78c8b19ae5b2234ddcb5174923eeded8.ppt
- Количество слайдов: 57
 ADENOVIRUS AS A VECTOR IN GENE THERAPY
ADENOVIRUS AS A VECTOR IN GENE THERAPY
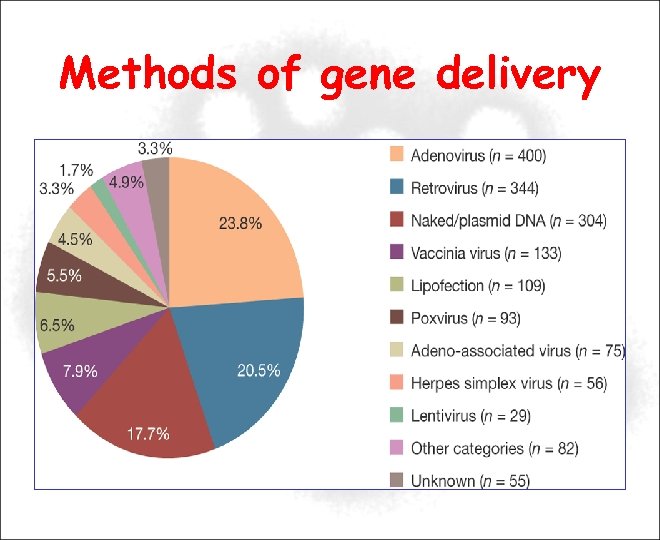 Methods of gene delivery
Methods of gene delivery
 Methods of gene delivery • Viral Vectors: – – – Adenovirus Retrovirus Lentivirus Adeno-associated virus (AAV) Herpes simplex virus (HSV) • Non-viral vector based – Naked DNA (plasmid DNA): injection or genegun – Liposomes (cationic lipids): mix with genes • Ex-vivo • In vivo
Methods of gene delivery • Viral Vectors: – – – Adenovirus Retrovirus Lentivirus Adeno-associated virus (AAV) Herpes simplex virus (HSV) • Non-viral vector based – Naked DNA (plasmid DNA): injection or genegun – Liposomes (cationic lipids): mix with genes • Ex-vivo • In vivo
 Why use viral vectors Virus at transferring viral DNA into host cells Specific target cells: depending on the viral attachment proteins (capsid or glycoproteins) Gene replacement: non-essential genes of virus are deleted and exogenous genes are inserted Very efficient
Why use viral vectors Virus at transferring viral DNA into host cells Specific target cells: depending on the viral attachment proteins (capsid or glycoproteins) Gene replacement: non-essential genes of virus are deleted and exogenous genes are inserted Very efficient
 The Ideal Vector for Gene Transfer High concentration of virus allowing many cells to be infected or transduced Convenience and reproducibility of production Ability to transduce dividing and non-dividing cells Ability to integrate into a site-specific location in the host chromosome, or to be successfully maintained as stable episome A transcriptional unit that can respond to manipulation of its regulatory elements Ability to target the desired type of cell No components that elicit an immune response
The Ideal Vector for Gene Transfer High concentration of virus allowing many cells to be infected or transduced Convenience and reproducibility of production Ability to transduce dividing and non-dividing cells Ability to integrate into a site-specific location in the host chromosome, or to be successfully maintained as stable episome A transcriptional unit that can respond to manipulation of its regulatory elements Ability to target the desired type of cell No components that elicit an immune response
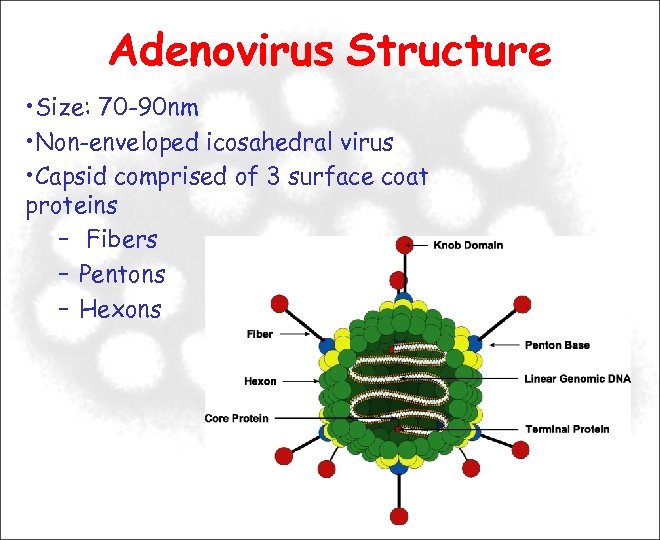 Adenovirus Structure • Size: 70 -90 nm • Non-enveloped icosahedral virus • Capsid comprised of 3 surface coat proteins – Fibers – Pentons – Hexons
Adenovirus Structure • Size: 70 -90 nm • Non-enveloped icosahedral virus • Capsid comprised of 3 surface coat proteins – Fibers – Pentons – Hexons
 Electron Micrograph of Purified Adenoviruses Particles
Electron Micrograph of Purified Adenoviruses Particles
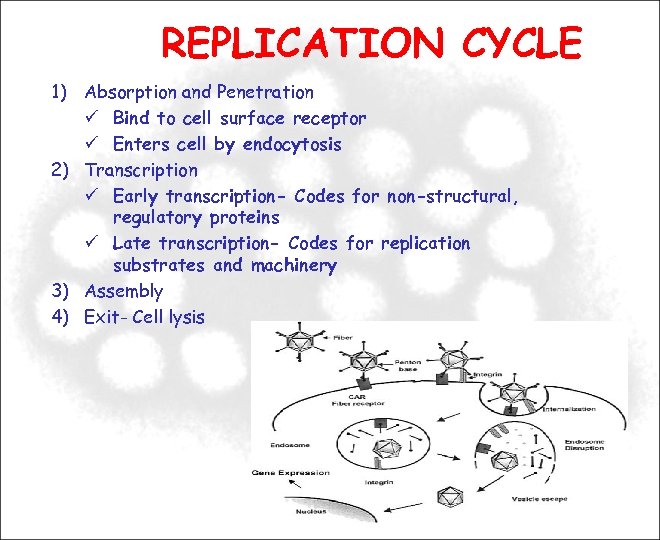 REPLICATION CYCLE 1) Absorption and Penetration Bind to cell surface receptor Enters cell by endocytosis 2) Transcription Early transcription- Codes for non-structural, regulatory proteins Late transcription- Codes for replication substrates and machinery 3) Assembly 4) Exit- Cell lysis
