9b2d4366fc8d24816e29635cbc8b6230.ppt
- Количество слайдов: 77

Advances in the treatment of Type 2 Diabetes The role of insulin glargine

Insulin glargine, marketed by Sanofi-Aventis under the name Lantus, is a long-acting basal insulin analogue, given once daily to help control the blood sugar level of those with diabetes. It consists of microcrystals that slowly release insulin, giving a long duration of action of 18 to 26 hours, with a "peakless" profile (according to the Lantus package insert). Pharmacokinetically, it resembles basal insulin secretion of non-diabetic pancreatic beta cells. Sometimes, in type 2 diabetes and in combination with a short acting sulfonylurea (drugs which stimulate the pancreas to make more insulin), it can offer moderate control of serum glucose levels. In the absence of endogenous insulin—Type 1 diabetes, depleted type two (in some cases) or latent autoimmune diabetes of adults in late stage—Lantus needs the support of fast acting insulin taken with food to reduce the effect of prandially derived glucose.

Benefit When standard NPH is administered at night, its peak of action can coincide with the lower serum glucose levels associated with nocturnal metabolism potentially setting the stage for nocturnal hypoglycaemia. Lantus is associated with a lower risk of nocturnal hypoglycaemia. Pharmacological specifications Mechanism of action (pharmacodynamics) Insulin glargine have substitution of glycine for asparagine at A 21 and two arginines added to the carboxy terminal of B chain. The arginine amino acids shift the isoelectric point from a p. H of 5. 4 to 6. 7, making the molecule more soluble at an acidic p. H, allowing for the subcutaneous injection of a clear solution. The asparagine substitution prevents deamidization of the acidsensitive glycine at acidic p. H. In the neutral subcutaneous space, higher-order aggregates form, resulting in a slow, peakless dissolution and absorption of insulin from the site of injection. It can achieve a peakless level for at least 24 hours. Acceptance and repartition in the body (pharmacokinetic) Lantus is formulated at an acidic p. H 4, where it is completely water soluble. After subcutaneous injection of the acidic solute (which can cause discomfort and a stinging sensation), when a physiologic p. H (approximately 7. 4) is achieved the increase in p. H causes the insulin to come out of solution resulting in the formation of higher order aggregates of insulin hexamers. The higher order aggregation slows the dissociation of the hexamers into insulin monomers, the functional and physiologically active unit of insulin. This gradual process ensures that small amounts of Lantus are released into the body continuously, giving an almost peakless profile.

Usage Mixing with other insulin preparations Unlike some other longer-acting insulins, Lantus must not be diluted or mixed with other insulin or solution in the same syringe However, this restriction has been successfully challenged in trials conducted by Kaplan, Rodriguez, Smith, Haymond, and Heptulla of Texas Childrens Hospital Other information Development The development of Lantus was conducted at Sanofi-Aventis's biotechnology competence center in Frankfurt-Höchst. Sanofi supplies the product to over 100 countries and more than 3, 5 million patients worldwide. This makes Lantus Germany's largest and most important export pharmaceutical product. Sanofi-Aventis increased its turn-over with Lantus around 28% to 2, 45 Billion €, therefrom 130 Million € in Germany, where approx. 1, 8 Mio. people with diabetes applied this preparation. In 2007 Lantus ranked place 15 on top-selling pharmaceutical products in Germany. The investment in the production of Lantus and insulin-pen-manufacturing at the location Frankfurt-Höchst lied at 700 Mio. €. In 2008 a new manufacturing plant was established for further insulin-pen with an investment sum of 150 Mio. €. At Sanofi-Aventis the production of Lantus created 3000 jobs in Berlin and Frankfurt-Höchst. On June 9, 2000 the European Commission approbated Sanofi-Aventis Germany Ltd. the launching of Lantus in the entire European Union. The admission was prolonged on June 9, 2005

Advantages International clinical studies have confirmed the advantages of insulin glargine in the treatment of heavy hypoglycaemia compared to standard NPH insulin. Insulin glargine reduces the risk of severe nocturnal hypoglycaemia. Extensive clinical studies (ACCORD) have confirmed the higher risk of mortality with higher incidence of severe hypoglycaemia. A comparison trial of insulin detemir and glargine proved that subjects randomized to detemir used slightly higher daily insulin doses, but gained less weight on average than glargine-treated subjects. Other systematic reviews corroborate the results of benefit of insulin glargine regarding lower incidence of severe hypoglycaemia. On June 13, 2009, Diabetologia, the journal of European Association for the Study of Diabetes (EASD), published the results of a 5 year long-term observational, retrospective analysis. During the study no other safety issues, such as unexpected adverse events for either insulin emerged. However, insulin glargine was associated with a lower incidence of severe hypoglycaemia compared with NPH insulin.

Possible cancer link On June 26, 2009, Diabetologia published the results of four large-scale registry studies from Sweden, Germany, Scotland the rest of the UK. The German study, of around 127, 000 insulin-treated patients from an insurance database, suggested a possible link between insulin glargine (Lantus) and increased risk of developing cancer. The risk of cancer was dose-dependent, with those taking higher doses of Lantus apparently at increased risk. Whilst the authors stressed the limitations of the study and recommended that patients prescribed Lantus continue to take the drug, the results led to the EASD making "an urgent call for more research into a possible link between use of insulin glargine (an insulin analogue, brand name Lantus) and increased risk of cancer. The European Medicines Agency (EMEA) responded, stating that the results of the four studies were inconsistent, and that a relationship between insulin glargine and cancer could neither be confirmed nor excluded. They announced that they would undertake further detailed assessment of the studies’ results and any other relevant information, including several potential confounding factors that had not been fully taken into account by the studies. Patients being treated with insulin glargine were advised to continue their treatment as normal. The following month, the EMEA reported back, concluding that "the available data does not provide a cause for concern and that changes to the prescribing advice are therefore not necessary. The American Diabetes Association (ADA) also responded to the Diabetologia report, describing the published registry studies as “conflicting and confusing” and “inconclusive”. They advised patients against discontinuing Lantus and warned against "over-reaction.

New study confirms cancer link Type 1 diabetics who used Lantus had a 2. 9 -fold greater chance of cancer, while those who took the generic drug metformin had an 8 percent lower risk, according to a study presented on 9 December 2011 at the San Antonio Breast Cancer Symposium. Researchers examined medical records of 23, 266 patients in southern Sweden. The researchers were unable to identify which types of cancer were most common among Lantus users, said Hakan Olsson, lead researcher and professor of oncology at Lund University. They plan to follow the patients, and investigate different forms of treatment for Type 1 diabetes, including Novo Nordisk A/S’s long- acting insulin Levemir, to tease out any differences, he said. “Women should be aware that diabetes and breast cancer may be related, ” Olsson said in a telephone interview. “The diabetes itself could play a role in the development of cancer and now data is emerging that drug therapy may also be important in relation to cancer.
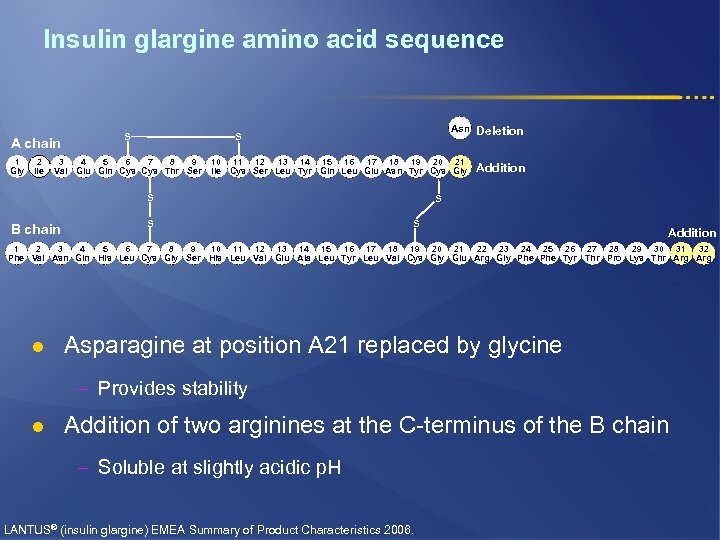
Insulin glargine amino acid sequence A chain 1 Gly 2 Ile Asn Deletion 10 11 12 13 14 15 16 17 18 19 20 21 Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr Cys Gly Addition S S 3 4 5 6 7 8 9 Val Glu Gln Cys Thr Ser S B chain S S S Addition 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Phe Val Asn Gln His Leu Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly Glu Arg Gly Phe Tyr Thr Pro Lys Thr Arg l Asparagine at position A 21 replaced by glycine – Provides stability l Addition of two arginines at the C-terminus of the B chain – Soluble at slightly acidic p. H LANTUS® (insulin glargine) EMEA Summary of Product Characteristics 2006.

Lantus® l Insulin glargine is the first basal insulin analogue l Its extensive clinical development program shows that once-daily insulin glargine consistently allows Hb. A 1 c levels to be reduced to <7% in patients with T 1 DM or T 2 DM 1– 6 l Evidence from randomized and ‘real life’ studies show that glycemic control is achieved with: § Reduced incidence of hypoglycemia compared with NPH 3, 7, 8 or premix 70/309 insulins § Smaller insulin dose (-77%) than detemir 16 § A minimal impact on weight 10– 14 l The clinical benefits of insulin glargine are attributed to its peakless, prolonged action profile that closely mimics endogenous insulin secretion and results in 24 hours of activity 1 1. Porcellati F et al. Diabet Med 2004; 21: 1213– 1220 2. Gerstein H et al. Diabet Med 2006; 23: 736– 742 3. Riddle M et al. Diabetes Care 2003; 26: 3080– 3086 4. Bretzel RG et al. Diabetes 2006; 55(suppl). Abstract 326–OR 5. Yki-Jarvinen H et al. Diabetes Care 2007; 30: 1364– 1369 6. Bergenstal R et al. Diabetes 2006; 55(suppl). Abstract 441–P 7. Rosenstock J et al. Diabetes Care 2005; 28: 950– 955 8. Mullins P et al. Clin Ther 2007; 29: 1607– 1619 9. Janka H et al. Diabetes Care 2005; 28: 254– 259 10. Schreiber S et al. Diabetes Obesity Metab 2007; 9: 31– 38 11. Yki-Jarvinen H et al. Diabetes Care 2000; 23: 1130– 1136 12. Fritsche A et al. Ann Int Med 2003; 138: 952– 959 13. Yki-Jarvinen H et al. Diabetologia 2006; 49: 442– 451 14. Rosenstock J et al. Diabetes Care 2006; 29: 554– 559 15. Lepore M et al. Diabetes 2000; 49: 2142– 2148 16. Rosenstock J et al. Diabetologia 2008; 51: 408– 416

Lantus® l These pharmacodynamic properties are clinically translated into: § Once-daily administration 1 § Effective lowering of FBG and PPBG over 24 hours when compared with NPH 2 and premix 70/303 insulins l Insulin glargine can be injected once a day either in the morning or at bedtime without affecting glucose control 4 l Insulin glargine is easy to titrate thanks to simple titration algorithms 5, 6 that facilitate patients’ contribution to the management of their diabetes 6 l These features allow efficient and safe initiation of insulin therapy in patients with T 2 DM uncontrolled on OHAs 5, 7, 3 1. Lantus Prescribing Information 2. Yki-Jarvinen H et al. Diabetes Care 2000; 23: 1130– 1136 3. Janka H et al. Diabetes Care 2005; 28: 254– 259 4. Standl E et al. Hormone Metab Res 2006; 38: 172– 177 5. Gerstein HC et al. Diabet Med 2006; 23: 736– 742 6. Davies M et al. Diabetes Care 2005; 28: 1282– 1288 7. Riddle M et al. Diabetes Care 2003; 26: 3080– 3086
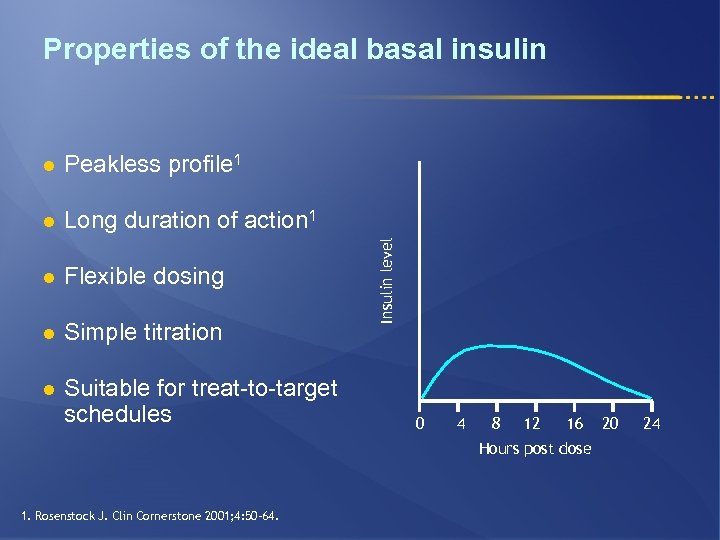
Properties of the ideal basal insulin Peakless profile 1 l Long duration of action 1 l Flexible dosing l Simple titration l Suitable for treat-to-target schedules Insulin level l 0 4 8 12 16 Hours post dose 1. Rosenstock J. Clin Cornerstone 2001; 4: 50– 64. 20 24
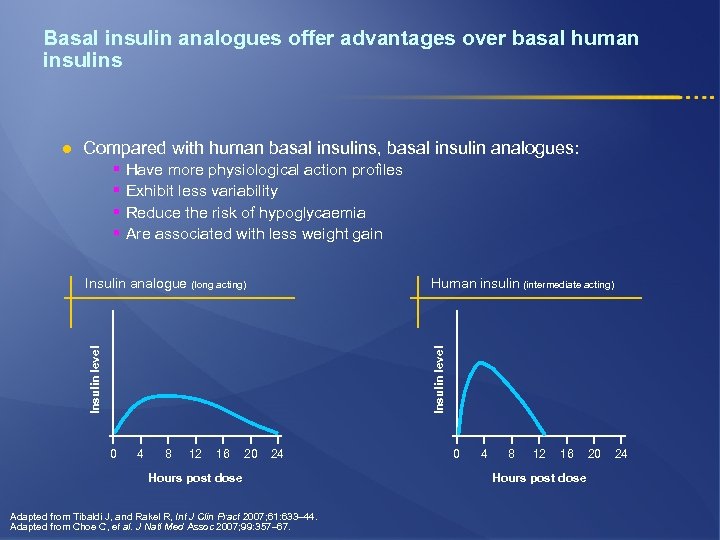
Basal insulin analogues offer advantages over basal human insulins l Compared with human basal insulins, basal insulin analogues: § § Have more physiological action profiles Exhibit less variability Reduce the risk of hypoglycaemia Are associated with less weight gain Insulin level Human insulin (intermediate acting) Insulin level Insulin analogue (long acting) 0 4 8 12 16 20 24 Hours post dose Adapted from Tibaldi J, and Rakel R, Int J Clin Pract 2007; 61: 633– 44. Adapted from Choe C, et al. J Natl Med Assoc 2007; 99: 357– 67. 0 4 8 12 16 Hours post dose 20 24

