140616d254e88933c8d9eb80c7f68f0b.ppt
- Количество слайдов: 54

Adhesives and Sealents • Adhesive= bonding agent – Binds two surfaces – Glue, cement, paste – Organic/inorganic components that hardens/set – Viscous liquid

Sealant • A material applied to a joint in paste or liquid form that hardens or cures in place, forming a barrier against gas or liquid entry.

Adhesives • Soft (connective) tissue adhesives (temporary) – External use: • colostomy bags – Internal use: • wound closure and sealing • Hard (calcified) tissue adhesives (permanent) – bond prosthetic materials to teeth and bone

What do we need? A material that will be – biocompatible – easy to manipulate – interact intimately with the tissue to form a strong bond • good bonding and gap-filling characteristics • join two dissimilar materials – subjected to a variety of physical, mechanical, chemical stresses – need good resistance to environmental degradative processes (biodegradation)

BIOMEDICAL GLUES Soft tissue 1. Cynaoacrylates 2. Fibrin 3. GRF

CYANOACRYLATES
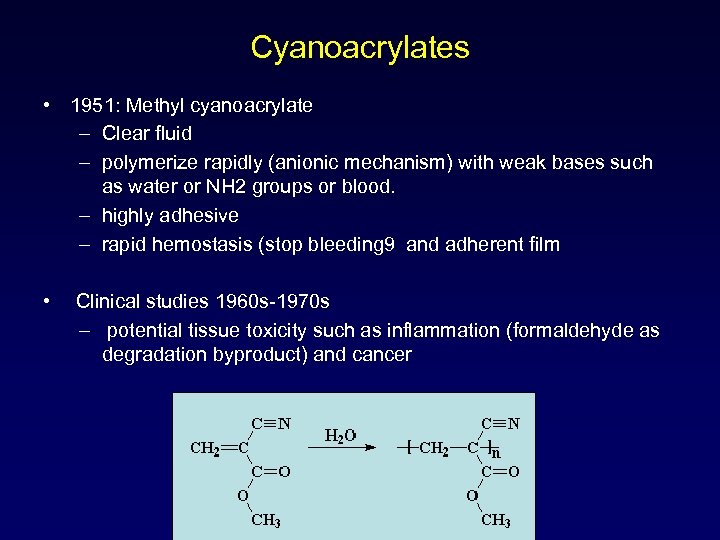
Cyanoacrylates • 1951: Methyl cyanoacrylate – Clear fluid – polymerize rapidly (anionic mechanism) with weak bases such as water or NH 2 groups or blood. – highly adhesive – rapid hemostasis (stop bleeding 9 and adherent film • Clinical studies 1960 s-1970 s – potential tissue toxicity such as inflammation (formaldehyde as degradation byproduct) and cancer

Cyanoacrylates • first used on wounded soldiers in Vietnam: spray over the wounds • Midwives used Super Glue successfully in lieu of suture to close the perineum. • Surgeons have used household cyanoacrylate adhesive to apply sutureless patches that stopped bleeding in critically injured patients with torn or ruptured myocardium. • Cyanoacrylates are also used in repairing corneas and retinas • AS synthetic skin in treating severe burns

Cyanoacrylates • Ethyl, isobutyl, octyl, R- cyanoacrylate adhesives – Spread more rapidly on wound surface – Polymerize more rapidly – Degrades more slowly over several weeks – Toxicity decreased

Cyanoacrylates • Medical grade products: – R = butyl, isobutyl or octyl esters. – Bacteriostatic and painless to apply – Break down by hydrolysis – Essentially inert once dry ? ! • Butyl products: – rigid when dry + provide a strong bond • Octyl products: – more flexible when dry + produce a weaker bond. • DMSO (dimethyl sulfoxide) or acetone serve as removers.

Cyanoacrylates – Brittle ! • May be dislodged on mobile tissue – Difficult for large wounds – NOT approved in USA (due to cancer occasions in animal studies) – APPROVED in Europe : R = n-Butyl
Butyl-2 -cyanoacrylate • effective in closing superficial lacerations under low tension • wound-breaking strength – equal to sutured wound at 5 -7 days – however, on day 1, approximately 10 -15% of that in a sutured wound • Brittle after polymerization – May break in skin creases or long incisions • Best if used – areas of low tension (limited to incision repair) • Good cosmetic outcomes for various plastic surgical procedures (eg, lid, facial skin closure, scalp wound closure).

