db0328ecc6e9167271dd209c431edd74.ppt
- Количество слайдов: 28
 Advanced Aseptic Manufacturing Solutions for Clinical Trial Material Next. Pharma Technologies Geneviève Motte, Ph. D VP Sterile Product Development Photo courtesy of Texwipe Paris, 26 – 27 January 2010
Advanced Aseptic Manufacturing Solutions for Clinical Trial Material Next. Pharma Technologies Geneviève Motte, Ph. D VP Sterile Product Development Photo courtesy of Texwipe Paris, 26 – 27 January 2010
 Next. Pharma Technologies Introduction § Market and Clinical Pipeline § Challenges of NCEs § Manufacturing Solutions
Next. Pharma Technologies Introduction § Market and Clinical Pipeline § Challenges of NCEs § Manufacturing Solutions
 Next. Pharma Technologies Market outlook § World pharmaceutical market forecast is $750 bn in 2009 Expected growth rate: 3 – 6% per year through 2013 § Weight of biomedicines: $127 bn by 2012 (more than 15%) Expected growth rate: 12% per year § Anticancer drugs: $80 bn by 2012 Expected growth rate: 12% per year § Injectables: $147 bn (20% of the entire market) Expected to grow at 11% per year through 2012 (26% of market)
Next. Pharma Technologies Market outlook § World pharmaceutical market forecast is $750 bn in 2009 Expected growth rate: 3 – 6% per year through 2013 § Weight of biomedicines: $127 bn by 2012 (more than 15%) Expected growth rate: 12% per year § Anticancer drugs: $80 bn by 2012 Expected growth rate: 12% per year § Injectables: $147 bn (20% of the entire market) Expected to grow at 11% per year through 2012 (26% of market)
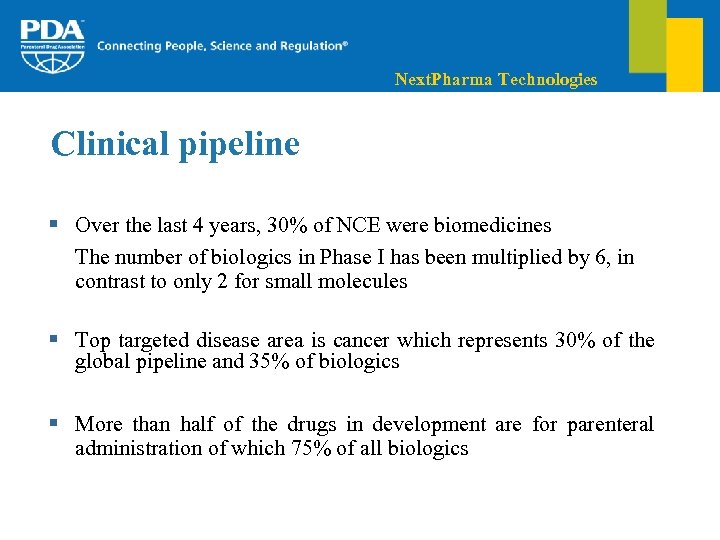 Next. Pharma Technologies Clinical pipeline § Over the last 4 years, 30% of NCE were biomedicines The number of biologics in Phase I has been multiplied by 6, in contrast to only 2 for small molecules § Top targeted disease area is cancer which represents 30% of the global pipeline and 35% of biologics § More than half of the drugs in development are for parenteral administration of which 75% of all biologics
Next. Pharma Technologies Clinical pipeline § Over the last 4 years, 30% of NCE were biomedicines The number of biologics in Phase I has been multiplied by 6, in contrast to only 2 for small molecules § Top targeted disease area is cancer which represents 30% of the global pipeline and 35% of biologics § More than half of the drugs in development are for parenteral administration of which 75% of all biologics
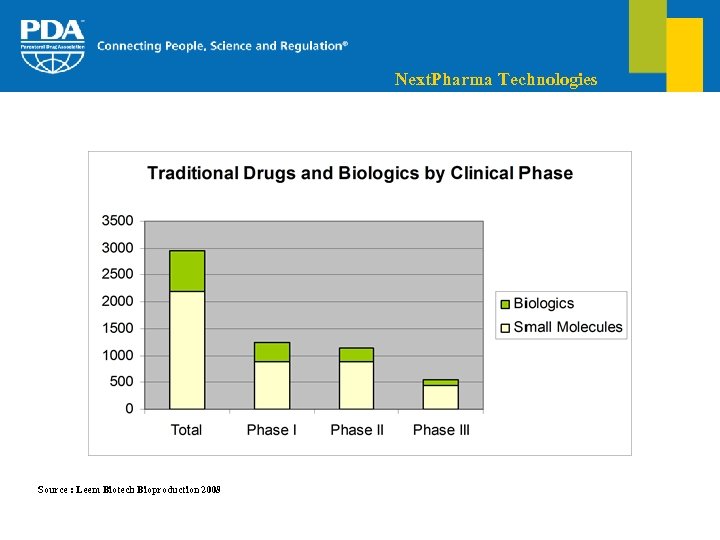 Next. Pharma Technologies Source : Leem Biotech Bioproduction 2008
