8d80ffbe97cae3f16bae3def2e4b1304.ppt
- Количество слайдов: 24
 Adsorption Reading: Chap. 12 • • • 2018/3/19 Physical vs chemical adsorption Sorbent materials Mechanism Isotherm Effects of humidity Fixed-bed systems Regeneration Rotary bed and fluidized bed systems Pressure drop Aerosol & Particulate Research Lab 1
Adsorption Reading: Chap. 12 • • • 2018/3/19 Physical vs chemical adsorption Sorbent materials Mechanism Isotherm Effects of humidity Fixed-bed systems Regeneration Rotary bed and fluidized bed systems Pressure drop Aerosol & Particulate Research Lab 1
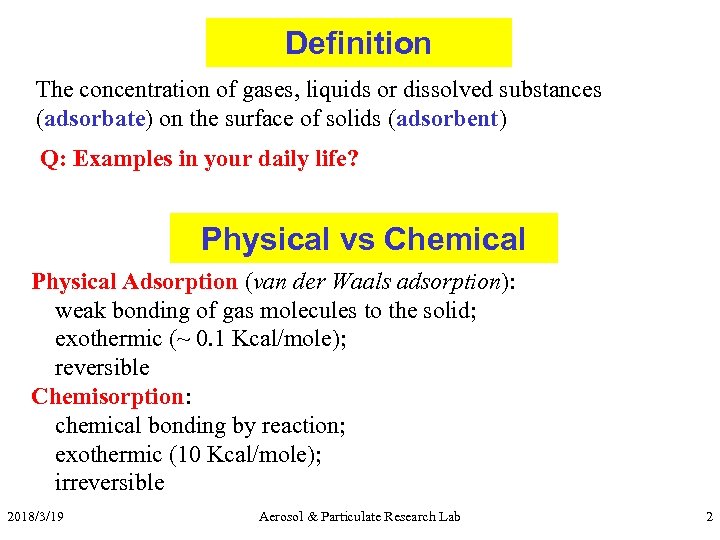 Definition The concentration of gases, liquids or dissolved substances (adsorbate) on the surface of solids (adsorbent) Q: Examples in your daily life? Physical vs Chemical Physical Adsorption (van der Waals adsorption): weak bonding of gas molecules to the solid; exothermic (~ 0. 1 Kcal/mole); reversible Chemisorption: chemical bonding by reaction; exothermic (10 Kcal/mole); irreversible 2018/3/19 Aerosol & Particulate Research Lab 2
Definition The concentration of gases, liquids or dissolved substances (adsorbate) on the surface of solids (adsorbent) Q: Examples in your daily life? Physical vs Chemical Physical Adsorption (van der Waals adsorption): weak bonding of gas molecules to the solid; exothermic (~ 0. 1 Kcal/mole); reversible Chemisorption: chemical bonding by reaction; exothermic (10 Kcal/mole); irreversible 2018/3/19 Aerosol & Particulate Research Lab 2
