emergence of VL 1995 Sero-survey with DAT El-Masum MA, IEDRC Go. B 53% sero-prevalence; age group mostly affected: 0 -20 years old DAT cutoff: 1: 3200 Male predominance 1999 -2004 Disease burden, risk factors Caryn Bern Yukiko Wagatsuma CDC, USA VL burden in Fulabria is very high VL is clustered within HH and village Cattle is protective against VL Mortality among women is higher VL makes poor families more poorer Malnutrition is associated with VL 2006 -2010 Incidence of Post-kala-azar Dermal Leishmaniasis Caryn Bern S. Luby CDC, USA Incidence of PKDL is high among VL patients treated with SSG Median time for development of PKDL is about 2 years since treatment 2006 -2007 VL Burden Dinesh Mondal TDR, WHO VL burden was 27 times higher than the elimination target Poor KAP of the community people about VL Average time for diagnosis was 4 months since onset of symptoms 2007 -2008 PKDL Dinesh Mondal TDR, WHO PKDL prevalence in a medium VL endemic area was 6 per 10, 000 people Treatment compliance with SSG was poor 2007 -2009 National survey for VL Dr. AKM Jamil Go. B Not published Since 1994 Surveillance Director(s), CDC, DGHS Go. B VL burden is coming down and currently it has been reduced by 98% compared to the VL burden in 2006 2011 -2013 VL case fatality study Mamun Huda TDR/SEA RO CFR was 1%-12%. Tribal population are at higher risk
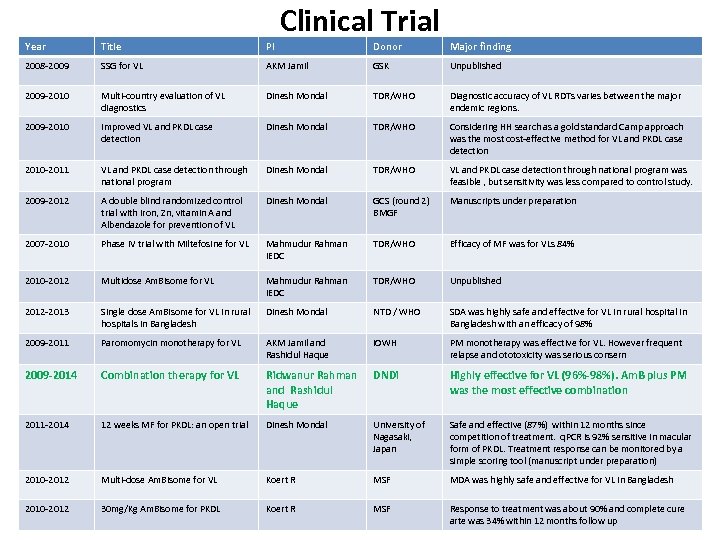 Clinical Trial Year Title PI Donor Major finding 2008 -2009 SSG for VL AKM Jamil GSK Unpublished 2009 -2010 Multi-country evaluation of VL diagnostics Dinesh Mondal TDR/WHO Diagnostic accuracy of VL RDTs varies between the major endemic regions. 2009 -2010 Improved VL and PKDL case detection Dinesh Mondal TDR/WHO Considering HH search as a gold standard Camp approach was the most cost-effective method for VL and PKDL case detection 2010 -2011 VL and PKDL case detection through national program Dinesh Mondal TDR/WHO VL and PKDL case detection through national program was feasible , but sensitivity was less compared to control study. 2009 -2012 A double blind randomized control trial with iron, Zn, vitamin A and Albendazole for prevention of VL Dinesh Mondal GCS (round 2) BMGF Manuscripts under preparation 2007 -2010 Phase IV trial with Miltefosine for VL Mahmudur Rahman IEDC TDR/WHO Efficacy of MF was for VLs 84% 2010 -2012 Multidose Am. Bisome for VL Mahmudur Rahman IEDC TDR/WHO Unpublished 2012 -2013 Single dose Am. Bisome for VL in rural hospitals in Bangladesh Dinesh Mondal NTD / WHO SDA was highly safe and effective for VL in rural hospital in Bangladesh with an efficacy of 98% 2009 -2011 Paromomycin monotherapy for VL AKM Jamil and Rashidul Haque i. OWH PM monotherapy was effective for VL. However frequent relapse and ototoxicity was serious consern 2009 -2014 Combination therapy for VL Ridwanur Rahman and Rashidul Haque DNDi Highly effective for VL (96%-98%). Am. B plus PM was the most effective combination 2011 -2014 12 weeks MF for PKDL: an open trial Dinesh Mondal University of Nagasaki, Japan Safe and effective (87%) within 12 months since competition of treatment. q. PCR is 92% sensitive in macular form of PKDL. Treatment response can be monitored by a simple scoring tool (manuscript under preparation) 2010 -2012 Multi-dose Am. Bisome for VL Koert R MSF MDA was highly safe and effective for VL in Bangladesh 2010 -2012 30 mg/Kg Am. Bisome for PKDL Koert R MSF Response to treatment was about 90% and complete cure arte was 34% within 12 months follow up
Clinical Trial Year Title PI Donor Major finding 2008 -2009 SSG for VL AKM Jamil GSK Unpublished 2009 -2010 Multi-country evaluation of VL diagnostics Dinesh Mondal TDR/WHO Diagnostic accuracy of VL RDTs varies between the major endemic regions. 2009 -2010 Improved VL and PKDL case detection Dinesh Mondal TDR/WHO Considering HH search as a gold standard Camp approach was the most cost-effective method for VL and PKDL case detection 2010 -2011 VL and PKDL case detection through national program Dinesh Mondal TDR/WHO VL and PKDL case detection through national program was feasible , but sensitivity was less compared to control study. 