• currently FDA approved adhesives suitable for use as suture alternatives are veterinary products – n-butyl- cyanoacrylate tissue adhesives: Vetbond (3 M) and Nexaband liquid – octyl- cyanoacrylate: Nexaband S/C (intended for topical skin closure when deep sutures have been placed). Canada for human use • Histoacryl Blue (butyl cyanoacrylate ) (Davis & Geck) : 2007 FDA approval • Tissu-Glu (isobutyl cyanoacrylate ) (Medi-West Pharmaceuticals) Canada for human use. http: //www. tissueseal. com/

n-Butyl cyanoacrylate • Histoacryl Blue® • middle ear surgery, bone and cartilage grafts, repair of cerebrospinal fluid leaks, and skin closure.

Histoacryl® topical skin adhesive • topical medication is applied to body surfaces such as the skin or mucous membranes such as the vagina, anus, throat, eyes and ears. • should be used only on wounds that have been thoroughly cleaned, debrided and have easily apposed wound edges. • small amount of heat during polymerization and should not be applied to tissues that may be affected by such heat. Heavy application may cause thermal damage to tissues, and delayed healing may result. • should not be applied to wet wounds. Excess moisture, such as water or alcohol, may accelerate polymerization, resulting in the generation of excess heat. • Application and/or migration (leak) of either version of the product below the surface of the skin will impair the healing process by forming a barrier between tissue edges.

• Uses: • surface wound dressing in dental surgery – especially in periodontics, • life-threatening applications such as brain arteriovenous malformations. • skin sutures in plastic surgery • treating simple lacerations in children • Side effects: • sarcomas in laboratory animals (Reiter, 1987), • Late complications after dura surgery (Chilla, 1987), • in vitro cytotoxicity (Ciapetti et al. , 1994),

Peri. Acryl® 90 CE Oral Tissue Adhesive • 2011 -Glu. Stitch introduced High Viscosity, a thicker formulation to provide more control during application. • butyl cyanoacrylate, 2 -octyl cyanoacrylate, and special proprietary additives • Uses: secure tissue grafts without the use of any dental sutures, as a liquid bandage over donor sites after harvesting tissue, as a means of secondary closure overtop of sutures, and to secure suture knots and threads. • Key Peri. Acryl® 90 Product Features • • • Biocompatible and non-toxic Two year shelf life Fast set time http: //www. glustitch. com/produ Violet colouring allows for better visibility cts. Detail. php? id=7®ion=Int ernational User friendly delivery system Unique bottle cap design helps keep the bottle opening free and clear, use after use
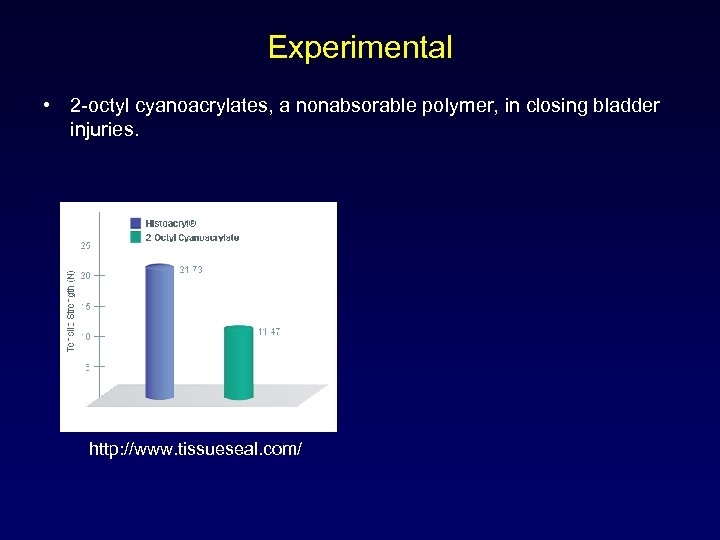
Experimental • 2 -octyl cyanoacrylates, a nonabsorable polymer, in closing bladder injuries. http: //www. tissueseal. com/