REPLICATION CYCLE 1) Absorption and Penetration Bind to cell surface receptor Enters cell by endocytosis 2) Transcription Early transcription- Codes for non-structural, regulatory proteins Late transcription- Codes for replication substrates and machinery 3) Assembly 4) Exit- Cell lysis
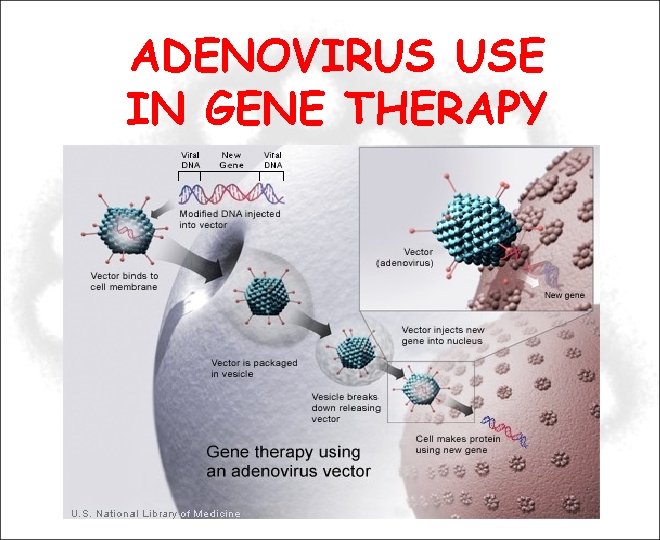 ADENOVIRUS USE IN GENE THERAPY
ADENOVIRUS USE IN GENE THERAPY
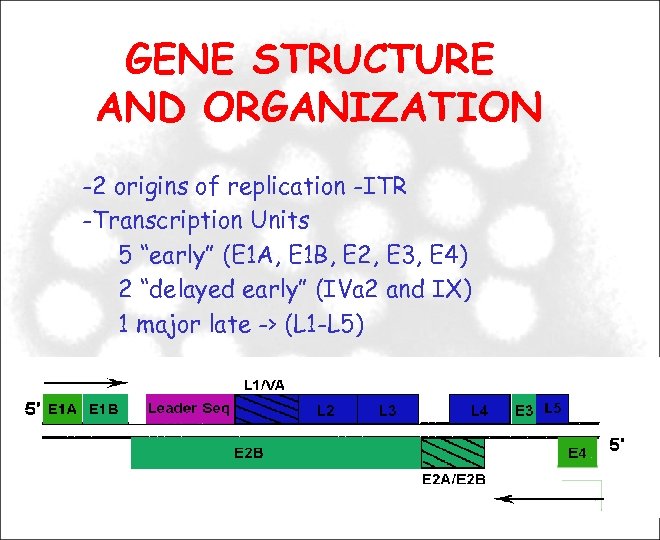 GENE STRUCTURE AND ORGANIZATION -2 origins of replication -ITR -Transcription Units 5 “early” (E 1 A, E 1 B, E 2, E 3, E 4) 2 “delayed early” (IVa 2 and IX) 1 major late -> (L 1 -L 5)
GENE STRUCTURE AND ORGANIZATION -2 origins of replication -ITR -Transcription Units 5 “early” (E 1 A, E 1 B, E 2, E 3, E 4) 2 “delayed early” (IVa 2 and IX) 1 major late -> (L 1 -L 5)
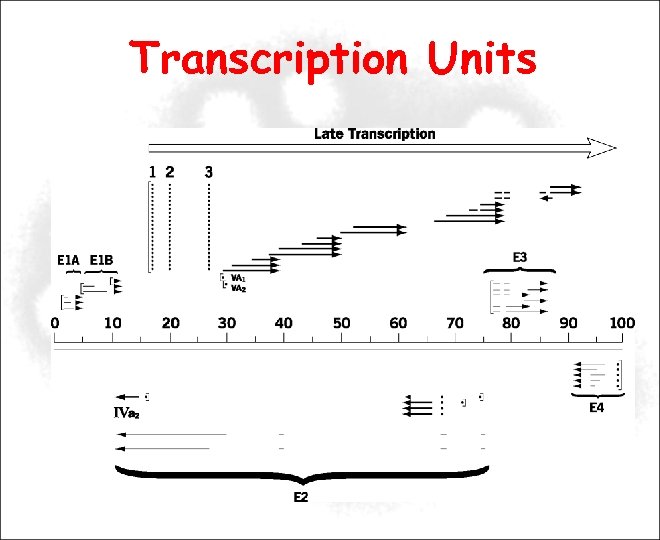 Transcription Units
Transcription Units
 Transcription Units E 1, E 2, E 4 Encode essential regulatory proteins involved in: Transactivation of viral and cellular promoters DNA replication Cell cycle Apoptosis Essential for replication in cell culture
Transcription Units E 1, E 2, E 4 Encode essential regulatory proteins involved in: Transactivation of viral and cellular promoters DNA replication Cell cycle Apoptosis Essential for replication in cell culture
 Transcription Units E 3 Escaping host immune defenses Preventing T cell cytotoxicity Tumor necrosis factor (TNF) action Dispensable for replication in cell culture
Transcription Units E 3 Escaping host immune defenses Preventing T cell cytotoxicity Tumor necrosis factor (TNF) action Dispensable for replication in cell culture
 Transcription Units Late genes Encodes proteins required for packaging the viral genome Essential for replication in cell culture
Transcription Units Late genes Encodes proteins required for packaging the viral genome Essential for replication in cell culture
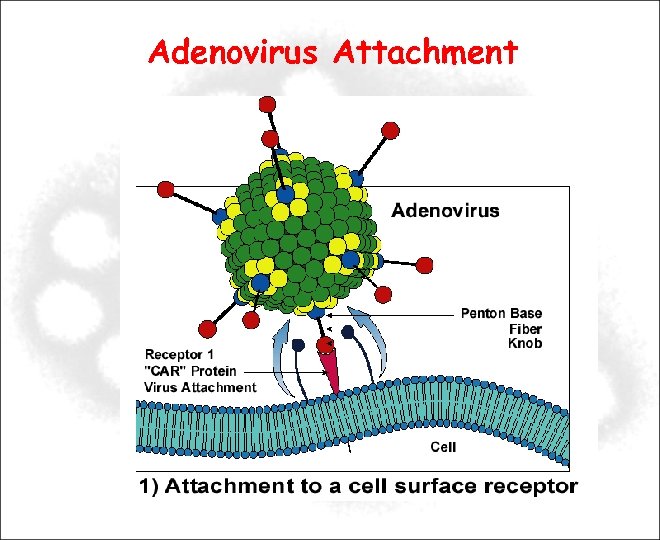 Adenovirus Attachment
Adenovirus Attachment
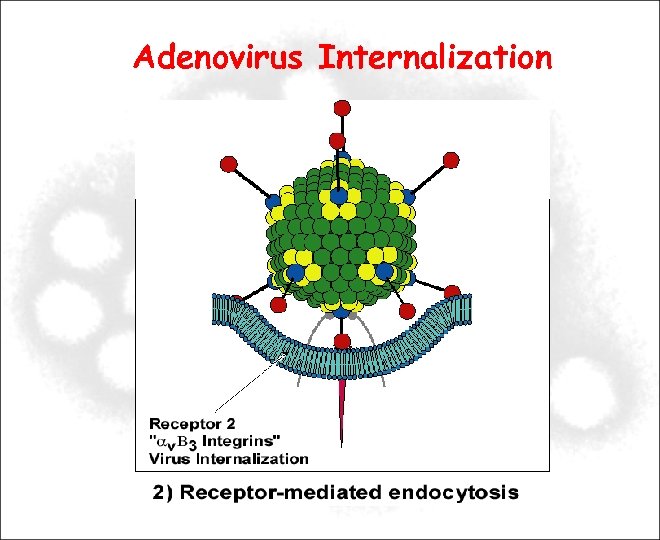 Adenovirus Internalization
Adenovirus Internalization
 WHY ADENOVIRUSES ARE GOOD VECTORS FOR GENE THERAPY Gene therapy works by manipulating viruses to contain “good genes” in which they can transport to the cells to code for needed protein/hormone/enzyme/etc They do not incorporate their genes into the host genome The Adenovirus is ubiquitous- it has been isolated from a large number of different species with over 100 known serotypes
WHY ADENOVIRUSES ARE GOOD VECTORS FOR GENE THERAPY Gene therapy works by manipulating viruses to contain “good genes” in which they can transport to the cells to code for needed protein/hormone/enzyme/etc They do not incorporate their genes into the host genome The Adenovirus is ubiquitous- it has been isolated from a large number of different species with over 100 known serotypes
