3e72ad9323c06b9c197b0d8cf0e04c4a.ppt
- Количество слайдов: 29
 Advanced Transport Phenomena Mass Transport: Two-Phase Flow Module 6 Lecture 27 Dr. R. Nagarajan Professor Dept of Chemical Engineering IIT Madras 1
Advanced Transport Phenomena Mass Transport: Two-Phase Flow Module 6 Lecture 27 Dr. R. Nagarajan Professor Dept of Chemical Engineering IIT Madras 1
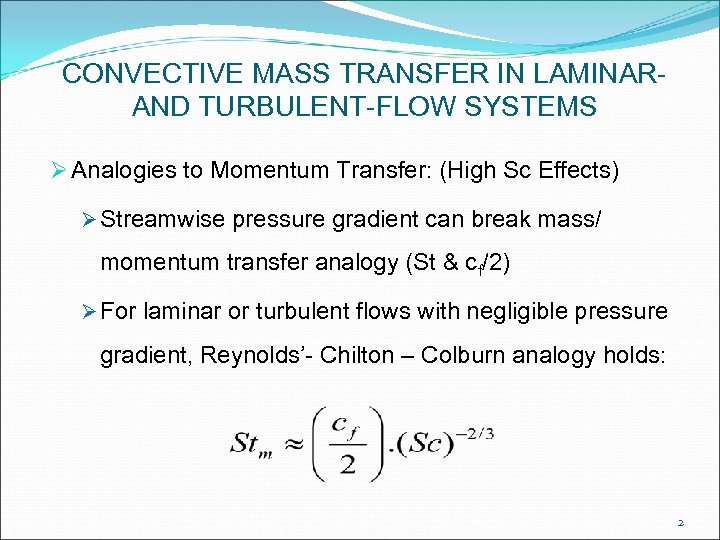 CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø Streamwise pressure gradient can break mass/ momentum transfer analogy (St & cf/2) Ø For laminar or turbulent flows with negligible pressure gradient, Reynolds’- Chilton – Colburn analogy holds: 2
CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø Streamwise pressure gradient can break mass/ momentum transfer analogy (St & cf/2) Ø For laminar or turbulent flows with negligible pressure gradient, Reynolds’- Chilton – Colburn analogy holds: 2
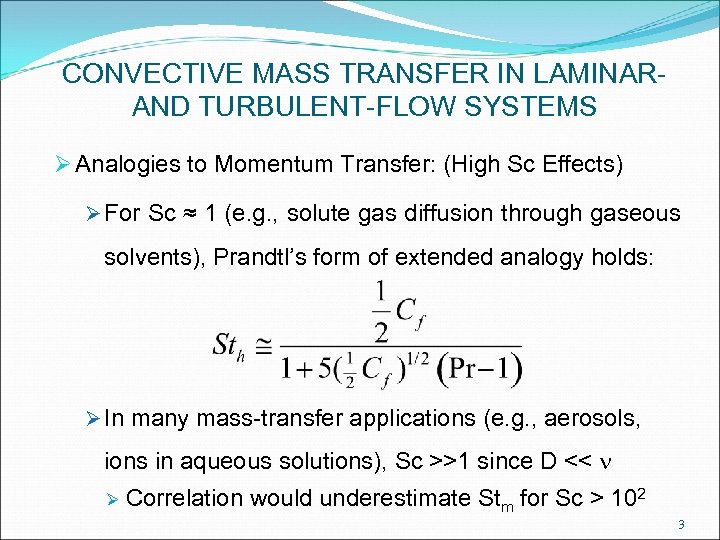 CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø For Sc ≈ 1 (e. g. , solute gas diffusion through gaseous solvents), Prandtl’s form of extended analogy holds: Ø In many mass-transfer applications (e. g. , aerosols, ions in aqueous solutions), Sc >>1 since D << n Ø Correlation would underestimate Stm for Sc > 102 3
CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø For Sc ≈ 1 (e. g. , solute gas diffusion through gaseous solvents), Prandtl’s form of extended analogy holds: Ø In many mass-transfer applications (e. g. , aerosols, ions in aqueous solutions), Sc >>1 since D << n Ø Correlation would underestimate Stm for Sc > 102 3
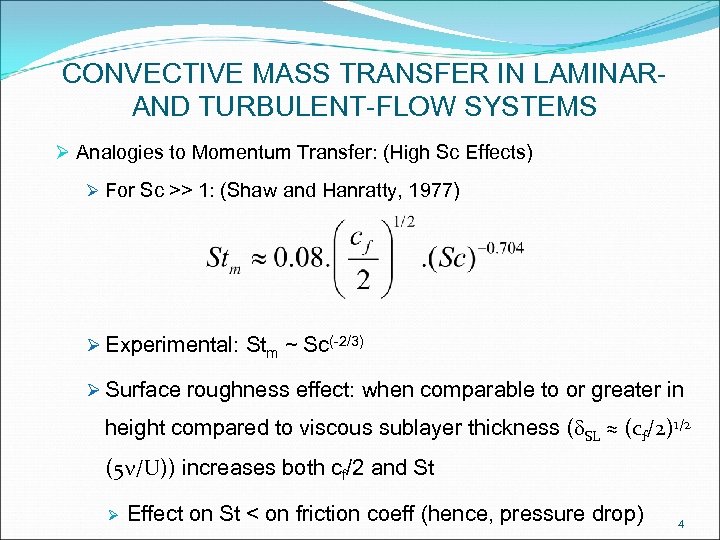 CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø For Sc >> 1: (Shaw and Hanratty, 1977) Ø Experimental: Stm ~ Sc(-2/3) Ø Surface roughness effect: when comparable to or greater in height compared to viscous sublayer thickness (d. SL ≈ (cf/2)1/2 (5 n/U)) increases both cf/2 and St Ø Effect on St < on friction coeff (hence, pressure drop) 4