2009 -2012 A double blind randomized control trial with iron, Zn, vitamin A and Albendazole for prevention of VL Dinesh Mondal GCS (round 2) BMGF Manuscripts under preparation 2007 -2010 Phase IV trial with Miltefosine for VL Mahmudur Rahman IEDC TDR/WHO Efficacy of MF was for VLs 84% 2010 -2012 Multidose Am. Bisome for VL Mahmudur Rahman IEDC TDR/WHO Unpublished 2012 -2013 Single dose Am. Bisome for VL in rural hospitals in Bangladesh Dinesh Mondal NTD / WHO SDA was highly safe and effective for VL in rural hospital in Bangladesh with an efficacy of 98% 2009 -2011 Paromomycin monotherapy for VL AKM Jamil and Rashidul Haque i. OWH PM monotherapy was effective for VL. However frequent relapse and ototoxicity was serious consern 2009 -2014 Combination therapy for VL Ridwanur Rahman and Rashidul Haque DNDi Highly effective for VL (96%-98%). Am. B plus PM was the most effective combination 2011 -2014 12 weeks MF for PKDL: an open trial Dinesh Mondal University of Nagasaki, Japan Safe and effective (87%) within 12 months since competition of treatment. q. PCR is 92% sensitive in macular form of PKDL. Treatment response can be monitored by a simple scoring tool (manuscript under preparation) 2010 -2012 Multi-dose Am. Bisome for VL Koert R MSF MDA was highly safe and effective for VL in Bangladesh 2010 -2012 30 mg/Kg Am. Bisome for PKDL Koert R MSF Response to treatment was about 90% and complete cure arte was 34% within 12 months follow up
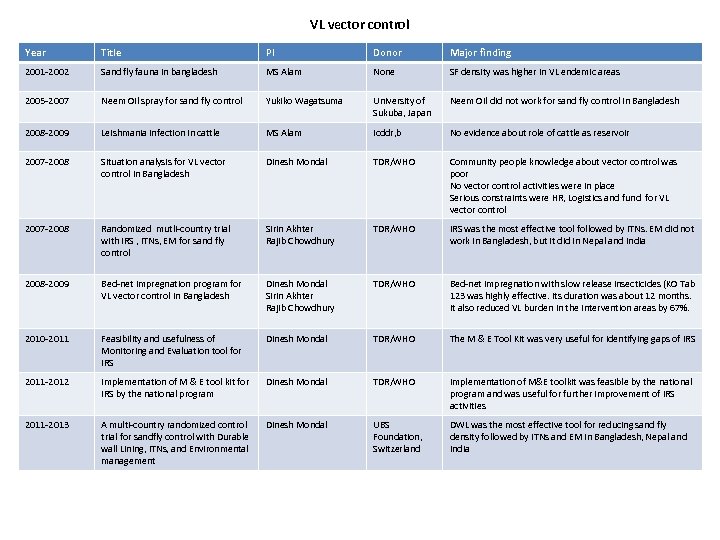 VL vector control Year Title PI Donor Major finding 2001 -2002 Sand fly fauna in bangladesh MS Alam None SF density was higher in VL endemic areas 2005 -2007 Neem Oil spray for sand fly control Yukiko Wagatsuma University of Sukuba, Japan Neem Oil did not work for sand fly control in Bangladesh 2008 -2009 Leishmania infection in cattle MS Alam Icddr, b No evidence about role of cattle as reservoir 2007 -2008 Situation analysis for VL vector control in Bangladesh Dinesh Mondal TDR/WHO Community people knowledge about vector control was poor No vector control activities were in place Serious constraints were HR, Logistics and fund for VL vector control 2007 -2008 Randomized mutli-country trial with IRS , ITNs, EM for sand fly control Sirin Akhter Rajib Chowdhury TDR/WHO IRS was the most effective tool followed by ITNs. EM did not work in Bangladesh, but it did in Nepal and India 2008 -2009 Bed-net impregnation program for VL vector control in Bangladesh Dinesh Mondal Sirin Akhter Rajib Chowdhury TDR/WHO Bed-net impregnation with slow release insecticides (KO Tab 123 was highly effective. Its duration was about 12 months. It also reduced VL burden in the intervention areas by 67%. 2010 -2011 Feasibility and usefulness of Monitoring and Evaluation tool for IRS Dinesh Mondal TDR/WHO The M & E Tool Kit was very useful for identifying gaps of IRS 2011 -2012 Implementation of M & E tool kit for IRS by the national program Dinesh Mondal TDR/WHO Implementation of M&E toolkit was feasible by the national program and was useful for further improvement of IRS activities 2011 -2013 A multi-country randomized control trial for sandfly control with Durable wall Lining, ITNs, and Environmental management Dinesh Mondal UBS Foundation, Switzerland DWL was the most effective tool for reducing sand fly density followed by ITNs and EM in Bangladesh, Nepal and India
VL vector control Year Title PI Donor Major finding 2001 -2002 Sand fly fauna in bangladesh MS Alam None SF density was higher in VL endemic areas 2005 -2007 Neem Oil spray for sand fly control Yukiko Wagatsuma University of Sukuba, Japan Neem Oil did not work for sand fly control in Bangladesh 2008 -2009 Leishmania infection in cattle MS Alam Icddr, b No evidence about role of cattle as reservoir 2007 -2008 Situation analysis for VL vector control in Bangladesh Dinesh Mondal TDR/WHO Community people knowledge about vector control was poor No vector control activities were in place Serious constraints were HR, Logistics and fund for VL vector control 2007 -2008 Randomized mutli-country trial with IRS , ITNs, EM for sand fly control Sirin Akhter Rajib Chowdhury TDR/WHO IRS was the most effective tool followed by ITNs. EM did not work in Bangladesh, but it did in Nepal and India 2008 -2009 Bed-net impregnation program for VL vector control in Bangladesh Dinesh Mondal Sirin Akhter Rajib Chowdhury TDR/WHO Bed-net impregnation with slow release insecticides (KO Tab 123 was highly effective. Its duration was about 12 months. It also reduced VL burden in the intervention areas by 67%. 2010 -2011 Feasibility and usefulness of Monitoring and Evaluation tool for IRS Dinesh Mondal TDR/WHO The M & E Tool Kit was very useful for identifying gaps of IRS 2011 -2012 Implementation of M & E tool kit for IRS by the national program Dinesh Mondal TDR/WHO Implementation of M&E toolkit was feasible by the national program and was useful for further improvement of IRS activities 2011 -2013 A multi-country randomized control trial for sandfly control with Durable wall Lining, ITNs, and Environmental management Dinesh Mondal UBS Foundation, Switzerland DWL was the most effective tool for reducing sand fly density followed by ITNs and EM in Bangladesh, Nepal and India
