4202f7457202d0b8d4751d7b02631028.ppt
- Количество слайдов: 13
 ADVAIR and FLOVENT DISKUS Supplements for the COPD indication: SUMMARY and QUESTIONS Mary Purucker, MD, Ph. D Medical Team Leader Division of Pulmonary and Allergy Drug Products CDER, USFDA Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ADVAIR and FLOVENT DISKUS Supplements for the COPD indication: SUMMARY and QUESTIONS Mary Purucker, MD, Ph. D Medical Team Leader Division of Pulmonary and Allergy Drug Products CDER, USFDA Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 OVERVIEW • Efficacy Summary • Safety Summary – Corticosteroid-related Issues • Pivotal Trials • Supportive Trials • Non-Application Data (with caveats) • Wrap-up • Discussion Points for PADAC Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
OVERVIEW • Efficacy Summary • Safety Summary – Corticosteroid-related Issues • Pivotal Trials • Supportive Trials • Non-Application Data (with caveats) • Wrap-up • Discussion Points for PADAC Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
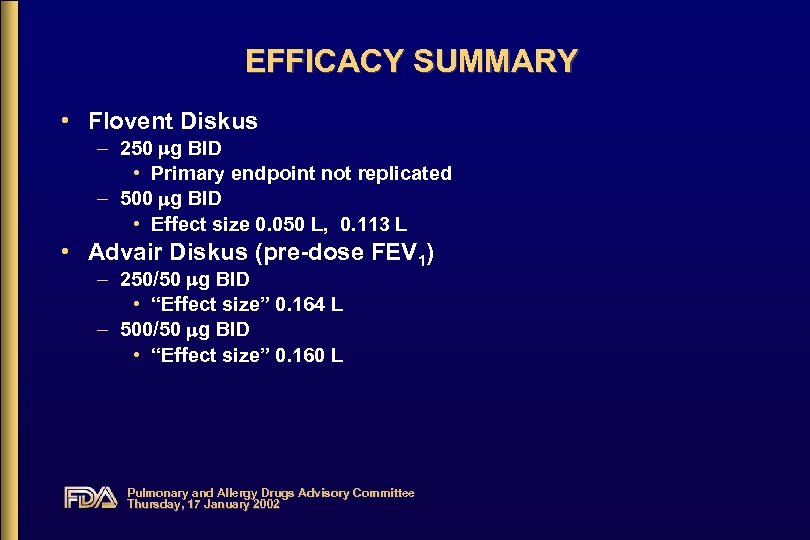 EFFICACY SUMMARY • Flovent Diskus – 250 g BID • Primary endpoint not replicated – 500 g BID • Effect size 0. 050 L, 0. 113 L • Advair Diskus (pre-dose FEV 1) – 250/50 g BID • “Effect size” 0. 164 L – 500/50 g BID • “Effect size” 0. 160 L Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
EFFICACY SUMMARY • Flovent Diskus – 250 g BID • Primary endpoint not replicated – 500 g BID • Effect size 0. 050 L, 0. 113 L • Advair Diskus (pre-dose FEV 1) – 250/50 g BID • “Effect size” 0. 164 L – 500/50 g BID • “Effect size” 0. 160 L Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 SAFETY ISSUES • Corticosteroid moiety common to the 2 products • Dose-related CS AE’s observed in pivotal trials • Systemic availability and activity demonstrated – PK studies show dose-related effect on HPA-axis • Potential for CS systemic effects should be assumed (on bone, eyes, connective tissue, and metabolism) • Pivotal and supportive studies not designed or powered to detect a difference in many aspects of CS systemic safety Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
SAFETY ISSUES • Corticosteroid moiety common to the 2 products • Dose-related CS AE’s observed in pivotal trials • Systemic availability and activity demonstrated – PK studies show dose-related effect on HPA-axis • Potential for CS systemic effects should be assumed (on bone, eyes, connective tissue, and metabolism) • Pivotal and supportive studies not designed or powered to detect a difference in many aspects of CS systemic safety Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
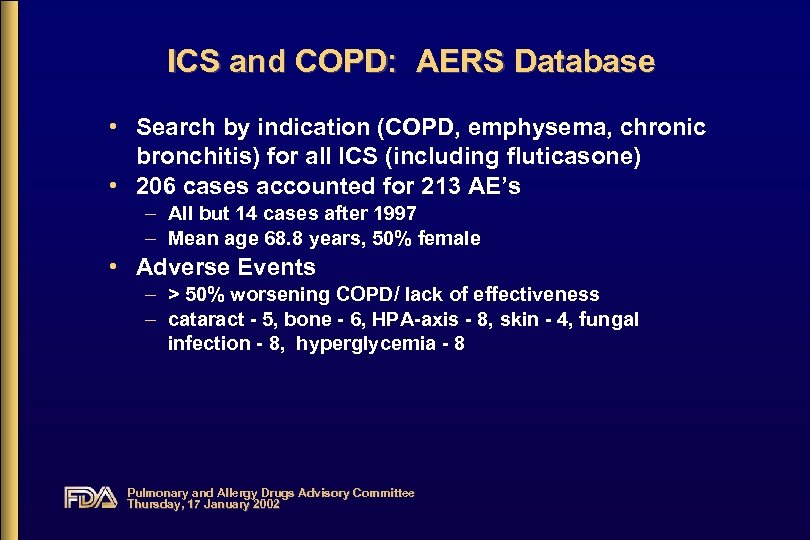 ICS and COPD: AERS Database • Search by indication (COPD, emphysema, chronic bronchitis) for all ICS (including fluticasone) • 206 cases accounted for 213 AE’s – All but 14 cases after 1997 – Mean age 68. 8 years, 50% female • Adverse Events – > 50% worsening COPD/ lack of effectiveness – cataract - 5, bone - 6, HPA-axis - 8, skin - 4, fungal infection - 8, hyperglycemia - 8 Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ICS and COPD: AERS Database • Search by indication (COPD, emphysema, chronic bronchitis) for all ICS (including fluticasone) • 206 cases accounted for 213 AE’s – All but 14 cases after 1997 – Mean age 68. 8 years, 50% female • Adverse Events – > 50% worsening COPD/ lack of effectiveness – cataract - 5, bone - 6, HPA-axis - 8, skin - 4, fungal infection - 8, hyperglycemia - 8 Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