CONVECTIVE MASS TRANSFER IN LAMINARAND TURBULENT-FLOW SYSTEMS Ø Analogies to Momentum Transfer: (High Sc Effects) Ø For Sc >> 1: (Shaw and Hanratty, 1977) Ø Experimental: Stm ~ Sc(-2/3) Ø Surface roughness effect: when comparable to or greater in height compared to viscous sublayer thickness (d. SL ≈ (cf/2)1/2 (5 n/U)) increases both cf/2 and St Ø Effect on St < on friction coeff (hence, pressure drop) 4
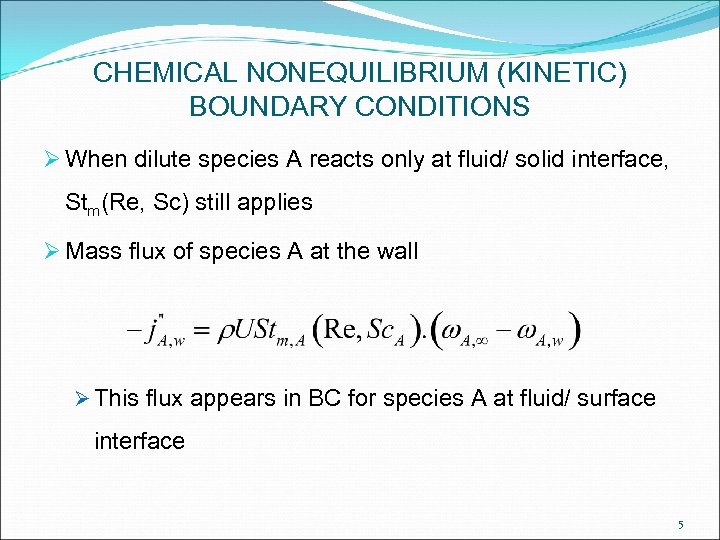 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø When dilute species A reacts only at fluid/ solid interface, Stm(Re, Sc) still applies Ø Mass flux of species A at the wall Ø This flux appears in BC for species A at fluid/ surface interface 5
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø When dilute species A reacts only at fluid/ solid interface, Stm(Re, Sc) still applies Ø Mass flux of species A at the wall Ø This flux appears in BC for species A at fluid/ surface interface 5
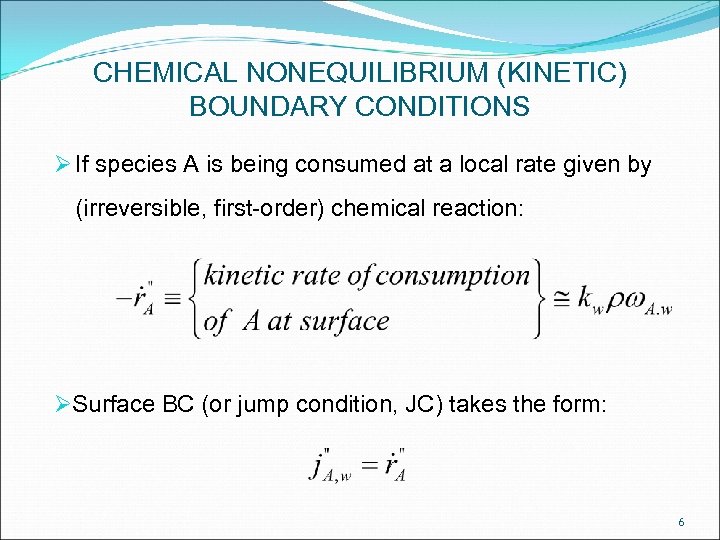 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø If species A is being consumed at a local rate given by (irreversible, first-order) chemical reaction: ØSurface BC (or jump condition, JC) takes the form: 6
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø If species A is being consumed at a local rate given by (irreversible, first-order) chemical reaction: ØSurface BC (or jump condition, JC) takes the form: 6
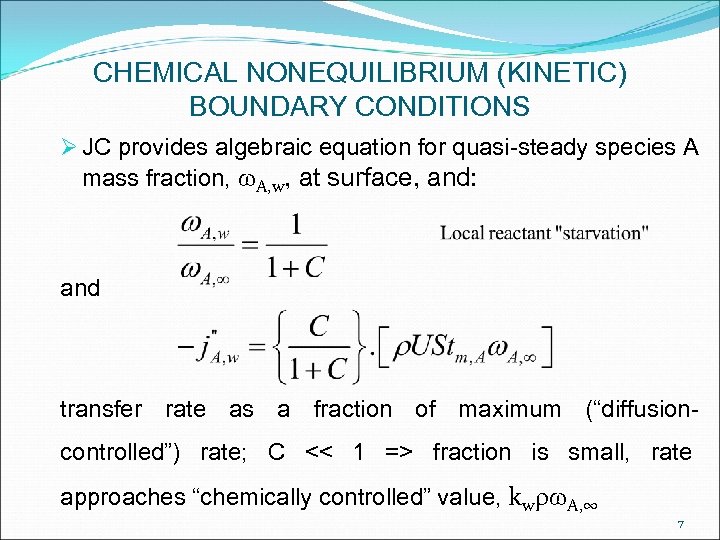 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø JC provides algebraic equation for quasi-steady species A mass fraction, w. A, w, at surface, and: and transfer rate as a fraction of maximum (“diffusioncontrolled”) rate; C << 1 => fraction is small, rate approaches “chemically controlled” value, kwrw. A, ∞ 7
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø JC provides algebraic equation for quasi-steady species A mass fraction, w. A, w, at surface, and: and transfer rate as a fraction of maximum (“diffusioncontrolled”) rate; C << 1 => fraction is small, rate approaches “chemically controlled” value, kwrw. A, ∞ 7
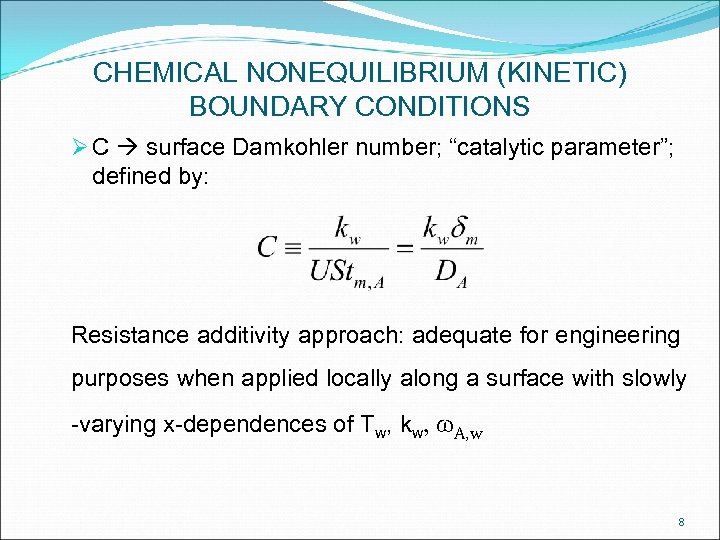 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø C surface Damkohler number; “catalytic parameter”; defined by: Resistance additivity approach: adequate for engineering purposes when applied locally along a surface with slowly -varying x-dependences of Tw, kw, w. A, w 8