Next. Pharma Technologies Source : Leem Biotech Bioproduction 2008
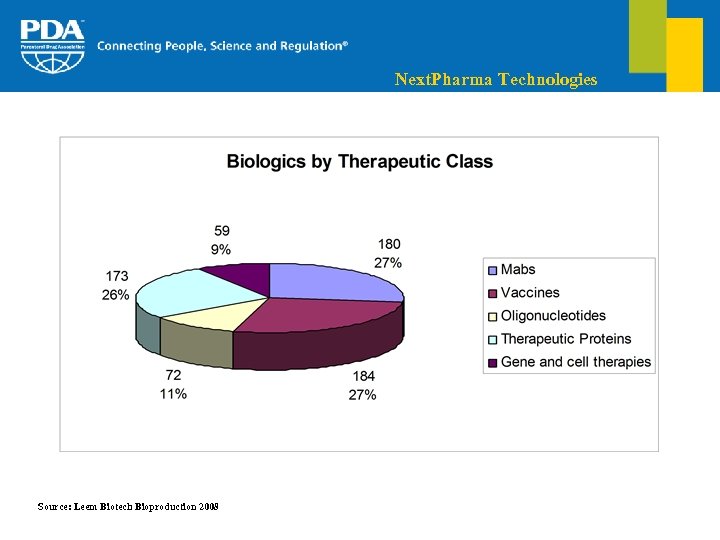 Next. Pharma Technologies Source: Leem Biotech Bioproduction 2008
Next. Pharma Technologies Source: Leem Biotech Bioproduction 2008
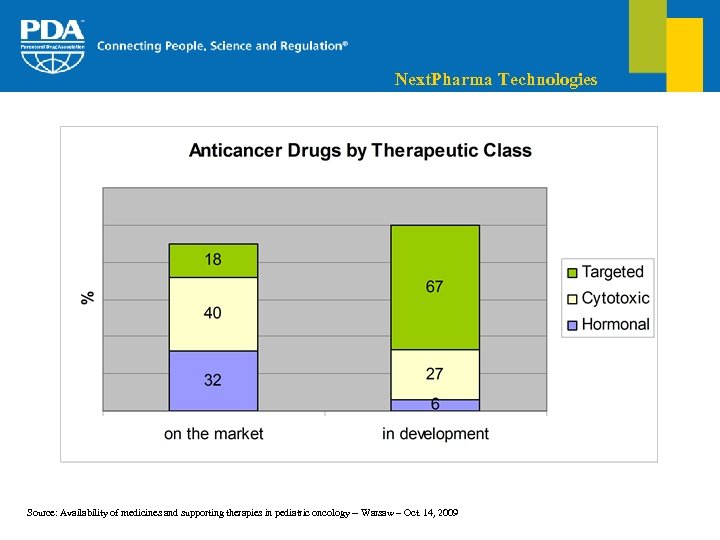 Next. Pharma Technologies Source: Availability of medicines and supporting therapies in pediatric oncology -- Warsaw – Oct. 14, 2009
Next. Pharma Technologies Source: Availability of medicines and supporting therapies in pediatric oncology -- Warsaw – Oct. 14, 2009
 Next. Pharma Technologies Challenges - Biologics § Minimal quantities of API available (g vs kg) § Unknown toxicity § Stability issue § Solubility issue § Controlled-release formulations to extend half-life and to reduce dose-related side-effects
Next. Pharma Technologies Challenges - Biologics § Minimal quantities of API available (g vs kg) § Unknown toxicity § Stability issue § Solubility issue § Controlled-release formulations to extend half-life and to reduce dose-related side-effects
 Next. Pharma Technologies Challenges - Anticancer drugs § Extremely high potency levels and/or toxicity § Tumor-targeting formulations § Fast-track development programs and high failure rates § Small batch size
Next. Pharma Technologies Challenges - Anticancer drugs § Extremely high potency levels and/or toxicity § Tumor-targeting formulations § Fast-track development programs and high failure rates § Small batch size
 Next. Pharma Technologies How to address these challenges? § Facility Design § Highly flexible manufacturing from Formulation Development to Clinical Trial Material and Commercial Supply § Innovative drug delivery systems
Next. Pharma Technologies How to address these challenges? § Facility Design § Highly flexible manufacturing from Formulation Development to Clinical Trial Material and Commercial Supply § Innovative drug delivery systems
 Next. Pharma Technologies Formulation labs at Next. Pharma Solubilization and stabilization Targeted and sustained drug delivery systems Lyo cycle development Process parameters
Next. Pharma Technologies Formulation labs at Next. Pharma Solubilization and stabilization Targeted and sustained drug delivery systems Lyo cycle development Process parameters
 Next. Pharma Technologies Development Center and Commercial Manufacturing and supply of Phase I to Phase III Clinical Trial Material Cytotoxic and biologics/conventional drugs in segregated units (Toxicity 4) Clinical Trial Packaging, labeling and storage Scaling-up, validation, registration and commercialization
