AML_lecture2studfeb06.ppt
- Количество слайдов: 41
 ACUTE MYELOID LEUKEMIA
ACUTE MYELOID LEUKEMIA
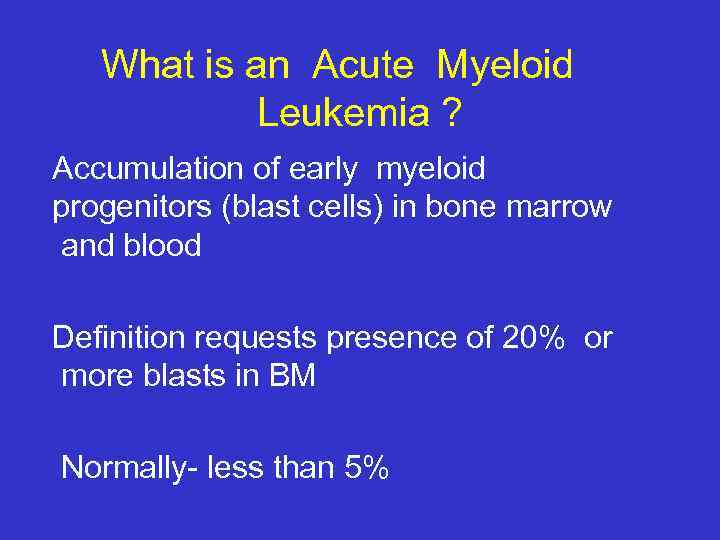 What is an Acute Myeloid Leukemia ? Accumulation of early myeloid progenitors (blast cells) in bone marrow and blood Definition requests presence of 20% or more blasts in BM Normally- less than 5%
What is an Acute Myeloid Leukemia ? Accumulation of early myeloid progenitors (blast cells) in bone marrow and blood Definition requests presence of 20% or more blasts in BM Normally- less than 5%
 ETIOLOGY • Environment: irradiation, chemotherapeutic agents, organic solvents – benzene etc. • Genetic diseases: neurofibromatosis, Wiscott-Aldrich synd. , defective DNA repair – Fanconi, Down synd. • Acquired disorders: Aplastic Anemia, PNH • MOST OF THE CASES APPEAR WITH NO APPARENT RISK FACTORS!!!