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø C surface Damkohler number; “catalytic parameter”; defined by: Resistance additivity approach: adequate for engineering purposes when applied locally along a surface with slowly -varying x-dependences of Tw, kw, w. A, w 8
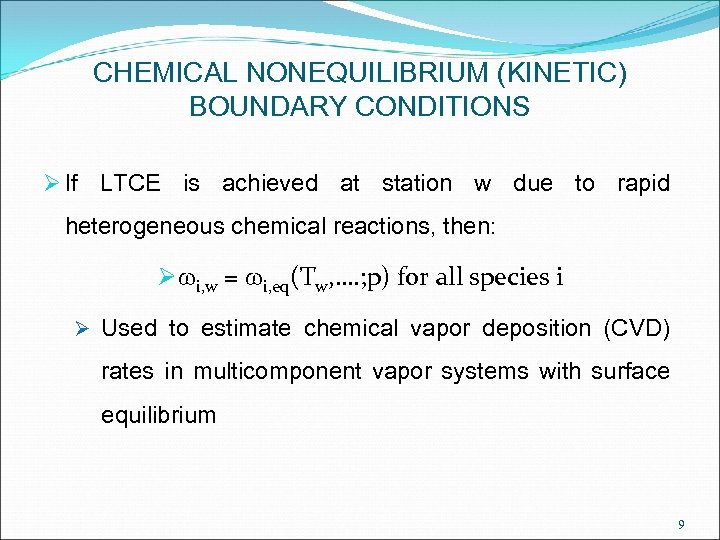 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø If LTCE is achieved at station w due to rapid heterogeneous chemical reactions, then: Ø wi, w = wi, eq(Tw, …. ; p) for all species i Ø Used to estimate chemical vapor deposition (CVD) rates in multicomponent vapor systems with surface equilibrium 9
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø If LTCE is achieved at station w due to rapid heterogeneous chemical reactions, then: Ø wi, w = wi, eq(Tw, …. ; p) for all species i Ø Used to estimate chemical vapor deposition (CVD) rates in multicomponent vapor systems with surface equilibrium 9
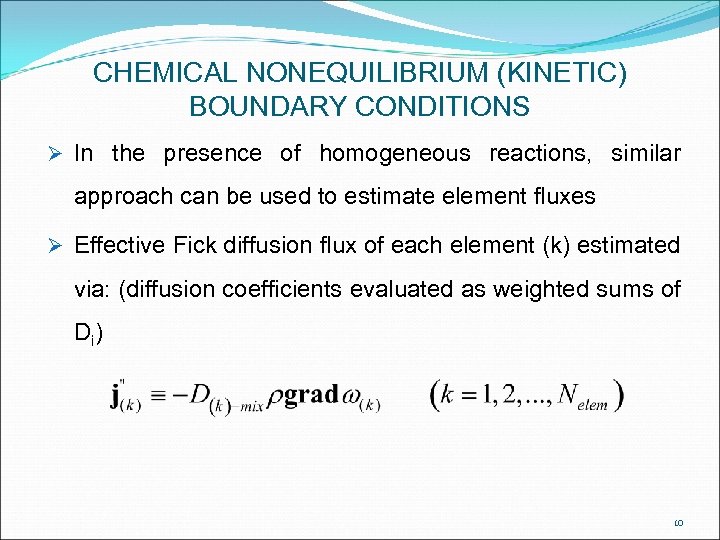 CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø In the presence of homogeneous reactions, similar approach can be used to estimate element fluxes Ø Effective Fick diffusion flux of each element (k) estimated via: (diffusion coefficients evaluated as weighted sums of D i) 10
CHEMICAL NONEQUILIBRIUM (KINETIC) BOUNDARY CONDITIONS Ø In the presence of homogeneous reactions, similar approach can be used to estimate element fluxes Ø Effective Fick diffusion flux of each element (k) estimated via: (diffusion coefficients evaluated as weighted sums of D i) 10
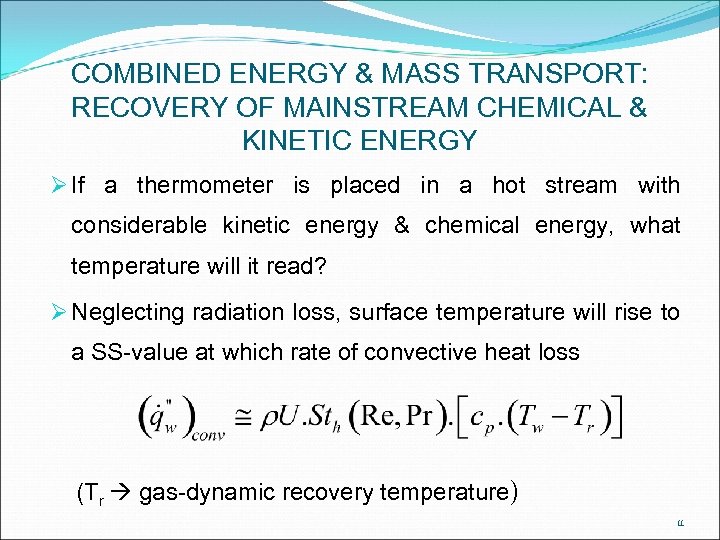 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø If a thermometer is placed in a hot stream with considerable kinetic energy & chemical energy, what temperature will it read? Ø Neglecting radiation loss, surface temperature will rise to a SS-value at which rate of convective heat loss (Tr gas-dynamic recovery temperature) 11