Next. Pharma Technologies Development Center and Commercial Manufacturing and supply of Phase I to Phase III Clinical Trial Material Cytotoxic and biologics/conventional drugs in segregated units (Toxicity 4) Clinical Trial Packaging, labeling and storage Scaling-up, validation, registration and commercialization
 Next. Pharma Technologies Flexibility § 2 -100 ml glass and plastic vials handled on standard trays Ø Washing machine Ø Oven Ø Filling line Ø Freeze-dryer Ø Capping § Automatic tool-free filling and stoppering machine ( 14. 25 – 52 mm)
Next. Pharma Technologies Flexibility § 2 -100 ml glass and plastic vials handled on standard trays Ø Washing machine Ø Oven Ø Filling line Ø Freeze-dryer Ø Capping § Automatic tool-free filling and stoppering machine ( 14. 25 – 52 mm)
 Next. Pharma Technologies Flexibility § Temperature control through the manufacturing process § Oxygen control in solution + head space § Light control in RABS § Compatibility with material Ø stainless steel or glass vessels, flex bags EVA et PE Ø PES, PVDF, Nylon® filters Ø Pt silicone, Teflon® tubings
Next. Pharma Technologies Flexibility § Temperature control through the manufacturing process § Oxygen control in solution + head space § Light control in RABS § Compatibility with material Ø stainless steel or glass vessels, flex bags EVA et PE Ø PES, PVDF, Nylon® filters Ø Pt silicone, Teflon® tubings
 Next. Pharma Technologies Innovative Drug Delivery Systems § Solvent-based formulations § Controlled release matrix § Nanoscale drug carriers § Conjugated APIs
Next. Pharma Technologies Innovative Drug Delivery Systems § Solvent-based formulations § Controlled release matrix § Nanoscale drug carriers § Conjugated APIs
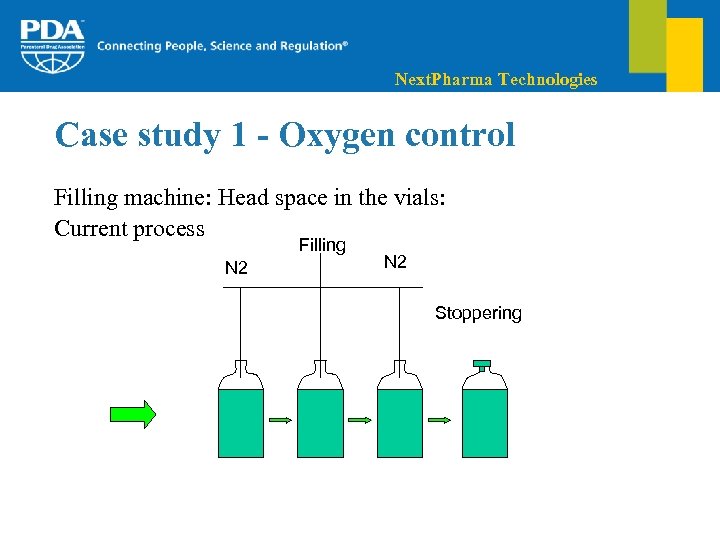 Next. Pharma Technologies Case study 1 - Oxygen control Filling machine: Head space in the vials: Current process Filling N 2 Stoppering
Next. Pharma Technologies Case study 1 - Oxygen control Filling machine: Head space in the vials: Current process Filling N 2 Stoppering
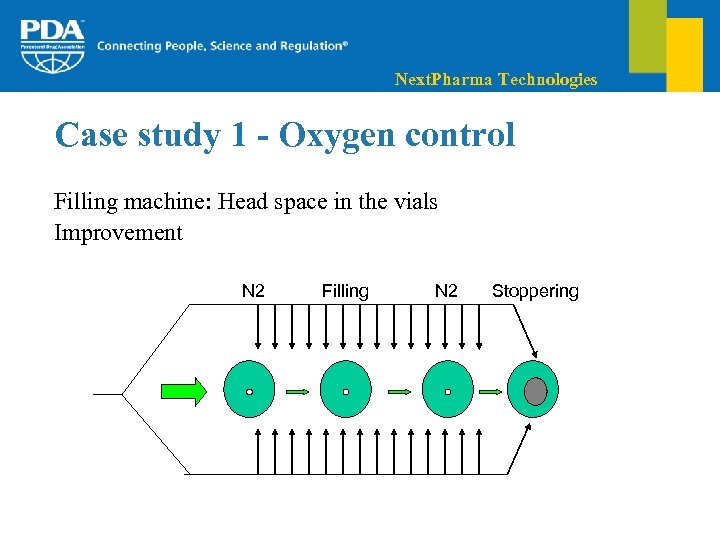 Next. Pharma Technologies Case study 1 - Oxygen control Filling machine: Head space in the vials Improvement N 2 Filling N 2 Stoppering
Next. Pharma Technologies Case study 1 - Oxygen control Filling machine: Head space in the vials Improvement N 2 Filling N 2 Stoppering