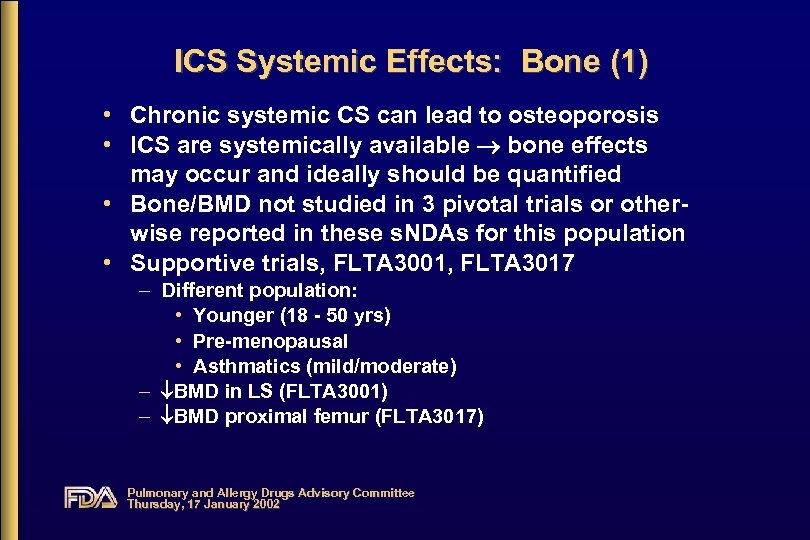 ICS Systemic Effects: Bone (1) • Chronic systemic CS can lead to osteoporosis • ICS are systemically available bone effects may occur and ideally should be quantified • Bone/BMD not studied in 3 pivotal trials or otherwise reported in these s. NDAs for this population • Supportive trials, FLTA 3001, FLTA 3017 – Different population: • Younger (18 - 50 yrs) • Pre-menopausal • Asthmatics (mild/moderate) – BMD in LS (FLTA 3001) – BMD proximal femur (FLTA 3017) Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ICS Systemic Effects: Bone (1) • Chronic systemic CS can lead to osteoporosis • ICS are systemically available bone effects may occur and ideally should be quantified • Bone/BMD not studied in 3 pivotal trials or otherwise reported in these s. NDAs for this population • Supportive trials, FLTA 3001, FLTA 3017 – Different population: • Younger (18 - 50 yrs) • Pre-menopausal • Asthmatics (mild/moderate) – BMD in LS (FLTA 3001) – BMD proximal femur (FLTA 3017) Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 ICS Systemic Effects: Bone (2) • LHS II showed decline in BMD over 3 years: – Lumbar spine (p=0. 007), N=328 – Femur (p<0. 001), N=359 • Asthma: – Israel, NEJM 2001; 3 -yr. prospective cohort study of premenopausal women dose-related BMD at hip – Wong, Lancet 2000; cross-sectional study of young adults, mean exposure 6 yrs cumulative dose-related decrease in BMD at hip and LS • IMPORTANT CAVEATS: – Different Moiety, Formulation (TAA vs. FP) – Different Populations Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ICS Systemic Effects: Bone (2) • LHS II showed decline in BMD over 3 years: – Lumbar spine (p=0. 007), N=328 – Femur (p<0. 001), N=359 • Asthma: – Israel, NEJM 2001; 3 -yr. prospective cohort study of premenopausal women dose-related BMD at hip – Wong, Lancet 2000; cross-sectional study of young adults, mean exposure 6 yrs cumulative dose-related decrease in BMD at hip and LS • IMPORTANT CAVEATS: – Different Moiety, Formulation (TAA vs. FP) – Different Populations Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 ICS Systemic Effects: HPA Axis • FLTA 3025: Serum Cortisol AUC 12 + PK (4 wks) – FP 250 g BID: 10% vs. placebo – FP 500 g BID: 21% vs. placebo • SFCA 3006, 3007: ACTH stimulation testing in a subset • ISOLDE (FLTA 78): AM Cortisol – FP 500 g BID X 36 months: 10 -15% mean AM Cortisol for FP vs. placebo at all time-points (p<0. 01) – 20% FP had Cortisol “shift” from N to L (9% placebo) • FLTA 1003 (PK/PD study): Urinary Cortisol – Single dose, crossover, normals – FP 1000 g single dose: 35% - 59% vs. pre-dose Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ICS Systemic Effects: HPA Axis • FLTA 3025: Serum Cortisol AUC 12 + PK (4 wks) – FP 250 g BID: 10% vs. placebo – FP 500 g BID: 21% vs. placebo • SFCA 3006, 3007: ACTH stimulation testing in a subset • ISOLDE (FLTA 78): AM Cortisol – FP 500 g BID X 36 months: 10 -15% mean AM Cortisol for FP vs. placebo at all time-points (p<0. 01) – 20% FP had Cortisol “shift” from N to L (9% placebo) • FLTA 1003 (PK/PD study): Urinary Cortisol – Single dose, crossover, normals – FP 1000 g single dose: 35% - 59% vs. pre-dose Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 ICS Systemic Effects: Ocular • CAVEATS: – Epidemiologic studies not RCT – ICS in general, not FP specifically – All cataracts not just PSC • Australian study (Cumming, NEJM 1997) – ICS users age 49 - 82 years – 2 -fold in PSC, further with cumulative lifetime dose • Canadian study: (Garbe, NEJM 1998) – ICS-users age 70 yrs with 3 years – 3 -fold in cataract extraction, dose response Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