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø If a thermometer is placed in a hot stream with considerable kinetic energy & chemical energy, what temperature will it read? Ø Neglecting radiation loss, surface temperature will rise to a SS-value at which rate of convective heat loss (Tr gas-dynamic recovery temperature) 11
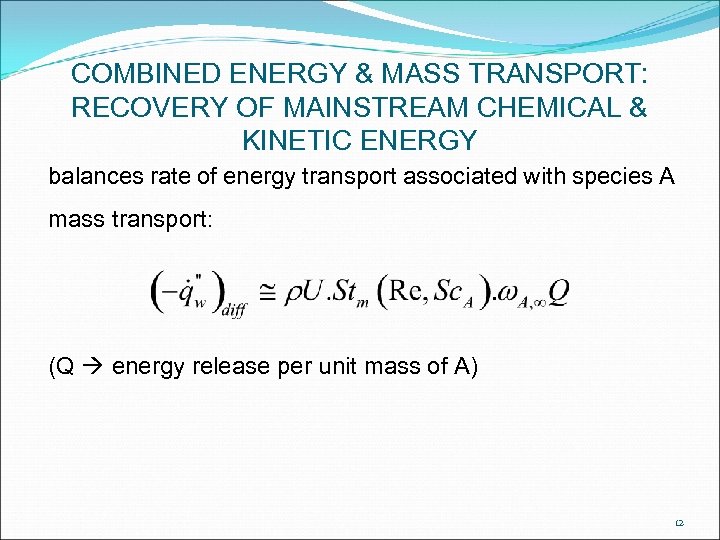 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY balances rate of energy transport associated with species A mass transport: (Q energy release per unit mass of A) 12
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY balances rate of energy transport associated with species A mass transport: (Q energy release per unit mass of A) 12
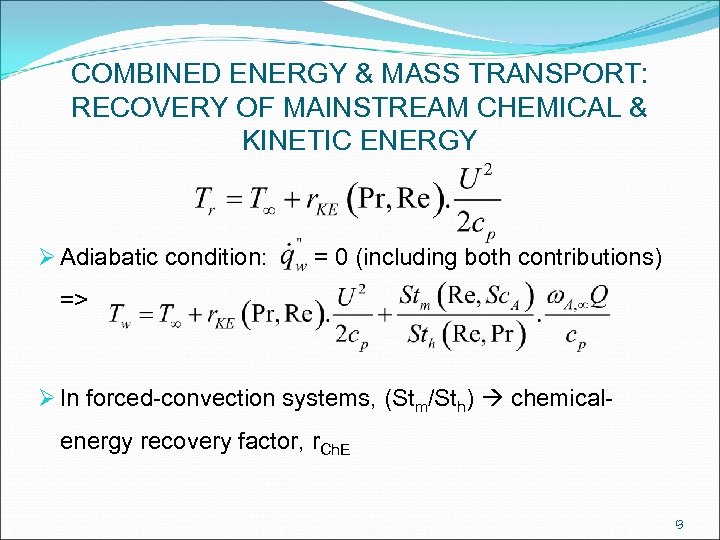 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø Adiabatic condition: = 0 (including both contributions) => Ø In forced-convection systems, (Stm/Sth) chemical- energy recovery factor, r. Ch. E 13
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø Adiabatic condition: = 0 (including both contributions) => Ø In forced-convection systems, (Stm/Sth) chemical- energy recovery factor, r. Ch. E 13
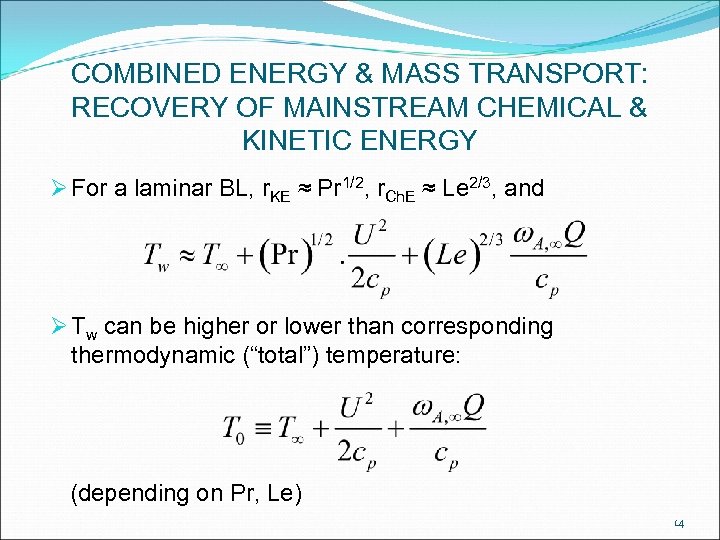 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø For a laminar BL, r. KE ≈ Pr 1/2, r. Ch. E ≈ Le 2/3, and Ø Tw can be higher or lower than corresponding thermodynamic (“total”) temperature: (depending on Pr, Le) 14
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø For a laminar BL, r. KE ≈ Pr 1/2, r. Ch. E ≈ Le 2/3, and Ø Tw can be higher or lower than corresponding thermodynamic (“total”) temperature: (depending on Pr, Le) 14
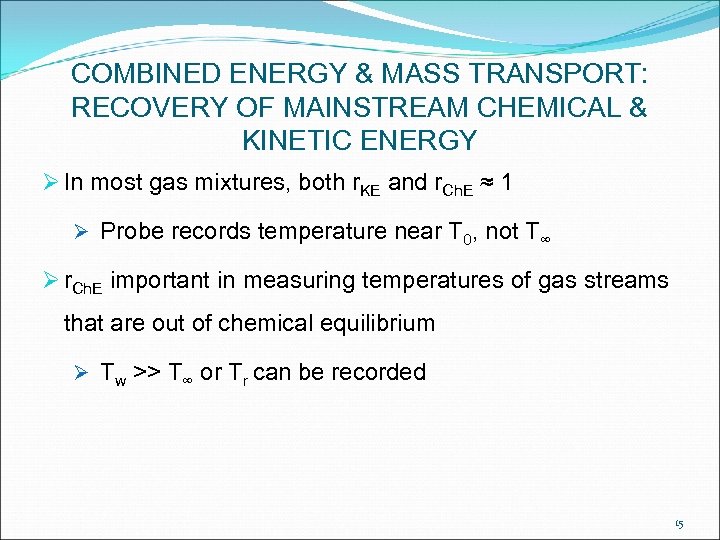 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø In most gas mixtures, both r. KE and r. Ch. E ≈ 1 Ø Probe records temperature near T 0, not T∞ Ø r. Ch. E important in measuring temperatures of gas streams that are out of chemical equilibrium Ø Tw >> T∞ or Tr can be recorded 15
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø In most gas mixtures, both r. KE and r. Ch. E ≈ 1 Ø Probe records temperature near T 0, not T∞ Ø r. Ch. E important in measuring temperatures of gas streams that are out of chemical equilibrium Ø Tw >> T∞ or Tr can be recorded 15