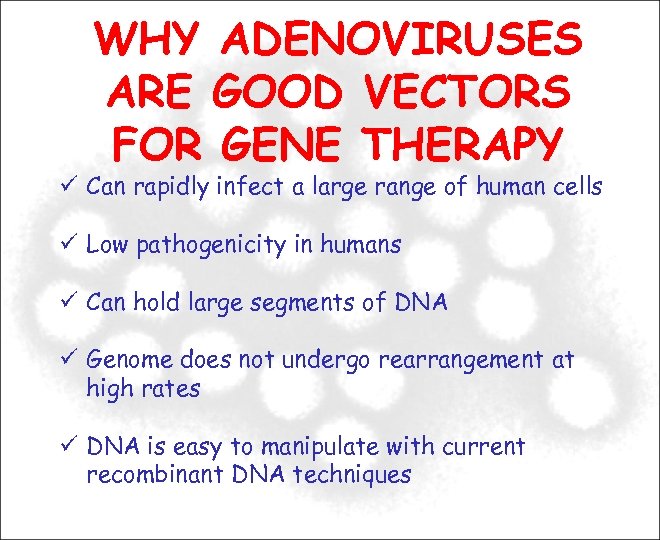 WHY ADENOVIRUSES ARE GOOD VECTORS FOR GENE THERAPY Can rapidly infect a large range of human cells Low pathogenicity in humans Can hold large segments of DNA Genome does not undergo rearrangement at high rates DNA is easy to manipulate with current recombinant DNA techniques
WHY ADENOVIRUSES ARE GOOD VECTORS FOR GENE THERAPY Can rapidly infect a large range of human cells Low pathogenicity in humans Can hold large segments of DNA Genome does not undergo rearrangement at high rates DNA is easy to manipulate with current recombinant DNA techniques
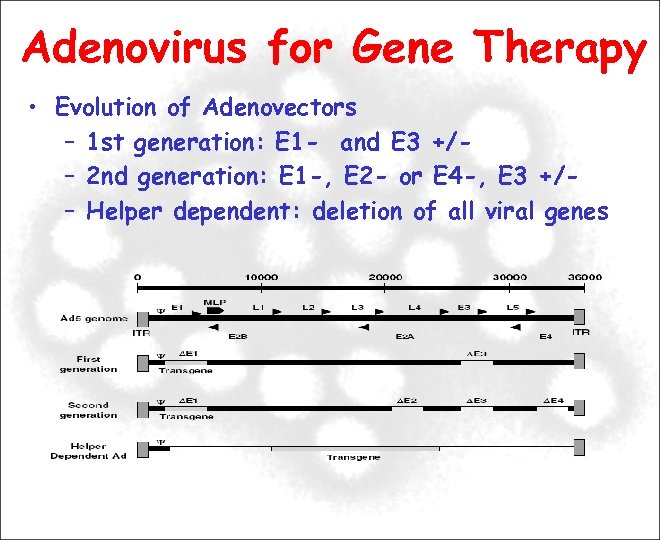 Adenovirus for Gene Therapy • Evolution of Adenovectors – 1 st generation: E 1 - and E 3 +/– 2 nd generation: E 1 -, E 2 - or E 4 -, E 3 +/– Helper dependent: deletion of all viral genes
Adenovirus for Gene Therapy • Evolution of Adenovectors – 1 st generation: E 1 - and E 3 +/– 2 nd generation: E 1 -, E 2 - or E 4 -, E 3 +/– Helper dependent: deletion of all viral genes
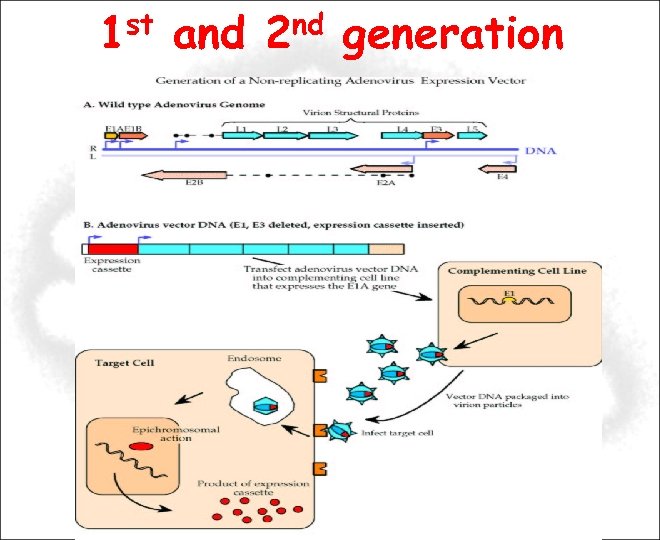 1 st and 2 nd generation
1 st and 2 nd generation
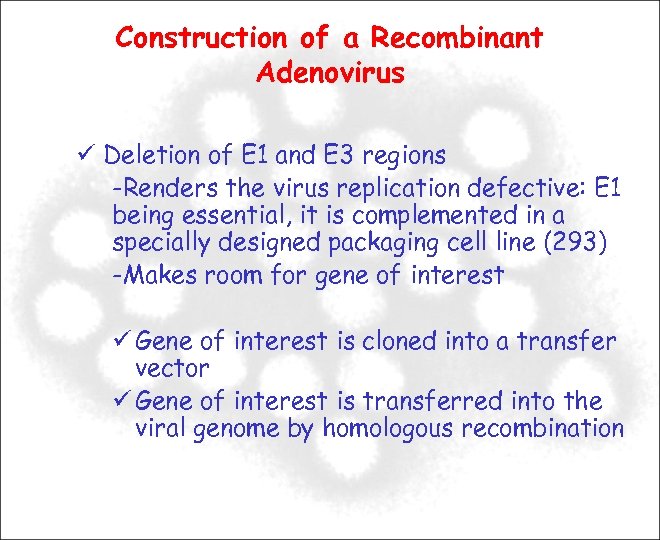 Construction of a Recombinant Adenovirus Deletion of E 1 and E 3 regions -Renders the virus replication defective: E 1 being essential, it is complemented in a specially designed packaging cell line (293) -Makes room for gene of interest Gene of interest is cloned into a transfer vector Gene of interest is transferred into the viral genome by homologous recombination
Construction of a Recombinant Adenovirus Deletion of E 1 and E 3 regions -Renders the virus replication defective: E 1 being essential, it is complemented in a specially designed packaging cell line (293) -Makes room for gene of interest Gene of interest is cloned into a transfer vector Gene of interest is transferred into the viral genome by homologous recombination
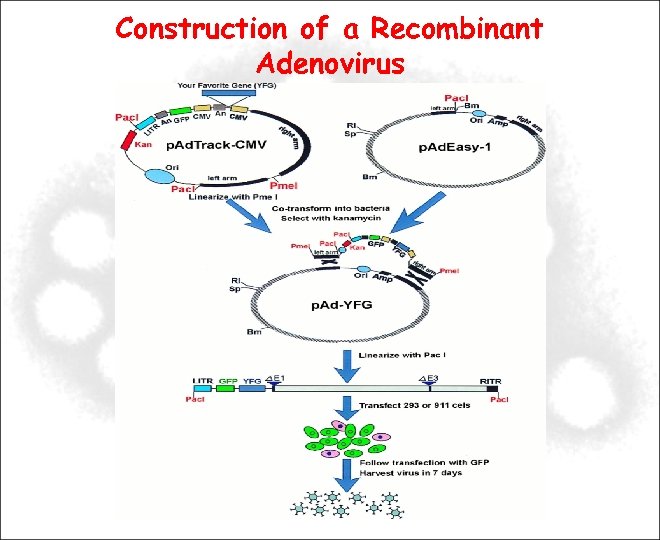 Construction of a Recombinant Adenovirus
Construction of a Recombinant Adenovirus

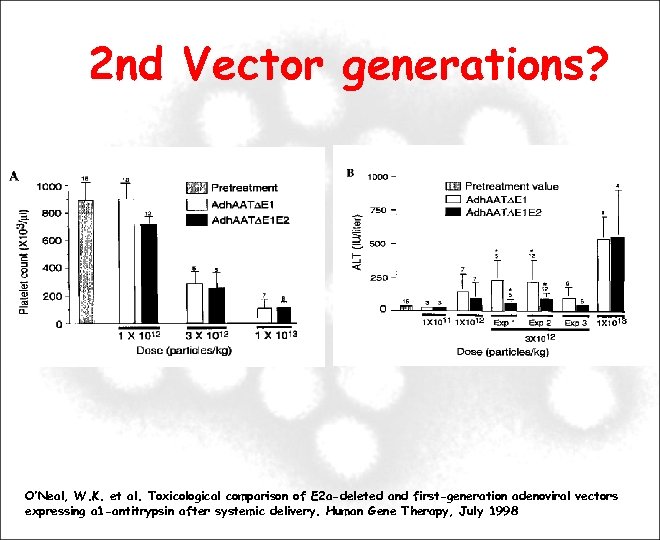 2 nd Vector generations? O’Neal, W. K. et al. Toxicological comparison of E 2 a-deleted and first-generation adenoviral vectors expressing a 1 -antitrypsin after systemic delivery. Human Gene Therapy, July 1998
2 nd Vector generations? O’Neal, W. K. et al. Toxicological comparison of E 2 a-deleted and first-generation adenoviral vectors expressing a 1 -antitrypsin after systemic delivery. Human Gene Therapy, July 1998
 Adenovirus for Gene Therapy Adenoviruses can be converted into efficient gene transfer vehicles Adenoviral vectors are not inherently dangerous Not all adenoviral vectors have equivalent toxicity profiles The dose of vector delivered is related to the toxicity observed Standardization of dose specification is necessary