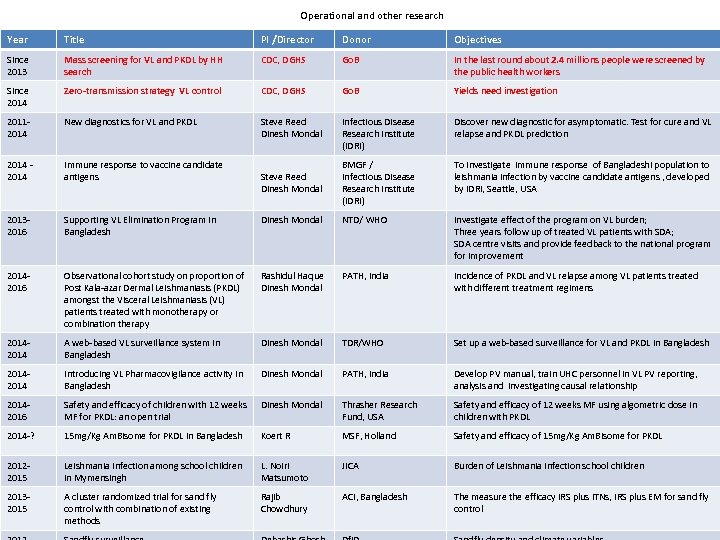 Operational and other research Year Title PI /Director Donor Objectives Since 2013 Mass screening for VL and PKDL by HH search CDC, DGHS Go. B In the last round about 2. 4 millions people were screened by the public health workers Since 2014 Zero-transmission strategy VL control CDC, DGHS Go. B Yields need investigation 20112014 New diagnostics for VL and PKDL Steve Reed Dinesh Mondal Infectious Disease Research Institute (IDRI) Discover new diagnostic for asymptomatic. Test for cure and VL relapse and PKDL prediction 2014 - 2014 Immune response to vaccine candidate antigens Steve Reed Dinesh Mondal BMGF / Infectious Disease Research Institute (IDRI) To investigate immune response of Bangladeshi population to leishmania infection by vaccine candidate antigens , developed by IDRI, Seattle, USA 20132016 Supporting VL Elimination Program in Bangladesh Dinesh Mondal NTD/ WHO Investigate effect of the program on VL burden; Three years follow up of treated VL patients with SDA; SDA centre visits and provide feedback to the national program for improvement 20142016 Observational cohort study on proportion of Post Kala-azar Dermal Leishmaniasis (PKDL) amongst the Visceral Leishmaniasis (VL) patients treated with monotherapy or combination therapy Rashidul Haque Dinesh Mondal PATH, India Incidence of PKDL and VL relapse among VL patients treated with different treatment regimens 2014 A web-based VL surveillance system in Bangladesh Dinesh Mondal TDR/WHO Set up a web-based surveillance for VL and PKDL in Bangladesh 2014 Introducing VL Pharmacovigilance activity in Bangladesh Dinesh Mondal PATH, India Develop PV manual, train UHC personnel in VL PV reporting, analysis and investigating causal relationship 20142016 Safety and efficacy of children with 12 weeks MF for PKDL: an open trial Dinesh Mondal Thrasher Research Fund, USA Safety and efficacy of 12 weeks MF using algometric dose in children with PKDL 2014 -? 15 mg/Kg Am. Bisome for PKDL in Bangladesh Koert R MSF, Holland Safety and efficacy of 15 mg/Kg Am. Bisome for PKDL 20122015 Leishmania Infection among school children in Mymensingh L. Noiri Matsumoto JICA Burden of Leishmania infection school children 20132015 A cluster randomized trial for sand fly control with combination of existing methods Rajib Chowdhury ACI, Bangladesh The measure the efficacy IRS plus ITNs, IRS plus EM for sand fly control
Operational and other research Year Title PI /Director Donor Objectives Since 2013 Mass screening for VL and PKDL by HH search CDC, DGHS Go. B In the last round about 2. 4 millions people were screened by the public health workers Since 2014 Zero-transmission strategy VL control CDC, DGHS Go. B Yields need investigation 20112014 New diagnostics for VL and PKDL Steve Reed Dinesh Mondal Infectious Disease Research Institute (IDRI) Discover new diagnostic for asymptomatic. Test for cure and VL relapse and PKDL prediction 2014 - 2014 Immune response to vaccine candidate antigens Steve Reed Dinesh Mondal BMGF / Infectious Disease Research Institute (IDRI) To investigate immune response of Bangladeshi population to leishmania infection by vaccine candidate antigens , developed by IDRI, Seattle, USA 20132016 Supporting VL Elimination Program in Bangladesh Dinesh Mondal NTD/ WHO Investigate effect of the program on VL burden; Three years follow up of treated VL patients with SDA; SDA centre visits and provide feedback to the national program for improvement 20142016 Observational cohort study on proportion of Post Kala-azar Dermal Leishmaniasis (PKDL) amongst the Visceral Leishmaniasis (VL) patients treated with monotherapy or combination therapy Rashidul Haque Dinesh Mondal PATH, India Incidence of PKDL and VL relapse among VL patients treated with different treatment regimens 2014 A web-based VL surveillance system in Bangladesh Dinesh Mondal TDR/WHO Set up a web-based surveillance for VL and PKDL in Bangladesh 2014 Introducing VL Pharmacovigilance activity in Bangladesh Dinesh Mondal PATH, India Develop PV manual, train UHC personnel in VL PV reporting, analysis and investigating causal relationship 20142016 Safety and efficacy of children with 12 weeks MF for PKDL: an open trial Dinesh Mondal Thrasher Research Fund, USA Safety and efficacy of 12 weeks MF using algometric dose in children with PKDL 2014 -? 15 mg/Kg Am. Bisome for PKDL in Bangladesh Koert R MSF, Holland Safety and efficacy of 15 mg/Kg Am. Bisome for PKDL 20122015 Leishmania Infection among school children in Mymensingh L. Noiri Matsumoto JICA Burden of Leishmania infection school children 20132015 A cluster randomized trial for sand fly control with combination of existing methods Rajib Chowdhury ACI, Bangladesh The measure the efficacy IRS plus ITNs, IRS plus EM for sand fly control