Don’t Forget Insulin Glargine l Better Control l Less Hypoglycemia l Less Weight Gain l Only One Shot a day.
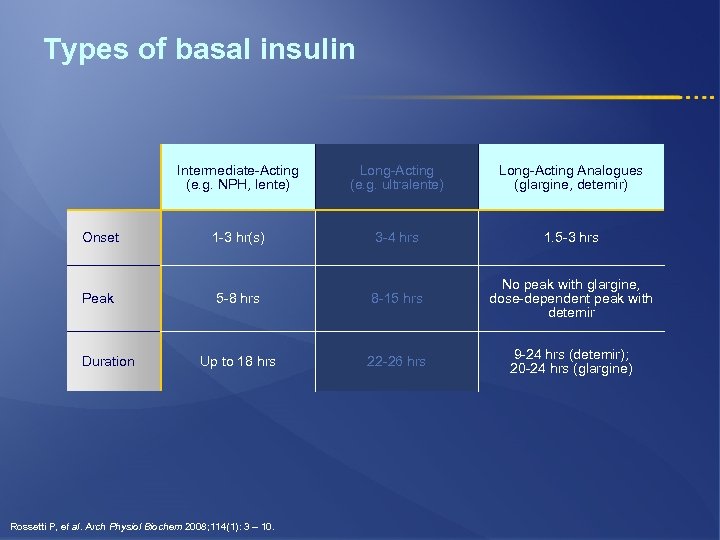
Types of basal insulin Intermediate-Acting (e. g. NPH, lente) Long-Acting (e. g. ultralente) Long-Acting Analogues (glargine, detemir) Onset 1 -3 hr(s) 3 -4 hrs 1. 5 -3 hrs Peak 5 -8 hrs 8 -15 hrs No peak with glargine, dose-dependent peak with detemir Up to 18 hrs 22 -26 hrs 9 -24 hrs (detemir); 20 -24 hrs (glargine) Duration Rossetti P, et al. Arch Physiol Biochem 2008; 114(1): 3 – 10.

Advantages of insulin therapy l Oldest medication, with most clinical experience l Most effective in lowering glycemia § Can decrease any level of elevated Hb. A 1 c § No maximum dose of insulin l Beneficial effects on triglyceride and HDL-c Nathan DM, et al. Diabetes Care 2009; 32 193 -203.