Adenovirus for Gene Therapy Adenoviruses can be converted into efficient gene transfer vehicles Adenoviral vectors are not inherently dangerous Not all adenoviral vectors have equivalent toxicity profiles The dose of vector delivered is related to the toxicity observed Standardization of dose specification is necessary
 Advantages of using a recombinant Adenovirus 1. Broad host range Can infect a broad range of mammalian cells Allows for the expression of recombinant proteins in most mammalian cell lines and tissues Have been used extensively to express human as well as non-human proteins
Advantages of using a recombinant Adenovirus 1. Broad host range Can infect a broad range of mammalian cells Allows for the expression of recombinant proteins in most mammalian cell lines and tissues Have been used extensively to express human as well as non-human proteins
 Advantages of using a recombinant Adenovirus 2. Infection and expression of genes in replicative and non-replicative cells Retroviruses can only infect replicative cells Transfection cannot be done in nonreplicative cells Best system to study gene expression in primary non-replicative cells Allows for a direct comparison of results obtained with transformed cell lines and primary cells
Advantages of using a recombinant Adenovirus 2. Infection and expression of genes in replicative and non-replicative cells Retroviruses can only infect replicative cells Transfection cannot be done in nonreplicative cells Best system to study gene expression in primary non-replicative cells Allows for a direct comparison of results obtained with transformed cell lines and primary cells
 Advantages of using a recombinant Adenovirus 3. Replicates efficiently to high titers Production of 1012 to 1013 VP/m. L
Advantages of using a recombinant Adenovirus 3. Replicates efficiently to high titers Production of 1012 to 1013 VP/m. L
 Advantages of using a recombinant Adenovirus 4. Helper independent Ad can accommodate up to 7. 5 kb of foreign DNA Ad can normally encapsidate a viral DNA molecule slightly bigger than the normal DNA (105%) To provide additional cloning space, the E 1 and E 3 early regions of Ad have been deleted
Advantages of using a recombinant Adenovirus 4. Helper independent Ad can accommodate up to 7. 5 kb of foreign DNA Ad can normally encapsidate a viral DNA molecule slightly bigger than the normal DNA (105%) To provide additional cloning space, the E 1 and E 3 early regions of Ad have been deleted
 Advantages of using a recombinant Adenovirus 5. No insertional mutagenesis; remains epichromosomal Retroviruses integrate randomly into the host chromosome and can inactivate genes or activate oncogenes Ad remains epichromosomal and therefore does not interfere with other host genes.
Advantages of using a recombinant Adenovirus 5. No insertional mutagenesis; remains epichromosomal Retroviruses integrate randomly into the host chromosome and can inactivate genes or activate oncogenes Ad remains epichromosomal and therefore does not interfere with other host genes.