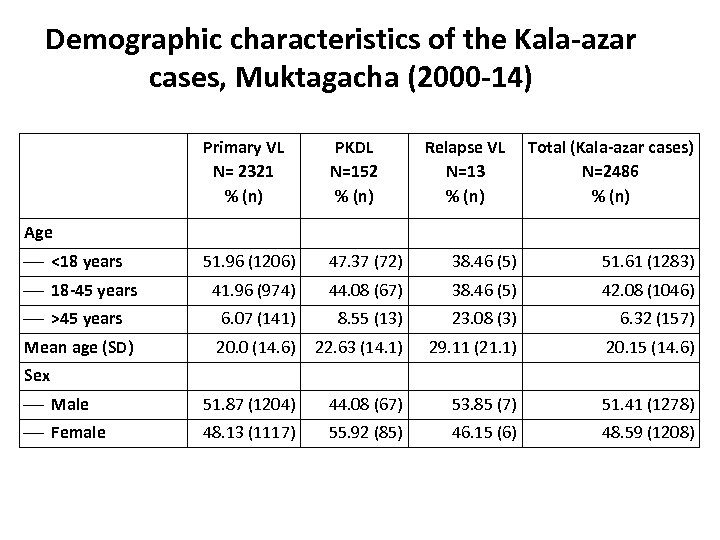 Demographic characteristics of the Kala-azar cases, Muktagacha (2000 -14) Primary VL N= 2321 % (n) PKDL N=152 % (n) Relapse VL N=13 % (n) Total (Kala-azar cases) N=2486 % (n) 51. 96 (1206) 47. 37 (72) 38. 46 (5) 51. 61 (1283) 41. 96 (974) 44. 08 (67) 38. 46 (5) 42. 08 (1046) >45 years 6. 07 (141) 8. 55 (13) 23. 08 (3) 6. 32 (157) Mean age (SD) 20. 0 (14. 6) 22. 63 (14. 1) 29. 11 (21. 1) 20. 15 (14. 6) Male 51. 87 (1204) 44. 08 (67) 53. 85 (7) 51. 41 (1278) Female 48. 13 (1117) 55. 92 (85) 46. 15 (6) 48. 59 (1208) Age <18 years 18 -45 years Sex
Demographic characteristics of the Kala-azar cases, Muktagacha (2000 -14) Primary VL N= 2321 % (n) PKDL N=152 % (n) Relapse VL N=13 % (n) Total (Kala-azar cases) N=2486 % (n) 51. 96 (1206) 47. 37 (72) 38. 46 (5) 51. 61 (1283) 41. 96 (974) 44. 08 (67) 38. 46 (5) 42. 08 (1046) >45 years 6. 07 (141) 8. 55 (13) 23. 08 (3) 6. 32 (157) Mean age (SD) 20. 0 (14. 6) 22. 63 (14. 1) 29. 11 (21. 1) 20. 15 (14. 6) Male 51. 87 (1204) 44. 08 (67) 53. 85 (7) 51. 41 (1278) Female 48. 13 (1117) 55. 92 (85) 46. 15 (6) 48. 59 (1208) Age <18 years 18 -45 years Sex
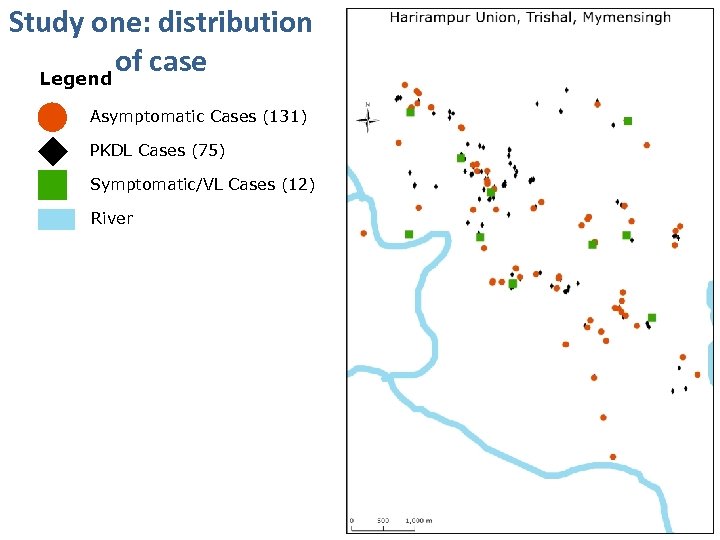 Study one: distribution of case Legend Asymptomatic Cases (131) PKDL Cases (75) Symptomatic/VL Cases (12) River
Study one: distribution of case Legend Asymptomatic Cases (131) PKDL Cases (75) Symptomatic/VL Cases (12) River
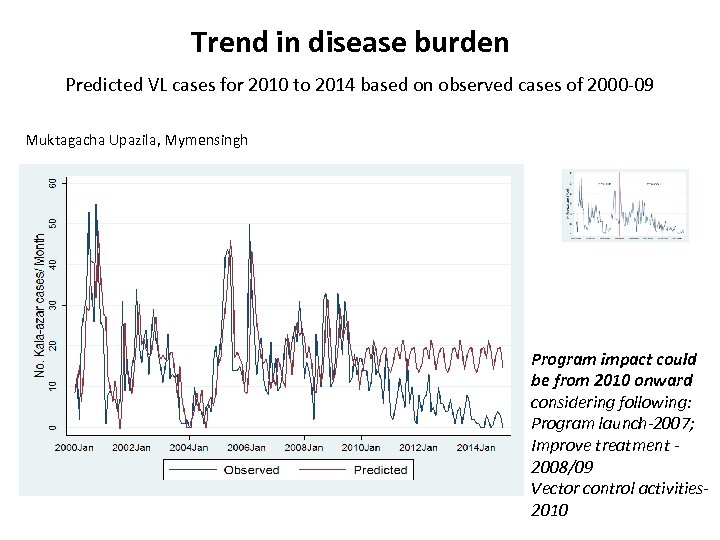 Trend in disease burden Predicted VL cases for 2010 to 2014 based on observed cases of 2000 -09 Muktagacha Upazila, Mymensingh Program impact could be from 2010 onward considering following: Program launch-2007; Improve treatment 2008/09 Vector control activities 2010
Trend in disease burden Predicted VL cases for 2010 to 2014 based on observed cases of 2000 -09 Muktagacha Upazila, Mymensingh Program impact could be from 2010 onward considering following: Program launch-2007; Improve treatment 2008/09 Vector control activities 2010
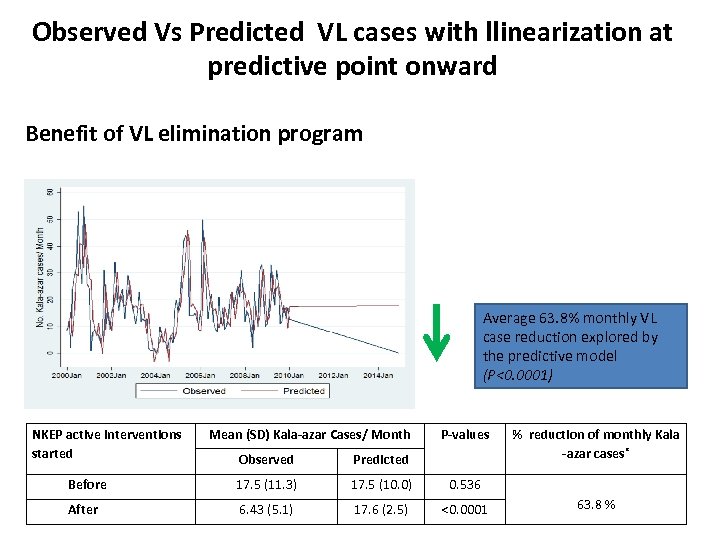 Observed Vs Predicted VL cases with llinearization at predictive point onward Benefit of VL elimination program Average 63. 8% monthly VL case reduction explored by the predictive model (P<0. 0001) NKEP active interventions started Mean (SD) Kala-azar Cases/ Month P-values Observed Predicted Before 17. 5 (11. 3) 17. 5 (10. 0) 0. 536 After 6. 43 (5. 1) 17. 6 (2. 5) <0. 0001 % reduction of monthly Kala -azar cases* 63. 8 %
Observed Vs Predicted VL cases with llinearization at predictive point onward Benefit of VL elimination program Average 63. 8% monthly VL case reduction explored by the predictive model (P<0. 0001) NKEP active interventions started Mean (SD) Kala-azar Cases/ Month P-values Observed Predicted Before 17. 5 (11. 3) 17. 5 (10. 0) 0. 536 After 6. 43 (5. 1) 17. 6 (2. 5) <0. 0001 % reduction of monthly Kala -azar cases* 63. 8 %
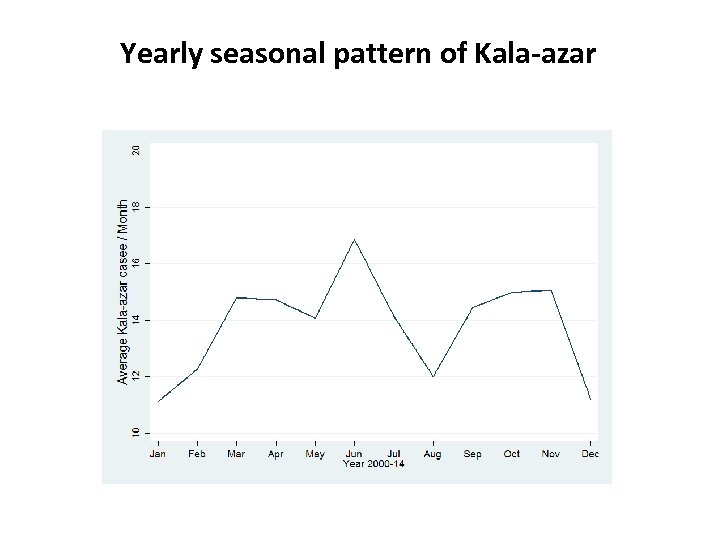 Yearly seasonal pattern of Kala-azar
Yearly seasonal pattern of Kala-azar
 Breeding Places of Sandfly
Breeding Places of Sandfly
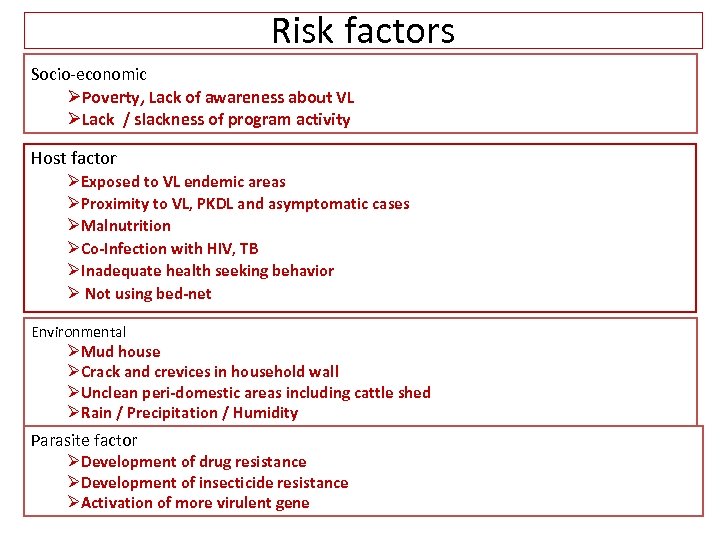 Risk factors Socio-economic ØPoverty, Lack of awareness about VL ØLack / slackness of program activity Host factor ØExposed to VL endemic areas ØProximity to VL, PKDL and asymptomatic cases ØMalnutrition ØCo-Infection with HIV, TB ØInadequate health seeking behavior Ø Not using bed-net Environmental ØMud house ØCrack and crevices in household wall ØUnclean peri-domestic areas including cattle shed ØRain / Precipitation / Humidity Parasite factor ØDevelopment of drug resistance ØDevelopment of insecticide resistance ØActivation of more virulent gene
Risk factors Socio-economic ØPoverty, Lack of awareness about VL ØLack / slackness of program activity Host factor ØExposed to VL endemic areas ØProximity to VL, PKDL and asymptomatic cases ØMalnutrition ØCo-Infection with HIV, TB ØInadequate health seeking behavior Ø Not using bed-net Environmental ØMud house ØCrack and crevices in household wall ØUnclean peri-domestic areas including cattle shed ØRain / Precipitation / Humidity Parasite factor ØDevelopment of drug resistance ØDevelopment of insecticide resistance ØActivation of more virulent gene
 Early VL and PKDL case detection
Early VL and PKDL case detection
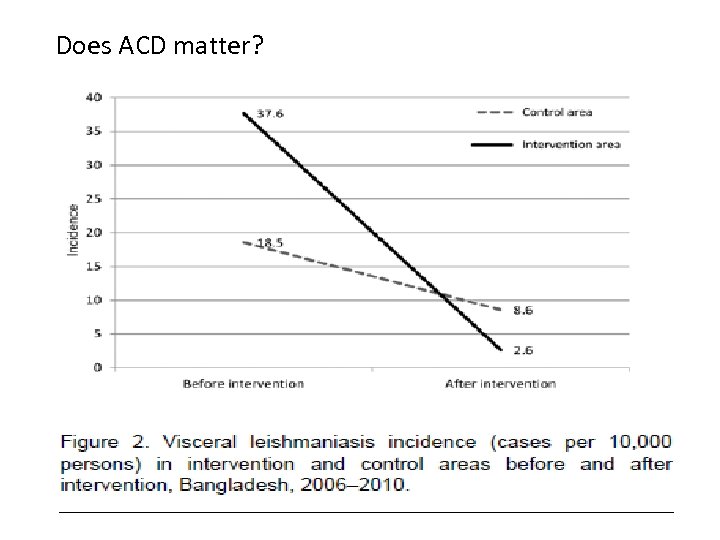 Does ACD matter?
Does ACD matter?