Dermabond® • The wound adhesive 2 -octyl cyanoacrylate is approved by the US Food and Drug Administration (FDA) for closure of incised skin. (2001) • surgical adhesive • barrier against common bacterial microbes

• • • Butyl-2 -cyanoacrylate manufacturers are as follows: Vetbond: 3 M (Maplewood, MN) Liquiband: Medlogic (Plymouth, UK) Peri. Acryl: Glu. Stitch (Delta, BC Canada) Xoin: Reevax Pharma (Hyderabad, India) Vet. Glu: Glu. Stitch (Delta, BC Canada) Liqui. Vet: Oasis Medical (Mettawa, IL) Indermil: Henkel (Dublin, Ireland) Histoacryl: B. Braun Medical (Bethlehem, PA) • • 2 -Octyl cyanoacrylate manufacturers are as follows: Dermabond: Johnson & Johnson, Ethicon, Inc (Somerville, NJ) Surgiseal: Adhezion Biomedical (Wyomissing, PA) Derma+flex QS: Chemence (Northampshire, UK) Surgi. Seal: Adhezion Biomedical (Wyomissing, PA) Flora. Seal: Adhezion Biomedical (Wyomissing, PA) Octylseal: Medline (Mundelein, IL) Nexaband: Abbott (Abbott Park, IL)
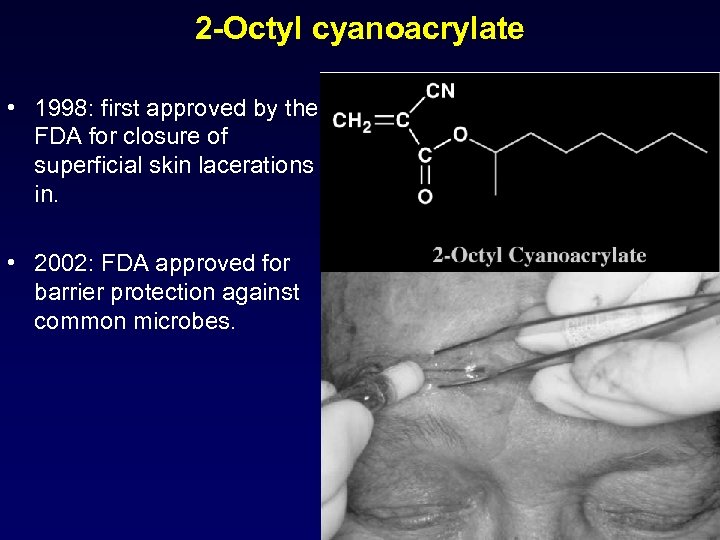
2 -Octyl cyanoacrylate • 1998: first approved by the FDA for closure of superficial skin lacerations in. • 2002: FDA approved for barrier protection against common microbes.

2 -Octyl cyanoacrylate • Its polymer is less reactive than the shorter-chain derivatives. • slower degradation may result in lower concentrations of the degradation byproducts in surrounding tissues • less inflammation. • Plasticizers: to produce a more pliable and tissue-compatible product that flexes with the skin and remains inherent for longer periods. • The 3 -dimensional (3 -D) breaking strength of 2 -octyl cyanoacrylate is 3 times that of butyl-2 -cyanoacrylate and is closer to that of a monofilament suture. • This stronger, flexible bond may allow its use on longer incisions. http: //emedicine. medscape. com/article/874047 -overview#a 3