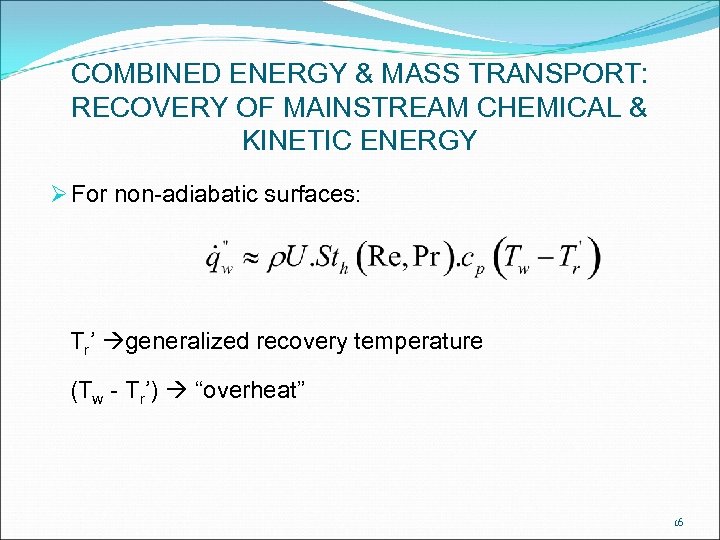 COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø For non-adiabatic surfaces: Tr’ generalized recovery temperature (Tw - Tr’) “overheat” 16
COMBINED ENERGY & MASS TRANSPORT: RECOVERY OF MAINSTREAM CHEMICAL & KINETIC ENERGY Ø For non-adiabatic surfaces: Tr’ generalized recovery temperature (Tw - Tr’) “overheat” 16
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø When dynamic coupling between suspended particles (or heavy solute molecules) & carrier fluid is weak consider particles as distinct phase Ø Distinction between two-phase flow & flow of ordinary mixtures Ø Quantified by Stokes’ number, Stk Ø Above critical value of Stk, 2 nd phase can inertially impact on target, even while host fluid is brought to rest 17
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø When dynamic coupling between suspended particles (or heavy solute molecules) & carrier fluid is weak consider particles as distinct phase Ø Distinction between two-phase flow & flow of ordinary mixtures Ø Quantified by Stokes’ number, Stk Ø Above critical value of Stk, 2 nd phase can inertially impact on target, even while host fluid is brought to rest 17
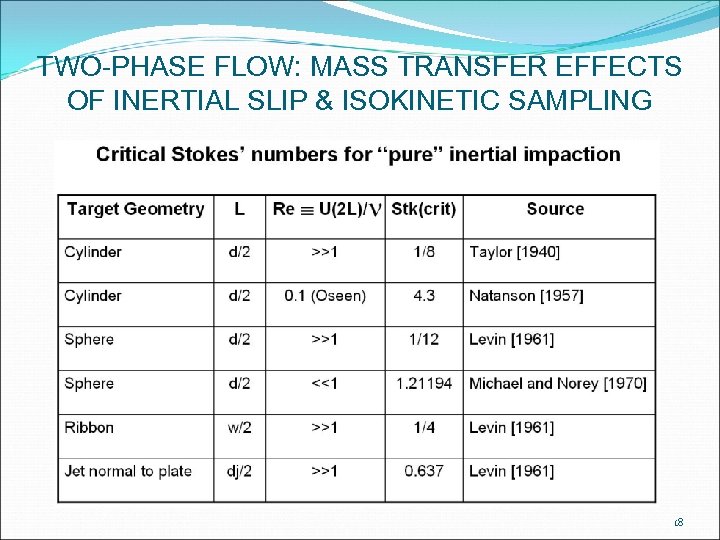 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING 18
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING 18
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Ø Particle-laden steady carrier flow of mainstream velocity, U Ø Suspended particles assumed to be: Ø Spherical (diameter dp << L) Ø Negligible mass loading & volume fraction Ø Large enough to neglect Dp, small enough to neglect gravitational sedimentation Ø Captured on impact (no rebound) 19
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Ø Particle-laden steady carrier flow of mainstream velocity, U Ø Suspended particles assumed to be: Ø Spherical (diameter dp << L) Ø Negligible mass loading & volume fraction Ø Large enough to neglect Dp, small enough to neglect gravitational sedimentation Ø Captured on impact (no rebound) 19