 Advantages of using a recombinant Adenovirus 6. Simultaneous expression of multiple genes Insertion of two genes in a double expression cassette Co-infection using different recombinant viruses each expressing a different protein Modulation of the expression by changing the MOI
Advantages of using a recombinant Adenovirus 6. Simultaneous expression of multiple genes Insertion of two genes in a double expression cassette Co-infection using different recombinant viruses each expressing a different protein Modulation of the expression by changing the MOI
 Disvantages of using a recombinant Adenovirus High toxicity Low transgene expression Replicant Competent Adenovirus (RCA)
Disvantages of using a recombinant Adenovirus High toxicity Low transgene expression Replicant Competent Adenovirus (RCA)
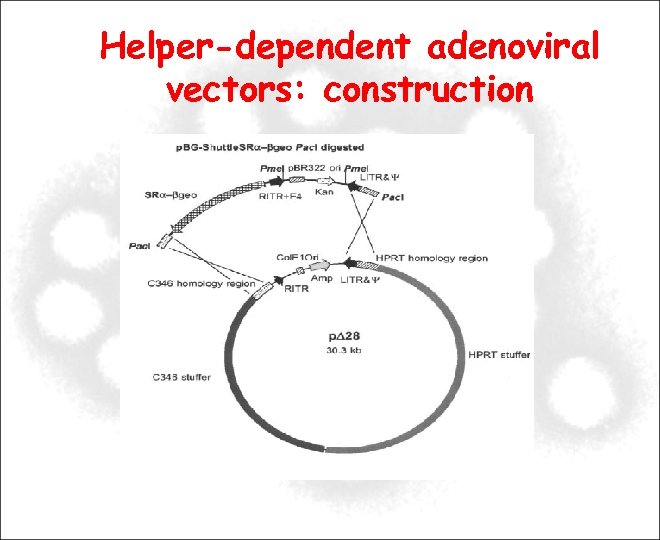 Helper-dependent adenoviral vectors: construction
Helper-dependent adenoviral vectors: construction
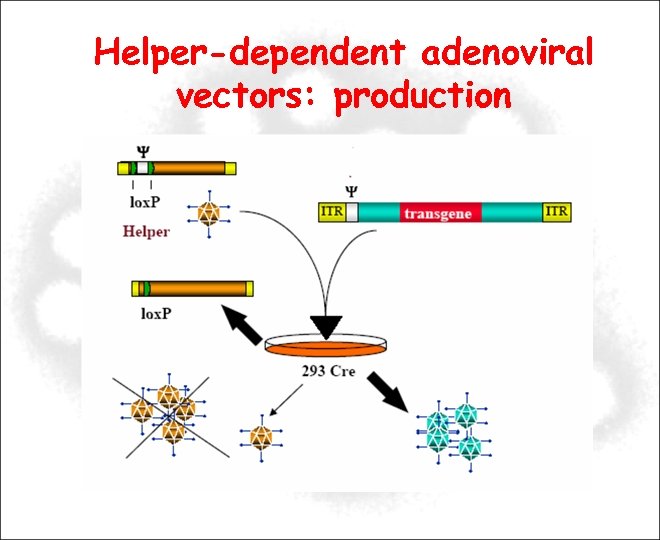 Helper-dependent adenoviral vectors: production
Helper-dependent adenoviral vectors: production
 Helper-dependent adenoviral vectors: advantages High-efficiency in vivo transduction High- level and long-term transgene expression Large cloning capacity ~ 37 kb Low toxicity profile
Helper-dependent adenoviral vectors: advantages High-efficiency in vivo transduction High- level and long-term transgene expression Large cloning capacity ~ 37 kb Low toxicity profile
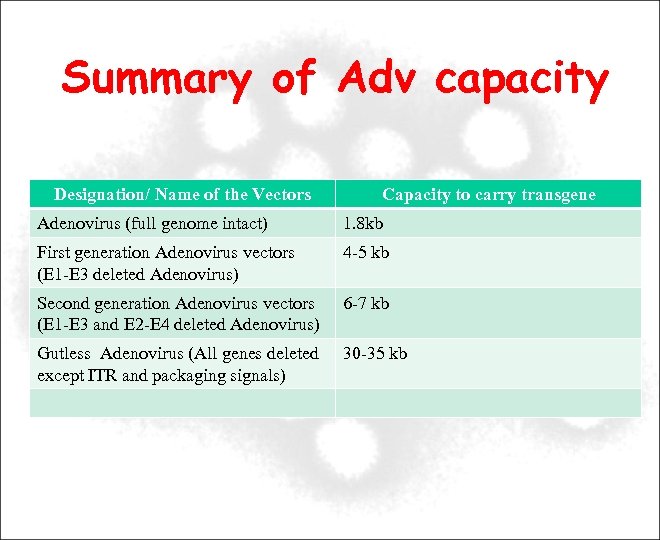 Summary of Adv capacity Designation/ Name of the Vectors Capacity to carry transgene Adenovirus (full genome intact) 1. 8 kb First generation Adenovirus vectors (E 1 -E 3 deleted Adenovirus) 4 -5 kb Second generation Adenovirus vectors (E 1 -E 3 and E 2 -E 4 deleted Adenovirus) 6 -7 kb Gutless Adenovirus (All genes deleted except ITR and packaging signals) 30 -35 kb
Summary of Adv capacity Designation/ Name of the Vectors Capacity to carry transgene Adenovirus (full genome intact) 1. 8 kb First generation Adenovirus vectors (E 1 -E 3 deleted Adenovirus) 4 -5 kb Second generation Adenovirus vectors (E 1 -E 3 and E 2 -E 4 deleted Adenovirus) 6 -7 kb Gutless Adenovirus (All genes deleted except ITR and packaging signals) 30 -35 kb
 Helper-dependent adenoviral vectors: amplification Viral prep Cs. Cl Triple flasks (293 cells )
Helper-dependent adenoviral vectors: amplification Viral prep Cs. Cl Triple flasks (293 cells )
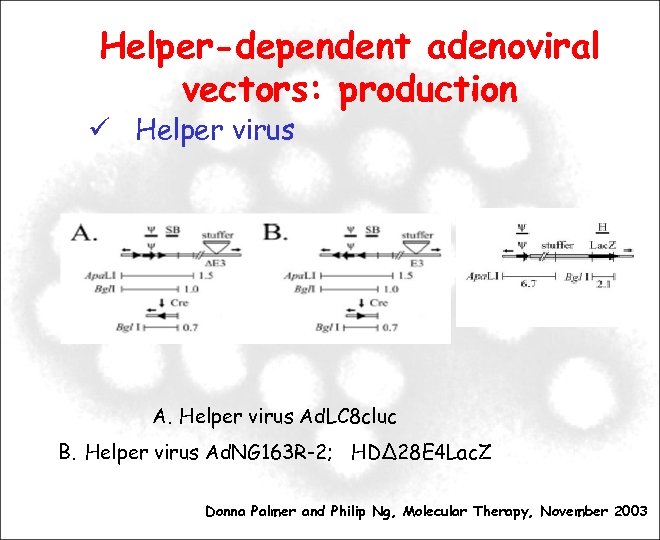 Helper-dependent adenoviral vectors: production Helper virus Ad. LC 8 cluc B. Helper virus Ad. NG 163 R-2; HDΔ 28 E 4 Lac. Z Donna Palmer and Philip Ng, Molecular Therapy, November 2003
Helper-dependent adenoviral vectors: production Helper virus Ad. LC 8 cluc B. Helper virus Ad. NG 163 R-2; HDΔ 28 E 4 Lac. Z Donna Palmer and Philip Ng, Molecular Therapy, November 2003
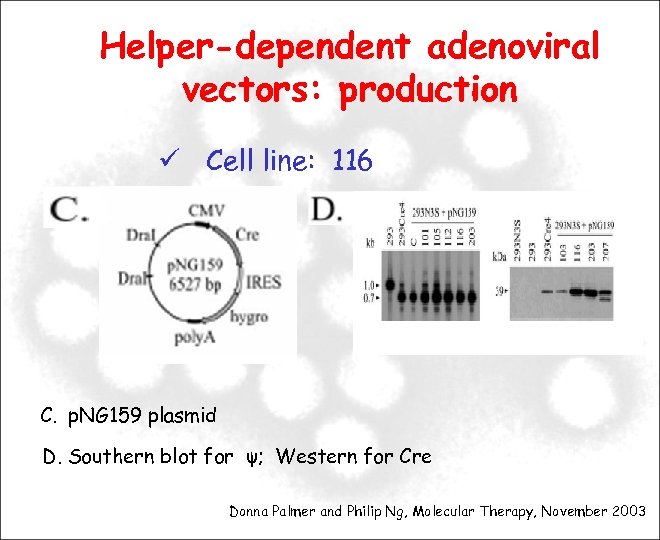 Helper-dependent adenoviral vectors: production Cell line: 116 C. p. NG 159 plasmid D. Southern blot for ψ; Western for Cre Donna Palmer and Philip Ng, Molecular Therapy, November 2003
Helper-dependent adenoviral vectors: production Cell line: 116 C. p. NG 159 plasmid D. Southern blot for ψ; Western for Cre Donna Palmer and Philip Ng, Molecular Therapy, November 2003
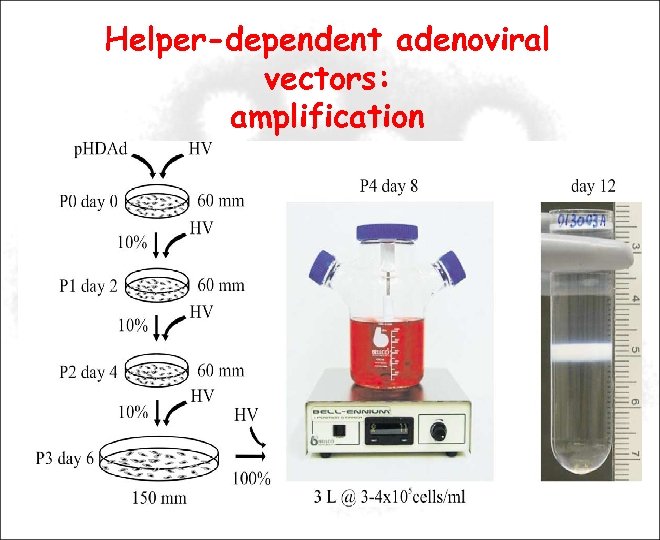 Helper-dependent adenoviral vectors: amplification
Helper-dependent adenoviral vectors: amplification