FIBRIN SEALANT

Fibrin sealants • Surgical haemostatic and adhesive agents derived from plasma products • Designed to reproduce the final steps of the physiological coagulation cascade and form a stable fibrin clot • Fibrin clot arrests blood loss, seals physiological compartments and aids normal wound healing • Clot degraded by naturally occurring fibrinolytic enzymes over several days to weeks
Fibrin Sealent • production of a synthetic fibrin clot as an adhesive and woundcovering agent. • The concept: 1909 • Matras et al. in 1972. • The first available commercial materials : – consisted of two solutions that are mixed immediately before application to provide a controlled fibrin deposition. • Later a “ready-to-use” formulation (Tisseel Duo) was introduced (Schlag and Redl, 1987).
Fibrin Sealants • Synthetic fibrin cloth Aprotinin, an inhibitor of fibrinolysis A. Solution A: – Fibrinogen (30 times higher conc than in human plasma: (∼ 70 mg/ml) – Factor XIII (plasma glutaminase) B. Solution B: – Thrombin initiates the polymerization of fibrinogen to – Ca. Cl 2 fibrin and fibrin cloth reabsorbed throughout the wound’s natural healing process Thorough mixing of the ingredients and application techniques or devices that allow uniform spreading are essential to success.
![Mechanism of action Fibrinogen Thrombin Fibrin clot Factor XIIIa Anti-fibrinolytic [Aprotinin, tranexamic acid] Cross-linked Mechanism of action Fibrinogen Thrombin Fibrin clot Factor XIIIa Anti-fibrinolytic [Aprotinin, tranexamic acid] Cross-linked](https://present5.com/presentation/140616d254e88933c8d9eb80c7f68f0b/image-27.jpg)
Mechanism of action Fibrinogen Thrombin Fibrin clot Factor XIIIa Anti-fibrinolytic [Aprotinin, tranexamic acid] Cross-linked fibrin clot X Fibrin degradation
Fibrin Sealants • Fibrinogen: – Initially from pooled plasma of selected donors – Health risk! – routinely screened for hepatitis and HIV. – Solution: • Autologous (reimplanted in the same individual as they come from) • Single blood donor • Approved in Europe, Japan, Canada • Recently approved in USA (1998) – high associated risk of hepatitis and acquired immune deficiency syndrome (AIDS) transmission from the pooled human plasma in the production of fibrinogen

Fibrin Sealents • Main advantages: – Hemostatic – Adhesive to soft tissue (weaker than cynoacrylates) – Promotes wound healing – Biodegradable with excellent tissue tolerance – Biocompatable: not associated with inflammation, foreign body reactions, tissue necrosis, or extensive fibrosis • Improvement needs: – Ease of application (commend cure) – Strength (by collagen) – Gelatin incorporation