 Next. Pharma Technologies Case study 2 - Quantity of API § Lyo cycle development and Phase I clinical trial material § 800 mg API available for lyo cycle development Ø Determination of freeze-drying parameters on small vials (2 R) o Freezing o Sublimation o Secondary drying Ø Transposition to the final dosage form (10 R vials) - adjustment of phases length only, with same pressure and t° parameters
Next. Pharma Technologies Case study 2 - Quantity of API § Lyo cycle development and Phase I clinical trial material § 800 mg API available for lyo cycle development Ø Determination of freeze-drying parameters on small vials (2 R) o Freezing o Sublimation o Secondary drying Ø Transposition to the final dosage form (10 R vials) - adjustment of phases length only, with same pressure and t° parameters
 Next. Pharma Technologies Case study 3 – Fast to Clinic § Freeze-dried drug product for First In Humans § Fast to clinic Ø Aseptic manufacturing and IPC Ø Clinical trial packaging and labeling Ø Final release testing Ø QP release 3 weeks after manufacturing date
Next. Pharma Technologies Case study 3 – Fast to Clinic § Freeze-dried drug product for First In Humans § Fast to clinic Ø Aseptic manufacturing and IPC Ø Clinical trial packaging and labeling Ø Final release testing Ø QP release 3 weeks after manufacturing date
 Next. Pharma Technologies Case study 4 - Prefilled syringes § Sterile depot formulation in PFS for intra-articular § administration Rheumatoid arthritis Ø Semi-automatic filling of syringes with peristaltic pump Ø Stopper placement unit under vacuum Ø 0. 5 -20 ml PFS
Next. Pharma Technologies Case study 4 - Prefilled syringes § Sterile depot formulation in PFS for intra-articular § administration Rheumatoid arthritis Ø Semi-automatic filling of syringes with peristaltic pump Ø Stopper placement unit under vacuum Ø 0. 5 -20 ml PFS
 Next. Pharma Technologies Case study 5 – Closed Vial Technology § Allow clients to use a new technology § Minimize investment costs § Offer key advantages for patient quality and ease of use to pharmaceutical companies Ø Create partnership with Aseptic Technologies Ø Leverage both EMEA approved / FDA registered facility and preliminary R&D setting
Next. Pharma Technologies Case study 5 – Closed Vial Technology § Allow clients to use a new technology § Minimize investment costs § Offer key advantages for patient quality and ease of use to pharmaceutical companies Ø Create partnership with Aseptic Technologies Ø Leverage both EMEA approved / FDA registered facility and preliminary R&D setting