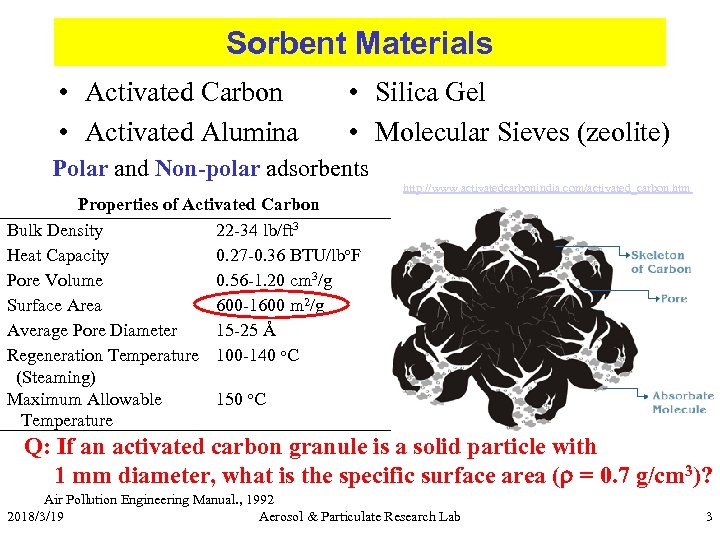 Sorbent Materials • Activated Carbon • Activated Alumina • Silica Gel • Molecular Sieves (zeolite) Polar and Non-polar adsorbents Properties of Activated Carbon Bulk Density 22 -34 lb/ft 3 Heat Capacity 0. 27 -0. 36 BTU/lbo. F Pore Volume 0. 56 -1. 20 cm 3/g Surface Area 600 -1600 m 2/g Average Pore Diameter 15 -25 Å Regeneration Temperature 100 -140 o. C (Steaming) Maximum Allowable 150 o. C Temperature http: //www. activatedcarbonindia. com/activated_carbon. htm Q: If an activated carbon granule is a solid particle with 1 mm diameter, what is the specific surface area (r = 0. 7 g/cm 3)? Air Pollution Engineering Manual. , 1992 2018/3/19 Aerosol & Particulate Research Lab 3
Sorbent Materials • Activated Carbon • Activated Alumina • Silica Gel • Molecular Sieves (zeolite) Polar and Non-polar adsorbents Properties of Activated Carbon Bulk Density 22 -34 lb/ft 3 Heat Capacity 0. 27 -0. 36 BTU/lbo. F Pore Volume 0. 56 -1. 20 cm 3/g Surface Area 600 -1600 m 2/g Average Pore Diameter 15 -25 Å Regeneration Temperature 100 -140 o. C (Steaming) Maximum Allowable 150 o. C Temperature http: //www. activatedcarbonindia. com/activated_carbon. htm Q: If an activated carbon granule is a solid particle with 1 mm diameter, what is the specific surface area (r = 0. 7 g/cm 3)? Air Pollution Engineering Manual. , 1992 2018/3/19 Aerosol & Particulate Research Lab 3
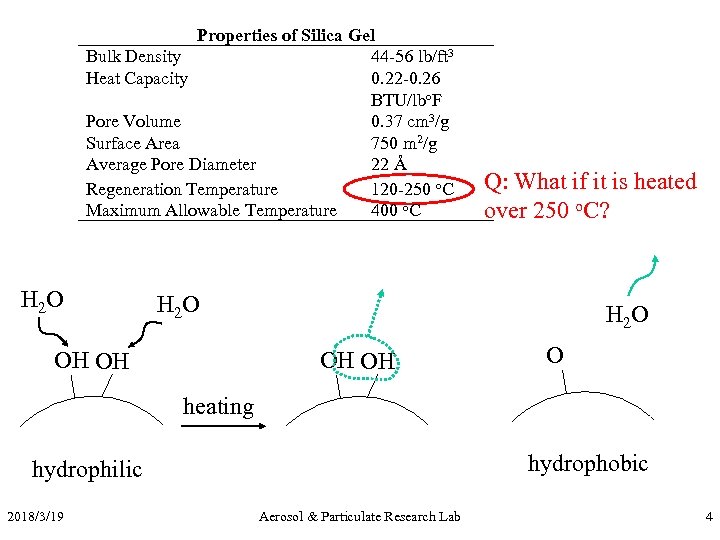 Properties of Silica Gel Bulk Density 44 -56 lb/ft 3 Heat Capacity 0. 22 -0. 26 BTU/lbo. F Pore Volume 0. 37 cm 3/g Surface Area 750 m 2/g Average Pore Diameter 22 Å Regeneration Temperature 120 -250 o. C Maximum Allowable Temperature 400 o. C H 2 O Q: What if it is heated over 250 o. C? H 2 O OH OH O heating hydrophobic hydrophilic 2018/3/19 Aerosol & Particulate Research Lab 4