Uses • • • thoracic–cardiovascular neurologic, plastic, ophthalmic surgery as a biodegradable adhesive scaffold for meshed skin grafts in burn patients
Fibrin Sealants • Tisseel VH kit and Hemaseel APR
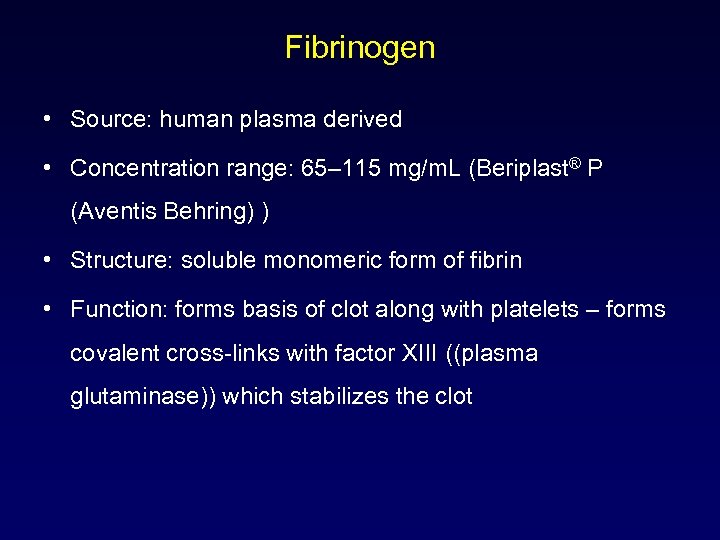
Fibrinogen • Source: human plasma derived • Concentration range: 65– 115 mg/m. L (Beriplast® P (Aventis Behring) ) • Structure: soluble monomeric form of fibrin • Function: forms basis of clot along with platelets – forms covalent cross-links with factor XIII ((plasma glutaminase)) which stabilizes the clot
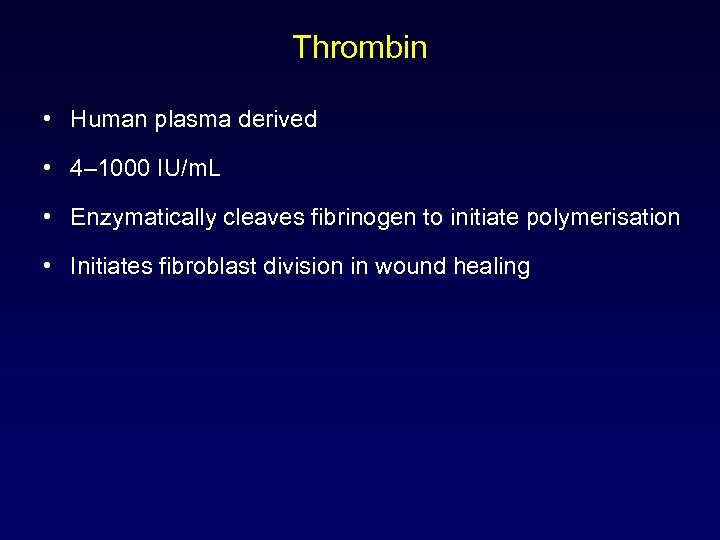
Thrombin • Human plasma derived • 4– 1000 IU/m. L • Enzymatically cleaves fibrinogen to initiate polymerisation • Initiates fibroblast division in wound healing
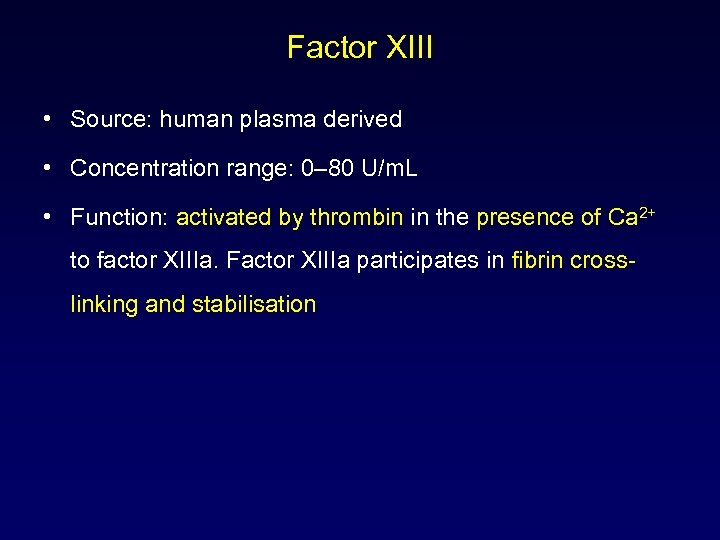
Factor XIII • Source: human plasma derived • Concentration range: 0– 80 U/m. L • Function: activated by thrombin in the presence of Ca 2+ to factor XIIIa. Factor XIIIa participates in fibrin crosslinking and stabilisation

Aprotinin • Some formulations have Aprotinin • Source: bovine lung derived • Concentration range: 0– 3000 KIU/m. L • Function: preventing fibrinolysis – prevents clot degradation

EVICEL® • May 2007 when FDA expanded the indication to include use during vascular surgery