ETIOLOGY • Environment: irradiation, chemotherapeutic agents, organic solvents – benzene etc. • Genetic diseases: neurofibromatosis, Wiscott-Aldrich synd. , defective DNA repair – Fanconi, Down synd. • Acquired disorders: Aplastic Anemia, PNH • MOST OF THE CASES APPEAR WITH NO APPARENT RISK FACTORS!!!
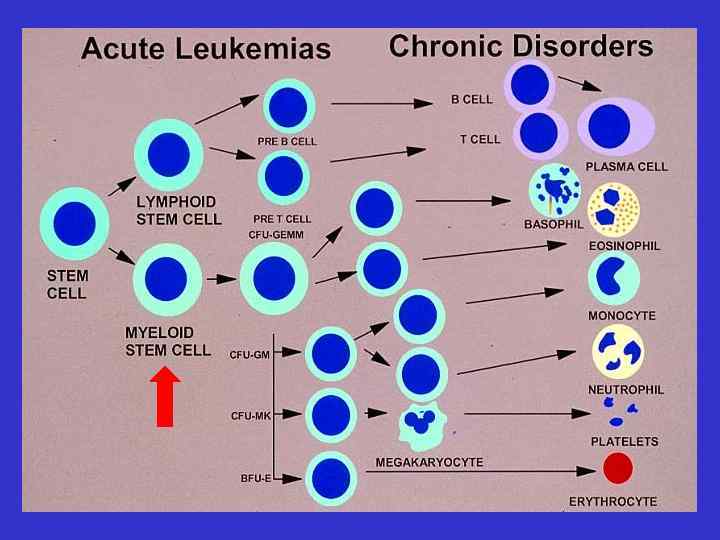
 AML Aggressive disease with an acute onset Can occur De Novo or following a known leukomogemic trigger (radiation, chemotherapy, diseases): Secondary AML
AML Aggressive disease with an acute onset Can occur De Novo or following a known leukomogemic trigger (radiation, chemotherapy, diseases): Secondary AML
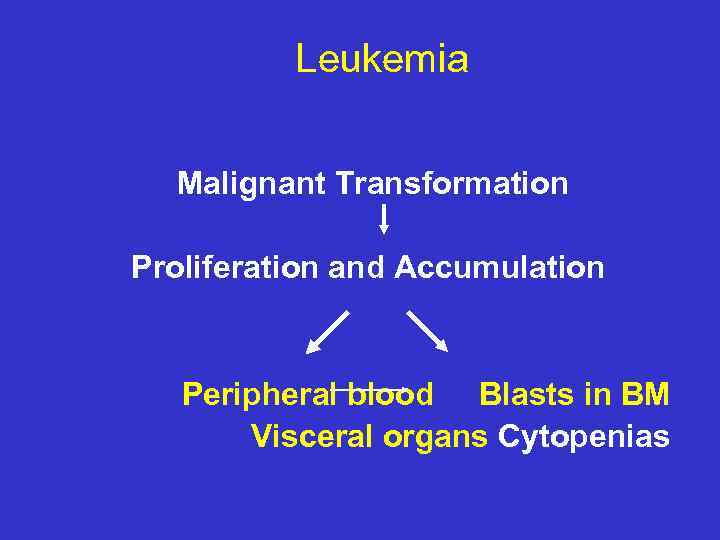 Leukemia Malignant Transformation Proliferation and Accumulation Peripheral blood Blasts in BM Visceral organs Cytopenias
Leukemia Malignant Transformation Proliferation and Accumulation Peripheral blood Blasts in BM Visceral organs Cytopenias