 How to do ACD? So far different types of ACD are proposed and investigated: 1. Blanket approach / House to House search (Gold Standard) 2. Camp approach (all HHs in a village are invited) 3. Focal approach / index case based approach (60 HHs around an index case) 4. Incentive based approach (private / NGO health worker based) 5. Accelerated ACD (all HHs in a village from where recent / current case is reported) 6. No Transmission Strategy / activity (index case-based approach plus deploying IRS and larvicide in 60 HHs) 7. Combined camp approach (camp approach for VL, PKDL, leprosy, filaria, TB, malaria etc plus vector control)
How to do ACD? So far different types of ACD are proposed and investigated: 1. Blanket approach / House to House search (Gold Standard) 2. Camp approach (all HHs in a village are invited) 3. Focal approach / index case based approach (60 HHs around an index case) 4. Incentive based approach (private / NGO health worker based) 5. Accelerated ACD (all HHs in a village from where recent / current case is reported) 6. No Transmission Strategy / activity (index case-based approach plus deploying IRS and larvicide in 60 HHs) 7. Combined camp approach (camp approach for VL, PKDL, leprosy, filaria, TB, malaria etc plus vector control)
 Referral Centre (Close to you is in red) Sl No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Treatment Center Fulbaria UHC Trishal UHC SKKRC Muktagaccha UHC Bhaluka UHC Gafforgaon UHC Modhupur UHC Sakhipur UHC Kalihati UHC Nagarpur UHC Ghatail UHC Madargonj UHC Bera UHC Chatmohor UHC Mohadebpur UHC Pirgonj UHC Ghoraghat UHC Parbatipur UHC Godagari UHC Tanore UHC Terokhada UHC Daulatpur UHC Pangsha UHC Contact No. 01730324521 01730324529 09165582 01730324527 01730324519 01730324523 01730324567 01730324569 01730324565 01730324568 01730324563 01730324490 01730324691 01730324672 01730324714 01730324640 01730324645 01730324704 01730324708 01730324601 01730324603 01730324550
Referral Centre (Close to you is in red) Sl No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Treatment Center Fulbaria UHC Trishal UHC SKKRC Muktagaccha UHC Bhaluka UHC Gafforgaon UHC Modhupur UHC Sakhipur UHC Kalihati UHC Nagarpur UHC Ghatail UHC Madargonj UHC Bera UHC Chatmohor UHC Mohadebpur UHC Pirgonj UHC Ghoraghat UHC Parbatipur UHC Godagari UHC Tanore UHC Terokhada UHC Daulatpur UHC Pangsha UHC Contact No. 01730324521 01730324529 09165582 01730324527 01730324519 01730324523 01730324567 01730324569 01730324565 01730324568 01730324563 01730324490 01730324691 01730324672 01730324714 01730324640 01730324645 01730324704 01730324708 01730324601 01730324603 01730324550
 Diagnostic tools q. Microscopy : Tissue aspirate; peripheral blood buffy coat q. Serological methods: r. K 39, r. K 28, r. KRP 42 by RDT / ELISA using serum, saliva, urine q. Molecular methods: Ln-PCR, q. PCR, LAMP, RPA
Diagnostic tools q. Microscopy : Tissue aspirate; peripheral blood buffy coat q. Serological methods: r. K 39, r. K 28, r. KRP 42 by RDT / ELISA using serum, saliva, urine q. Molecular methods: Ln-PCR, q. PCR, LAMP, RPA