Disadvantages of insulin therapy l Weight gain ~ 2 -4 kg § ± proportional to the correction of glycemia § Predominantly the result of glycosuria l Hypoglycemia § Rates of severe hypoglycemia in patients with T 2 DM are low in treat-to-target clinical trials (compared to T 1 DM): - Type 1 DM: 61 events per 100 patient-years Type 2 DM: 1 to 3 events per 100 patient-years Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
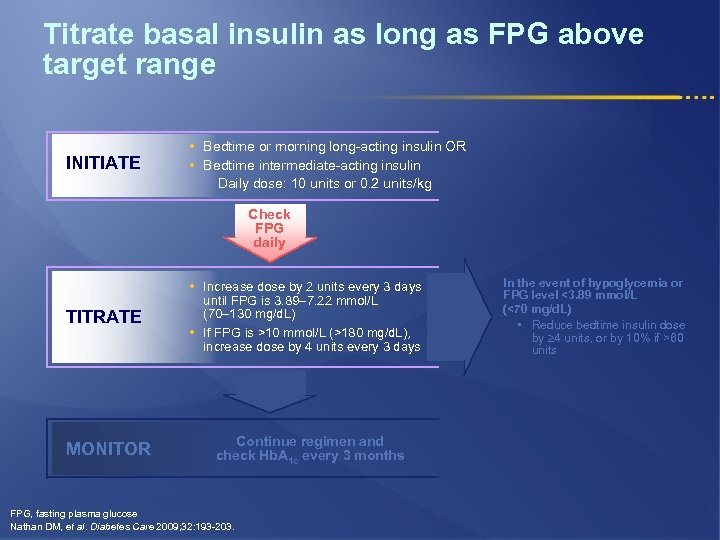
Titrate basal insulin as long as FPG above target range INITIATE • Bedtime or morning long-acting insulin OR • Bedtime intermediate-acting insulin Daily dose: 10 units or 0. 2 units/kg Check FPG daily • Increase dose by 2 units every 3 days TITRATE until FPG is 3. 89– 7. 22 mmol/L (70– 130 mg/d. L) • If FPG is >10 mmol/L (>180 mg/d. L), increase dose by 4 units every 3 days MONITOR Continue regimen and check Hb. A 1 c every 3 months FPG, fasting plasma glucose Nathan DM, et al. Diabetes Care 2009; 32: 193 -203. In the event of hypoglycemia or FPG level <3. 89 mmol/L (<70 mg/d. L) • Reduce bedtime insulin dose by 4 units, or by 10% if >60 units
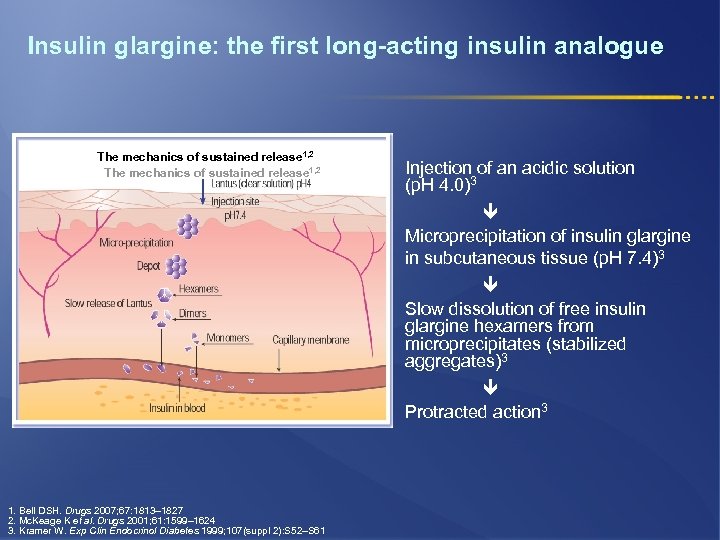
Insulin glargine: the first long-acting insulin analogue The mechanics of sustained release 1, 2 Injection of an acidic solution (p. H 4. 0)3 Microprecipitation of insulin glargine in subcutaneous tissue (p. H 7. 4)3 Slow dissolution of free insulin glargine hexamers from microprecipitates (stabilized aggregates)3 Protracted action 3 1. Bell DSH. Drugs 2007; 67: 1813– 1827 2. Mc. Keage K et al. Drugs 2001; 61: 1599– 1624 3. Kramer W. Exp Clin Endocrinol Diabetes 1999; 107(suppl 2): S 52–S 61
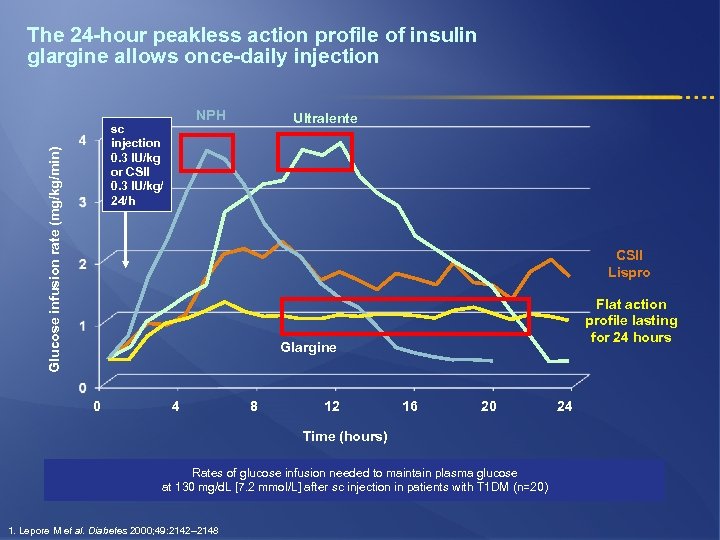
The 24 -hour peakless action profile of insulin glargine allows once-daily injection NPH Glucose infusion rate (mg/kg/min) sc injection 0. 3 IU/kg or CSII 0. 3 IU/kg/ 24/h Ultralente CSII Lispro Flat action profile lasting for 24 hours Glargine 0 4 8 12 16 20 Time (hours) Rates of glucose infusion needed to maintain plasma glucose at 130 mg/d. L [7. 2 mmol/L] after sc injection in patients with T 1 DM (n=20) 1. Lepore M et al. Diabetes 2000; 49: 2142– 2148 24
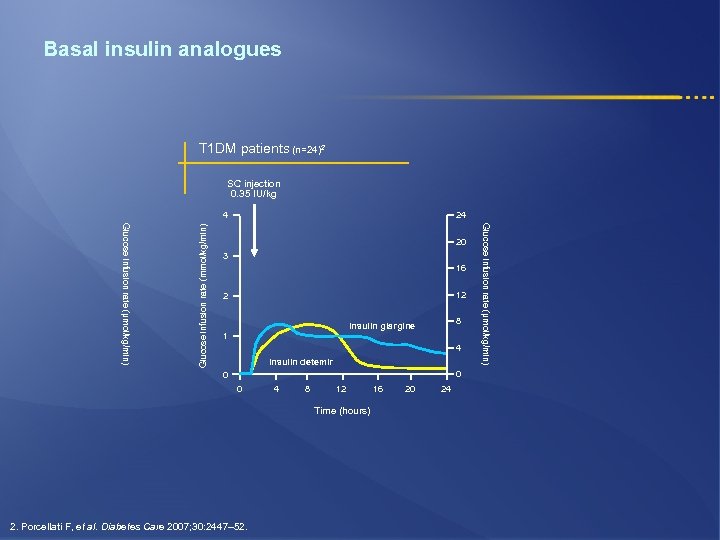
Basal insulin analogues T 1 DM patients (n=24)2 SC injection 0. 35 IU/kg 24 20 3 16 12 2 8 Insulin glargine 1 4 Insulin detemir 0 0 0 4 8 12 Time (hours) 2. Porcellati F, et al. Diabetes Care 2007; 30: 2447– 52. 16 20 24 Glucose infusion rate (µmol/kg/min) Glucose infusion rate (mmol/kg/min) 4
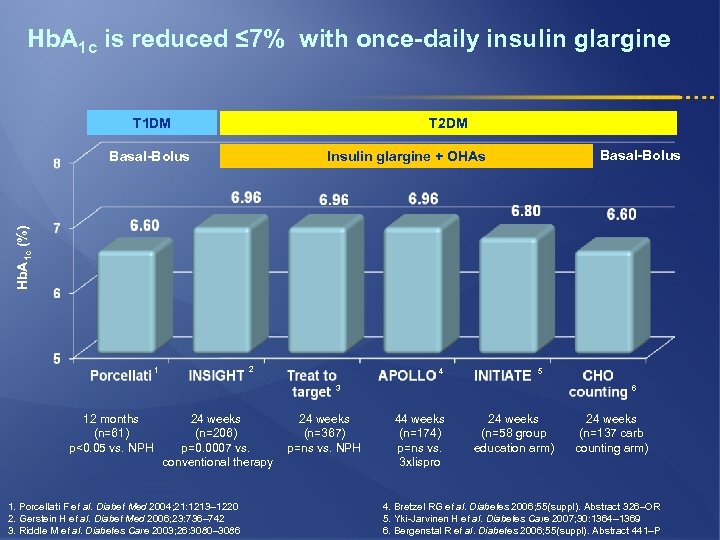
Hb. A 1 c is reduced ≤ 7% with once-daily insulin glargine T 1 DM T 2 DM Basal-Bolus Insulin glargine + OHAs Hb. A 1 c (%) Basal-Bolus 2 1 4 5 3 12 months (n=61) p<0. 05 vs. NPH 24 weeks (n=206) p=0. 0007 vs. conventional therapy 1. Porcellati F et al. Diabet Med 2004; 21: 1213– 1220 2. Gerstein H et al. Diabet Med 2006; 23: 736– 742 3. Riddle M et al. Diabetes Care 2003; 26: 3080– 3086 24 weeks (n=367) p=ns vs. NPH 6 44 weeks (n=174) p=ns vs. 3 xlispro 24 weeks (n=58 group education arm) 24 weeks (n=137 carb counting arm) 4. Bretzel RG et al. Diabetes 2006; 55(suppl). Abstract 326–OR 5. Yki-Jarvinen H et al. Diabetes Care 2007; 30: 1364– 1369 6. Bergenstal R et al. Diabetes 2006; 55(suppl). Abstract 441–P
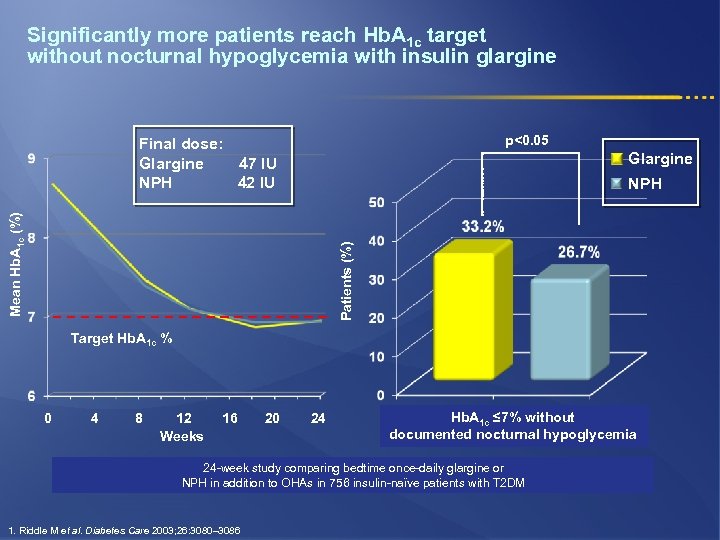
Significantly more patients reach Hb. A 1 c target without nocturnal hypoglycemia with insulin glargine p<0. 05 Final dose: Glargine 47 IU NPH 42 IU Glargine Patients (%) Mean Hb. A 1 c (%) NPH Target Hb. A 1 c % 0 4 8 12 Weeks 16 20 24 Hb. A 1 c ≤ 7% without documented nocturnal hypoglycemia 24 -week study comparing bedtime once-daily glargine or NPH in addition to OHAs in 756 insulin-naϊve patients with T 2 DM 1. Riddle M et al. Diabetes Care 2003; 26: 3080– 3086
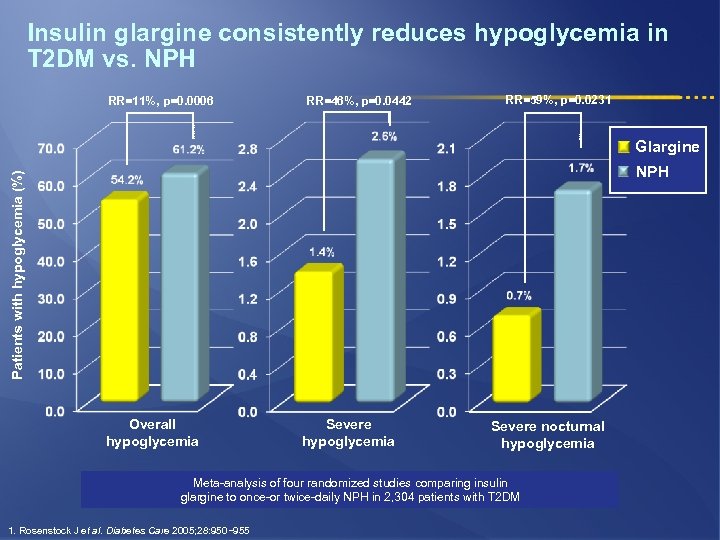
Insulin glargine consistently reduces hypoglycemia in T 2 DM vs. NPH RR=11%, p=0. 0006 RR=46%, p=0. 0442 RR=59%, p=0. 0231 Glargine Patients with hypoglycemia (%) NPH Overall hypoglycemia Severe nocturnal hypoglycemia Meta-analysis of four randomized studies comparing insulin glargine to once-or twice-daily NPH in 2, 304 patients with T 2 DM 1. Rosenstock J et al. Diabetes Care 2005; 28: 950− 955
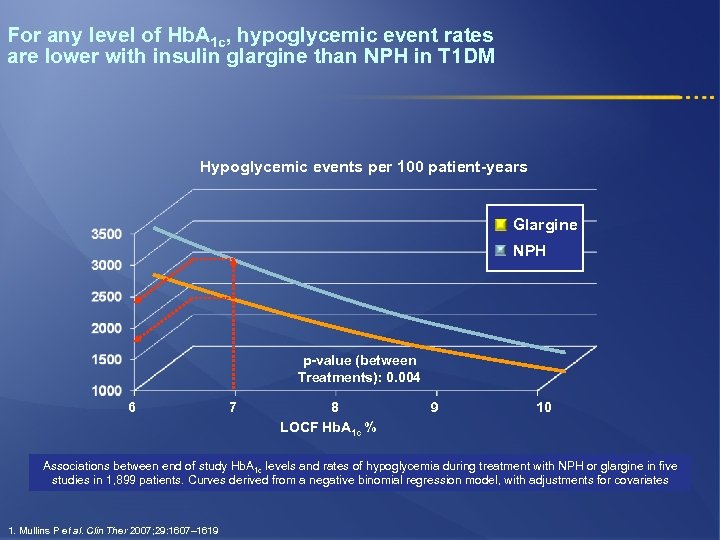
For any level of Hb. A 1 c, hypoglycemic event rates are lower with insulin glargine than NPH in T 1 DM Hypoglycemic events per 100 patient-years Glargine NPH p-value (between Treatments): 0. 004 6 7 8 LOCF Hb. A 1 c % 9 10 Associations between end of study Hb. A 1 c levels and rates of hypoglycemia during treatment with NPH or glargine in five studies in 1, 899 patients. Curves derived from a negative binomial regression model, with adjustments for covariates 1. Mullins P et al. Clin Ther 2007; 29: 1607– 1619
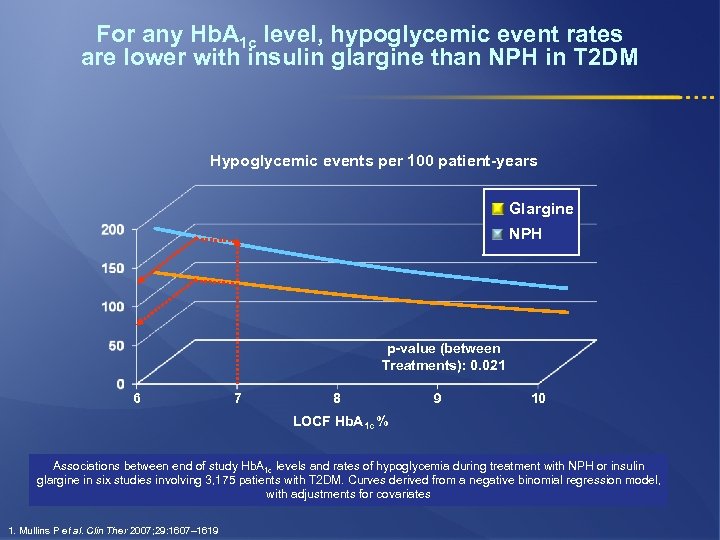
For any Hb. A 1 c level, hypoglycemic event rates are lower with insulin glargine than NPH in T 2 DM Hypoglycemic events per 100 patient-years Glargine NPH p-value (between Treatments): 0. 021 6 7 8 9 10 LOCF Hb. A 1 c % Associations between end of study Hb. A 1 c levels and rates of hypoglycemia during treatment with NPH or insulin glargine in six studies involving 3, 175 patients with T 2 DM. Curves derived from a negative binomial regression model, with adjustments for covariates 1. Mullins P et al. Clin Ther 2007; 29: 1607– 1619
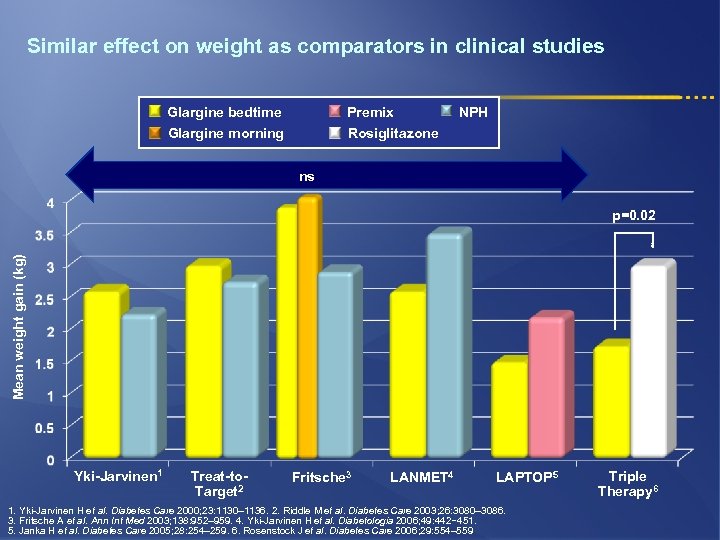
Similar effect on weight as comparators in clinical studies Glargine bedtime Glargine morning Premix Rosiglitazone NPH ns Mean weight gain (kg) p=0. 02 Yki-Jarvinen 1 Treat-to. Target 2 Fritsche 3 LANMET 4 LAPTOP 5 1. Yki-Jarvinen H et al. Diabetes Care 2000; 23: 1130– 1136. 2. Riddle M et al. Diabetes Care 2003; 26: 3080– 3086. 3. Fritsche A et al. Ann Int Med 2003; 138: 952– 959. 4. Yki-Jarvinen H et al. Diabetologia 2006; 49: 442− 451. 5. Janka H et al. Diabetes Care 2005; 28: 254– 259. 6. Rosenstock J et al. Diabetes Care 2006; 29: 554– 559 Triple Therapy 6
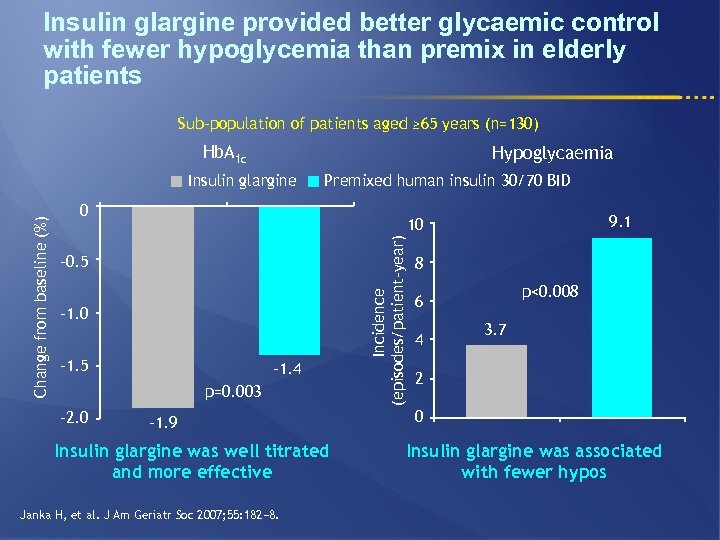
Insulin glargine provided better glycaemic control with fewer hypoglycemia than premix in elderly patients Sub-population of patients aged ≥ 65 years (n=130) Hb. A 1 c Hypoglycaemia Premixed human insulin 30/70 BID 0 – 0. 5 – 1. 0 – 1. 5 – 1. 4 p=0. 003 – 2. 0 9. 1 10 – 1. 9 Insulin glargine was well titrated and more effective Janka H, et al. J Am Geriatr Soc 2007; 55: 182− 8. Incidence (episodes/patient-year) Change from baseline (%) Insulin glargine 8 p<0. 008 6 4 3. 7 2 0 Insulin glargine was associated with fewer hypos
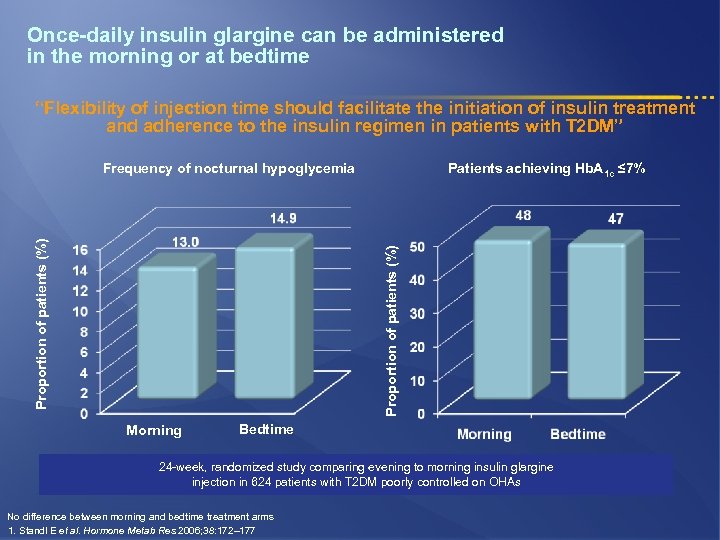
Once-daily insulin glargine can be administered in the morning or at bedtime “Flexibility of injection time should facilitate the initiation of insulin treatment and adherence to the insulin regimen in patients with T 2 DM” Patients achieving Hb. A 1 c ≤ 7% Proportion of patients (%) Frequency of nocturnal hypoglycemia Morning Bedtime 24 -week, randomized study comparing evening to morning insulin glargine injection in 624 patients with T 2 DM poorly controlled on OHAs No difference between morning and bedtime treatment arms 1. Standl E et al. Hormone Metab Res 2006; 38: 172– 177
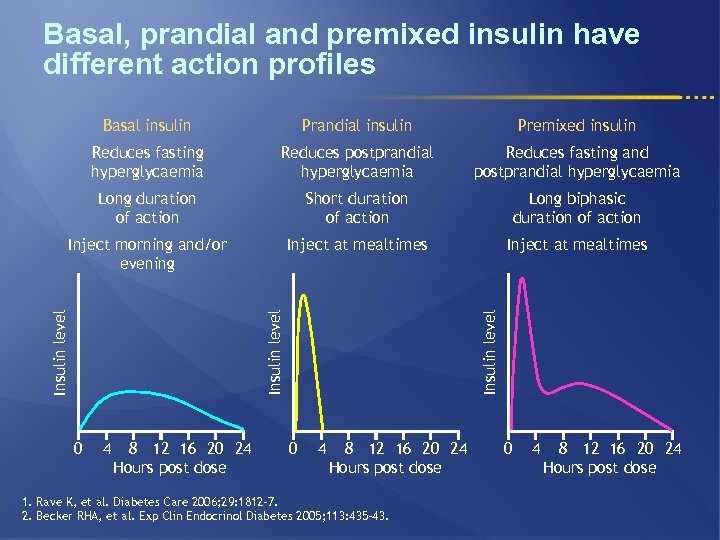
Basal, prandial and premixed insulin have different action profiles Prandial insulin Premixed insulin Reduces fasting hyperglycaemia Reduces postprandial hyperglycaemia Reduces fasting and postprandial hyperglycaemia Long duration of action Short duration of action Long biphasic duration of action Inject morning and/or evening Inject at mealtimes 0 4 8 12 16 20 24 Hours post dose Insulin level Basal insulin 0 4 8 12 16 20 24 Hours post dose 1. Rave K, et al. Diabetes Care 2006; 29: 1812– 7. 2. Becker RHA, et al. Exp Clin Endocrinol Diabetes 2005; 113: 435– 43. 0 4 8 12 16 20 24 Hours post dose
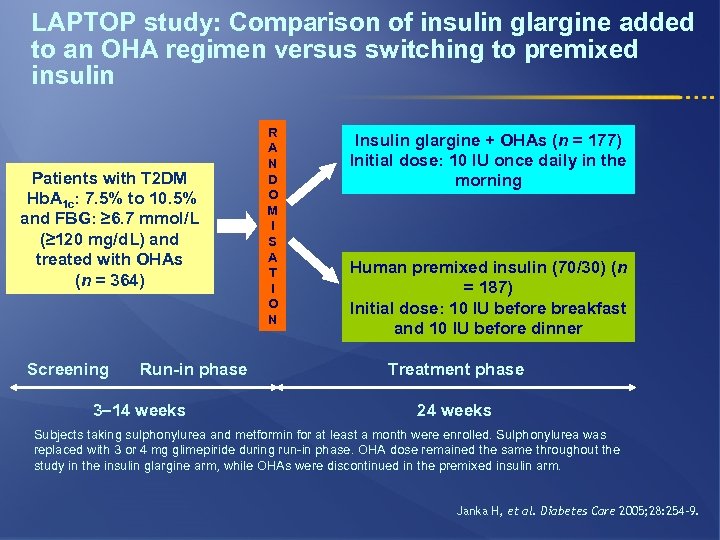
LAPTOP study: Comparison of insulin glargine added to an OHA regimen versus switching to premixed insulin Patients with T 2 DM Hb. A 1 c: 7. 5% to 10. 5% and FBG: ≥ 6. 7 mmol/L (≥ 120 mg/d. L) and treated with OHAs (n = 364) Screening Run-in phase 3– 14 weeks R A N D O M I S A T I O N Insulin glargine + OHAs (n = 177) Initial dose: 10 IU once daily in the morning Human premixed insulin (70/30) (n = 187) Initial dose: 10 IU before breakfast and 10 IU before dinner Treatment phase 24 weeks Subjects taking sulphonylurea and metformin for at least a month were enrolled. Sulphonylurea was replaced with 3 or 4 mg glimepiride during run-in phase. OHA dose remained the same throughout the study in the insulin glargine arm, while OHAs were discontinued in the premixed insulin arm. Janka H, et al. Diabetes Care 2005; 28: 254– 9.
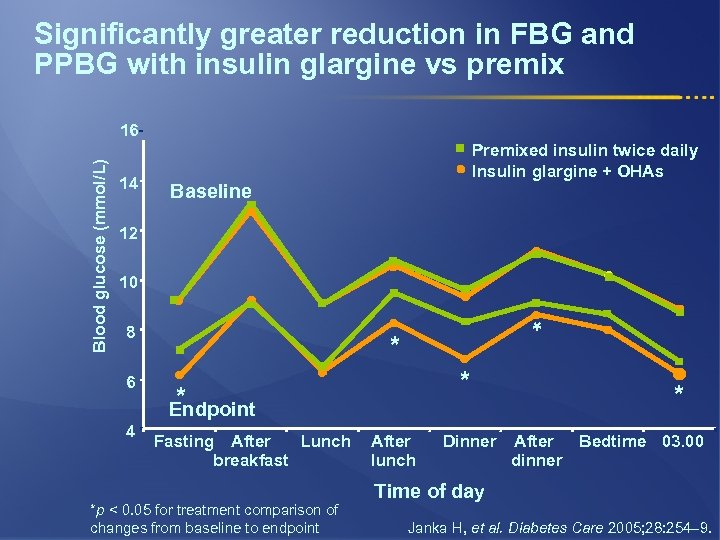
Significantly greater reduction in FBG and PPBG with insulin glargine vs premix 14 Premixed insulin twice daily Insulin glargine + OHAs Baseline 12 10 8 6 4 * Blood glucose (mmol/L) 16 * * * Endpoint Fasting After Lunch breakfast *p < 0. 05 for treatment comparison of changes from baseline to endpoint After lunch Dinner * After Bedtime 03. 00 dinner Time of day Janka H, et al. Diabetes Care 2005; 28: 254– 9.
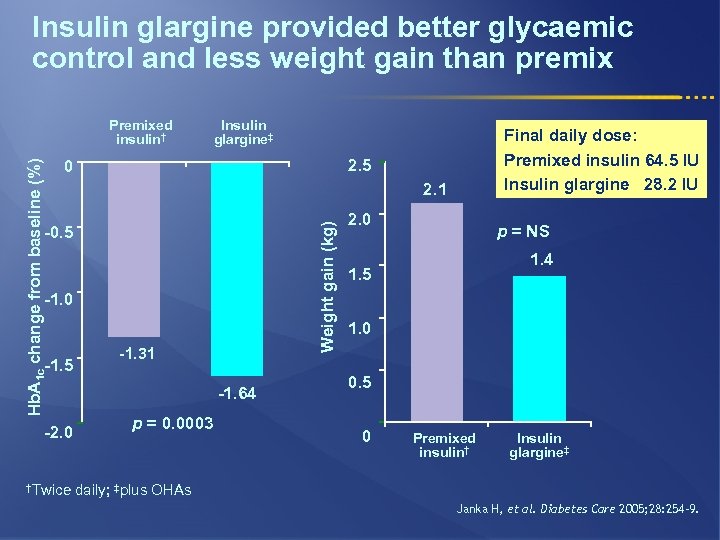
Insulin glargine provided better glycaemic control and less weight gain than premix Insulin glargine‡ 2. 1 -0. 5 -1. 0 -1. 5 -2. 0 †Twice Final daily dose: Premixed insulin 64. 5 IU Insulin glargine 28. 2 IU 2. 5 0 Weight gain (kg) Hb. A 1 c change from baseline (%) Premixed insulin† -1. 31 -1. 64 p = 0. 0003 2. 0 p = NS 1. 4 1. 5 1. 0 0. 5 0 Premixed insulin† Insulin glargine‡ daily; ‡plus OHAs Janka H, et al. Diabetes Care 2005; 28: 254– 9.
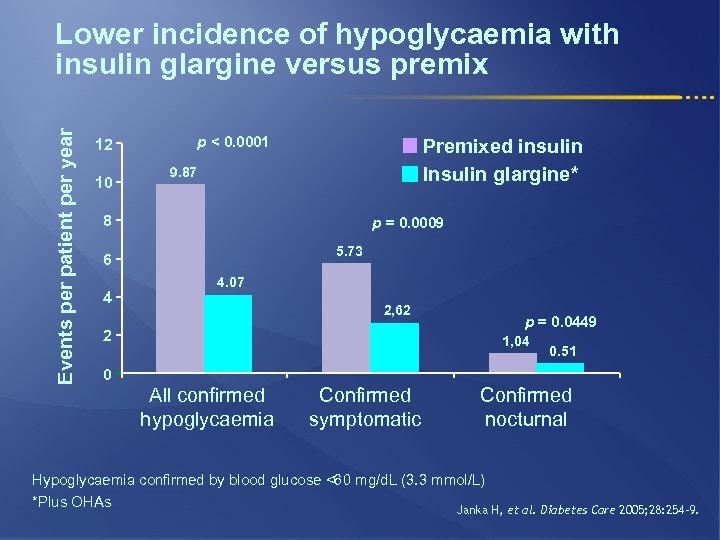
Events per patient per year Lower incidence of hypoglycaemia with insulin glargine versus premix p < 0. 0001 12 10 Premixed insulin Insulin glargine* 9. 87 8 p = 0. 0009 5. 73 6 4 4. 07 2, 62 2 p = 0. 0449 1, 04 0. 51 0 All confirmed hypoglycaemia Confirmed symptomatic Confirmed nocturnal Hypoglycaemia confirmed by blood glucose <60 mg/d. L (3. 3 mmol/L) *Plus OHAs Janka H, et al. Diabetes Care 2005; 28: 254– 9.
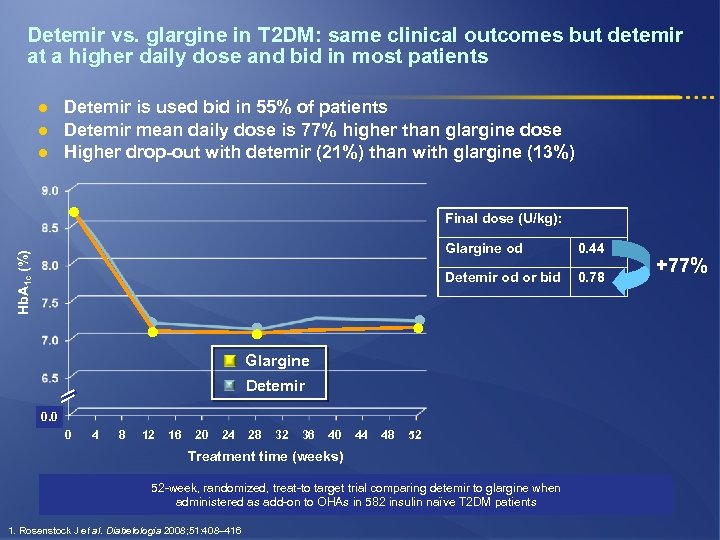
Detemir vs. glargine in T 2 DM: same clinical outcomes but detemir at a higher daily dose and bid in most patients l l l Detemir is used bid in 55% of patients Detemir mean daily dose is 77% higher than glargine dose Higher drop-out with detemir (21%) than with glargine (13%) Final dose (U/kg): 0. 44 Detemir od or bid Hb. A 1 c (%) Glargine od 0. 78 Glargine Detemir 0. 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 Treatment time (weeks) 52 -week, randomized, treat-to target trial comparing detemir to glargine when administered as add-on to OHAs in 582 insulin naïve T 2 DM patients 1. Rosenstock J et al. Diabetologia 2008; 51: 408– 416 +77%
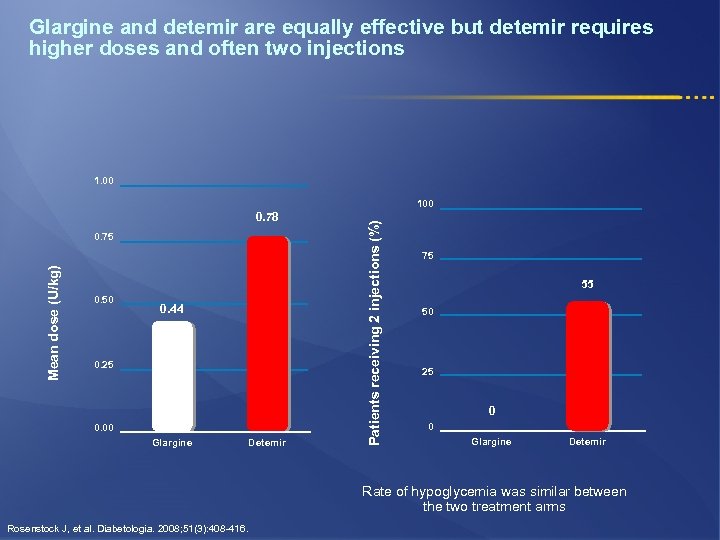
Glargine and detemir are equally effective but detemir requires higher doses and often two injections 1. 00 0. 78 Mean dose (U/kg) 0. 75 0. 50 0. 44 0. 25 0. 00 Glargine Detemir Patients receiving 2 injections (%) 100 75 55 50 25 0 0 Glargine Detemir Rate of hypoglycemia was similar between the two treatment arms Rosenstock J, et al. Diabetologia. 2008; 51(3): 408 -416.
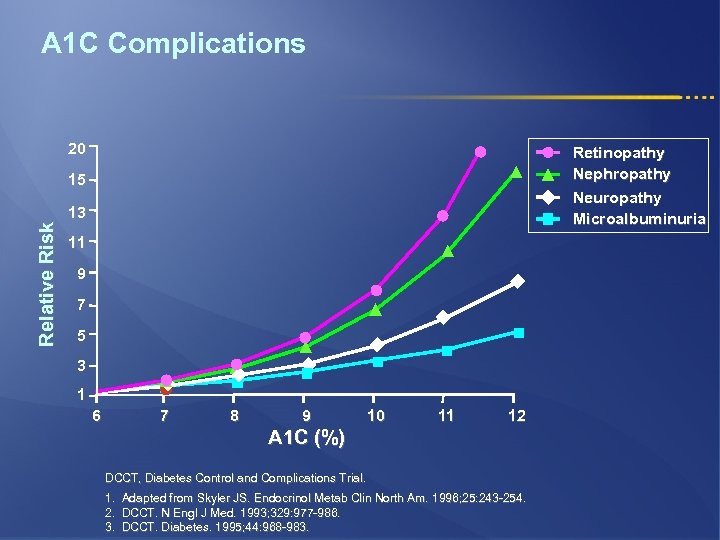
A 1 C Complications 20 Retinopathy Nephropathy Neuropathy Microalbuminuria Relative Risk 15 13 11 9 7 5 3 1 6 7 8 9 10 11 12 A 1 C (%) DCCT, Diabetes Control and Complications Trial. 1. Adapted from Skyler JS. Endocrinol Metab Clin North Am. 1996; 25: 243 -254. 2. DCCT. N Engl J Med. 1993; 329: 977 -986. 3. DCCT. Diabetes. 1995; 44: 968 -983.
Initial Insulin Delivery Device