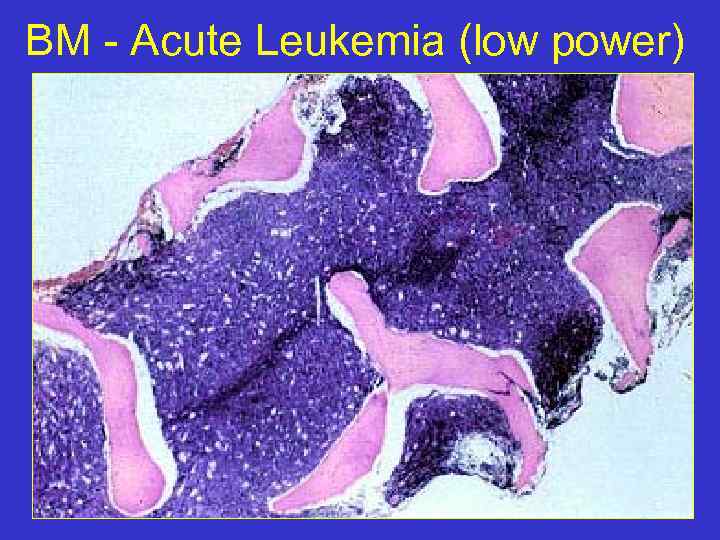 BM - Acute Leukemia (low power)
BM - Acute Leukemia (low power)
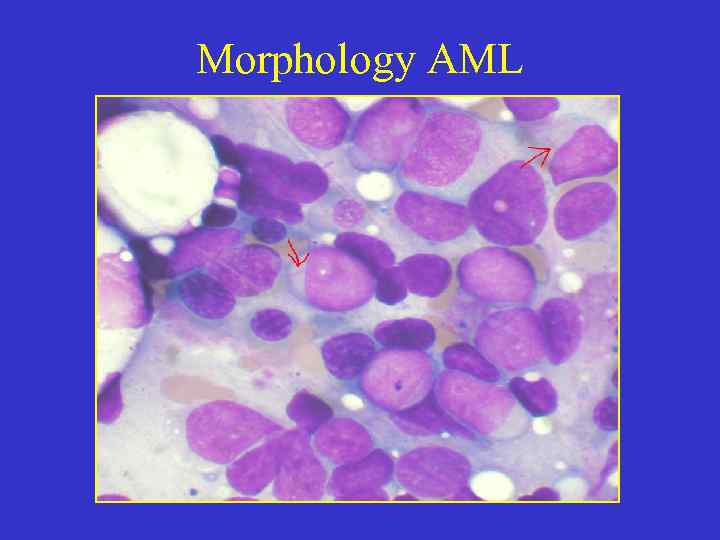 Morphology AML
Morphology AML
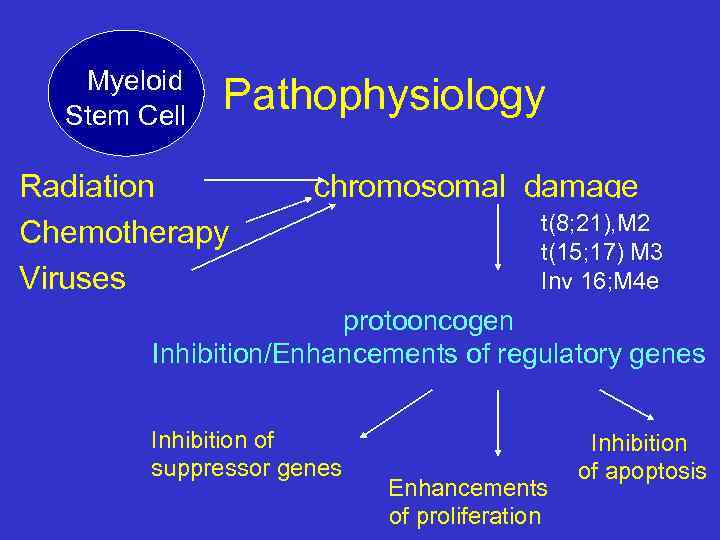 Myeloid Stem Cell Pathophysiology Radiation Chemotherapy Viruses chromosomal damage t(8; 21), M 2 t(15; 17) M 3 Inv 16; M 4 e protooncogen Inhibition/Enhancements of regulatory genes Inhibition of suppressor genes Enhancements of proliferation Inhibition of apoptosis
Myeloid Stem Cell Pathophysiology Radiation Chemotherapy Viruses chromosomal damage t(8; 21), M 2 t(15; 17) M 3 Inv 16; M 4 e protooncogen Inhibition/Enhancements of regulatory genes Inhibition of suppressor genes Enhancements of proliferation Inhibition of apoptosis
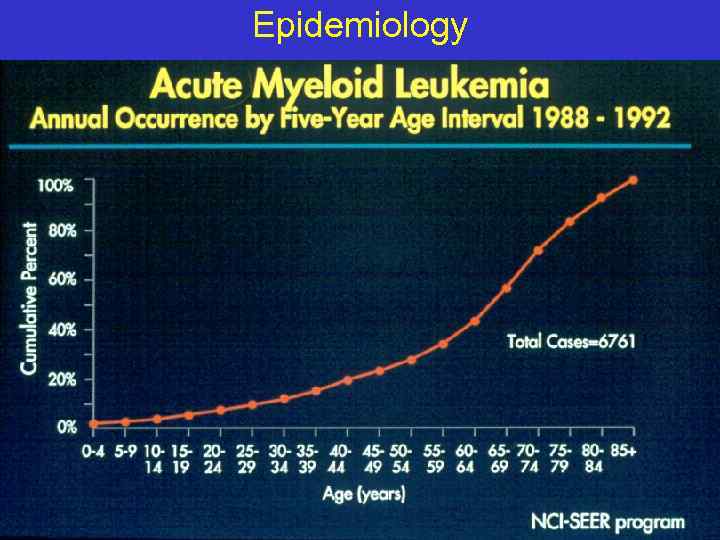 Epidemiology
Epidemiology