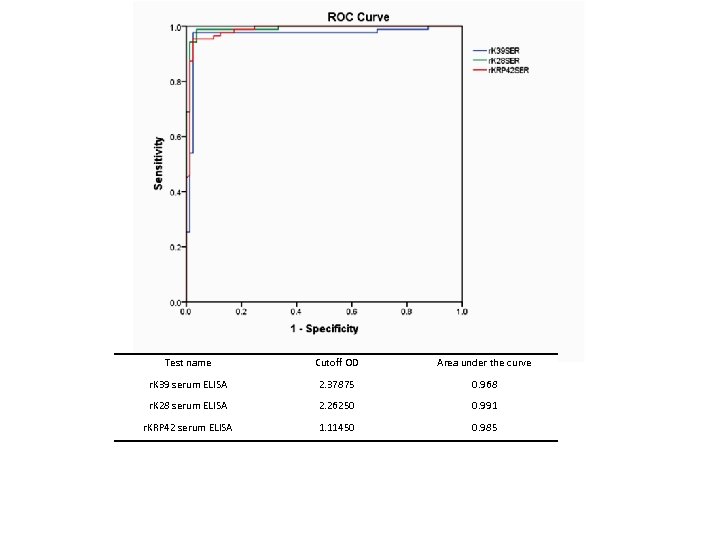 Test name Cutoff OD Area under the curve r. K 39 serum ELISA 2. 37875 0. 968 r. K 28 serum ELISA 2. 26250 0. 991 r. KRP 42 serum ELISA 1. 11450 0. 985
Test name Cutoff OD Area under the curve r. K 39 serum ELISA 2. 37875 0. 968 r. K 28 serum ELISA 2. 26250 0. 991 r. KRP 42 serum ELISA 1. 11450 0. 985
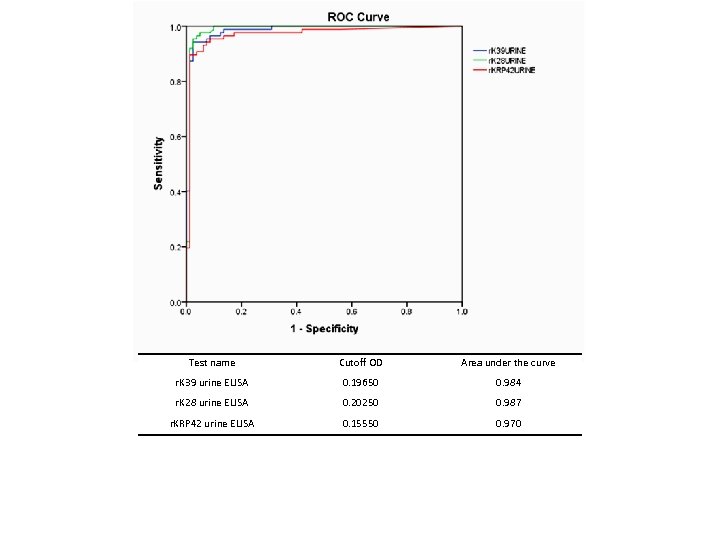 Test name Cutoff OD Area under the curve r. K 39 urine ELISA 0. 19650 0. 984 r. K 28 urine ELISA 0. 20250 0. 987 r. KRP 42 urine ELISA 0. 15550 0. 970
Test name Cutoff OD Area under the curve r. K 39 urine ELISA 0. 19650 0. 984 r. K 28 urine ELISA 0. 20250 0. 987 r. KRP 42 urine ELISA 0. 15550 0. 970
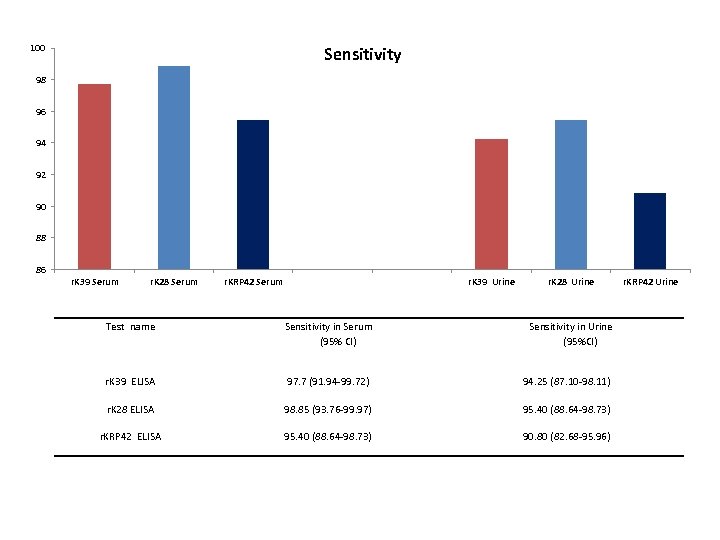 100 Sensitivity 98 96 94 92 90 88 86 r. K 39 Serum r. K 28 Serum r. KRP 42 Serum r. K 39 Urine r. K 28 Urine Test name Sensitivity in Serum (95% CI) Sensitivity in Urine (95%CI) r. K 39 ELISA 97. 7 (91. 94 -99. 72) 94. 25 (87. 10 -98. 11) r. K 28 ELISA 98. 85 (93. 76 -99. 97) 95. 40 (88. 64 -98. 73) r. KRP 42 ELISA 95. 40 (88. 64 -98. 73) 90. 80 (82. 68 -95. 96) r. KRP 42 Urine
100 Sensitivity 98 96 94 92 90 88 86 r. K 39 Serum r. K 28 Serum r. KRP 42 Serum r. K 39 Urine r. K 28 Urine Test name Sensitivity in Serum (95% CI) Sensitivity in Urine (95%CI) r. K 39 ELISA 97. 7 (91. 94 -99. 72) 94. 25 (87. 10 -98. 11) r. K 28 ELISA 98. 85 (93. 76 -99. 97) 95. 40 (88. 64 -98. 73) r. KRP 42 ELISA 95. 40 (88. 64 -98. 73) 90. 80 (82. 68 -95. 96) r. KRP 42 Urine
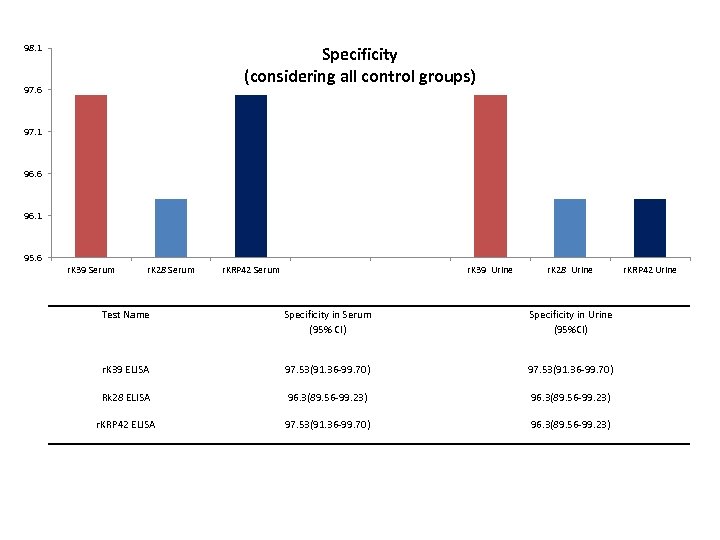 98. 1 Specificity (considering all control groups) 97. 6 97. 1 96. 6 96. 1 95. 6 r. K 39 Serum r. K 28 Serum r. KRP 42 Serum r. K 39 Urine r. K 28 Urine Test Name Specificity in Serum (95% CI) Specificity in Urine (95%CI) r. K 39 ELISA 97. 53(91. 36 -99. 70) Rk 28 ELISA 96. 3(89. 56 -99. 23) r. KRP 42 ELISA 97. 53(91. 36 -99. 70) 96. 3(89. 56 -99. 23) r. KRP 42 Urine
98. 1 Specificity (considering all control groups) 97. 6 97. 1 96. 6 96. 1 95. 6 r. K 39 Serum r. K 28 Serum r. KRP 42 Serum r. K 39 Urine r. K 28 Urine Test Name Specificity in Serum (95% CI) Specificity in Urine (95%CI) r. K 39 ELISA 97. 53(91. 36 -99. 70) Rk 28 ELISA 96. 3(89. 56 -99. 23) r. KRP 42 ELISA 97. 53(91. 36 -99. 70) 96. 3(89. 56 -99. 23) r. KRP 42 Urine
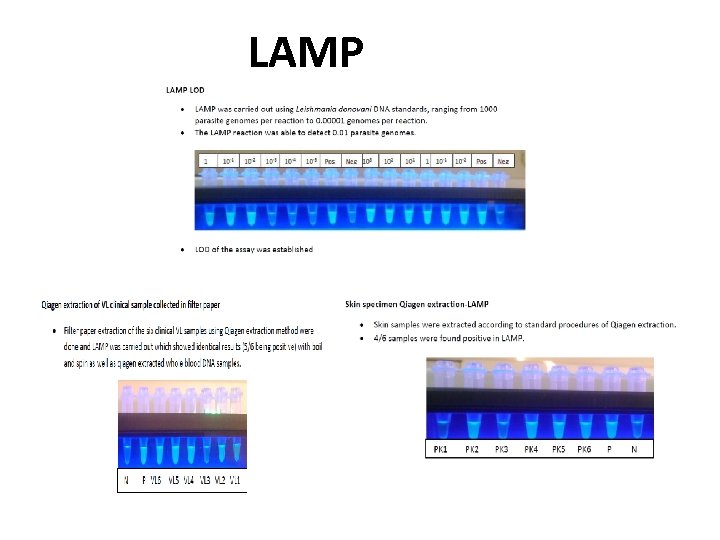 LAMP
LAMP
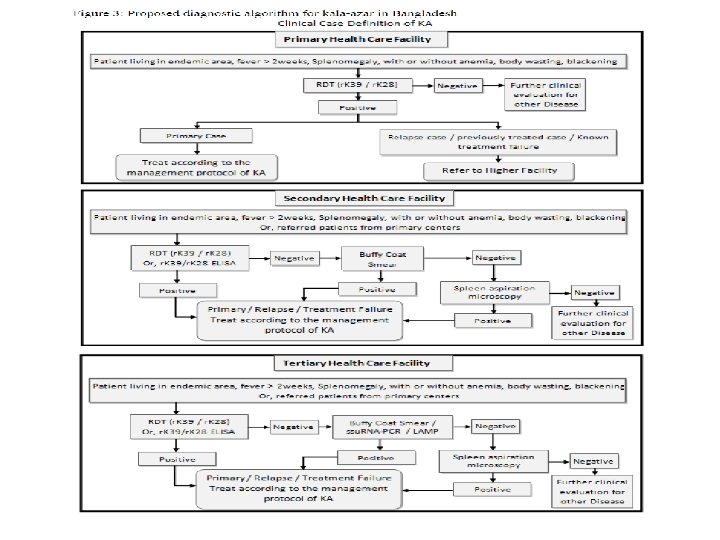
 Suitcase Lab
Suitcase Lab
 Gloves automatic pipette s ettes automatic pip Mobile Suitcase Lab Gloves Tubescanner Tips 100 µl microtube rack Disinfectant Vortex Waste Centrifuge Tips 10 µl Centrifuge Magnet Tips 100 µl Heatblock Waste Tips 1000 µl Vortex Disinfectant Scissors microtube rack extraction using the Speed. Xtract amplification and detection using the RPA assay 15 -20 minutes 15 minutes
Gloves automatic pipette s ettes automatic pip Mobile Suitcase Lab Gloves Tubescanner Tips 100 µl microtube rack Disinfectant Vortex Waste Centrifuge Tips 10 µl Centrifuge Magnet Tips 100 µl Heatblock Waste Tips 1000 µl Vortex Disinfectant Scissors microtube rack extraction using the Speed. Xtract amplification and detection using the RPA assay 15 -20 minutes 15 minutes
 TREATMENT
TREATMENT