Lantus Solo. Star
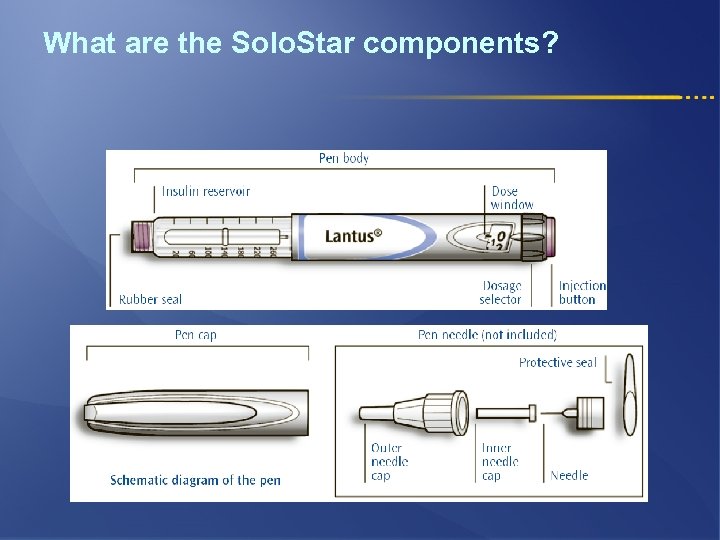
What are the Solo. Star components?
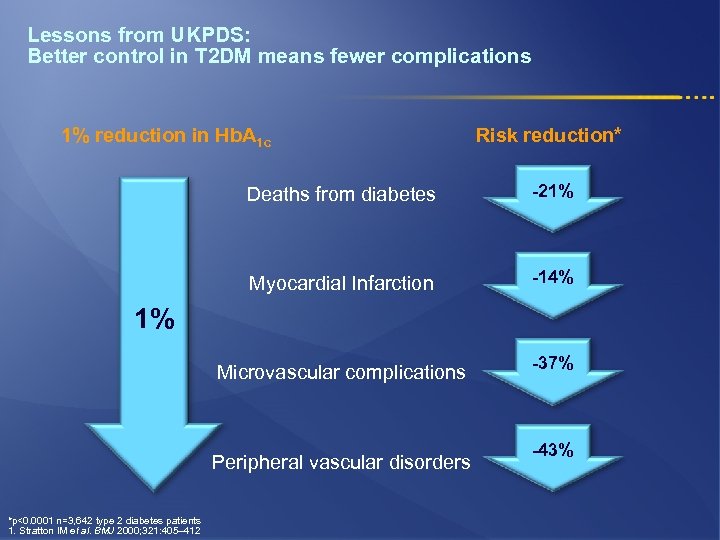
Lessons from UKPDS: Better control in T 2 DM means fewer complications 1% reduction in Hb. A 1 c Risk reduction* Deaths from diabetes -21% Myocardial Infarction -14% Microvascular complications -37% 1% Peripheral vascular disorders *p<0. 0001 n=3, 642 type 2 diabetes patients 1. Stratton IM et al. BMJ 2000; 321: 405– 412 -43%
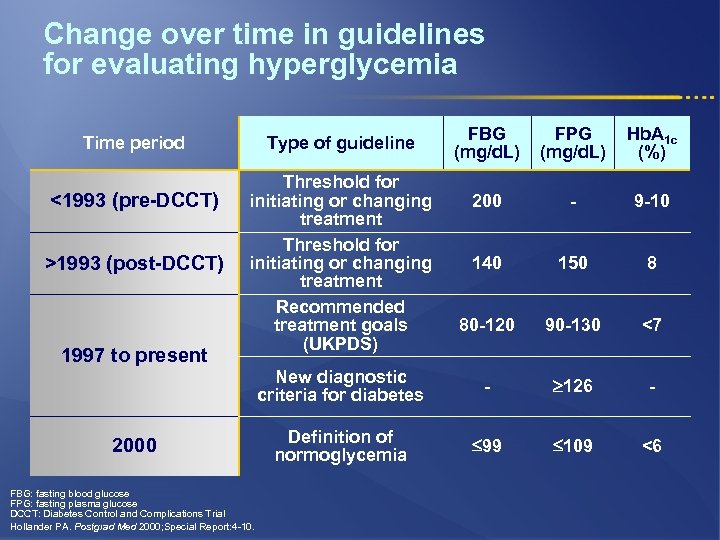
Change over time in guidelines for evaluating hyperglycemia <1993 (pre-DCCT) >1993 (post-DCCT) 1997 to present FBG (mg/d. L) FPG (mg/d. L) Hb. A 1 c (%) 200 - 9 -10 140 150 8 80 -120 90 -130 <7 New diagnostic criteria for diabetes Time period - 126 - Definition of normoglycemia 99 109 <6 Type of guideline Threshold for initiating or changing treatment Recommended treatment goals (UKPDS) 2000 FBG: fasting blood glucose FPG: fasting plasma glucose DCCT: Diabetes Control and Complications Trial Hollander PA. Postgrad Med 2000; Special Report: 4 -10.
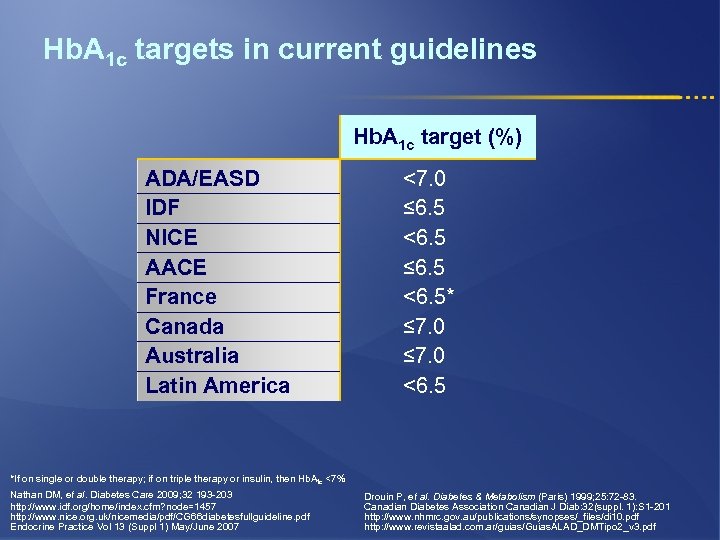
Hb. A 1 c targets in current guidelines Hb. A 1 c target (%) ADA/EASD IDF NICE AACE France Canada Australia Latin America <7. 0 ≤ 6. 5 <6. 5* ≤ 7. 0 <6. 5 *If on single or double therapy; if on triple therapy or insulin, then Hb. A 1 c <7% Nathan DM, et al. Diabetes Care 2009; 32 193 -203 http: //www. idf. org/home/index. cfm? node=1457 http: //www. nice. org. uk/nicemedia/pdf/CG 66 diabetesfullguideline. pdf Endocrine Practice Vol 13 (Suppl 1) May/June 2007 Drouin P, et al. Diabetes & Metabolism (Paris) 1999; 25: 72 -83. Canadian Diabetes Association Canadian J Diab: 32(suppl. 1): S 1 -201 http: //www. nhmrc. gov. au/publications/synopses/_files/di 10. pdf http: //www. revistaalad. com. ar/guias/Guias. ALAD_DMTipo 2_v 3. pdf