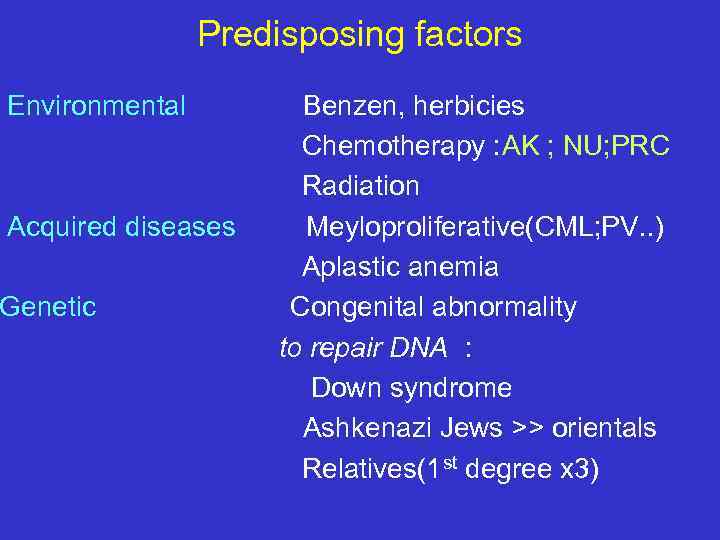 Predisposing factors Environmental Acquired diseases Genetic Benzen, herbicies Chemotherapy : AK ; NU; PRC Radiation Meyloproliferative(CML; PV. . ) Aplastic anemia Congenital abnormality to repair DNA : Down syndrome Ashkenazi Jews >> orientals Relatives(1 st degree x 3)
Predisposing factors Environmental Acquired diseases Genetic Benzen, herbicies Chemotherapy : AK ; NU; PRC Radiation Meyloproliferative(CML; PV. . ) Aplastic anemia Congenital abnormality to repair DNA : Down syndrome Ashkenazi Jews >> orientals Relatives(1 st degree x 3)
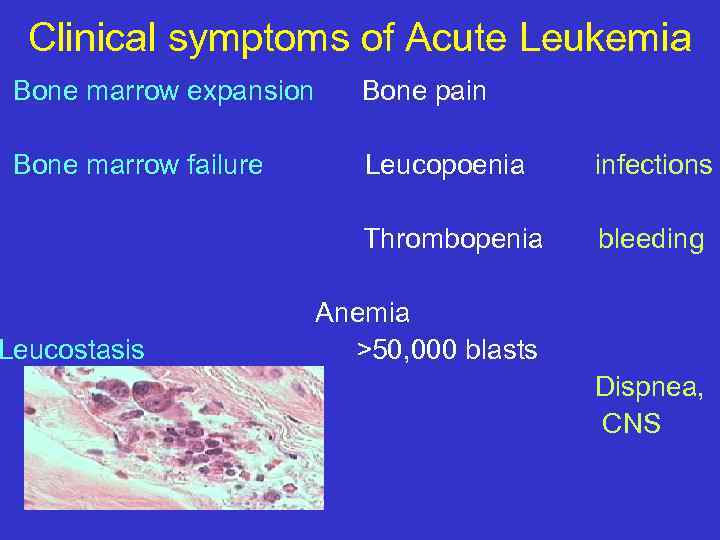 Clinical symptoms of Acute Leukemia Bone marrow expansion Bone pain Bone marrow failure Leucopoenia infections Thrombopenia bleeding Leucostasis Anemia >50, 000 blasts Dispnea, CNS
Clinical symptoms of Acute Leukemia Bone marrow expansion Bone pain Bone marrow failure Leucopoenia infections Thrombopenia bleeding Leucostasis Anemia >50, 000 blasts Dispnea, CNS