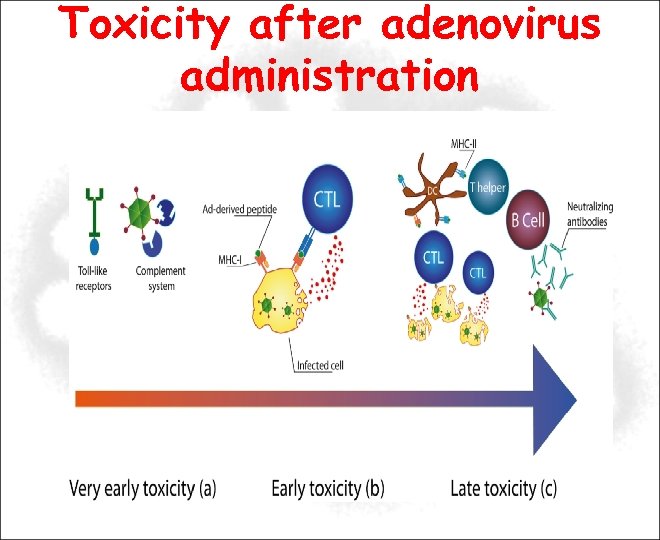
 Toxicity after adenovirus administration Immediate toxicity (Acute) Inflammatory/innate immunity Early toxicity Hepatitis, coagulopathy; CTL vs. viral products Late toxicity (Chronic) CTL/Ab response to virus/transgene; accumulation of viral proteins
Toxicity after adenovirus administration Immediate toxicity (Acute) Inflammatory/innate immunity Early toxicity Hepatitis, coagulopathy; CTL vs. viral products Late toxicity (Chronic) CTL/Ab response to virus/transgene; accumulation of viral proteins
 Toxicity after adenovirus administration
Toxicity after adenovirus administration
 Immediate toxicity Time: 1 -96 hours after treatment Characterized by: elevation of several cytokines (IL-6, IL-12, TNFα) and signs of thrombocytopenia. Involved cells: Macrophage, Kupffer cells Effect: elimination of ~90% vector from liver within first 24 h The immediate toxicity is indipendent of viral gene expression and it is due to an innate response to viral capsid proteins
Immediate toxicity Time: 1 -96 hours after treatment Characterized by: elevation of several cytokines (IL-6, IL-12, TNFα) and signs of thrombocytopenia. Involved cells: Macrophage, Kupffer cells Effect: elimination of ~90% vector from liver within first 24 h The immediate toxicity is indipendent of viral gene expression and it is due to an innate response to viral capsid proteins
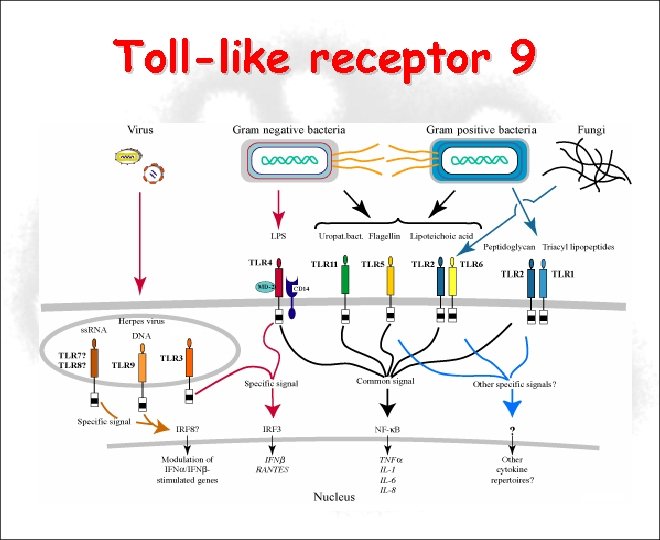 Toll-like receptor 9
Toll-like receptor 9
 Strategies to prevent adenovirus-induced immune response Circumventing the adaptive immune response by adaptations of the patients: Immunomodulation or immunosuppression Circumventing the adaptive immune response by adaptating adenoviral vectors: Altering native Ad vector and cell surface receptor interactions Vector microencapsulation Serotype switching Helper-virus dependent vectors Use of nonhuman Ad vectors
Strategies to prevent adenovirus-induced immune response Circumventing the adaptive immune response by adaptations of the patients: Immunomodulation or immunosuppression Circumventing the adaptive immune response by adaptating adenoviral vectors: Altering native Ad vector and cell surface receptor interactions Vector microencapsulation Serotype switching Helper-virus dependent vectors Use of nonhuman Ad vectors
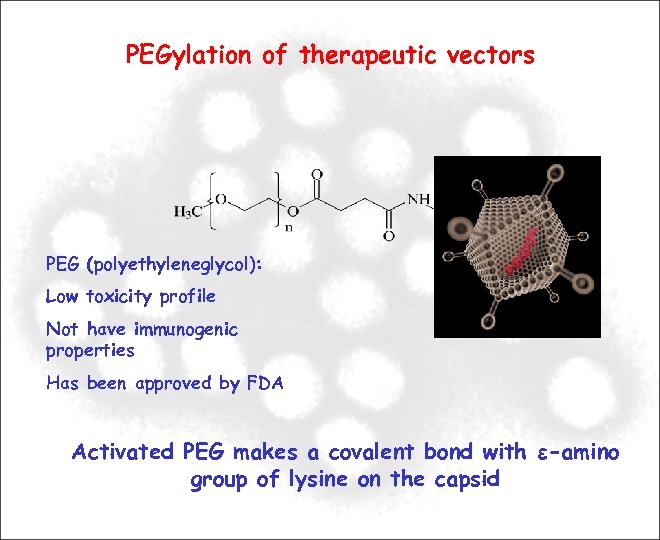 PEGylation of therapeutic vectors PEG (polyethyleneglycol): Low toxicity profile Not have immunogenic properties Has been approved by FDA Activated PEG makes a covalent bond with ε-amino group of lysine on the capsid
PEGylation of therapeutic vectors PEG (polyethyleneglycol): Low toxicity profile Not have immunogenic properties Has been approved by FDA Activated PEG makes a covalent bond with ε-amino group of lysine on the capsid
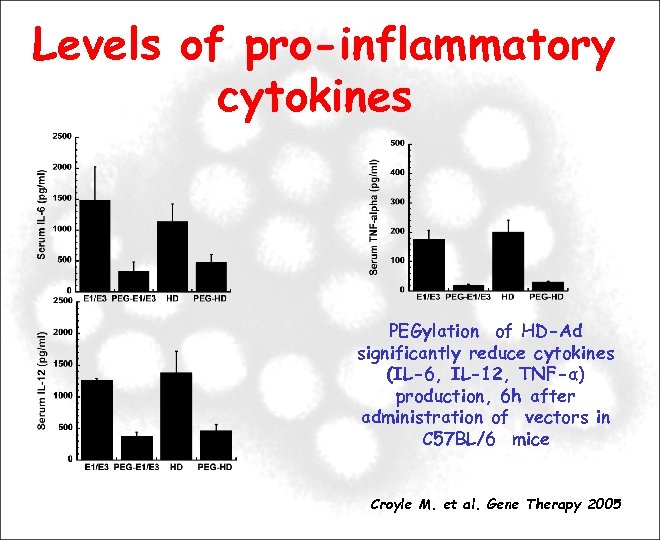 Levels of pro-inflammatory cytokines PEGylation of HD-Ad significantly reduce cytokines (IL-6, IL-12, TNF-α) production, 6 h after administration of vectors in C 57 BL/6 mice Croyle M. et al. Gene Therapy 2005
Levels of pro-inflammatory cytokines PEGylation of HD-Ad significantly reduce cytokines (IL-6, IL-12, TNF-α) production, 6 h after administration of vectors in C 57 BL/6 mice Croyle M. et al. Gene Therapy 2005
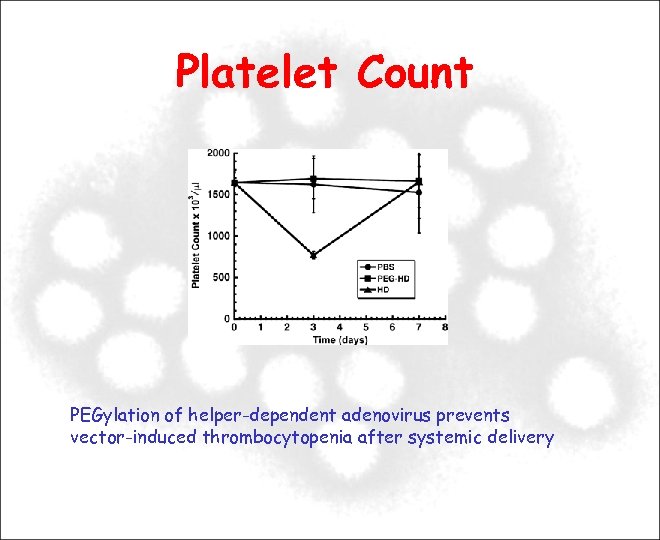 Platelet Count PEGylation of helper-dependent adenovirus prevents vector-induced thrombocytopenia after systemic delivery