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Ø Each particle moves along trajectory determined by host- fluid velocity field & its drag at prevailing Re (based on local slip velocity) Ø Capture efficiency function Ø Calculated from limiting-particle trajectories (upstream locations of particles whose trajectories become tangent to target) 20
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Ø Each particle moves along trajectory determined by host- fluid velocity field & its drag at prevailing Re (based on local slip velocity) Ø Capture efficiency function Ø Calculated from limiting-particle trajectories (upstream locations of particles whose trajectories become tangent to target) 20
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø hcapture = 0 for Stk < Stkcrit Ø Capture occurs only above a critical Stokes’ number (for idealized model of particle capture from a twophase flow) 21
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø hcapture = 0 for Stk < Stkcrit Ø Capture occurs only above a critical Stokes’ number (for idealized model of particle capture from a twophase flow) 21
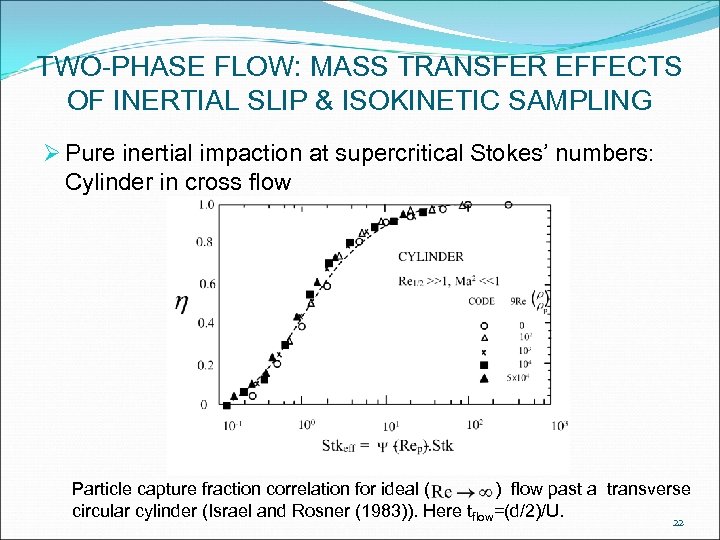 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Particle capture fraction correlation for ideal ( ) flow past a transverse circular cylinder (Israel and Rosner (1983)). Here tflow=(d/2)/U. 22
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Particle capture fraction correlation for ideal ( ) flow past a transverse circular cylinder (Israel and Rosner (1983)). Here tflow=(d/2)/U. 22
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø In practice, some deposition occurs even at Stk < Stkcrit Ø Due to non-zero Brownian diffusivity, thermophoresis, etc. Ø Rates still influenced by Stk since particle fluid is compressible (even while host carrier is subsonic) Ø Inertial enrichment (pile-up) of particles in forward stagnation region, centrifugal depletion downstream Ø Net effect: can be a reduction below diffusional deposition rate 23