Properties of Silica Gel Bulk Density 44 -56 lb/ft 3 Heat Capacity 0. 22 -0. 26 BTU/lbo. F Pore Volume 0. 37 cm 3/g Surface Area 750 m 2/g Average Pore Diameter 22 Å Regeneration Temperature 120 -250 o. C Maximum Allowable Temperature 400 o. C H 2 O Q: What if it is heated over 250 o. C? H 2 O OH OH O heating hydrophobic hydrophilic 2018/3/19 Aerosol & Particulate Research Lab 4
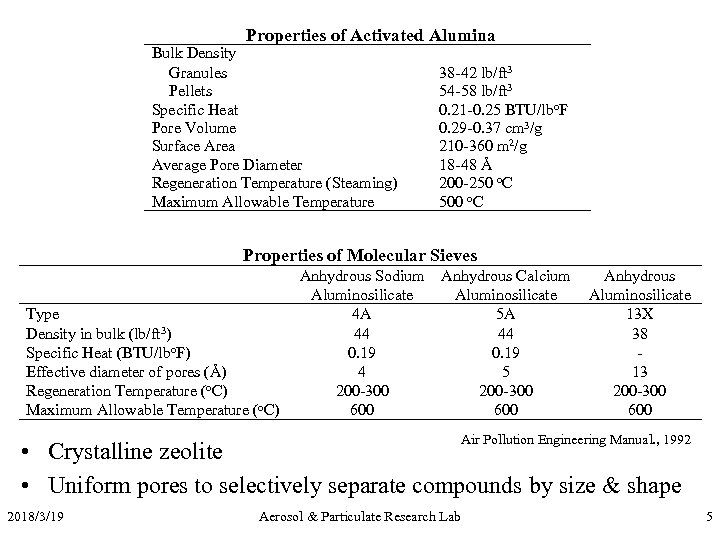 Properties of Activated Alumina Bulk Density Granules Pellets Specific Heat Pore Volume Surface Area Average Pore Diameter Regeneration Temperature (Steaming) Maximum Allowable Temperature 38 -42 lb/ft 3 54 -58 lb/ft 3 0. 21 -0. 25 BTU/lbo. F 0. 29 -0. 37 cm 3/g 210 -360 m 2/g 18 -48 Å 200 -250 o. C 500 o. C Properties of Molecular Sieves Type Density in bulk (lb/ft 3) Specific Heat (BTU/lbo. F) Effective diameter of pores (Å) Regeneration Temperature (o. C) Maximum Allowable Temperature (o. C) Anhydrous Sodium Aluminosilicate 4 A 44 0. 19 4 200 -300 600 Anhydrous Calcium Aluminosilicate 5 A 44 0. 19 5 200 -300 600 Anhydrous Aluminosilicate 13 X 38 13 200 -300 600 Air Pollution Engineering Manual. , 1992 • Crystalline zeolite • Uniform pores to selectively separate compounds by size & shape 2018/3/19 Aerosol & Particulate Research Lab 5
Properties of Activated Alumina Bulk Density Granules Pellets Specific Heat Pore Volume Surface Area Average Pore Diameter Regeneration Temperature (Steaming) Maximum Allowable Temperature 38 -42 lb/ft 3 54 -58 lb/ft 3 0. 21 -0. 25 BTU/lbo. F 0. 29 -0. 37 cm 3/g 210 -360 m 2/g 18 -48 Å 200 -250 o. C 500 o. C Properties of Molecular Sieves Type Density in bulk (lb/ft 3) Specific Heat (BTU/lbo. F) Effective diameter of pores (Å) Regeneration Temperature (o. C) Maximum Allowable Temperature (o. C) Anhydrous Sodium Aluminosilicate 4 A 44 0. 19 4 200 -300 600 Anhydrous Calcium Aluminosilicate 5 A 44 0. 19 5 200 -300 600 Anhydrous Aluminosilicate 13 X 38 13 200 -300 600 Air Pollution Engineering Manual. , 1992 • Crystalline zeolite • Uniform pores to selectively separate compounds by size & shape 2018/3/19 Aerosol & Particulate Research Lab 5