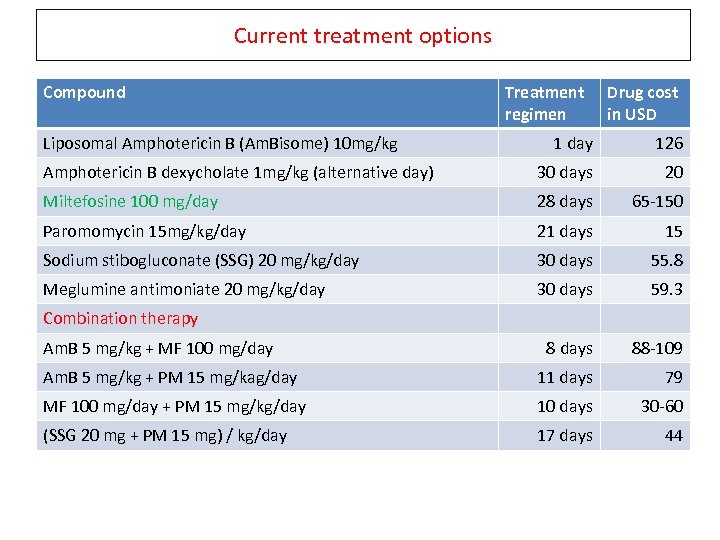 Current treatment options Compound Liposomal Amphotericin B (Am. Bisome) 10 mg/kg Treatment regimen Drug cost in USD 1 day 126 Amphotericin B dexycholate 1 mg/kg (alternative day) 30 days 20 Miltefosine 100 mg/day 28 days 65 -150 Paromomycin 15 mg/kg/day 21 days 15 Sodium stibogluconate (SSG) 20 mg/kg/day 30 days 55. 8 Meglumine antimoniate 20 mg/kg/day 30 days 59. 3 8 days 88 -109 Am. B 5 mg/kg + PM 15 mg/kag/day 11 days 79 MF 100 mg/day + PM 15 mg/kg/day 10 days 30 -60 (SSG 20 mg + PM 15 mg) / kg/day 17 days 44 Combination therapy Am. B 5 mg/kg + MF 100 mg/day
Current treatment options Compound Liposomal Amphotericin B (Am. Bisome) 10 mg/kg Treatment regimen Drug cost in USD 1 day 126 Amphotericin B dexycholate 1 mg/kg (alternative day) 30 days 20 Miltefosine 100 mg/day 28 days 65 -150 Paromomycin 15 mg/kg/day 21 days 15 Sodium stibogluconate (SSG) 20 mg/kg/day 30 days 55. 8 Meglumine antimoniate 20 mg/kg/day 30 days 59. 3 8 days 88 -109 Am. B 5 mg/kg + PM 15 mg/kag/day 11 days 79 MF 100 mg/day + PM 15 mg/kg/day 10 days 30 -60 (SSG 20 mg + PM 15 mg) / kg/day 17 days 44 Combination therapy Am. B 5 mg/kg + MF 100 mg/day
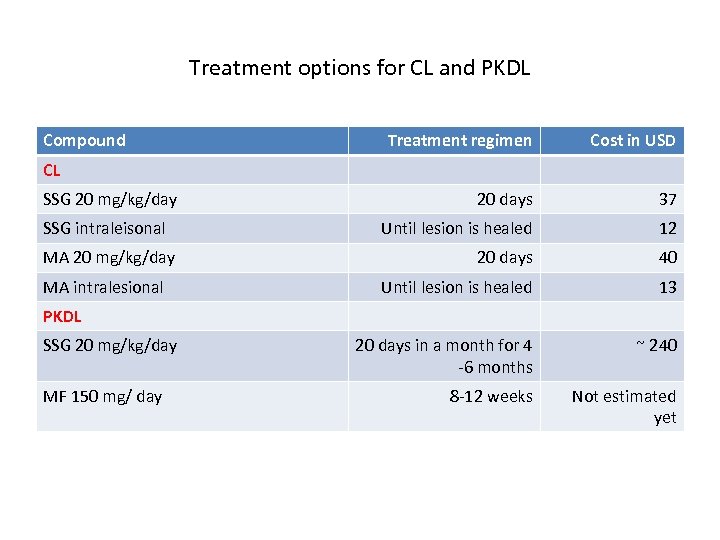 Treatment options for CL and PKDL Compound Treatment regimen Cost in USD 20 days 37 Until lesion is healed 12 20 days 40 Until lesion is healed 13 20 days in a month for 4 -6 months ~ 240 8 -12 weeks Not estimated yet CL SSG 20 mg/kg/day SSG intraleisonal MA 20 mg/kg/day MA intralesional PKDL SSG 20 mg/kg/day MF 150 mg/ day
Treatment options for CL and PKDL Compound Treatment regimen Cost in USD 20 days 37 Until lesion is healed 12 20 days 40 Until lesion is healed 13 20 days in a month for 4 -6 months ~ 240 8 -12 weeks Not estimated yet CL SSG 20 mg/kg/day SSG intraleisonal MA 20 mg/kg/day MA intralesional PKDL SSG 20 mg/kg/day MF 150 mg/ day


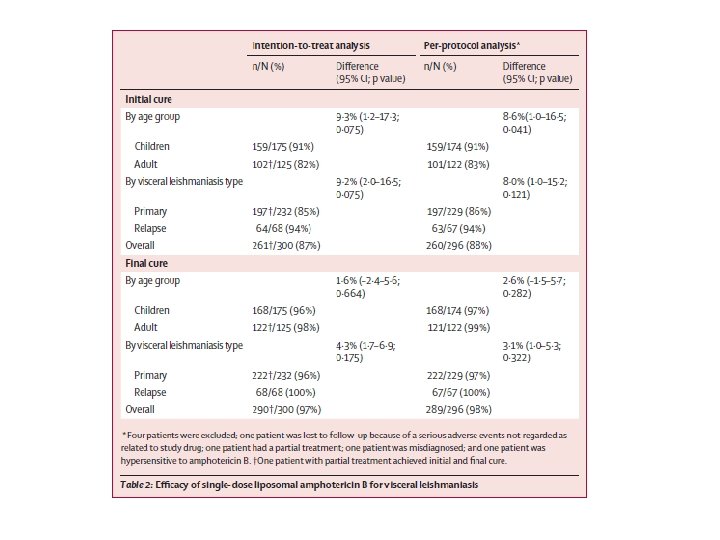
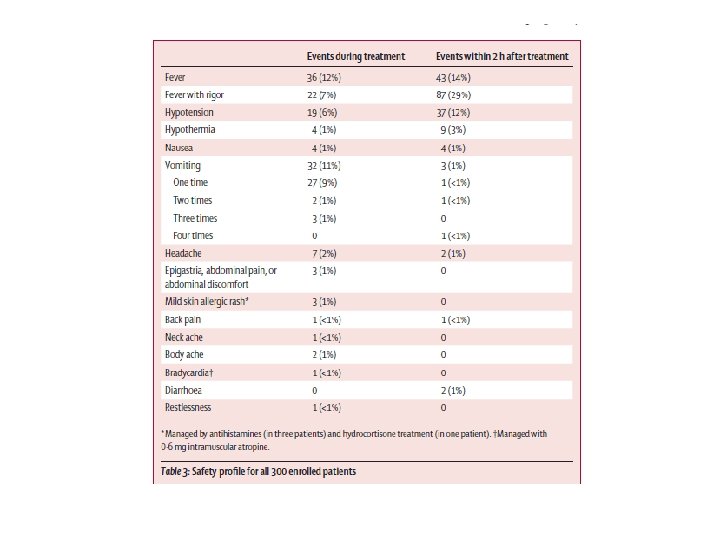
 FOLLOW UP
FOLLOW UP
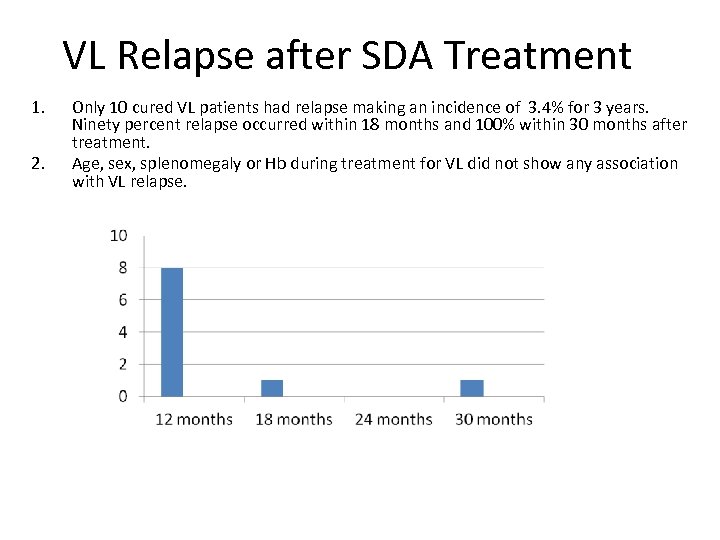 VL Relapse after SDA Treatment 1. 2. Only 10 cured VL patients had relapse making an incidence of 3. 4% for 3 years. Ninety percent relapse occurred within 18 months and 100% within 30 months after treatment. Age, sex, splenomegaly or Hb during treatment for VL did not show any association with VL relapse.
VL Relapse after SDA Treatment 1. 2. Only 10 cured VL patients had relapse making an incidence of 3. 4% for 3 years. Ninety percent relapse occurred within 18 months and 100% within 30 months after treatment. Age, sex, splenomegaly or Hb during treatment for VL did not show any association with VL relapse.
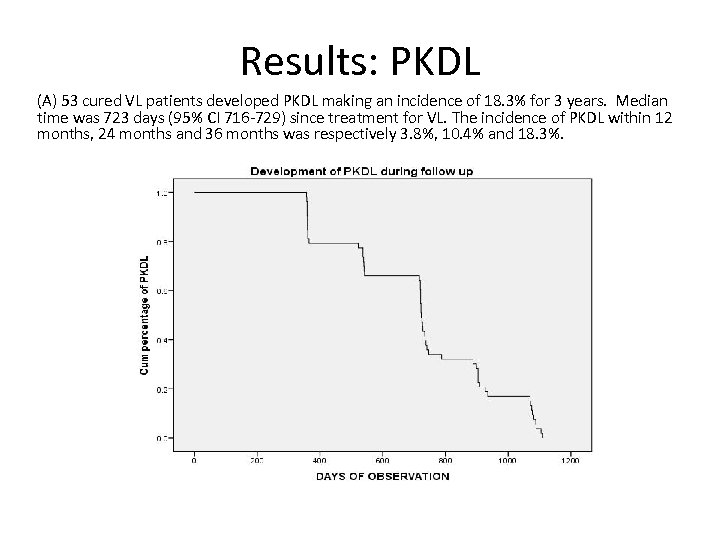 Results: PKDL (A) 53 cured VL patients developed PKDL making an incidence of 18. 3% for 3 years. Median time was 723 days (95% CI 716 -729) since treatment for VL. The incidence of PKDL within 12 months, 24 months and 36 months was respectively 3. 8%, 10. 4% and 18. 3%.
Results: PKDL (A) 53 cured VL patients developed PKDL making an incidence of 18. 3% for 3 years. Median time was 723 days (95% CI 716 -729) since treatment for VL. The incidence of PKDL within 12 months, 24 months and 36 months was respectively 3. 8%, 10. 4% and 18. 3%.