Platelet Count PEGylation of helper-dependent adenovirus prevents vector-induced thrombocytopenia after systemic delivery
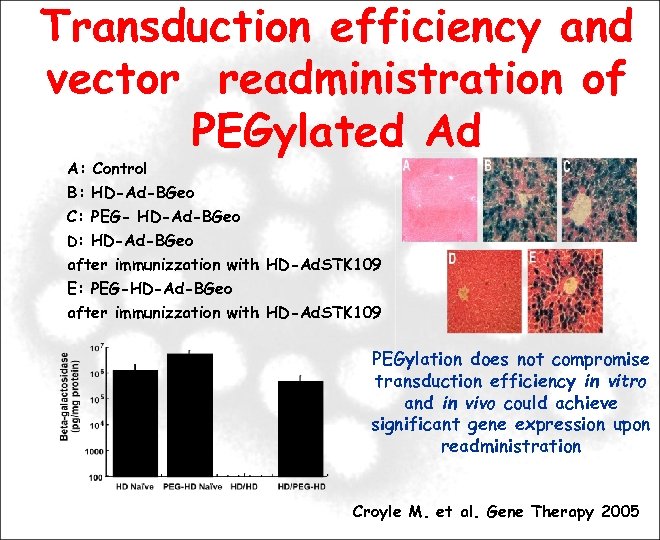 Transduction efficiency and vector readministration of PEGylated Ad A: Control B: HD-Ad-BGeo C: PEG- HD-Ad-BGeo D: HD-Ad-BGeo after immunizzation with HD-Ad. STK 109 E: PEG-HD-Ad-BGeo after immunizzation with HD-Ad. STK 109 PEGylation does not compromise transduction efficiency in vitro and in vivo could achieve significant gene expression upon readministration Croyle M. et al. Gene Therapy 2005
Transduction efficiency and vector readministration of PEGylated Ad A: Control B: HD-Ad-BGeo C: PEG- HD-Ad-BGeo D: HD-Ad-BGeo after immunizzation with HD-Ad. STK 109 E: PEG-HD-Ad-BGeo after immunizzation with HD-Ad. STK 109 PEGylation does not compromise transduction efficiency in vitro and in vivo could achieve significant gene expression upon readministration Croyle M. et al. Gene Therapy 2005
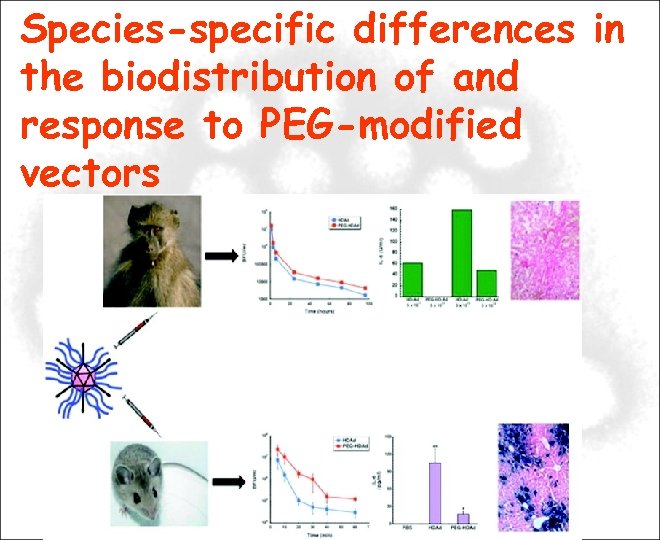 Species-specific differences in the biodistribution of and response to PEG-modified vectors
Species-specific differences in the biodistribution of and response to PEG-modified vectors
 PEGylation of HD-Ad: Reduces vector-induced tissue damage Decreases prolongation of clotting time Prevents vector-induced cytokine release Does not change transduction efficiency of vectors
PEGylation of HD-Ad: Reduces vector-induced tissue damage Decreases prolongation of clotting time Prevents vector-induced cytokine release Does not change transduction efficiency of vectors
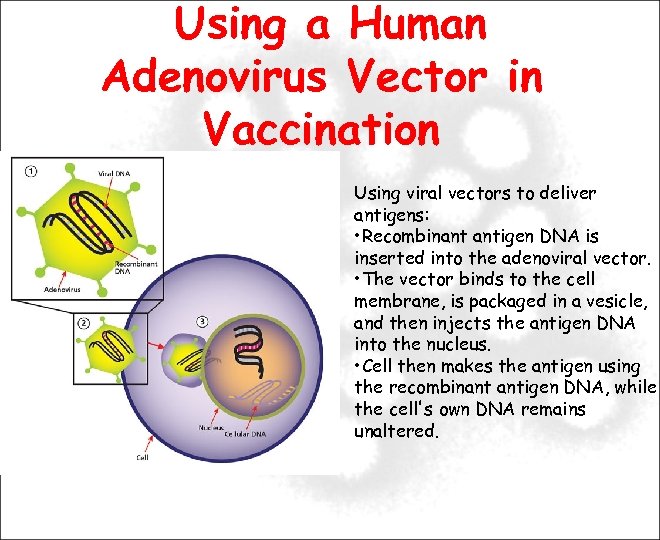 Using a Human Adenovirus Vector in Vaccination Using viral vectors to deliver antigens: • Recombinant antigen DNA is inserted into the adenoviral vector. • The vector binds to the cell membrane, is packaged in a vesicle, and then injects the antigen DNA into the nucleus. • Cell then makes the antigen using the recombinant antigen DNA, while the cell's own DNA remains unaltered.
Using a Human Adenovirus Vector in Vaccination Using viral vectors to deliver antigens: • Recombinant antigen DNA is inserted into the adenoviral vector. • The vector binds to the cell membrane, is packaged in a vesicle, and then injects the antigen DNA into the nucleus. • Cell then makes the antigen using the recombinant antigen DNA, while the cell's own DNA remains unaltered.
 Using a Human Adenovirus Vector in Vaccination Primary criticism is the issue of pre-existing immunity 35 -55% of the Ad population has neutralizing Abs, in particular against Ad 5 These Abs might limit Ad based vaccines vector’s efficacy Use of alternate serotypes that are antigenically different and thus cannot be neutralized by these Abs is an option in this respect However still conflicting data exists in this regard Incase of Merck Ad-5 based HIV vaccine, the effect of neutralizing Abs can be overcome by increasing the dose of the vaccine in clinical trials
Using a Human Adenovirus Vector in Vaccination Primary criticism is the issue of pre-existing immunity 35 -55% of the Ad population has neutralizing Abs, in particular against Ad 5 These Abs might limit Ad based vaccines vector’s efficacy Use of alternate serotypes that are antigenically different and thus cannot be neutralized by these Abs is an option in this respect However still conflicting data exists in this regard Incase of Merck Ad-5 based HIV vaccine, the effect of neutralizing Abs can be overcome by increasing the dose of the vaccine in clinical trials
