2ac53b77bcbc5b9328331bf01dce0fde.ppt
- Количество слайдов: 24


Advancements in Aseptic Processing: Isolators and RABS Dr. James E. Akers President Akers Kennedy and Associates, Technical Advisor Shibuya Kogyo, Co. LTD © 2012 PDA 2

What is Advanced Aseptic Processing? • Isolators (known as aseptic chambers in the food industry) and RABS are both types of advanced aseptic processing. • Advanced aseptic processing has been defined as the complete elimination of direct interventions by gowned employees. © 2012 PDA 3

Humans are the only significant source of contamination in aseptic processing. • So, if humans are effectively separated from the work environment contamination risk is inherently low and products manufactured therein are innately safe. • In my experience any method that succeeds at eliminating human contamination input will also succeed in assuring product safety in aseptic processing. 4

There are two principal ways that advanced aseptic processing can be achieved. • Complete process automation so that human interventions are unnecessary (hard to do now, but achievable as technology evolves). • Separation by physical or aerodynamic means. • Separative devices are specified in ISO 146447: 2004 and include UAF Hoods, glove boxes, isolators, and mini-environments. • RABS is not specifically mentioned but obviously is a separative devise. 5
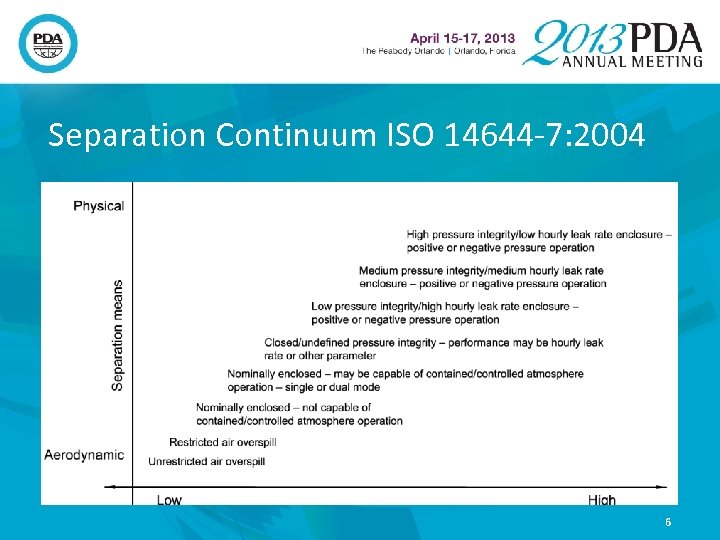
Separation Continuum ISO 14644 -7: 2004 6

The ISO Separation Continuum Considers Chemical as well as Microbiological/Particulate Contamination • The requirements for chemical contamination control/separation are different than microbiological. • Microbes are far larger in terms of physical size and mass than volatile chemicals. • The contamination sources for these disinctly different forms of contamination are very different as well. • Microbial contamination is principally a people problem. 7

Contamination Control: More Separation results in better performance

Open RABS vs. Closed RABS • Both are capable of very high levels of product safety. • Closed (c. RABS) can have the same separation capability as an isolator/aseptic chamber. 9 • Open RABS (o. RABS) is not advanced aseptic processing technology because it allows direct intervention by gowned operators (less separation). • o. RABs can be an excellent way to update the contamination control performance of existing aseptic filling lines

Advanced Technologies and Contamination Control- What the Future Holds • What the future holds depends very much on how intelligently we as an industry consider new advancements. • We have reached a point of financial and performance diminishing returns. • It is unlikely that even massive additional investment will reduce product and patient risk. • Risk to the patient is already too low to measure - it is literally lost in the statistical noise. 10

If we are already at aseptically safe- what would perfect cost? Patient benefits? ? ? 11

Have we built the requirements for advanced technology on the wrong foundation. • 25 years ago it was often said that “Isolators are nothing more than a small clean room”. • Is that really true? One could argue its not. • If it isn’t true what are the implications of acting as though it is? • The answer is systems engineered to meet the wrong requirements, based on incorrect user requirement specifications. 12