Rationale for glycemic goals l Glycemic goals of therapy are based on: § Clinical studies - Type 1: DCCT, Stockholm Diabetes Intervention Study Type 2: UKPDS, Kumamoto § Epidemiological data l "Normal" Hb. A 1 c § Upper limit of nondiabetic range: 6. 1% l Goals of therapy in DCCT and UKPDS § Neither study was able to maintain Hb. A 1 c level in the nondiabetic range § Hb. A 1 c ~ 7% in intensive treatment groups - i. e. , 4 SD above nondiabetic mean DCCT Research Group. N. Eng. J. Med. 1993; 329: 977 -986. Raichard P, et al. Acta Medica Sandinavica 224(2): 115 -122. UKPDS Group Lancet 1998; 352: 837 -53. Ohkubo Y, et al. Diabetes Res Clin Pract 1995; 28: 103– 117. Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
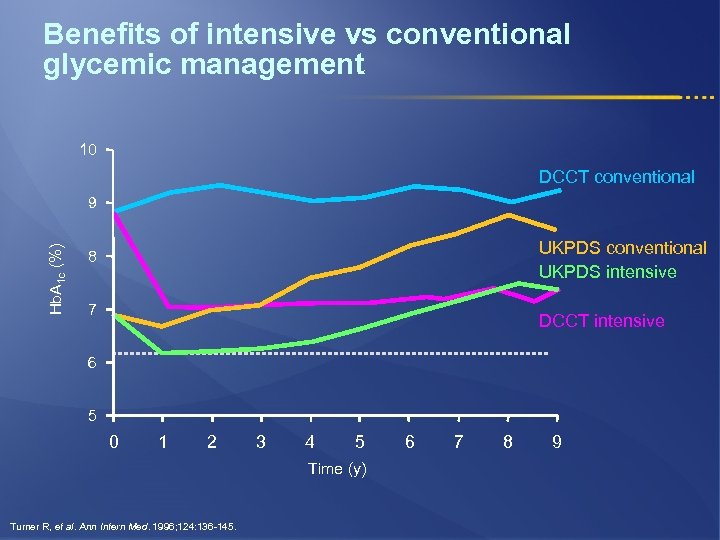
Benefits of intensive vs conventional glycemic management 10 DCCT conventional Hb. A 1 c (%) 9 UKPDS conventional UKPDS intensive 8 7 DCCT intensive 6 5 0 1 2 3 4 5 Time (y) Turner R, et al. Ann Intern Med. 1996; 124: 136 -145. 6 7 8 9
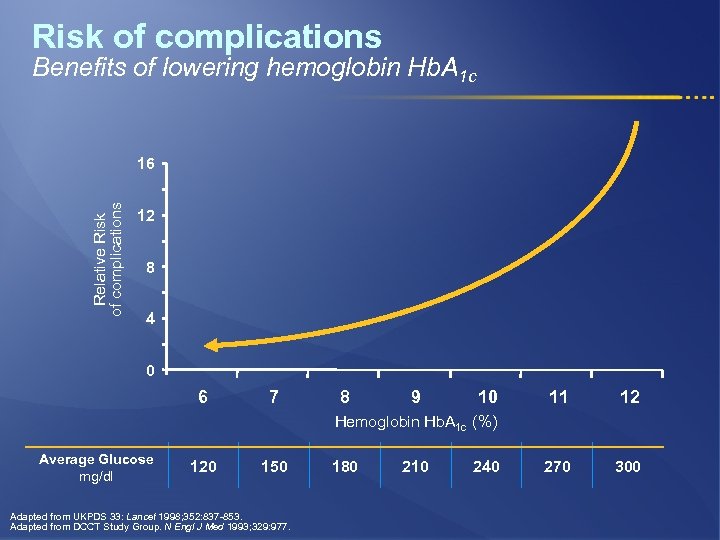
Risk of complications Benefits of lowering hemoglobin Hb. A 1 c Relative Risk of complications 16 12 8 4 0 6 7 8 9 10 11 12 270 300 Hemoglobin Hb. A 1 c (%) Average Glucose mg/dl 120 150 Adapted from UKPDS 33: Lancet 1998; 352: 837 -853. Adapted from DCCT Study Group. N Engl J Med 1993; 329: 977. 180 210 240

Why guidelines for the treatment of T 2 DM? l Diabetes is a complex and progressive disease, requiring timely treatment escalation l Guidelines interpret existing evidence in order to help all physicians l The increase in the number of available therapies has increased treatment options l Guidelines should be revised as new evidence accrues l Guidelines do not replace clinical judgement in the individual patient Nathan DM, et al. Diabetes Care 2009; 32 193 -203.

