3a3d99d5d3b8c319144f641d74f59e60.ppt
- Количество слайдов: 17

Additives and Ingredients Subcommittee Food Advisory Committee The Office of Food Additive Safety George Pauli Associate Director for Science and Policy Office of Food Additive Safety August 26, 2003

MISSION STATEMENT FOOD INGREDIENT SAFETY PROGRAM To assure that the use of food ingredients is safe by: § § § Evaluating new applications efficiently & effectively Expediting applications that mitigate food hazards Meeting high performance standards with strong science and modern infrastructure § Directing resources to issues of greater public health importance while anticipating future trends § Maintaining data to monitor safety over time § Conducting research that supports the FDA regulatory agenda


Food Safety Decision Framework U. S. law provides different approaches for different segments or components of the food supply; i. e. , whole foods, generally recognized as safe food ingredients, food additives, color additives, food contact substances, dietary ingredients in dietary supplements, contaminants, etc.

Food Additive Safety Decision Framework “…the steps (i. e. , scientific basis) by means of which the agency deduces (under the applicable statutory construct) whether a particular use of a food additive is safe. ” > Statutory standards > Scientific principles

Food, Drug, and Cosmetic Act (as amended, ‘ 58, ‘ 60, ‘ 94, ‘ 97) Defines “food additive, ” w/ GRAS exemption Requires premarket approval of new uses of food additives Establishes the standard of review Establishes the standard of safety Establishes formal rulemaking procedures – Petition or Agency Initiative ------since FDAMA of 1997: ---------Defines “food contact substance” (FCS) Establishes a premarket notification program for FCSs

Statutory Definition of “Food Additive” FD&C Act Section 201(s) “The term ‘food additive’ means any substance the intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food… …if such substance is not generally recognized…as safe…. ”

Food “Ingredient” Universe Direct Food Ingredients Processing Aids Sweeteners; Preservatives; Nutrients; Fat substitutes; Texturizers (thickeners, emulsifiers, etc. ); Flavors Antimicrobials (meat and poultry processing); Defoamers; Ion exchange resins; Color Additives Food Irradiation Equipment In food, animal feed, drugs, cosmetics, and medical devices (i. e. , sutures and contact lenses) To Process food “GRAS” Ingredient uses Enzymes; Fibers; Proteins; Lipids; Sugars; MSG; Antimicrobials; Phytosterols/stanols; Flavors; Infant formula ingredients Foods/Ingredients produced using modern biotechnology Plants w/ herbicide resistance or insect resistence; delayed ripening, etc. To Inspect food Food Packaging / Food Contact Substances. Coatings (paper, metal, etc. ); New/recycled plastics including both polymers and monomers; Paper; Adhesives; Ingredients in pkgs. (i. e. , colorants; antimicrobials; antioxidants, etc. ); Packaging materials for use during food irradiation; Food packaging “formulations”

House of Representatives, Report No. 2284, “Food Additives Amendment of 1958” Committee on Interstate & Foreign Commerce, 85 th Congress, 2 nd Session, July 28, 1958 “The committee feels that the Secretary’s findings of fact and orders should not be based on isolated evidence in the record, which evidence in and of itself may be considered substantial without taking account of the contradictory evidence of equal or even greater substance. . ”

REASONABLE CERTAINTY OF NO HARM (Legislative History of the FD&C Act) “The concept of safety used in this legislation involves the question of whether a substance is hazardous to the health of man or animal. Safety requires proof of a reasonable certainty that no harm will result from the proposed use of an additive. ” “It does not -- and cannot -- require proof beyond any possible doubt that no harm will result under any conceivable circumstance. ” H. R. Report No. 2284, 85 th Congress 1958

Standard of Safety for New Food Additives The petitioner has the burden to demonstrate a “reasonable certainty of no harm” from the intended use of the additive This requires that the FDA assess whether it has received adequately documented answers to appropriate questions of probative value.

General Approach for Food Safety Assessment Goal: “New” Food Must Be as Safe as “Today’s” Food

The “olden days: ” “ONE FLAVOR” § Food and Color Additive Petitions § GRAS Affirmation Petitions

FOOD ADDITIVE PETITION REVIEW THE SAFETY DECISION What the safety evaluation is NOT: n It is NOT an academic inquiry or academic research n It is NOT a search for “complete knowledge” n n n It is NOT intended to ensure, nor is it possible to ensure safety with absolute certainty: (“Reasonable Certainty of No Harm” rather than “Certainty of No Theoretical Possibility of Harm”) Does NOT weigh risks and benefits It is NOT intended to enforce or limit consumer or producer choices among safe foods (e. g. , Need is NOT a Criterion)

FOOD ADDITIVE PETITION REVIEW THE SAFETY DECISION • It DOES, in fact, ensure safety • It IS a consensus decision, made under uncertainty, that provides a fair evaluation of all the data of record … - That must protect the public health - That is made in the absence of complete knowledge - That will withstand scientific, procedural, and legal challenge from all sides - Where the residual uncertainty is not out-of-line with what has been previously tolerated in the context of all previous similar safety decisions

Notification Programs… the shape of the future § Bioengineered Foods Consultations § GRAS Notices § Food Contact Substance Notifications
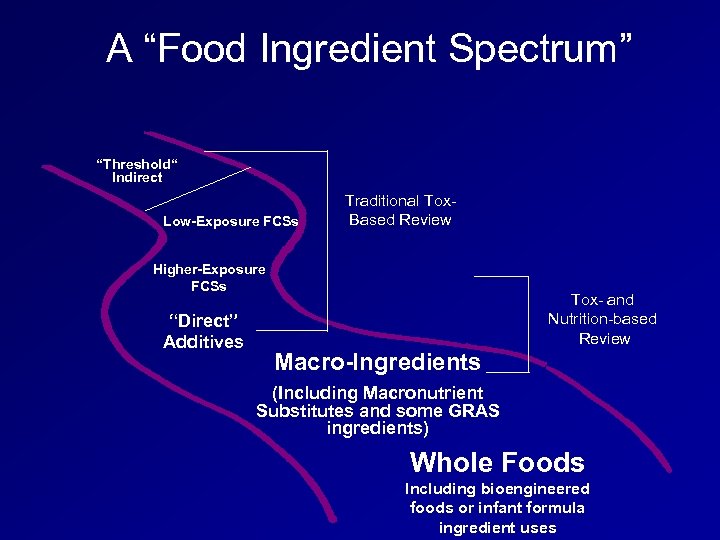
A “Food Ingredient Spectrum” “Threshold“ Indirect Low-Exposure FCSs Traditional Tox. Based Review Higher-Exposure FCSs “Direct” Additives Tox- and Nutrition-based Review Macro-Ingredients (Including Macronutrient Substitutes and some GRAS ingredients) Whole Foods Including bioengineered foods or infant formula ingredient uses
3a3d99d5d3b8c319144f641d74f59e60.ppt