 Using a Human Adenovirus Vector in Vaccination Evidence also exist that alternative route of administration (oral or intranasal) instead of injection can overcome pre-existing vector immunity The issue of pre-existing Ad vector immunity is a source of frequent debate and experimental data exist for support on both sides of the argument.
Using a Human Adenovirus Vector in Vaccination Evidence also exist that alternative route of administration (oral or intranasal) instead of injection can overcome pre-existing vector immunity The issue of pre-existing Ad vector immunity is a source of frequent debate and experimental data exist for support on both sides of the argument.
 Future work using adenovirus as a vector The majority of clinical trials done using the adenovirus in gene therapy have been phase 1 Phase 1 trials are used to determine safety, feasibility, and toxicity of the process The Phase 1 trials have set the stage for future work with the next generation of Adeno vectors that will show less stimulation of the host immune system and can be selectively targeted to specific tissues. Live recombinant adenovirus vaccine are being developed Provides hope for practitioners in using a more economical vaccine that provides a longer lasting immunity. Ongoing research is aimed at determining effectiveness of recombinant vaccines
Future work using adenovirus as a vector The majority of clinical trials done using the adenovirus in gene therapy have been phase 1 Phase 1 trials are used to determine safety, feasibility, and toxicity of the process The Phase 1 trials have set the stage for future work with the next generation of Adeno vectors that will show less stimulation of the host immune system and can be selectively targeted to specific tissues. Live recombinant adenovirus vaccine are being developed Provides hope for practitioners in using a more economical vaccine that provides a longer lasting immunity. Ongoing research is aimed at determining effectiveness of recombinant vaccines
 REFERENCES Palmer, D. ; Ng, P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003, 8, 846– 852 Lasaro, M. O. ; Ertl, H. C. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009, 17, 1333– 1339. Brunetti-Pierri, N. ; Ng, P. Progress towards the clinical application of helperdependent adenoviral vectors for liver and lung gene therapy. Curr. Opin. Mol. Ther. 2006, 8, 446– 454. Wonganan P, Clemens CC, Brasky K, Pastore L, Croyle MA Species differences in the pharmacology and toxicology of PEGylated helperdependent adenovirus. Mol Pharm. 2011 Feb 7; 8(1): 78 -92.
REFERENCES Palmer, D. ; Ng, P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003, 8, 846– 852 Lasaro, M. O. ; Ertl, H. C. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009, 17, 1333– 1339. Brunetti-Pierri, N. ; Ng, P. Progress towards the clinical application of helperdependent adenoviral vectors for liver and lung gene therapy. Curr. Opin. Mol. Ther. 2006, 8, 446– 454. Wonganan P, Clemens CC, Brasky K, Pastore L, Croyle MA Species differences in the pharmacology and toxicology of PEGylated helperdependent adenovirus. Mol Pharm. 2011 Feb 7; 8(1): 78 -92.