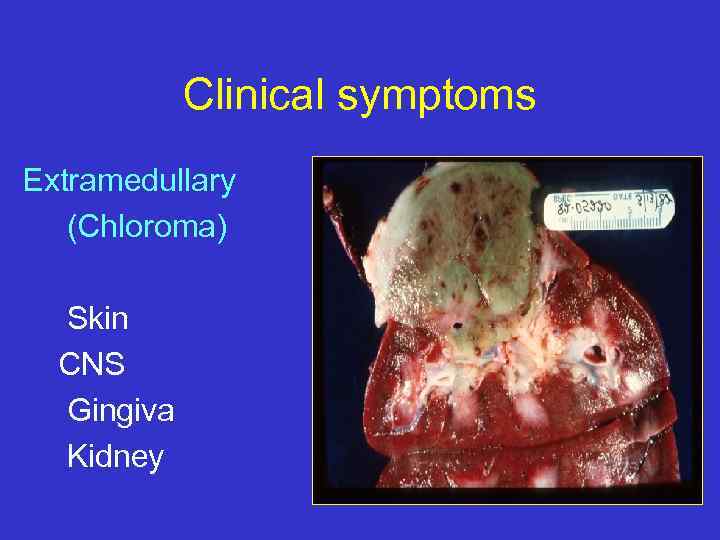 Clinical symptoms Extramedullary (Chloroma) Skin CNS Gingiva Kidney
Clinical symptoms Extramedullary (Chloroma) Skin CNS Gingiva Kidney
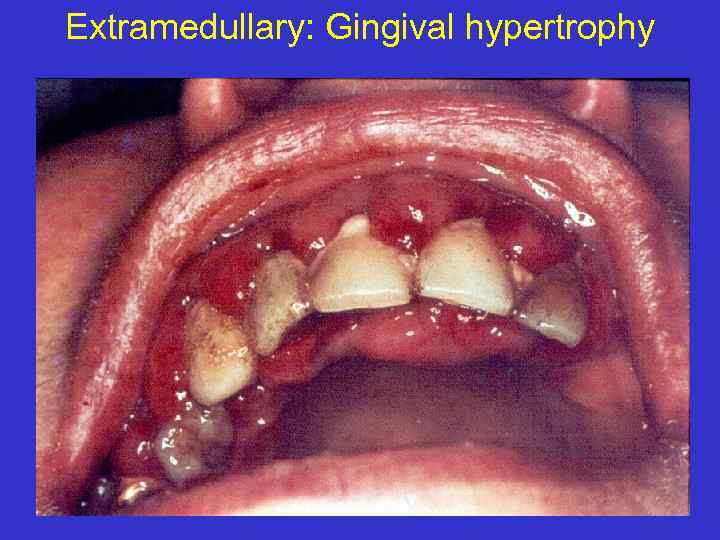 Extramedullary: Gingival hypertrophy
Extramedullary: Gingival hypertrophy
 Clinical symptomes DIC Bleeding Thrombosis Metabolic Hyperuricemia Tumor lysis syndrome K, phosphor, Ca Uric Acid
Clinical symptomes DIC Bleeding Thrombosis Metabolic Hyperuricemia Tumor lysis syndrome K, phosphor, Ca Uric Acid
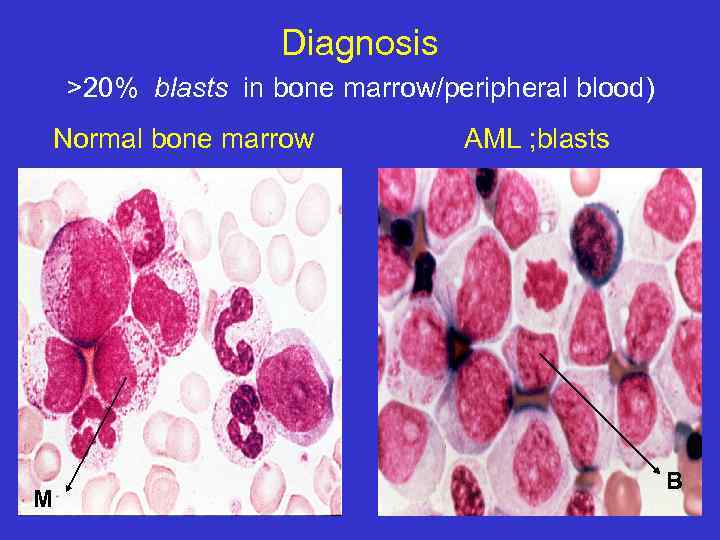 Diagnosis >20% blasts in bone marrow/peripheral blood) Normal bone marrow M AML ; blasts B
Diagnosis >20% blasts in bone marrow/peripheral blood) Normal bone marrow M AML ; blasts B
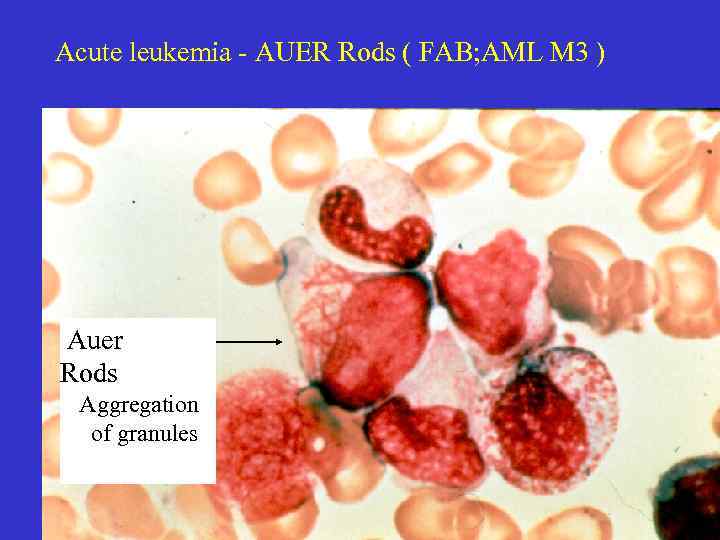 Acute leukemia - AUER Rods ( FAB; AML M 3 ) Auer Rods Aggregation of granules