History of ADA/EASD consensus algorithm l First Consensus algorithm § August 20061 l 1 st Update § January 2008: Update regarding thiazolidinediones 2 l 2 nd Update § January 20093 1. Nathan DM, et al. Diabetes Care 2006; 29(8): 1963 -72. 2. Nathan DM, et al. Diabetes Care 2008; 31(1): 173 -5. 3. Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.

Hb. A 1 c targets should be individualized l Goal of therapy § In general: Hb. A 1 c <7% § In the individual patient: Hb. A 1 c as close to 6% as possible without significant hypoglycemia l Call to action: Hb. A 1 c 7% l Less stringent goals may be appropriate for: § Patients with a history of severe hypoglycemia § Patients with limited life expectancies § Very young children or older adults § Individuals with co-morbid conditions Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
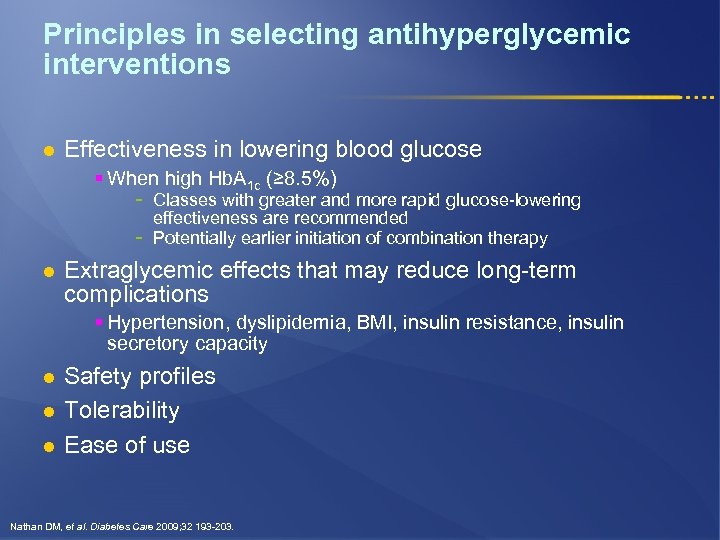
Principles in selecting antihyperglycemic interventions l Effectiveness in lowering blood glucose § When high Hb. A 1 c (≥ 8. 5%) - l Classes with greater and more rapid glucose-lowering effectiveness are recommended Potentially earlier initiation of combination therapy Extraglycemic effects that may reduce long-term complications § Hypertension, dyslipidemia, BMI, insulin resistance, insulin secretory capacity l l l Safety profiles Tolerability Ease of use Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
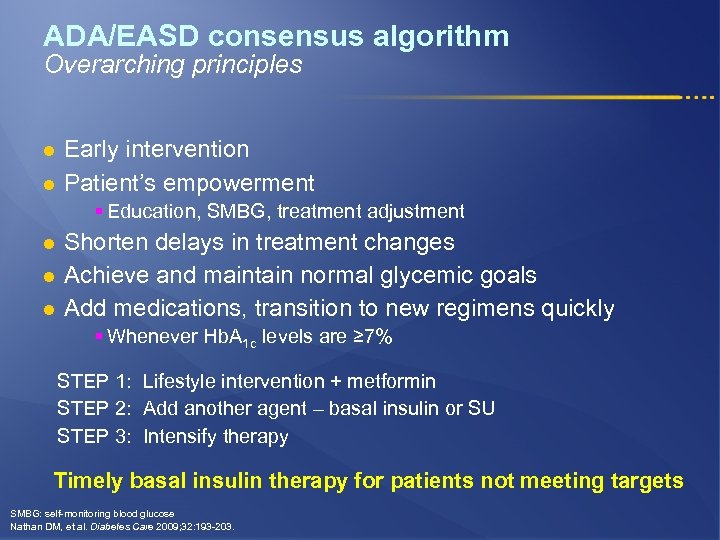
ADA/EASD consensus algorithm Overarching principles l l Early intervention Patient’s empowerment § Education, SMBG, treatment adjustment l l l Shorten delays in treatment changes Achieve and maintain normal glycemic goals Add medications, transition to new regimens quickly § Whenever Hb. A 1 c levels are ≥ 7% STEP 1: Lifestyle intervention + metformin STEP 2: Add another agent – basal insulin or SU STEP 3: Intensify therapy Timely basal insulin therapy for patients not meeting targets SMBG: self-monitoring blood glucose Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.
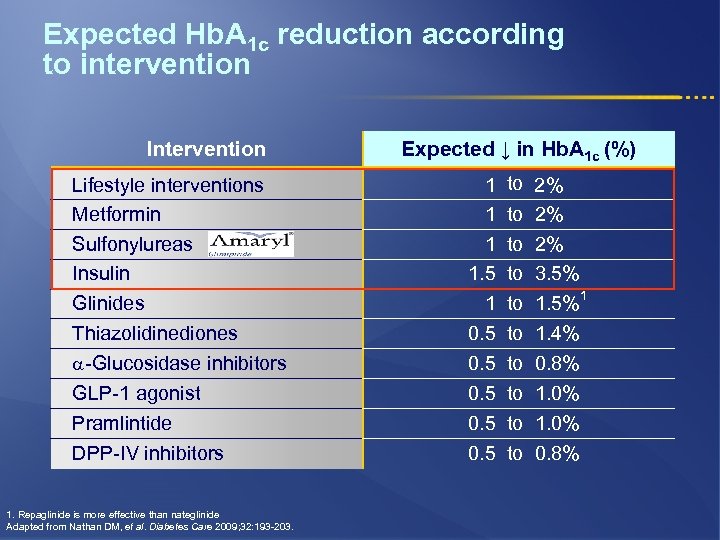
Expected Hb. A 1 c reduction according to intervention Intervention Lifestyle interventions Metformin Sulfonylureas Insulin Glinides Thiazolidinediones -Glucosidase inhibitors GLP-1 agonist Pramlintide DPP-IV inhibitors 1. Repaglinide is more effective than nateglinide Adapted from Nathan DM, et al. Diabetes Care 2009; 32: 193 -203. Expected ↓ in Hb. A 1 c (%) 1 1. 5 1 0. 5 0. 5 to 2% to 3. 5% 1 to 1. 5% to 1. 4% to 0. 8% to 1. 0% to 0. 8%
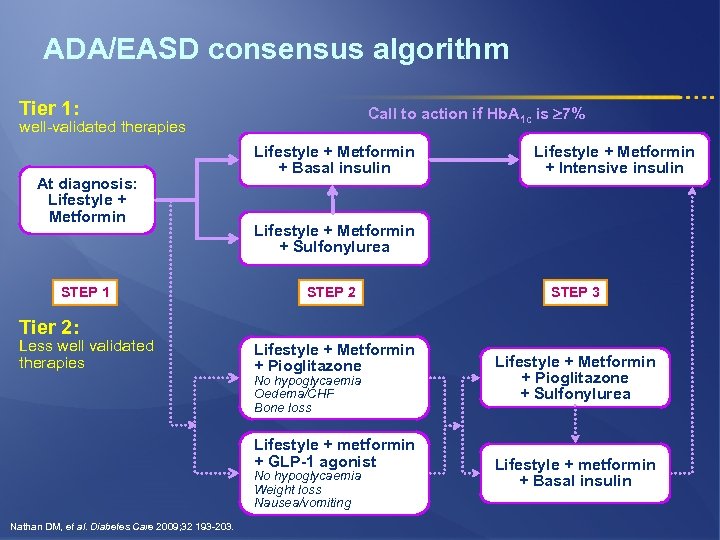
ADA/EASD consensus algorithm Tier 1: Call to action if Hb. A 1 c is 7% well-validated therapies At diagnosis: Lifestyle + Metformin STEP 1 Lifestyle + Metformin + Basal insulin Lifestyle + Metformin + Intensive insulin Lifestyle + Metformin + Sulfonylurea STEP 2 STEP 3 Tier 2: Less well validated therapies Lifestyle + Metformin + Pioglitazone No hypoglycaemia Oedema/CHF Bone loss Lifestyle + metformin + GLP-1 agonist No hypoglycaemia Weight loss Nausea/vomiting Nathan DM, et al. Diabetes Care 2009; 32 193 -203. Lifestyle + Metformin + Pioglitazone + Sulfonylurea Lifestyle + metformin + Basal insulin
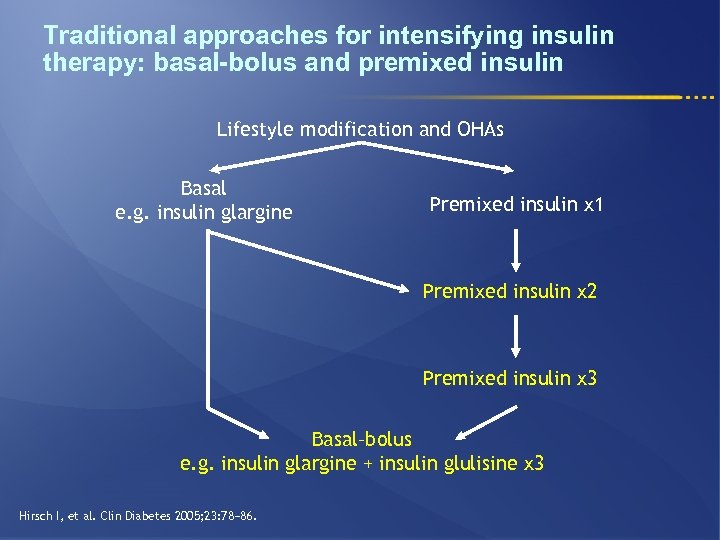
Traditional approaches for intensifying insulin therapy: basal-bolus and premixed insulin Lifestyle modification and OHAs Basal e. g. insulin glargine Premixed insulin x 1 Premixed insulin x 2 Premixed insulin x 3 Basal–bolus e. g. insulin glargine + insulin glulisine x 3 Hirsch I, et al. Clin Diabetes 2005; 23: 78− 86.
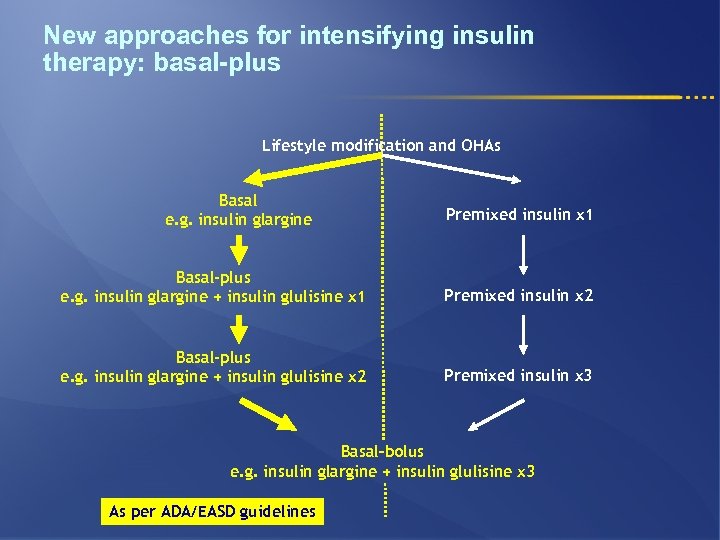
New approaches for intensifying insulin therapy: basal-plus Lifestyle modification and OHAs Basal e. g. insulin glargine Premixed insulin x 1 Basal-plus e. g. insulin glargine + insulin glulisine x 1 Premixed insulin x 2 Basal-plus e. g. insulin glargine + insulin glulisine x 2 Premixed insulin x 3 Basal–bolus e. g. insulin glargine + insulin glulisine x 3 As per ADA/EASD guidelines
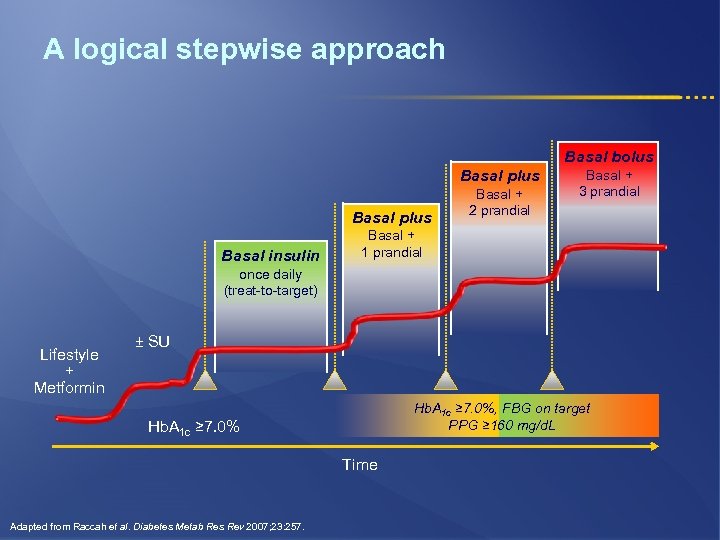
A logical stepwise approach Basal bolus Basal plus Basal insulin Basal + 2 prandial Basal + 3 prandial Basal + 1 prandial once daily (treat-to-target) Lifestyle + Metformin ± SU Hb. A 1 c ≥ 7. 0%, FBG on target PPG ≥ 160 mg/d. L Hb. A 1 c ≥ 7. 0% Time Adapted from Raccah et al. Diabetes Metab Res Rev 2007; 23: 257.
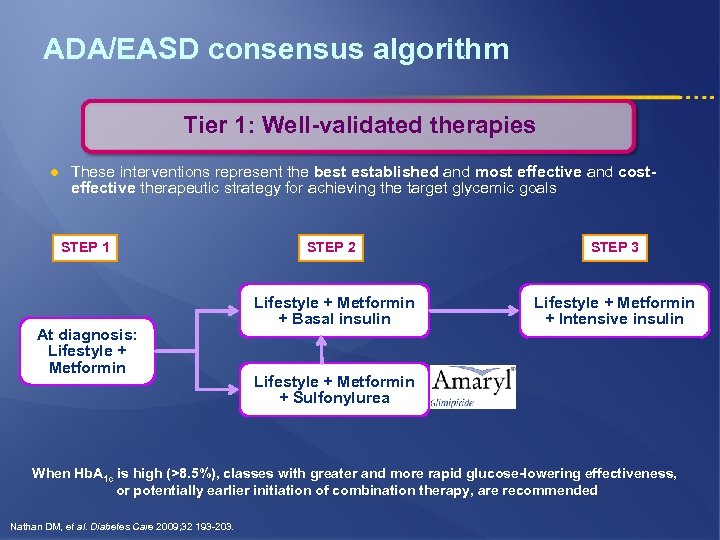
ADA/EASD consensus algorithm Tier 1: Well-validated therapies l These interventions represent the best established and most effective and costeffective therapeutic strategy for achieving the target glycemic goals STEP 1 At diagnosis: Lifestyle + Metformin STEP 2 STEP 3 Lifestyle + Metformin + Basal insulin Lifestyle + Metformin + Intensive insulin Lifestyle + Metformin + Sulfonylurea When Hb. A 1 c is high (>8. 5%), classes with greater and more rapid glucose-lowering effectiveness, or potentially earlier initiation of combination therapy, are recommended Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
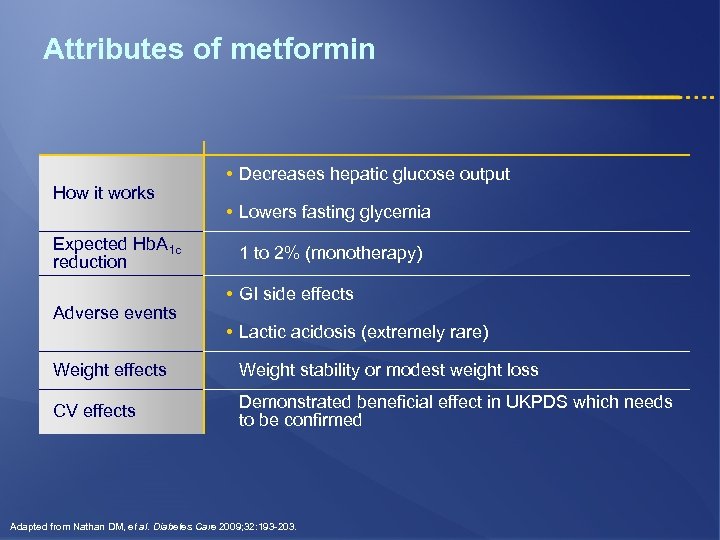
Attributes of metformin How it works Expected Hb. A 1 c reduction Adverse events • Decreases hepatic glucose output • Lowers fasting glycemia 1 to 2% (monotherapy) • GI side effects • Lactic acidosis (extremely rare) Weight effects Weight stability or modest weight loss CV effects Demonstrated beneficial effect in UKPDS which needs to be confirmed Adapted from Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.
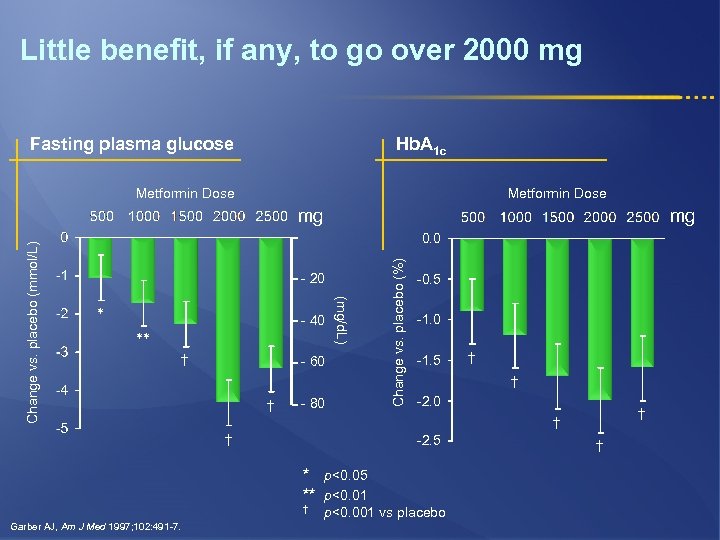
Little benefit, if any, to go over 2000 mg Fasting plasma glucose Hb. A 1 c Metformin Dose mg - 20 * - 40 ** † - 60 † - 80 Change vs. placebo (%) 0. 0 (mg/d. L) Change vs. placebo (mmol/L) mg -0. 5 -1. 0 -1. 5 † -2. 0 † † † -2. 5 * p<0. 05 ** p<0. 01 † Garber AJ, Am J Med 1997; 102: 491 -7. † p<0. 001 vs placebo †
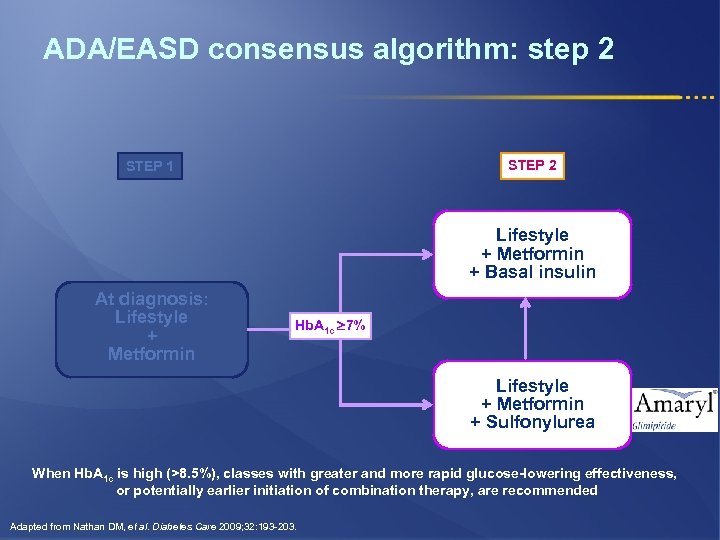
ADA/EASD consensus algorithm: step 2 STEP 1 Lifestyle + Metformin + Basal insulin At diagnosis: Lifestyle + Metformin Hb. A 1 c 7% Lifestyle + Metformin + Sulfonylurea When Hb. A 1 c is high (>8. 5%), classes with greater and more rapid glucose-lowering effectiveness, or potentially earlier initiation of combination therapy, are recommended Adapted from Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.