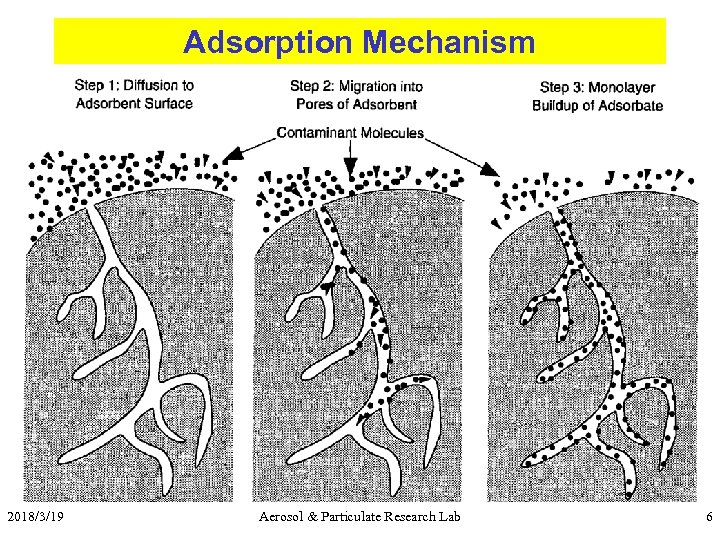 Adsorption Mechanism 2018/3/19 Aerosol & Particulate Research Lab 6
Adsorption Mechanism 2018/3/19 Aerosol & Particulate Research Lab 6
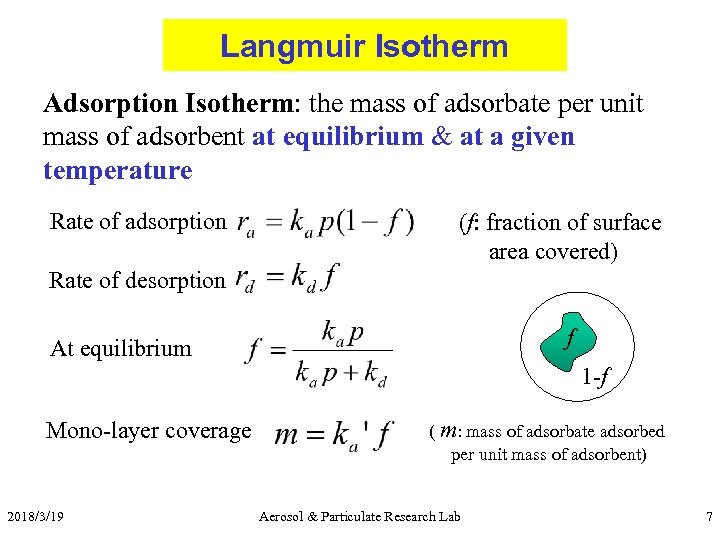 Langmuir Isotherm Adsorption Isotherm: the mass of adsorbate per unit mass of adsorbent at equilibrium & at a given temperature Rate of adsorption (f: fraction of surface area covered) Rate of desorption f At equilibrium 1 -f Mono-layer coverage 2018/3/19 ( m: mass of adsorbate adsorbed per unit mass of adsorbent) Aerosol & Particulate Research Lab 7
Langmuir Isotherm Adsorption Isotherm: the mass of adsorbate per unit mass of adsorbent at equilibrium & at a given temperature Rate of adsorption (f: fraction of surface area covered) Rate of desorption f At equilibrium 1 -f Mono-layer coverage 2018/3/19 ( m: mass of adsorbate adsorbed per unit mass of adsorbent) Aerosol & Particulate Research Lab 7
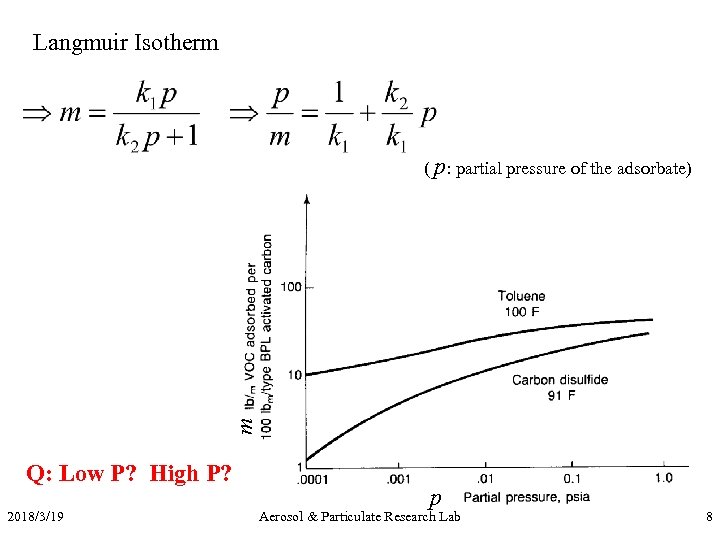 Langmuir Isotherm m ( p: partial pressure of the adsorbate) Q: Low P? High P? 2018/3/19 p Aerosol & Particulate Research Lab 8
Langmuir Isotherm m ( p: partial pressure of the adsorbate) Q: Low P? High P? 2018/3/19 p Aerosol & Particulate Research Lab 8
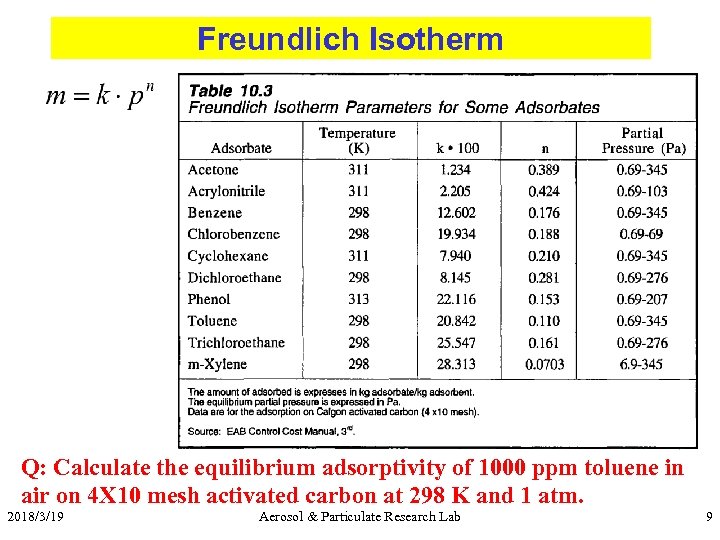 Freundlich Isotherm Q: Calculate the equilibrium adsorptivity of 1000 ppm toluene in air on 4 X 10 mesh activated carbon at 298 K and 1 atm. 2018/3/19 Aerosol & Particulate Research Lab 9