Acute leukemia - AUER Rods ( FAB; AML M 3 ) Auer Rods Aggregation of granules
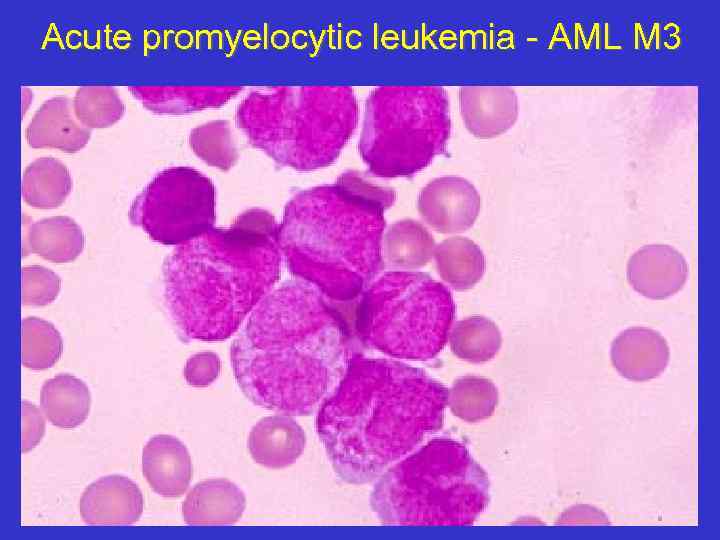 Acute promyelocytic leukemia - AML M 3
Acute promyelocytic leukemia - AML M 3
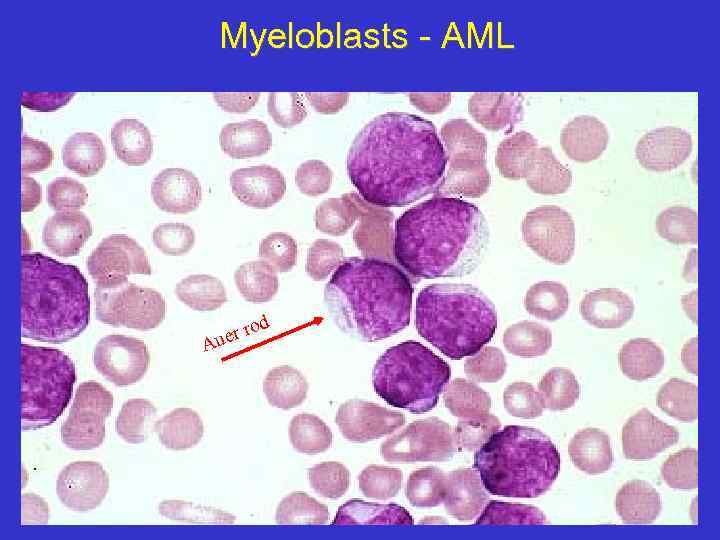 Myeloblasts - AML d ro uer A
Myeloblasts - AML d ro uer A
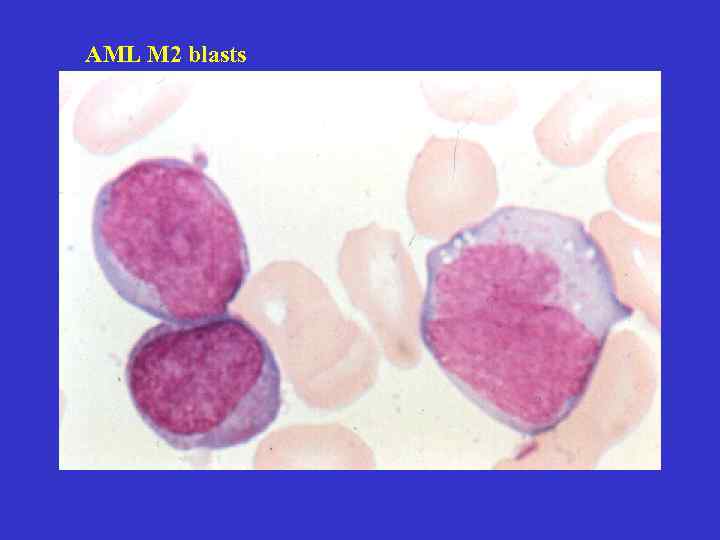 AML M 2 blasts
AML M 2 blasts
 French American British (FAB) classification -Based on morphology and staining (cytochemistry) -Divides patients into 7 AML subtypes -A morphological rather than biological classification -Correlation between morphological and biological characteristics may exist , but not always
French American British (FAB) classification -Based on morphology and staining (cytochemistry) -Divides patients into 7 AML subtypes -A morphological rather than biological classification -Correlation between morphological and biological characteristics may exist , but not always