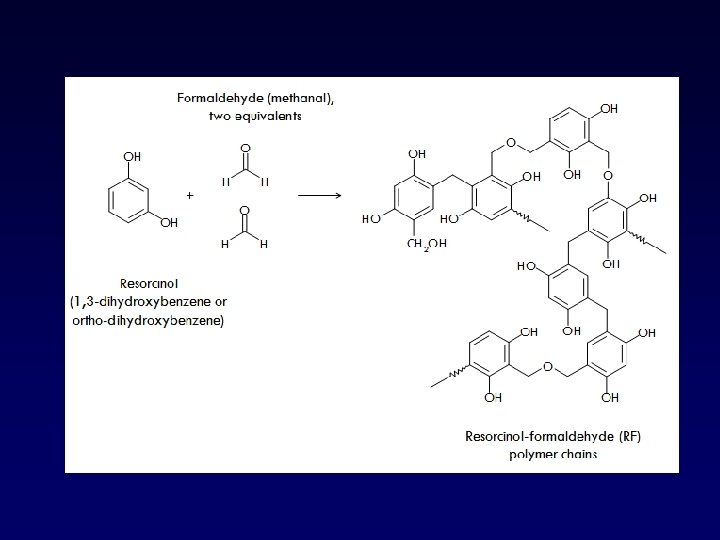
GELATIN-RESORCINOLFORMALDEHYDE (GRF)
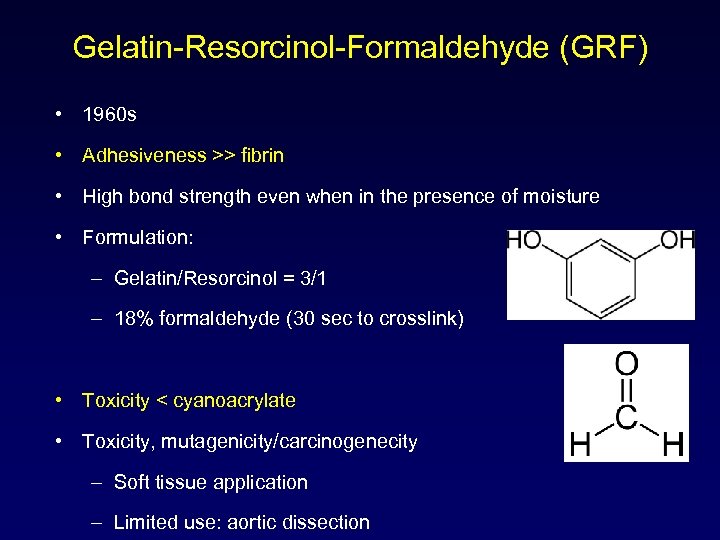
Gelatin-Resorcinol-Formaldehyde (GRF) • 1960 s • Adhesiveness >> fibrin • High bond strength even when in the presence of moisture • Formulation: – Gelatin/Resorcinol = 3/1 – 18% formaldehyde (30 sec to crosslink) • Toxicity < cyanoacrylate • Toxicity, mutagenicity/carcinogenecity – Soft tissue application – Limited use: aortic dissection