ICS Systemic Effects: Ocular • CAVEATS: – Epidemiologic studies not RCT – ICS in general, not FP specifically – All cataracts not just PSC • Australian study (Cumming, NEJM 1997) – ICS users age 49 - 82 years – 2 -fold in PSC, further with cumulative lifetime dose • Canadian study: (Garbe, NEJM 1998) – ICS-users age 70 yrs with 3 years – 3 -fold in cataract extraction, dose response Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 Conclusions • Efficacy has been closely studied, substantial data, open to clinical interpretation. If approval recommended, labeling issues remain but are not insurmountable • Safety database for this population is limited in describing long-term risks. How to adequately label for the potential long-term effects, particularly with regard to bone? Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
Conclusions • Efficacy has been closely studied, substantial data, open to clinical interpretation. If approval recommended, labeling issues remain but are not insurmountable • Safety database for this population is limited in describing long-term risks. How to adequately label for the potential long-term effects, particularly with regard to bone? Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 Agency Concerns for Discussion by PADAC: EFFICACY • Patient population – Representative of COPD population? Reversibility – Supportive of broad indication of “…long-term twicedaily maintenance treatment of COPD (including chronic bronchitis and emphysema)”? • Primary Endpoint: Change from baseline in FEV 1 – Clinical relevance with regard to meaningful benefit to the patients? – Adequately supported by secondary endpoints? • Not just spirometry • COPD exacerbation • HRQL Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
Agency Concerns for Discussion by PADAC: EFFICACY • Patient population – Representative of COPD population? Reversibility – Supportive of broad indication of “…long-term twicedaily maintenance treatment of COPD (including chronic bronchitis and emphysema)”? • Primary Endpoint: Change from baseline in FEV 1 – Clinical relevance with regard to meaningful benefit to the patients? – Adequately supported by secondary endpoints? • Not just spirometry • COPD exacerbation • HRQL Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 Agency Concerns for Discussion by PADAC: SAFETY • Fluticasone is systemically available (by PK) and impact on HPA axis can be measured after single dose in normals, 4 wks in COPD – Other systemic CS effects should be assumed • Are data sufficient for labeling with regard to impact on: – – Bone (BMD or fractures)? Ocular structures (PSC/cataracts, IOP)? HPA-axis? Connective tissue, metabolic, or other systemic events? Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
Agency Concerns for Discussion by PADAC: SAFETY • Fluticasone is systemically available (by PK) and impact on HPA axis can be measured after single dose in normals, 4 wks in COPD – Other systemic CS effects should be assumed • Are data sufficient for labeling with regard to impact on: – – Bone (BMD or fractures)? Ocular structures (PSC/cataracts, IOP)? HPA-axis? Connective tissue, metabolic, or other systemic events? Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
 Acknowledgements • • • Don Collier Tayo Fadiran James Gebert Lydia Gilbert-Mc. Clain Ted Guo Ladan Jafari Claudia Karwoski Charles Lee Robert Meyer Sandra Suarez Kimberly Topper Joyce Weaver Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002
Acknowledgements • • • Don Collier Tayo Fadiran James Gebert Lydia Gilbert-Mc. Clain Ted Guo Ladan Jafari Claudia Karwoski Charles Lee Robert Meyer Sandra Suarez Kimberly Topper Joyce Weaver Pulmonary and Allergy Drugs Advisory Committee Thursday, 17 January 2002