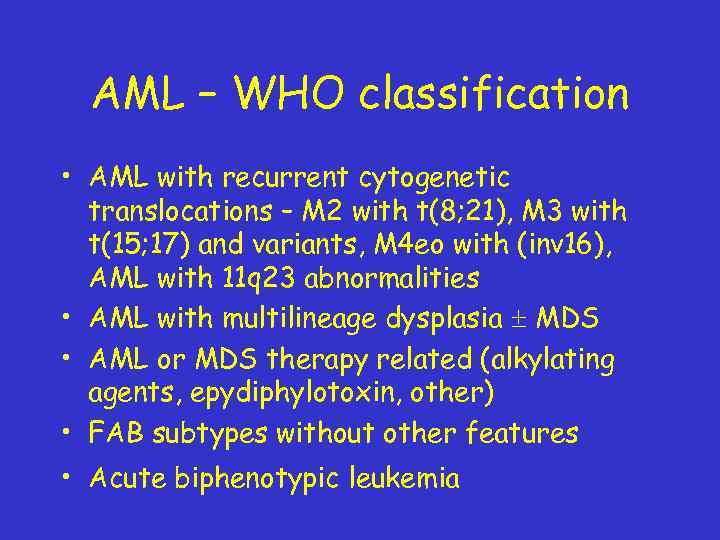 AML – WHO classification • AML with recurrent cytogenetic translocations – M 2 with t(8; 21), M 3 with t(15; 17) and variants, M 4 eo with (inv 16), AML with 11 q 23 abnormalities • AML with multilineage dysplasia MDS • AML or MDS therapy related (alkylating agents, epydiphylotoxin, other) • FAB subtypes without other features • Acute biphenotypic leukemia
AML – WHO classification • AML with recurrent cytogenetic translocations – M 2 with t(8; 21), M 3 with t(15; 17) and variants, M 4 eo with (inv 16), AML with 11 q 23 abnormalities • AML with multilineage dysplasia MDS • AML or MDS therapy related (alkylating agents, epydiphylotoxin, other) • FAB subtypes without other features • Acute biphenotypic leukemia
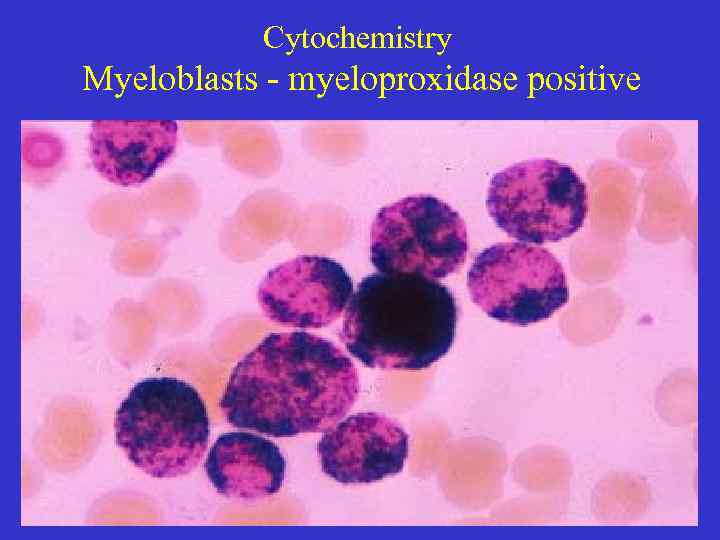 Cytochemistry Myeloblasts - myeloproxidase positive