Freundlich Isotherm Q: Calculate the equilibrium adsorptivity of 1000 ppm toluene in air on 4 X 10 mesh activated carbon at 298 K and 1 atm. 2018/3/19 Aerosol & Particulate Research Lab 9
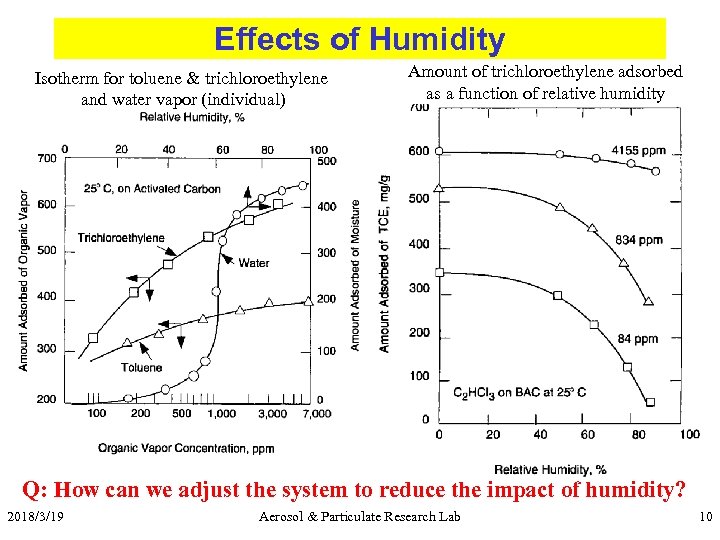 Effects of Humidity Isotherm for toluene & trichloroethylene and water vapor (individual) Amount of trichloroethylene adsorbed as a function of relative humidity Q: How can we adjust the system to reduce the impact of humidity? 2018/3/19 Aerosol & Particulate Research Lab 10
Effects of Humidity Isotherm for toluene & trichloroethylene and water vapor (individual) Amount of trichloroethylene adsorbed as a function of relative humidity Q: How can we adjust the system to reduce the impact of humidity? 2018/3/19 Aerosol & Particulate Research Lab 10
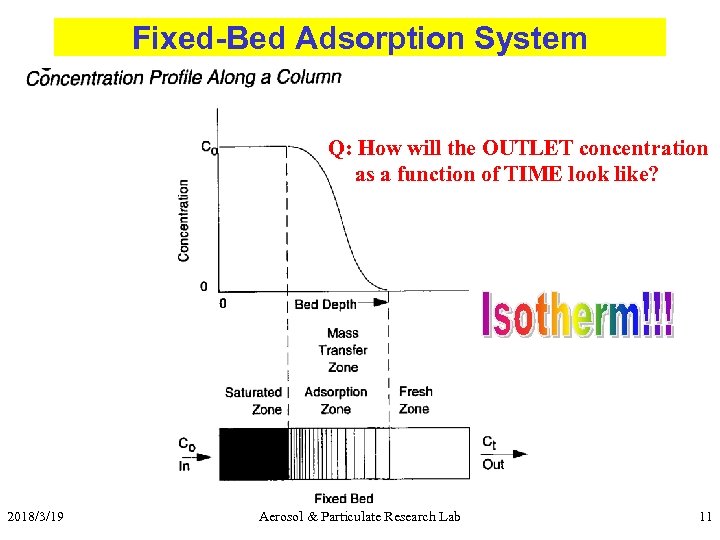 Fixed-Bed Adsorption System Q: How will the OUTLET concentration as a function of TIME look like? 2018/3/19 Aerosol & Particulate Research Lab 11
Fixed-Bed Adsorption System Q: How will the OUTLET concentration as a function of TIME look like? 2018/3/19 Aerosol & Particulate Research Lab 11
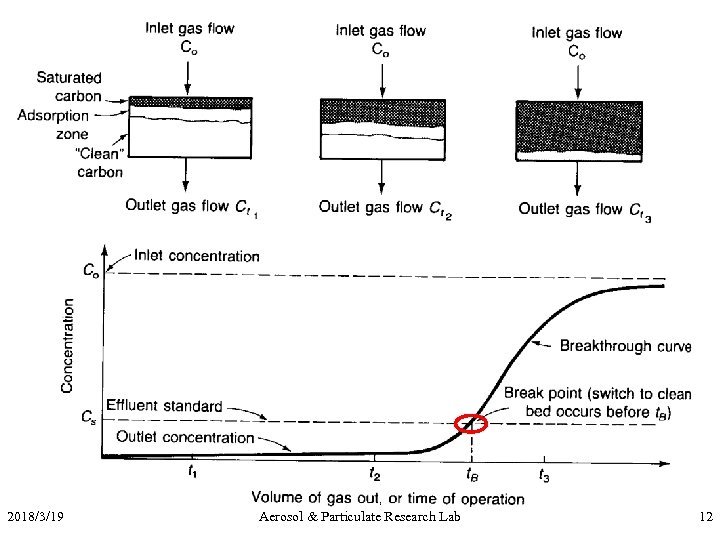 2018/3/19 Aerosol & Particulate Research Lab 12
2018/3/19 Aerosol & Particulate Research Lab 12
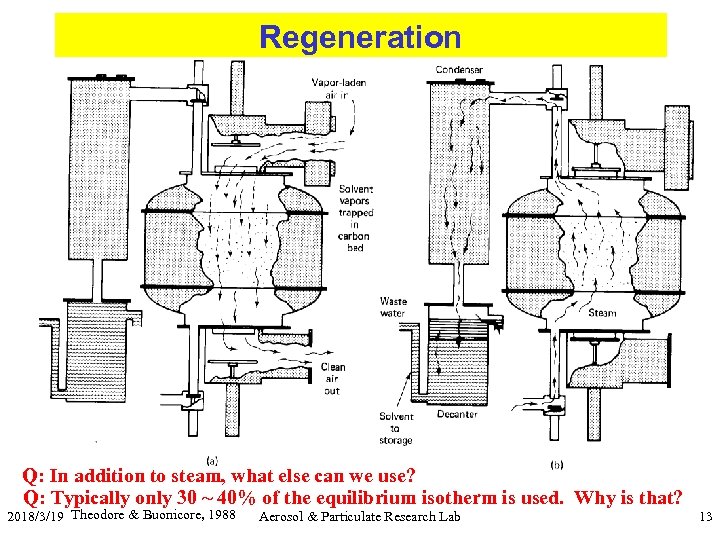 Regeneration Q: In addition to steam, what else can we use? Q: Typically only 30 ~ 40% of the equilibrium isotherm is used. Why is that? 2018/3/19 Theodore & Buonicore, 1988 Aerosol & Particulate Research Lab 13
Regeneration Q: In addition to steam, what else can we use? Q: Typically only 30 ~ 40% of the equilibrium isotherm is used. Why is that? 2018/3/19 Theodore & Buonicore, 1988 Aerosol & Particulate Research Lab 13