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø In practice, some deposition occurs even at Stk < Stkcrit Ø Due to non-zero Brownian diffusivity, thermophoresis, etc. Ø Rates still influenced by Stk since particle fluid is compressible (even while host carrier is subsonic) Ø Inertial enrichment (pile-up) of particles in forward stagnation region, centrifugal depletion downstream Ø Net effect: can be a reduction below diffusional deposition rate 23
 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø Combustion application: sampling of particle-laden (e. g. , sooty) combustion gases using a small suction probe Ø Sampling rate too great => capture efficiency for host gas > that of particles => under-estimation; and vice versa Ø Sampling rate at which both are equal isokinetic condition (particle size dependent) 24
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in crossflow Ø Combustion application: sampling of particle-laden (e. g. , sooty) combustion gases using a small suction probe Ø Sampling rate too great => capture efficiency for host gas > that of particles => under-estimation; and vice versa Ø Sampling rate at which both are equal isokinetic condition (particle size dependent) 24
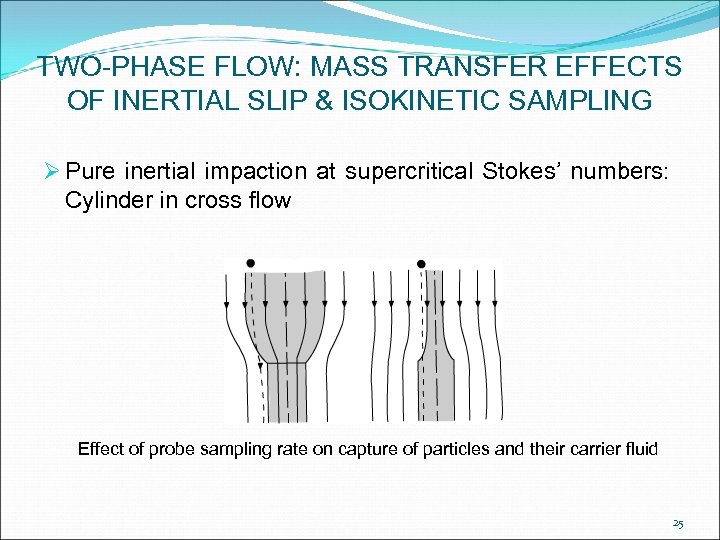 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Effect of probe sampling rate on capture of particles and their carrier fluid 25
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Pure inertial impaction at supercritical Stokes’ numbers: Cylinder in cross flow Effect of probe sampling rate on capture of particles and their carrier fluid 25
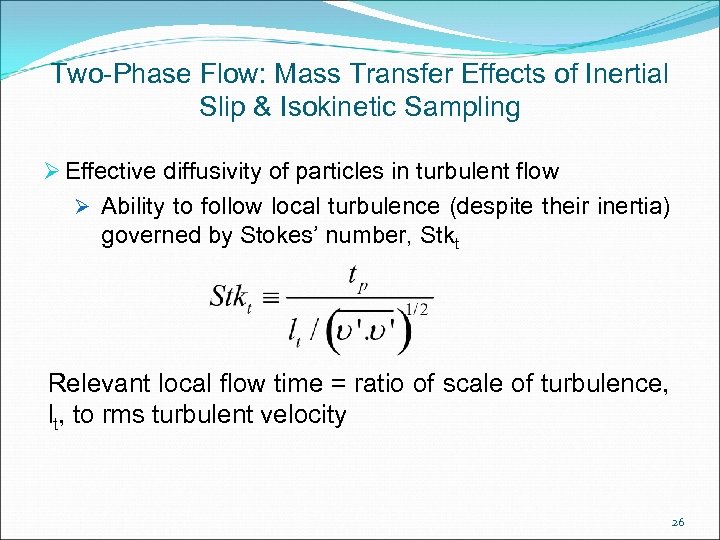 Two-Phase Flow: Mass Transfer Effects of Inertial Slip & Isokinetic Sampling Ø Effective diffusivity of particles in turbulent flow Ø Ability to follow local turbulence (despite their inertia) governed by Stokes’ number, Stkt Relevant local flow time = ratio of scale of turbulence, lt, to rms turbulent velocity 26
Two-Phase Flow: Mass Transfer Effects of Inertial Slip & Isokinetic Sampling Ø Effective diffusivity of particles in turbulent flow Ø Ability to follow local turbulence (despite their inertia) governed by Stokes’ number, Stkt Relevant local flow time = ratio of scale of turbulence, lt, to rms turbulent velocity 26