gelatin • prepared by thermal denaturation of collagen, • COLLAGEN is isolated from animal skin and bones, with very dilute acid. – It can also be extracted from fish skins. • Gelatin contains many glycine (almost 1 in 3 residues, arranged every third residue), proline and 4 -hydroxyproline residues. A typical structure is -Ala-Gly-Pro-Arg-Gly-Glu-4 Hyp-Gly-Pro-. • Gelatin is a heterogeneous mixture of single or multi-stranded polypeptides, each with extended left-handed proline helix conformations and containing between 50 - 1000 amino acids.
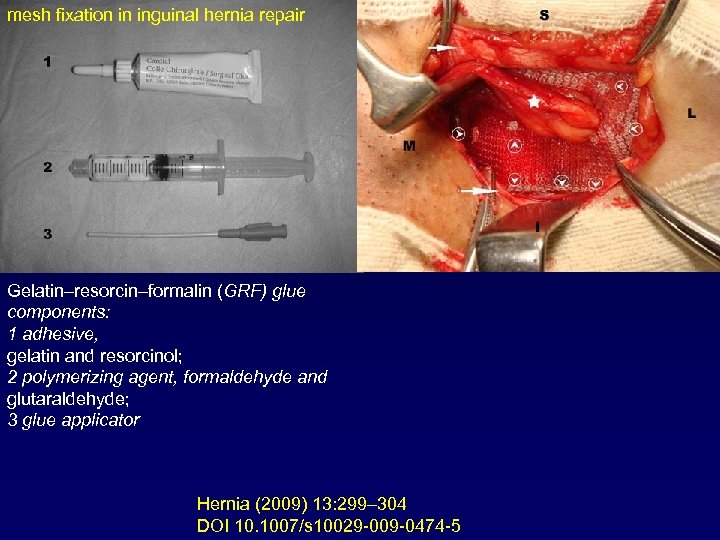
mesh fixation in inguinal hernia repair Gelatin–resorcin–formalin (GRF) glue components: 1 adhesive, gelatin and resorcinol; 2 polymerizing agent, formaldehyde and glutaraldehyde; 3 glue applicator Hernia (2009) 13: 299– 304 DOI 10. 1007/s 10029 -009 -0474 -5
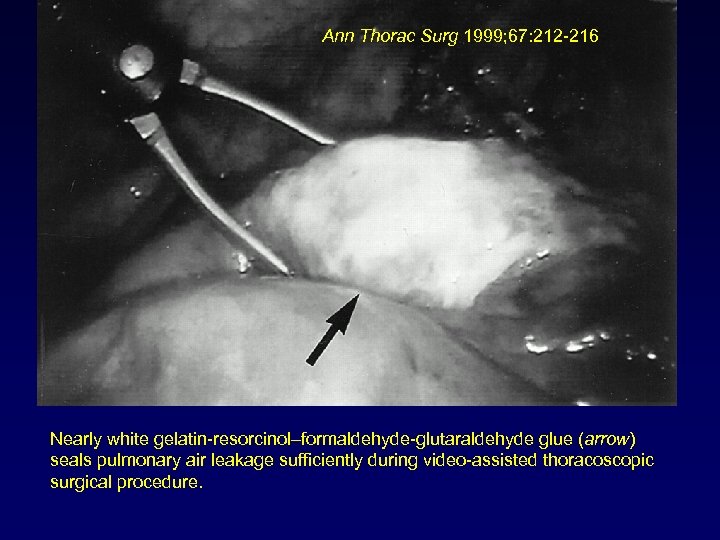
Ann Thorac Surg 1999; 67: 212 -216 Nearly white gelatin-resorcinol–formaldehyde-glutaraldehyde glue (arrow) seals pulmonary air leakage sufficiently during video-assisted thoracoscopic surgical procedure.
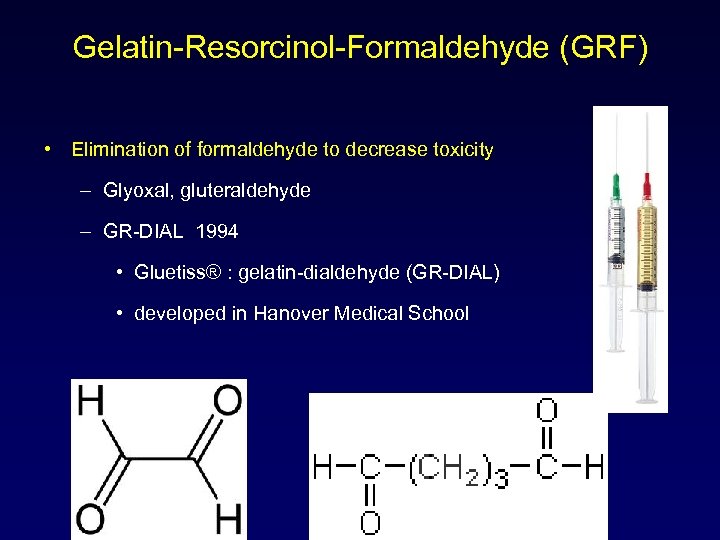
Gelatin-Resorcinol-Formaldehyde (GRF) • Elimination of formaldehyde to decrease toxicity – Glyoxal, gluteraldehyde – GR-DIAL 1994 • Gluetiss® : gelatin-dialdehyde (GR-DIAL) • developed in Hanover Medical School
Bio. Glue • The agent received FDA approval for general use as a hemostatic adjunct in cardiac and vascular surgery in December 2001. • Composition: – doses of glutaraldehyde (10%) and 45% albumin J CARD SURG 2003; 18: 500 -503
Hydrogel Sealents 1. Surgical sealants 2. barrier coatings 3. drug delivery Ø Biodegradable Ø Biocompatable Ø Synthetic