ADA/EASD consensus algorithm: step 2 l If step 1 fails to achieve or sustain Hb. A 1 c <7%, another medication should be added within 2 -3 months l The Hb. A 1 c level will determine (in part) which agent is selected next: § Most of newly diagnosed Type 2 Diabetic patients will usually respond to sulfonylurea* § Basal insulin if Hb. A 1 c >8. 5% or symptoms of hyperglycemia * Sulfonylureas other than glybenclamide (glyburide) or chlorpropamide Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.
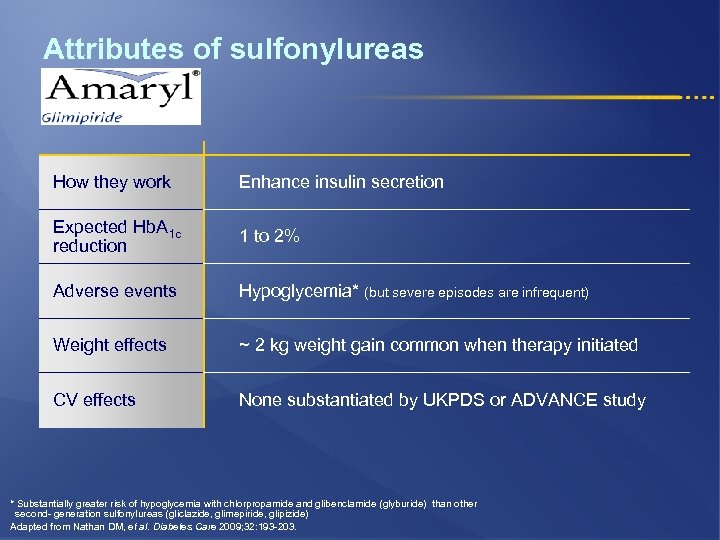
Attributes of sulfonylureas How they work Enhance insulin secretion Expected Hb. A 1 c reduction 1 to 2% Adverse events Hypoglycemia* (but severe episodes are infrequent) Weight effects ~ 2 kg weight gain common when therapy initiated CV effects None substantiated by UKPDS or ADVANCE study * Substantially greater risk of hypoglycemia with chlorpropamide and glibenclamide (glyburide) than other second- generation sulfonylureas (gliclazide, glimepiride, glipizide) Adapted from Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.
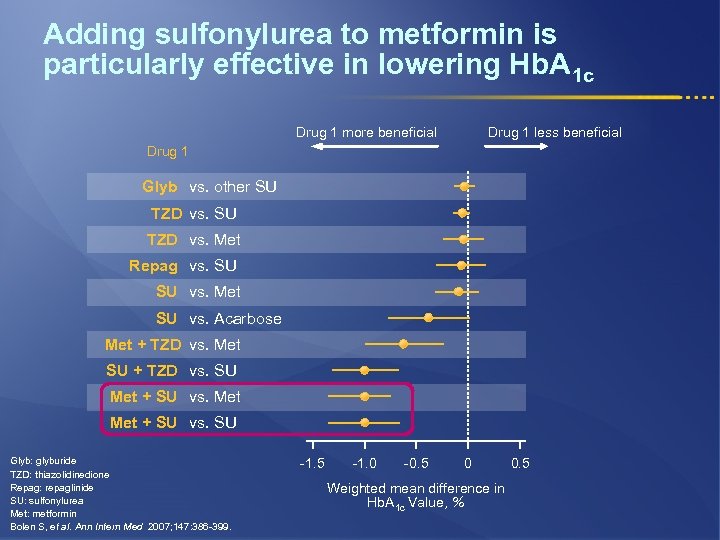
Adding sulfonylurea to metformin is particularly effective in lowering Hb. A 1 c Drug 1 more beneficial Drug 1 less beneficial Drug 1 Glyb vs. other SU TZD vs. Met Repag vs. SU SU vs. Met SU vs. Acarbose Met + TZD vs. Met SU + TZD vs. SU Met + SU vs. SU Glyb: glyburide TZD: thiazolidinedione Repag: repaglinide SU: sulfonylurea Met: metformin Bolen S, et al. Ann Intern Med 2007; 147: 386 -399. -1. 5 -1. 0 -0. 5 0 Weighted mean difference in Hb. A 1 c Value, % 0. 5
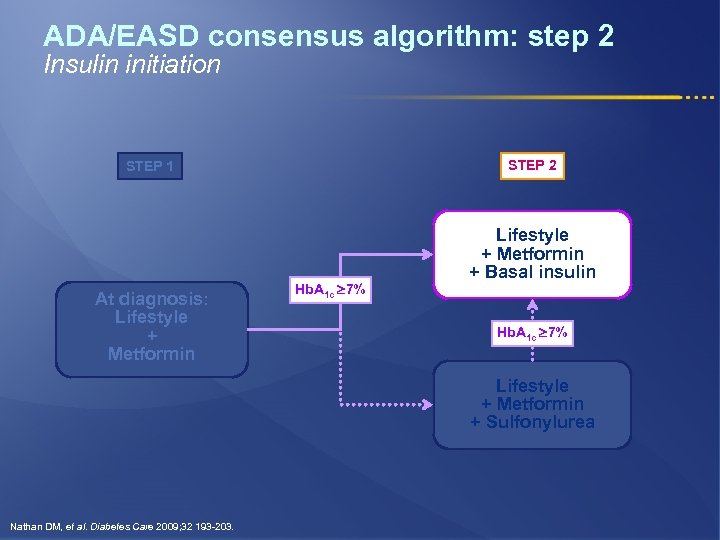
ADA/EASD consensus algorithm: step 2 Insulin initiation STEP 2 STEP 1 At diagnosis: Lifestyle + Metformin Hb. A 1 c 7% Lifestyle + Metformin + Basal insulin Hb. A 1 c 7% Lifestyle + Metformin + Sulfonylurea Nathan DM, et al. Diabetes Care 2009; 32 193 -203.
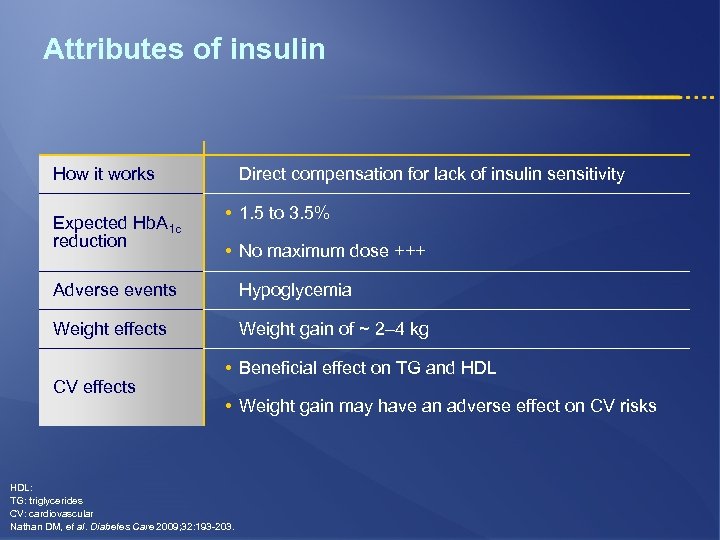
Attributes of insulin How it works Expected Hb. A 1 c reduction Direct compensation for lack of insulin sensitivity • 1. 5 to 3. 5% • No maximum dose +++ Adverse events Hypoglycemia Weight effects Weight gain of ~ 2– 4 kg CV effects • Beneficial effect on TG and HDL • Weight gain may have an adverse effect on CV risks HDL: TG: triglycerides CV: cardiovascular Nathan DM, et al. Diabetes Care 2009; 32: 193 -203.
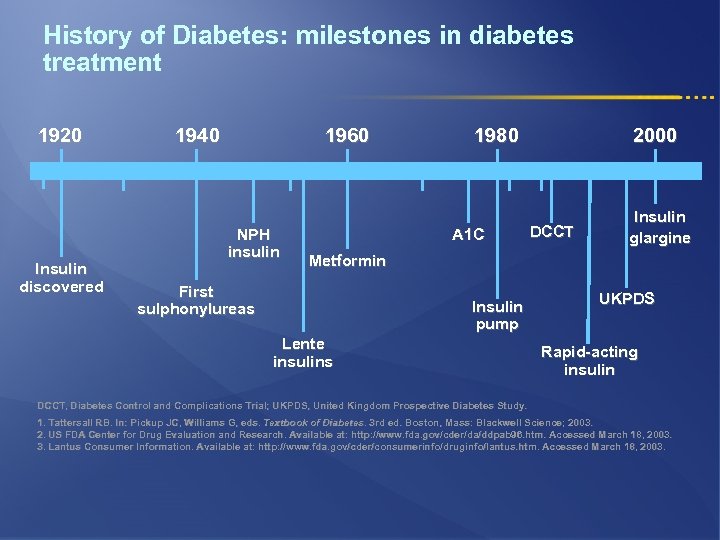
History of Diabetes: milestones in diabetes treatment 1920 Insulin discovered 1940 1960 NPH insulin 1980 A 1 C 2000 DCCT Insulin glargine Metformin First sulphonylureas Insulin pump Lente insulins UKPDS Rapid-acting insulin DCCT, Diabetes Control and Complications Trial; UKPDS, United Kingdom Prospective Diabetes Study. 1. Tattersall RB. In: Pickup JC, Williams G, eds. Textbook of Diabetes. 3 rd ed. Boston, Mass: Blackwell Science; 2003. Diabetes. 2. US FDA Center for Drug Evaluation and Research. Available at: http: //www. fda. gov/cder/da/ddpab 96. htm. Accessed March 18, 2003. 3. Lantus Consumer Information. Available at: http: //www. fda. gov/cder/consumerinfo/druginfo/lantus. htm. Accessed March 18, 2003.
History of Sanofi in Diabetes: 1923 First Announcement of Insulin Hoechst. .