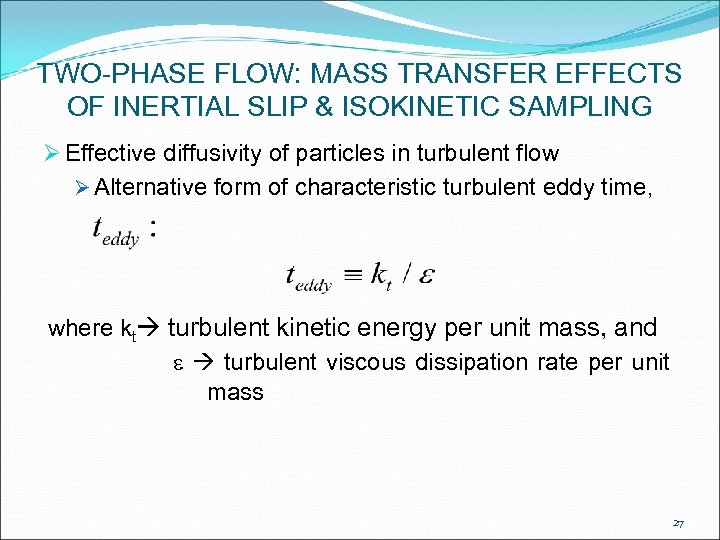 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Effective diffusivity of particles in turbulent flow Ø Alternative form of characteristic turbulent eddy time, where kt turbulent kinetic energy per unit mass, and e turbulent viscous dissipation rate per unit mass 27
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Effective diffusivity of particles in turbulent flow Ø Alternative form of characteristic turbulent eddy time, where kt turbulent kinetic energy per unit mass, and e turbulent viscous dissipation rate per unit mass 27
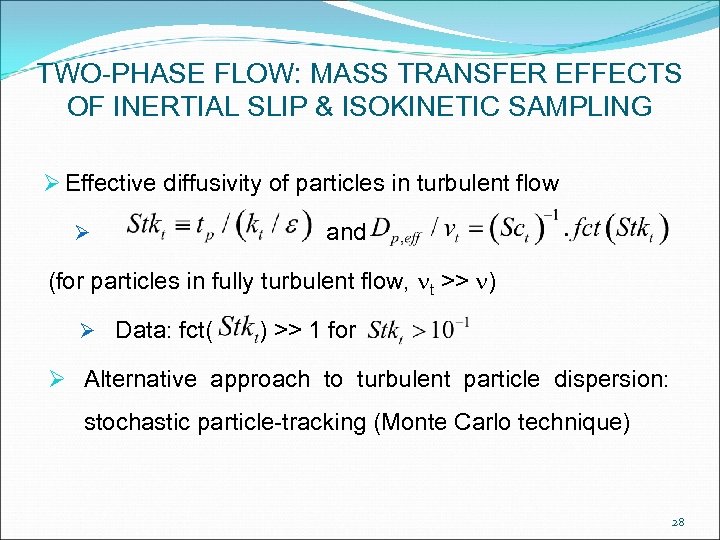 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Effective diffusivity of particles in turbulent flow Ø and (for particles in fully turbulent flow, nt >> n) Ø Data: fct( ) >> 1 for Ø Alternative approach to turbulent particle dispersion: stochastic particle-tracking (Monte Carlo technique) 28
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Effective diffusivity of particles in turbulent flow Ø and (for particles in fully turbulent flow, nt >> n) Ø Data: fct( ) >> 1 for Ø Alternative approach to turbulent particle dispersion: stochastic particle-tracking (Monte Carlo technique) 28
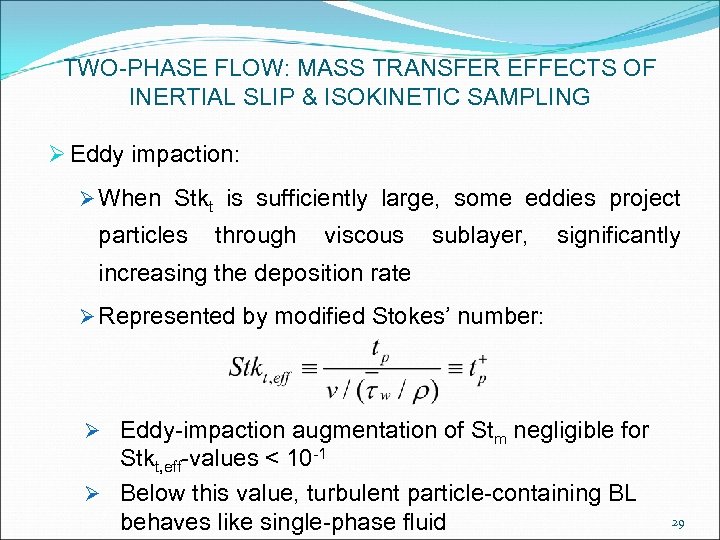 TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Eddy impaction: Ø When Stkt is sufficiently large, some eddies project particles through viscous sublayer, significantly increasing the deposition rate Ø Represented by modified Stokes’ number: Ø Eddy-impaction augmentation of Stm negligible for Stkt, eff-values < 10 -1 Ø Below this value, turbulent particle-containing BL behaves like single-phase fluid 29
TWO-PHASE FLOW: MASS TRANSFER EFFECTS OF INERTIAL SLIP & ISOKINETIC SAMPLING Ø Eddy impaction: Ø When Stkt is sufficiently large, some eddies project particles through viscous sublayer, significantly increasing the deposition rate Ø Represented by modified Stokes’ number: Ø Eddy-impaction augmentation of Stm negligible for Stkt, eff-values < 10 -1 Ø Below this value, turbulent particle-containing BL behaves like single-phase fluid 29