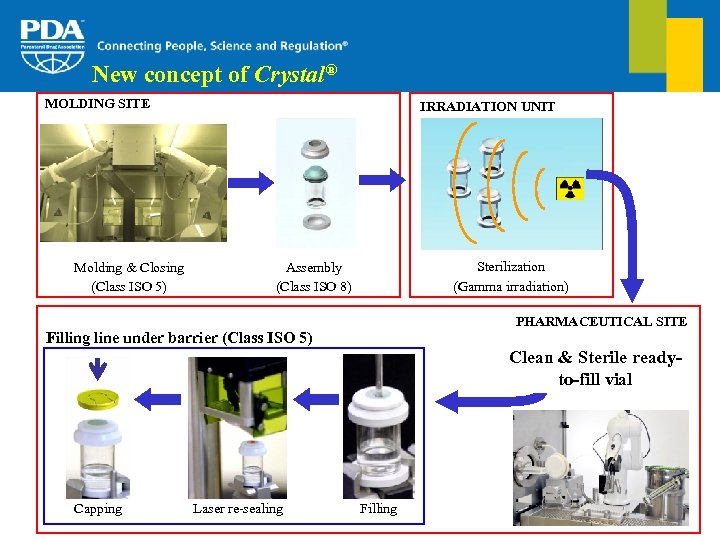 New concept of Crystal® MOLDING SITE Molding & Closing (Class ISO 5) IRRADIATION UNIT Sterilization (Gamma irradiation) Assembly (Class ISO 8) PHARMACEUTICAL SITE Filling line under barrier (Class ISO 5) Clean & Sterile readyto-fill vial Capping Laser re-sealing Filling
New concept of Crystal® MOLDING SITE Molding & Closing (Class ISO 5) IRRADIATION UNIT Sterilization (Gamma irradiation) Assembly (Class ISO 8) PHARMACEUTICAL SITE Filling line under barrier (Class ISO 5) Clean & Sterile readyto-fill vial Capping Laser re-sealing Filling
 Authority approved filling facility Offer in-house ad-hoc contract manufacturing for small quantities of GMP material (e. g. , stability batches), especially to client interested to test the concept EMEA approved 2 DMF filed at FDA Approved for recombinant viruses Clinical line in Class ISO 8 at Aseptic Technologies
Authority approved filling facility Offer in-house ad-hoc contract manufacturing for small quantities of GMP material (e. g. , stability batches), especially to client interested to test the concept EMEA approved 2 DMF filed at FDA Approved for recombinant viruses Clinical line in Class ISO 8 at Aseptic Technologies
 Preliminary R&D filling facility Offer in-house ad-hoc contract manufacturing for small non-GMP quantities to perform R&D investigation Clinical line and lyo unit in Class ISO 8 at Aseptic Technologies
Preliminary R&D filling facility Offer in-house ad-hoc contract manufacturing for small non-GMP quantities to perform R&D investigation Clinical line and lyo unit in Class ISO 8 at Aseptic Technologies
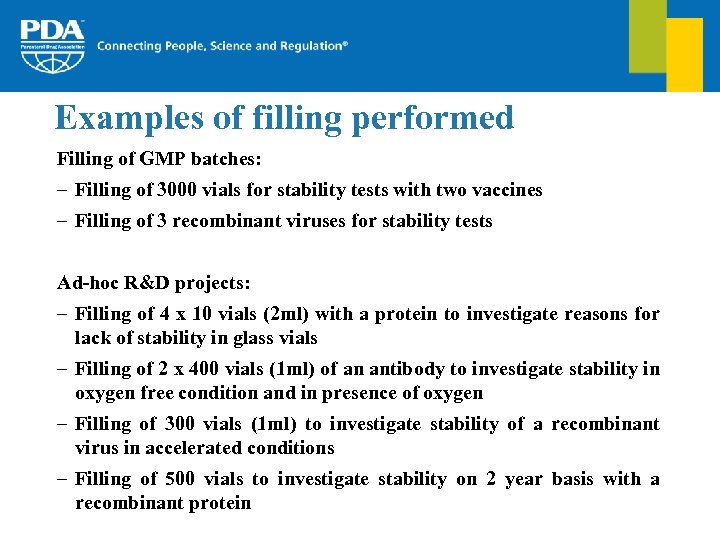 Examples of filling performed Filling of GMP batches: – Filling of 3000 vials for stability tests with two vaccines – Filling of 3 recombinant viruses for stability tests Ad-hoc R&D projects: – Filling of 4 x 10 vials (2 ml) with a protein to investigate reasons for lack of stability in glass vials – Filling of 2 x 400 vials (1 ml) of an antibody to investigate stability in oxygen free condition and in presence of oxygen – Filling of 300 vials (1 ml) to investigate stability of a recombinant virus in accelerated conditions – Filling of 500 vials to investigate stability on 2 year basis with a recombinant protein