History of Sanofi in Diabetes: Insulin production 1939. . . and Today

Sanofi today: developing agents to bring A 1 C under control Rapid-acting insulin analog Once-daily basal insulin Oral anti-diabetes agent
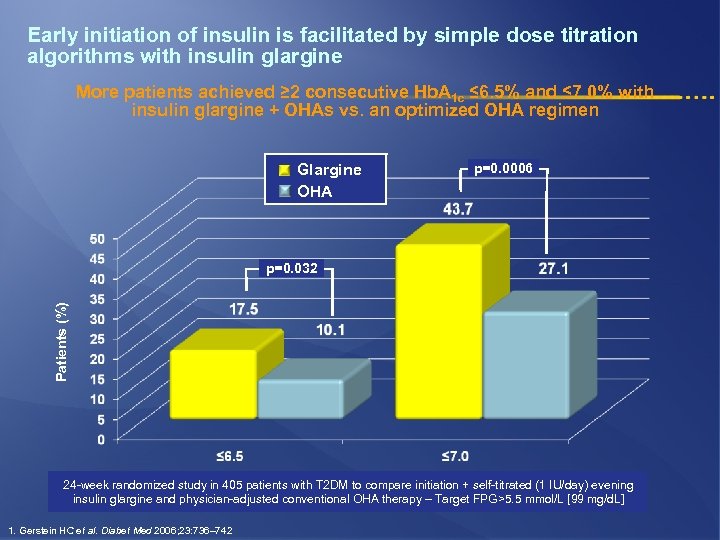
Early initiation of insulin is facilitated by simple dose titration algorithms with insulin glargine More patients achieved ≥ 2 consecutive Hb. A 1 c ≤ 6. 5% and ≤ 7. 0% with insulin glargine + OHAs vs. an optimized OHA regimen Glargine OHA p=0. 0006 Patients (%) p=0. 032 24 -week randomized study in 405 patients with T 2 DM to compare initiation + self-titrated (1 IU/day) evening insulin glargine and physician-adjusted conventional OHA therapy – Target FPG>5. 5 mmol/L [99 mg/d. L] 1. Gerstein HC et al. Diabet Med 2006; 23: 736– 742

Place of premixed insulins l Premixed insulins are not recommended § For initiation or during adjustment of doses l If the proportion of rapid- and intermediate-acting insulin is similar to the fixed proportions available § Can be used before breakfast and/or dinner Nathan DM, et al. Diabetes Care 2009; 32 193 -203.

Potential limitations of premixed insulin analogues in clinical practice l Lack of flexibility: ratio of the 2 insulin components cannot be adjusted separately § Structured meal content and timing needed l No flexible regimen of self-titration l Regimens based on carbohydrate counting difficult to devise l Insulin coverage may not address early-morning and/or postlunch hyperglycemia l Not suitable when food intake is held (eg, in the inpatient setting) Rizvi AA, et al. Insulin 2007; 2: 68– 79.

What are the Solo. Star key features? l Disposable pen l Easy to give large doses, with 80 units maximum dose l Dose setting in 1 unit steps l Easy to set and adjust l Easy to read dose numbers l Easy to feel and hear clicks when dialing l Easy to push, soft and gentle injection l Easy to confirm dose delivery, dose window returns to ‘ 0’ confirming the dose is delivered l Easy to differentiate Lantus and Apidra, for increased safety

So little can go wrong with Solo. Star that the first check in most cases of problems should be to change the needle and trying a safety test. Since needle re-use is common in many markets, blocked needles will very likely be the biggest source of user problems!
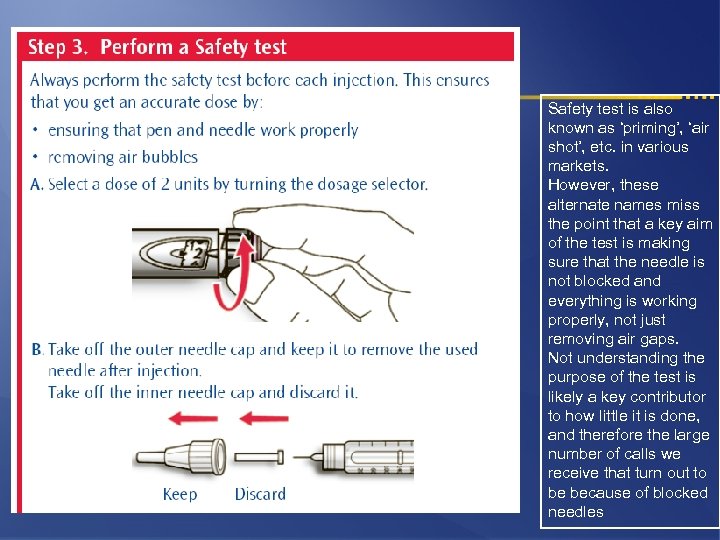
Safety test is also known as ‘priming’, ‘air shot’, etc. in various markets. However, these alternate names miss the point that a key aim of the test is making sure that the needle is not blocked and everything is working properly, not just removing air gaps. Not understanding the purpose of the test is likely a key contributor to how little it is done, and therefore the large number of calls we receive that turn out to be because of blocked needles
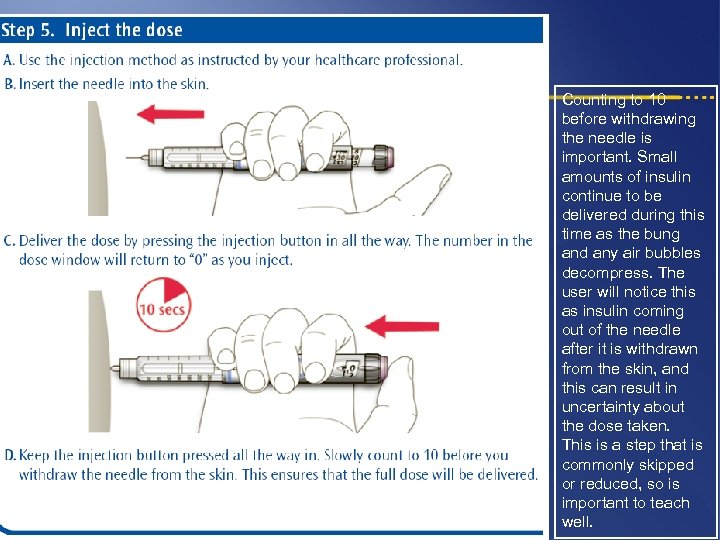
Counting to 10 before withdrawing the needle is important. Small amounts of insulin continue to be delivered during this time as the bung and any air bubbles decompress. The user will notice this as insulin coming out of the needle after it is withdrawn from the skin, and this can result in uncertainty about the dose taken. This is a step that is commonly skipped or reduced, so is important to teach well.
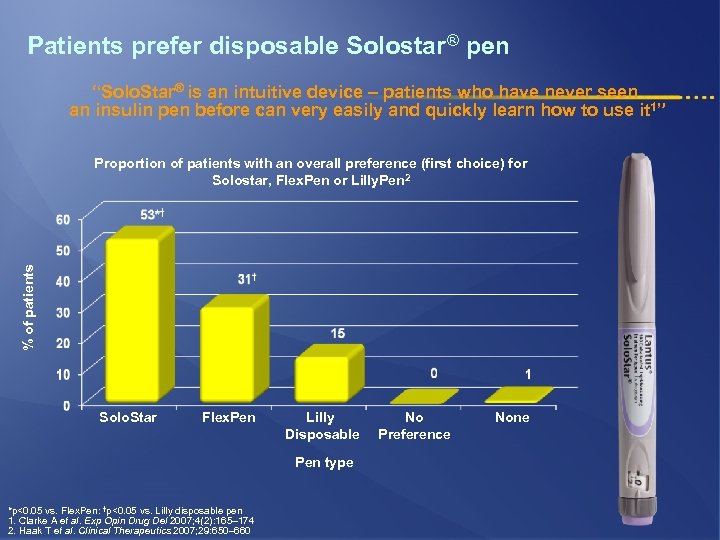
Patients prefer disposable Solostar® pen “Solo. Star® is an intuitive device – patients who have never seen an insulin pen before can very easily and quickly learn how to use it 1” % of patients Proportion of patients with an overall preference (first choice) for Solostar, Flex. Pen or Lilly. Pen 2 Solo. Star Flex. Pen Lilly Disposable Pen type *p<0. 05 vs. Flex. Pen: †p<0. 05 vs. Lilly disposable pen 1. Clarke A et al. Exp Opin Drug Del 2007; 4(2): 165– 174 2. Haak T et al. Clinical Therapeutics 2007; 29: 650– 660 No Preference None

THANK YOU
9b2d4366fc8d24816e29635cbc8b6230.ppt