 PREVENTION
PREVENTION
 Integrated Vector Management (IVM) Indoor Residual Spraying Larvicide Spraying
Integrated Vector Management (IVM) Indoor Residual Spraying Larvicide Spraying
 WALL LINING
WALL LINING
 Bed-net Impregnation
Bed-net Impregnation
 Wall plastering with Lime
Wall plastering with Lime
 VACCINE
VACCINE
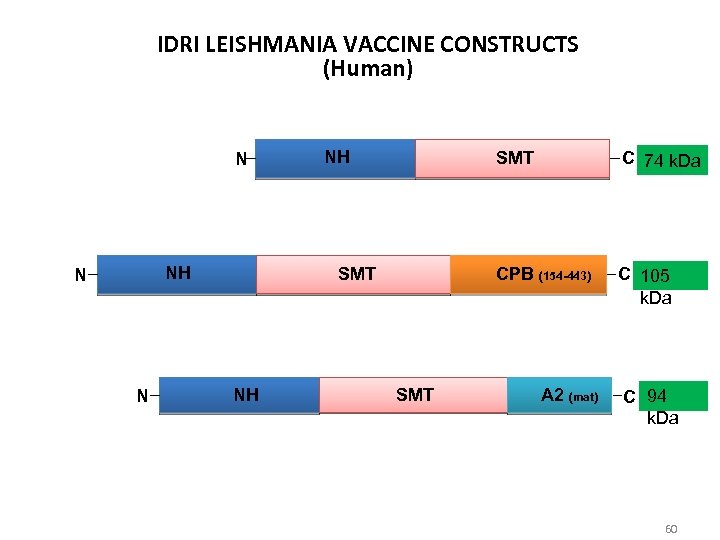 IDRI LEISHMANIA VACCINE CONSTRUCTS (Human) N NH N N NH SMT CPB (154 -443) SMT NH SMT C 74 k. Da C 105 k. Da A 2 (mat) C 94 k. Da 60
IDRI LEISHMANIA VACCINE CONSTRUCTS (Human) N NH N N NH SMT CPB (154 -443) SMT NH SMT C 74 k. Da C 105 k. Da A 2 (mat) C 94 k. Da 60
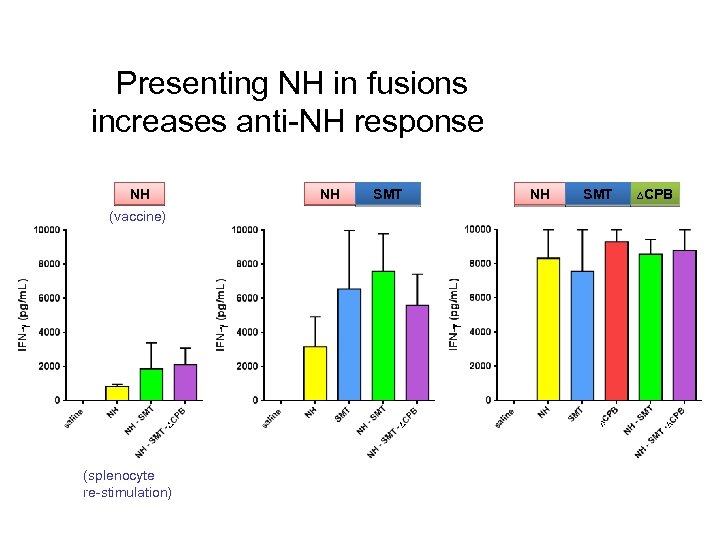 Presenting NH in fusions increases anti-NH response NH NH SMT ∆CPB (vaccine) (splenocyte re-stimulation) CONFIDENTIAL 61
Presenting NH in fusions increases anti-NH response NH NH SMT ∆CPB (vaccine) (splenocyte re-stimulation) CONFIDENTIAL 61
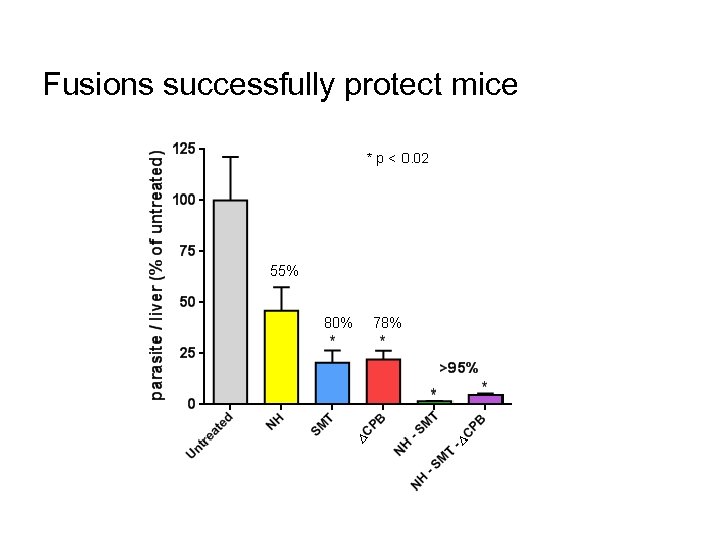 Fusions successfully protect mice CONFIDENTIAL 62
Fusions successfully protect mice CONFIDENTIAL 62
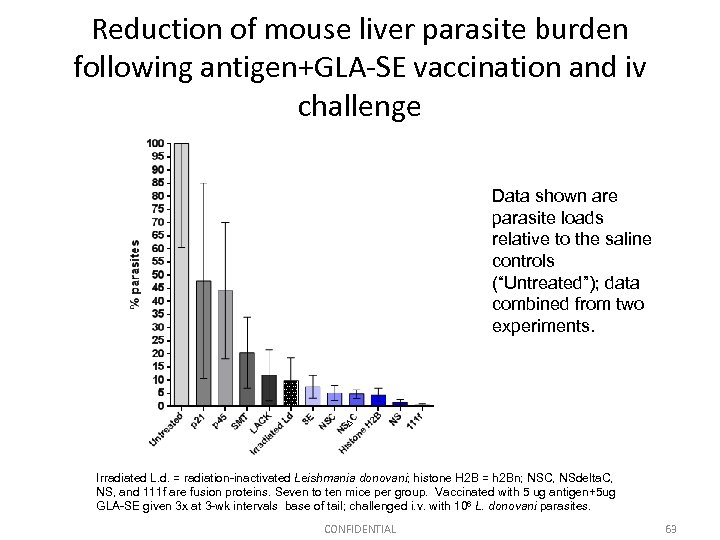 Reduction of mouse liver parasite burden following antigen+GLA-SE vaccination and iv challenge Data shown are parasite loads relative to the saline controls (“Untreated”); data combined from two experiments. Irradiated L. d. = radiation-inactivated Leishmania donovani; histone H 2 B = h 2 Bn; NSC, NSdelta. C, NS, and 111 f are fusion proteins. Seven to ten mice per group. Vaccinated with 5 ug antigen+5 ug GLA-SE given 3 x at 3 -wk intervals base of tail; challenged i. v. with 106 L. donovani parasites. CONFIDENTIAL 63
Reduction of mouse liver parasite burden following antigen+GLA-SE vaccination and iv challenge Data shown are parasite loads relative to the saline controls (“Untreated”); data combined from two experiments. Irradiated L. d. = radiation-inactivated Leishmania donovani; histone H 2 B = h 2 Bn; NSC, NSdelta. C, NS, and 111 f are fusion proteins. Seven to ten mice per group. Vaccinated with 5 ug antigen+5 ug GLA-SE given 3 x at 3 -wk intervals base of tail; challenged i. v. with 106 L. donovani parasites. CONFIDENTIAL 63
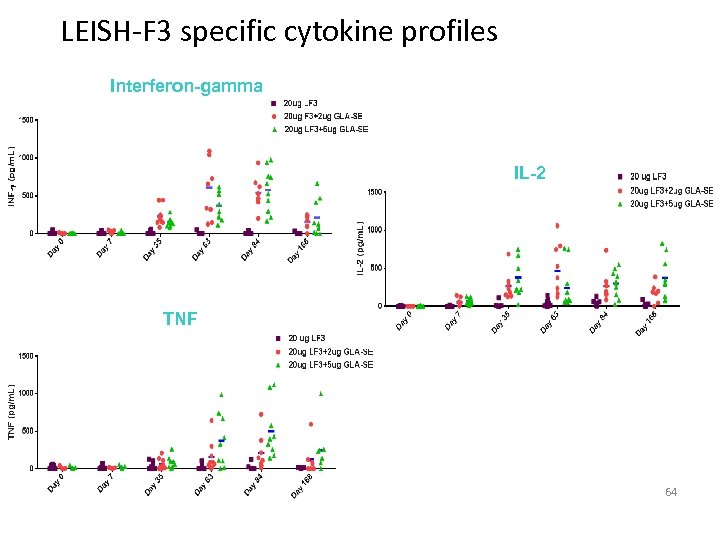 LEISH-F 3 specific cytokine profiles Interferon-gamma IL-2 TNF 64
LEISH-F 3 specific cytokine profiles Interferon-gamma IL-2 TNF 64
 THANK YOU!
THANK YOU!