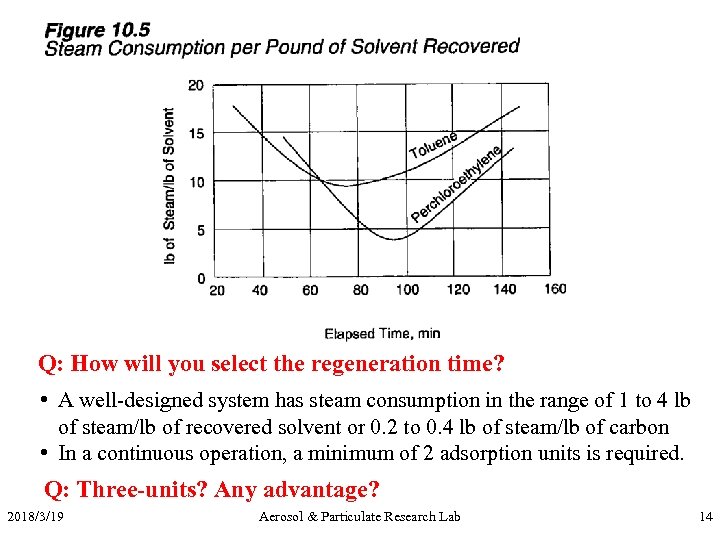 Q: How will you select the regeneration time? • A well-designed system has steam consumption in the range of 1 to 4 lb of steam/lb of recovered solvent or 0. 2 to 0. 4 lb of steam/lb of carbon • In a continuous operation, a minimum of 2 adsorption units is required. Q: Three-units? Any advantage? 2018/3/19 Aerosol & Particulate Research Lab 14
Q: How will you select the regeneration time? • A well-designed system has steam consumption in the range of 1 to 4 lb of steam/lb of recovered solvent or 0. 2 to 0. 4 lb of steam/lb of carbon • In a continuous operation, a minimum of 2 adsorption units is required. Q: Three-units? Any advantage? 2018/3/19 Aerosol & Particulate Research Lab 14
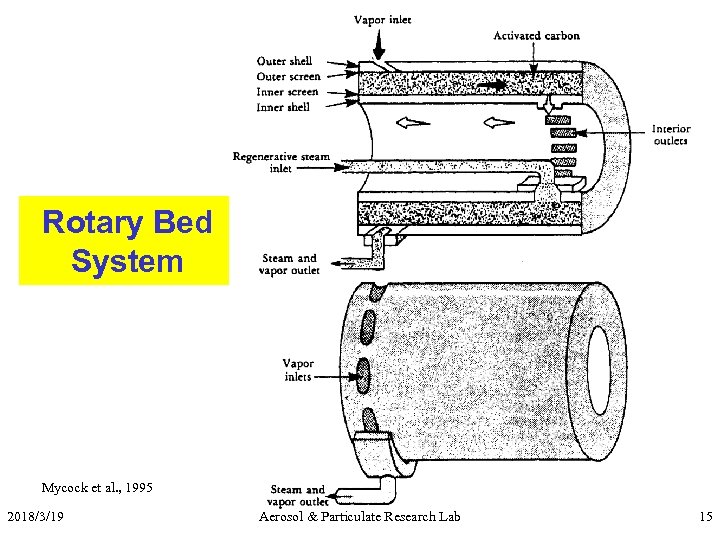 Rotary Bed System Mycock et al. , 1995 2018/3/19 Aerosol & Particulate Research Lab 15
Rotary Bed System Mycock et al. , 1995 2018/3/19 Aerosol & Particulate Research Lab 15
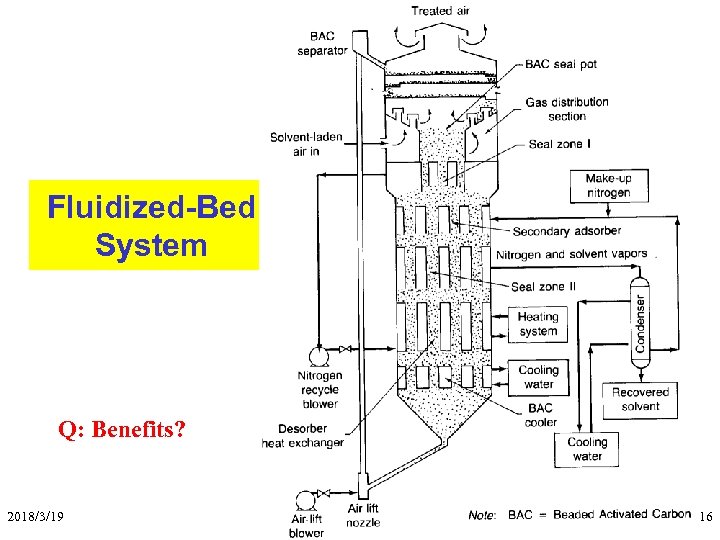 Fluidized-Bed System Q: Benefits? 2018/3/19 Aerosol & Particulate Research Lab 16
Fluidized-Bed System Q: Benefits? 2018/3/19 Aerosol & Particulate Research Lab 16
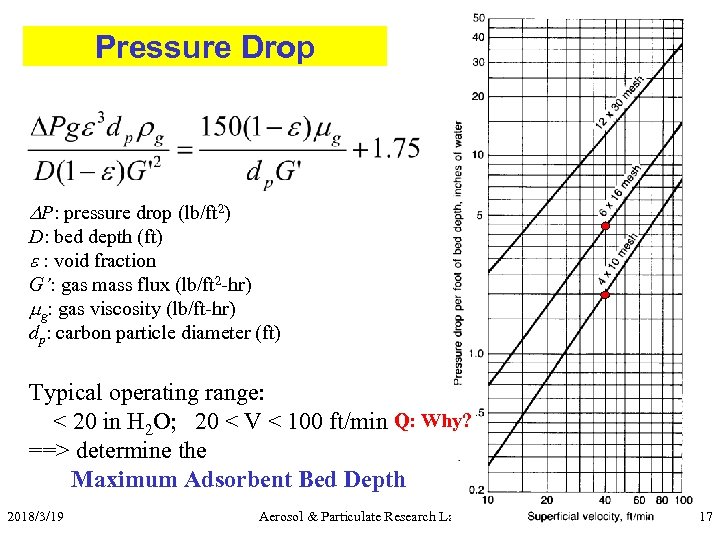 Pressure Drop P: pressure drop (lb/ft 2) D: bed depth (ft) e : void fraction G’: gas mass flux (lb/ft 2 -hr) mg: gas viscosity (lb/ft-hr) dp: carbon particle diameter (ft) Typical operating range: < 20 in H 2 O; 20 < V < 100 ft/min Q: Why? ==> determine the Maximum Adsorbent Bed Depth 2018/3/19 Aerosol & Particulate Research Lab 17
Pressure Drop P: pressure drop (lb/ft 2) D: bed depth (ft) e : void fraction G’: gas mass flux (lb/ft 2 -hr) mg: gas viscosity (lb/ft-hr) dp: carbon particle diameter (ft) Typical operating range: < 20 in H 2 O; 20 < V < 100 ft/min Q: Why? ==> determine the Maximum Adsorbent Bed Depth 2018/3/19 Aerosol & Particulate Research Lab 17