Examples of filling performed Filling of GMP batches: – Filling of 3000 vials for stability tests with two vaccines – Filling of 3 recombinant viruses for stability tests Ad-hoc R&D projects: – Filling of 4 x 10 vials (2 ml) with a protein to investigate reasons for lack of stability in glass vials – Filling of 2 x 400 vials (1 ml) of an antibody to investigate stability in oxygen free condition and in presence of oxygen – Filling of 300 vials (1 ml) to investigate stability of a recombinant virus in accelerated conditions – Filling of 500 vials to investigate stability on 2 year basis with a recombinant protein
 Next. Pharma Technologies Case study 6 - Controlled release matrix § § Injectable depot formulation for Phase I clinical trial Lipid-based sterile solution Cyclic peptide 800 vials – 1, 4 ml fill volume Ø High sensitivity to water => o Anhydrous formulation o Ethanol rinse of equipments and pipes o Nitrogen flush Ø High viscosity of excipients => o Compounding with viscosity-controlled stirring o Larger tubing for dispensing
Next. Pharma Technologies Case study 6 - Controlled release matrix § § Injectable depot formulation for Phase I clinical trial Lipid-based sterile solution Cyclic peptide 800 vials – 1, 4 ml fill volume Ø High sensitivity to water => o Anhydrous formulation o Ethanol rinse of equipments and pipes o Nitrogen flush Ø High viscosity of excipients => o Compounding with viscosity-controlled stirring o Larger tubing for dispensing
 Next. Pharma Technologies Case study 7 - Nanoparticles § Injectable nanoscale drug carrier of a cytotoxic drug for Phase II § § clinical trial API entrapped in nanoparticles Freeze-dried sterile formulation Ø Ø Dissolution of API and sterile filtration of the solution Aseptic addition of monomer => emulsion (isolator) In situ polymerization – control of particle size! Aseptic filling and freeze-drying
Next. Pharma Technologies Case study 7 - Nanoparticles § Injectable nanoscale drug carrier of a cytotoxic drug for Phase II § § clinical trial API entrapped in nanoparticles Freeze-dried sterile formulation Ø Ø Dissolution of API and sterile filtration of the solution Aseptic addition of monomer => emulsion (isolator) In situ polymerization – control of particle size! Aseptic filling and freeze-drying
 Next. Pharma Technologies Conclusions § Facility Design and equipments adapted to highly sensitive and highly potent drugs § Cost and Time efficiency § Advanced manufacturing solutions
Next. Pharma Technologies Conclusions § Facility Design and equipments adapted to highly sensitive and highly potent drugs § Cost and Time efficiency § Advanced manufacturing solutions