What if we had ask engineers to design isolators based upon contamination control requirements rather than clean room precedence? 13

Why Clean Rooms and Isolators are very different devices. • Isolators use physical separation to bar the entry of human contamination. • As a result they have zero human occupancy. • Clean rooms use high air dilution rates (large air changes/hour) to dilute microbial pollution. • Isolators have a small fraction of the total volume of a typical aseptic fill suite- the ratio is in some cases much greater than 50: 1 14

Current isolators tend to use clean room design specifications anyway • Air flow rates of 0. 45 m/s or even more, since more is often thought of as better. • This means large blowers and full HEPA coverage. • Isolators and c. RABS could be designed with lower air flow rates and much less elaborate air handling systems while meeting or exceeding current contamination control capability. • Complexity is not always better, but it is always more expensive to buy and maintain. • We did studies 12 years ago in which we discovered that lower air flow rates did not result in high total particulate air counts with modern production equipment. • We found that we could easily reduce airflow to 50% of less of typical clean room design criteria with no impact on particle level. • Microbial contamination was zero at all flow rates as one would expect. 15

We ignore the evidence in terms of isolator and c. RABS contamination control performance • We do not reduce microbiological evaluation effort even though the absence of direct human interventions clearly means we could. • We still do roughly the same EM sampling we’d do in a clean room. • We still do media fills at the same intervals as we always have. • We still believe there is a correlation between process time and contamination build up, even though the data from clean rooms doesn’t support this belief. 16

Decontamination Requirements • We treat an isolator as though it were an autoclave and insist upon using BIs with a population of 106 spores/coupon even though this costly and time consuming effort has nothing to do with microbiological safety. • So in the one area where we should have treated an isolator as though it were a clean room we failed to do so. • The fact is the decontamination requirements imposed are an insignificant contributor to microbiological safety. • In fact one of the major drivers for c. RABS is that it can get an organization to advanced aseptic processing technology using separative technology without isolator decontamination. • Looked at from a high level, this situation seems very strange indeed. 17

Separative Technology and the Future • Isolators can be used for a variety of new products and delivery systems. • Isolators are now being used for cell-processing in regenerative medicine. • Work by the Japan Society for the Promotion of Science suggests that isolator based cell factories can provide cost savings of up to 100 fold over conventional manned clean room alternatives. • The use of advanced decontamination capable robots in robust isolator designs could substantially reduce risk in pharmacy compounding 18

Modular Cell Processor Isolator with Double Armed VPHP Compatible Robot. 19

Modular Isolator for Cell Processing • Gloveless design in main module • Robot controllable by PLC or manually operated with trainability. • Modules can be connected to accommodate incubators, centrifuges, media supply, analysis and product transport. • Modules can be connected and removed while retaining asepsis. 20

Cell Processing Isolator System Advantages: 1. Reduces initial capital outlay ・Costly clean room, CPC are not required ・Same equipment can be used from research and development to fully commercial production 2. Reduces running cost ・Operator is not required all the time ・Regular sanitation of the clean room is not necessary 3. Established Product Quality Control ・Ensures production of the highest quality ・Ease of validation and implementation * Minimizes therapeutic costs * Provides optimized patient safety with GMP compliance - 21 -

State of the Art Aseptic Bottling • Gloveless primary operation. • Speeds of 600 -1300 units/m depending on fill volume and container/closure system sterilization. • 100% non-destructive fill accuracy checks. • Accommodations for in process control specification analysis. • Optimized up-time 48 -50 weeks per year on campaign basis. 22

Other likely future directions • Green lower energy consumption aseptic processing. • Ability to handle innovative drug delivery systems and complex aseptic packages. • Greater reliance on automation trending toward lights-out operation. • Evolution of more isolator friendly materials transport designs to accommodate more modularized operations for certain products, drug delivery systems. 23

Thank you for your attention! 800/minute CIP/SIP filler in gloveless Isolator. 24
2ac53b77bcbc5b9328331bf01dce0fde.ppt