Hydrogel Sealents • In situ formation • Aq. Formulation of macromers • Initiator: eosin Y–triethanolamine • Photopolymerization to crosslink • Formulation: macromer + photoinitiator (visible range 450 -550 nm) Macromer: • water-soluble core such as polyoxyethylene • biodegradable oligomers such as poly(lactic acid) or poly(trimethylene carbonate) end blocks • polymerizable end caps such as acrylate esters
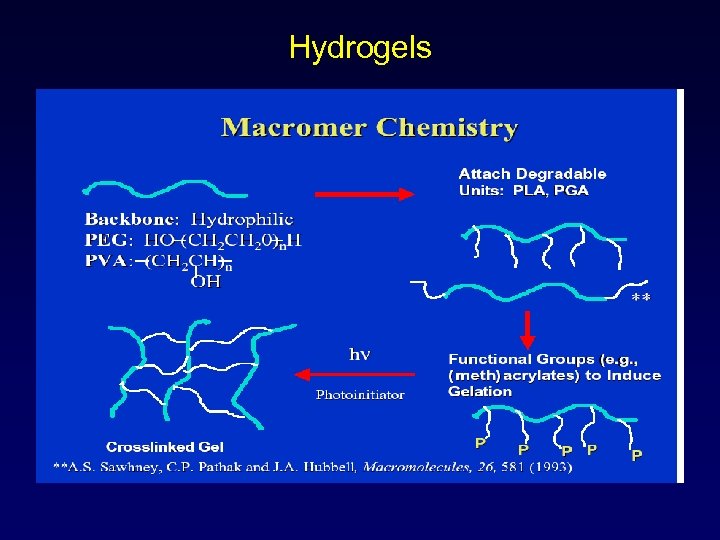
Hydrogels
Hydrogel sealents • Strong bonding: – two-part sealant system: primer and topcoat. • Strong, durable bonding to a wide variety of internal tissues has been demonstrated (Coury et al. , 1999). – sealants for the lung (Ranger et al. , 1997), – blood vessels (Tanaka et al. , 1999; Moody et al. , 1996) – local drug-delivery depots(Lovich et al. , 1998).
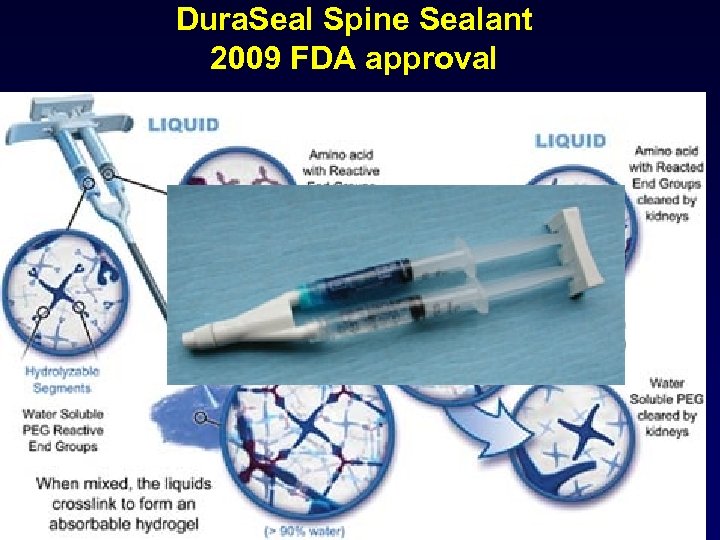
Dura. Seal Spine Sealant 2009 FDA approval

Progel: 2010 approval
![J Bone Joint Surg [Br] 2006; 88 -B: 238 -42. Facial Plast Surg Clin J Bone Joint Surg [Br] 2006; 88 -B: 238 -42. Facial Plast Surg Clin](https://present5.com/presentation/140616d254e88933c8d9eb80c7f68f0b/image-53.jpg)
J Bone Joint Surg [Br] 2006; 88 -B: 238 -42. Facial Plast Surg Clin N Am 10 (2002) 147– 154 • TECHNOLOGY STATUS EVALUATION REPORT Tissue adhesives and fibrin glues, NOVEMBER 2003 Hernia (2009) 13: 299– 304 Facial Plast Surg Clin N Am 10 (2002) 147– 154

Progel: 2010 approval • surgical sealants and adhesion barriers • human serum albumin protein (HSA) or recombinant human albumin (r. HA)* • polyethylene glycol (PEG) functionalize for cross-linking receptors. • The resultant PEG molecule is ideal forming strong, flexible bonds with the HSA/r. HA protein that encourage adherence to tissue surfaces like the visceral pleural of the lungs. These special bonds can be chemically engineered and developed to remain intact and provide strength during the critical phases of healing, but are designed to resorb at key time intervals depending on the type of tissue targeted and the speed and mechanism by which it repairs itself.
140616d254e88933c8d9eb80c7f68f0b.ppt