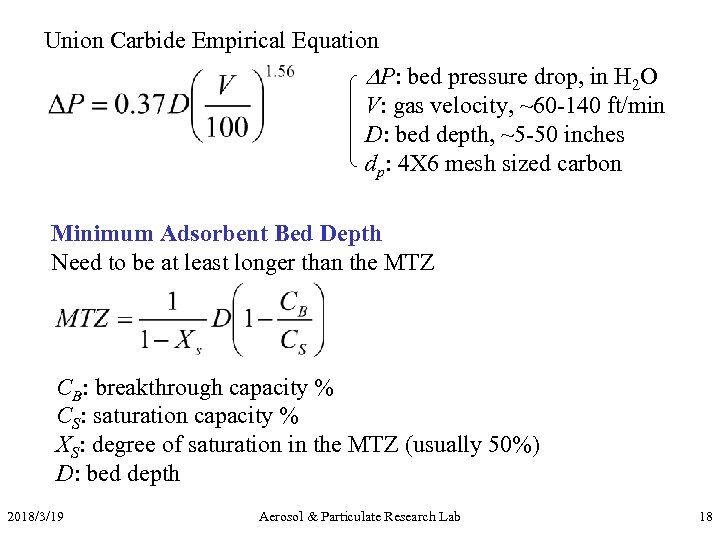 Union Carbide Empirical Equation P: bed pressure drop, in H 2 O V: gas velocity, ~60 -140 ft/min D: bed depth, ~5 -50 inches dp: 4 X 6 mesh sized carbon Minimum Adsorbent Bed Depth Need to be at least longer than the MTZ CB: breakthrough capacity % CS: saturation capacity % XS: degree of saturation in the MTZ (usually 50%) D: bed depth 2018/3/19 Aerosol & Particulate Research Lab 18
Union Carbide Empirical Equation P: bed pressure drop, in H 2 O V: gas velocity, ~60 -140 ft/min D: bed depth, ~5 -50 inches dp: 4 X 6 mesh sized carbon Minimum Adsorbent Bed Depth Need to be at least longer than the MTZ CB: breakthrough capacity % CS: saturation capacity % XS: degree of saturation in the MTZ (usually 50%) D: bed depth 2018/3/19 Aerosol & Particulate Research Lab 18
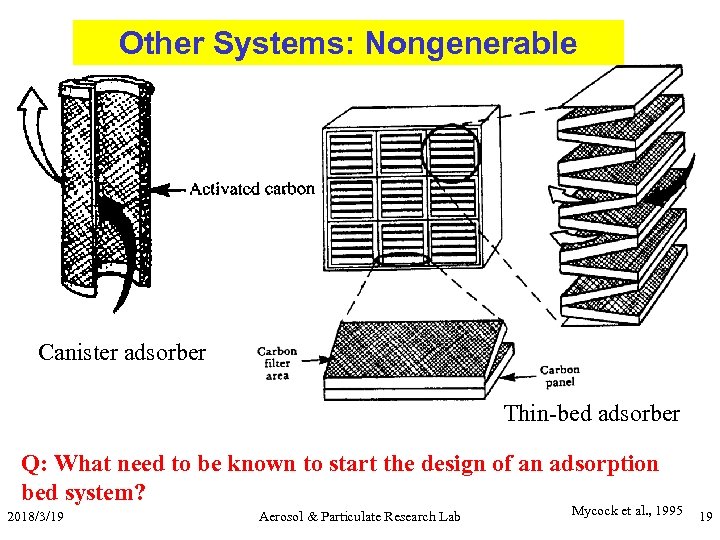 Other Systems: Nongenerable Canister adsorber Thin-bed adsorber Q: What need to be known to start the design of an adsorption bed system? 2018/3/19 Aerosol & Particulate Research Lab Mycock et al. , 1995 19
Other Systems: Nongenerable Canister adsorber Thin-bed adsorber Q: What need to be known to start the design of an adsorption bed system? 2018/3/19 Aerosol & Particulate Research Lab Mycock et al. , 1995 19
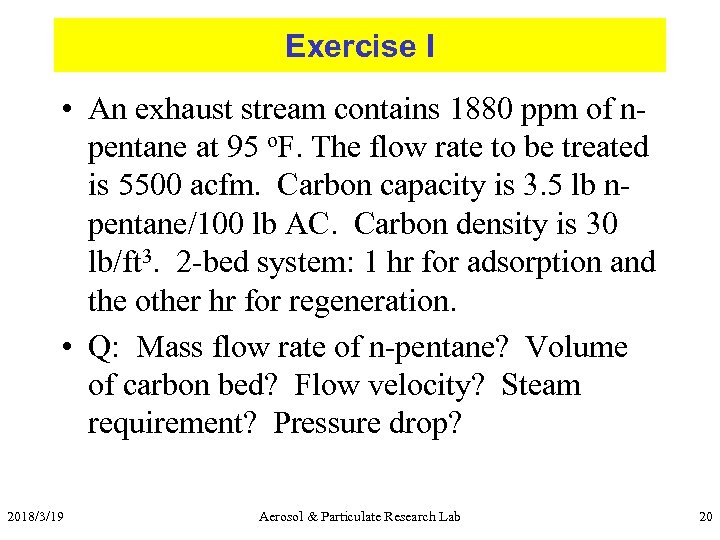 Exercise I • An exhaust stream contains 1880 ppm of npentane at 95 o. F. The flow rate to be treated is 5500 acfm. Carbon capacity is 3. 5 lb npentane/100 lb AC. Carbon density is 30 lb/ft 3. 2 -bed system: 1 hr for adsorption and the other hr for regeneration. • Q: Mass flow rate of n-pentane? Volume of carbon bed? Flow velocity? Steam requirement? Pressure drop? 2018/3/19 Aerosol & Particulate Research Lab 20
Exercise I • An exhaust stream contains 1880 ppm of npentane at 95 o. F. The flow rate to be treated is 5500 acfm. Carbon capacity is 3. 5 lb npentane/100 lb AC. Carbon density is 30 lb/ft 3. 2 -bed system: 1 hr for adsorption and the other hr for regeneration. • Q: Mass flow rate of n-pentane? Volume of carbon bed? Flow velocity? Steam requirement? Pressure drop? 2018/3/19 Aerosol & Particulate Research Lab 20
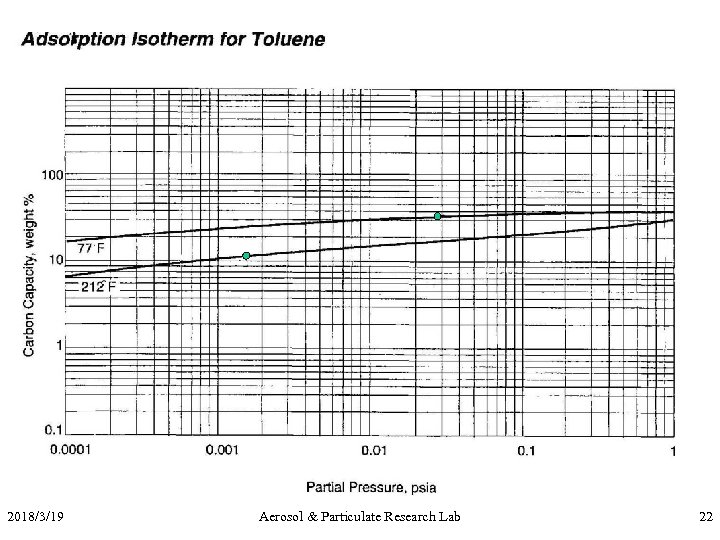
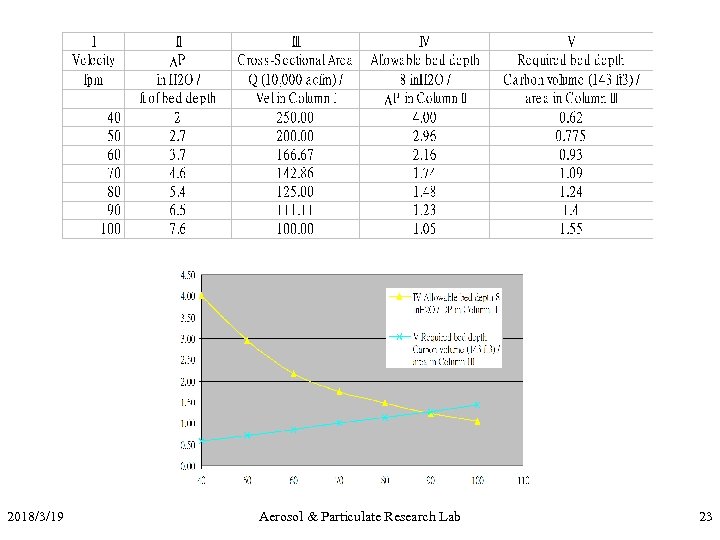
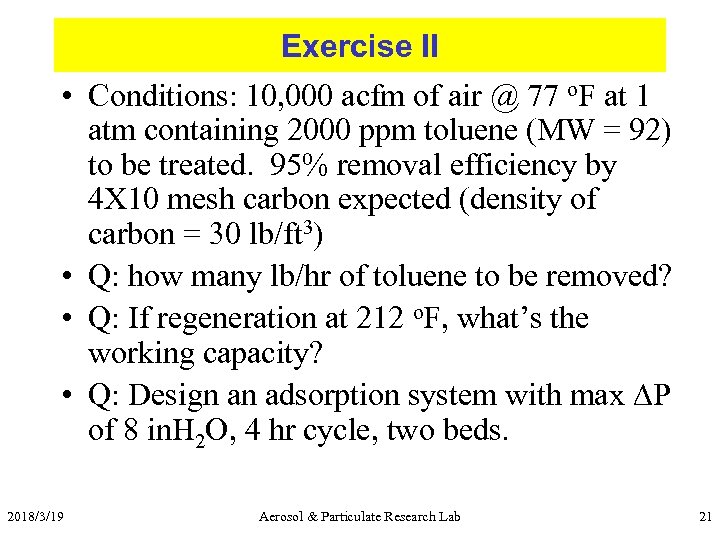 Exercise II • Conditions: 10, 000 acfm of air @ 77 o. F at 1 atm containing 2000 ppm toluene (MW = 92) to be treated. 95% removal efficiency by 4 X 10 mesh carbon expected (density of carbon = 30 lb/ft 3) • Q: how many lb/hr of toluene to be removed? • Q: If regeneration at 212 o. F, what’s the working capacity? • Q: Design an adsorption system with max DP of 8 in. H 2 O, 4 hr cycle, two beds. 2018/3/19 Aerosol & Particulate Research Lab 21
Exercise II • Conditions: 10, 000 acfm of air @ 77 o. F at 1 atm containing 2000 ppm toluene (MW = 92) to be treated. 95% removal efficiency by 4 X 10 mesh carbon expected (density of carbon = 30 lb/ft 3) • Q: how many lb/hr of toluene to be removed? • Q: If regeneration at 212 o. F, what’s the working capacity? • Q: Design an adsorption system with max DP of 8 in. H 2 O, 4 hr cycle, two beds. 2018/3/19 Aerosol & Particulate Research Lab 21
 2018/3/19 Aerosol & Particulate Research Lab 22
2018/3/19 Aerosol & Particulate Research Lab 22
 2018/3/19 Aerosol & Particulate Research Lab 23
2018/3/19 Aerosol & Particulate Research Lab 23
 Quick Reflection 2018/3/19 Aerosol & Particulate Research Lab 24
Quick Reflection 2018/3/19 Aerosol & Particulate Research Lab 24