Cytochemistry Myeloblasts - myeloproxidase positive
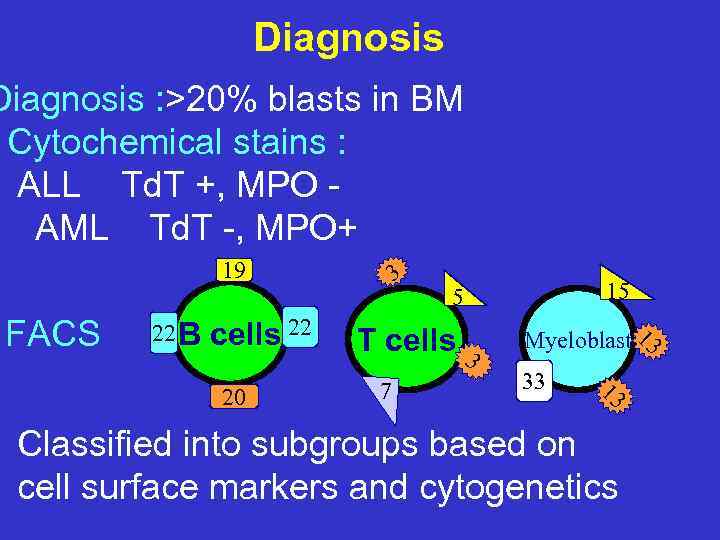 Diagnosis : >20% blasts in BM Cytochemical stains : ALL Td. T +, MPO AML Td. T -, MPO+ 19 FACS 22 B cells 22 20 3 T cells 3 7 15 5 Myeloblast 13 33 13 Classified into subgroups based on cell surface markers and cytogenetics
Diagnosis : >20% blasts in BM Cytochemical stains : ALL Td. T +, MPO AML Td. T -, MPO+ 19 FACS 22 B cells 22 20 3 T cells 3 7 15 5 Myeloblast 13 33 13 Classified into subgroups based on cell surface markers and cytogenetics
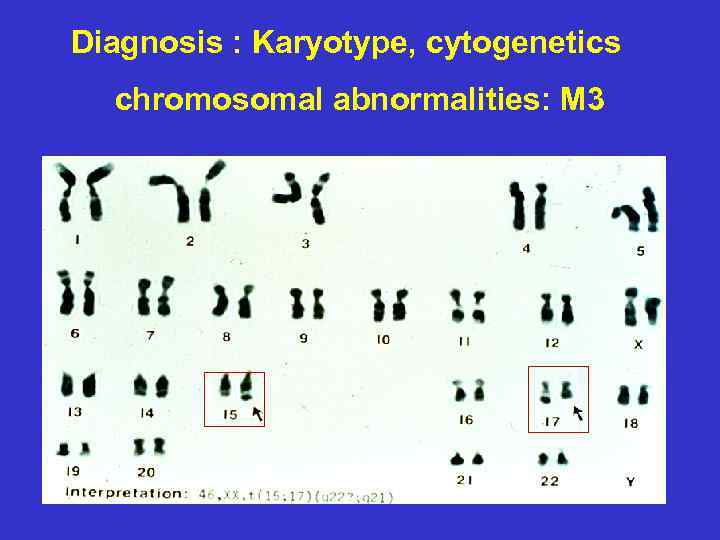 Diagnosis : Karyotype, cytogenetics chromosomal abnormalities: M 3
Diagnosis : Karyotype, cytogenetics chromosomal abnormalities: M 3
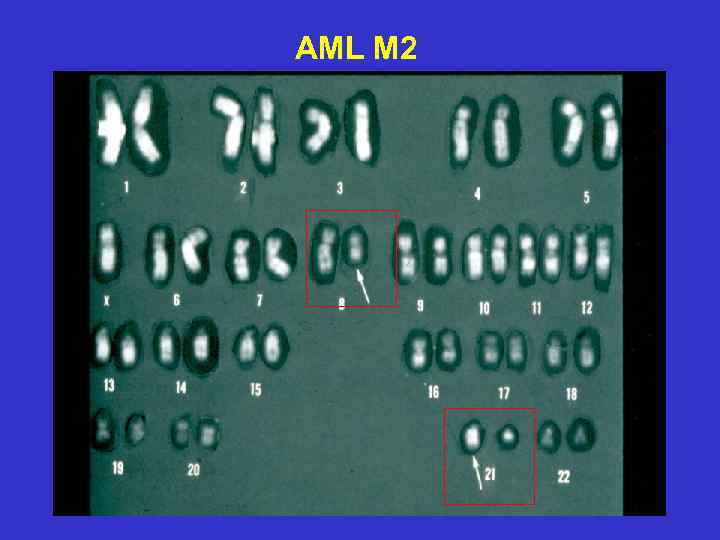 AML M 2
AML M 2
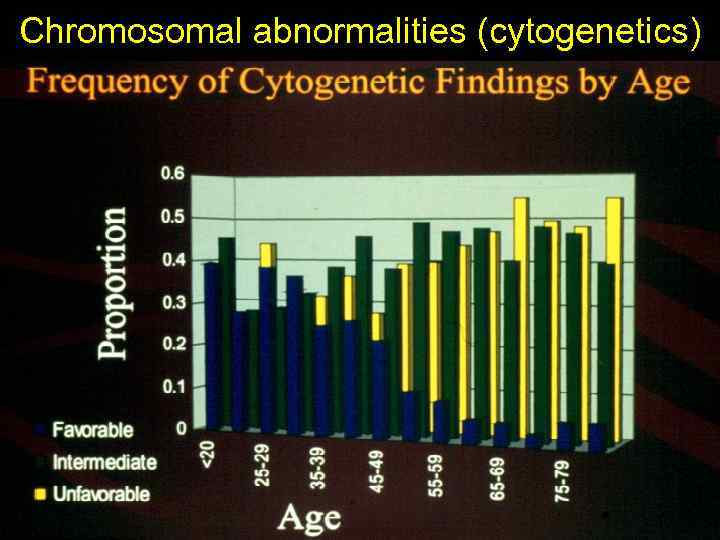 Chromosomal abnormalities (cytogenetics)
Chromosomal abnormalities (cytogenetics)
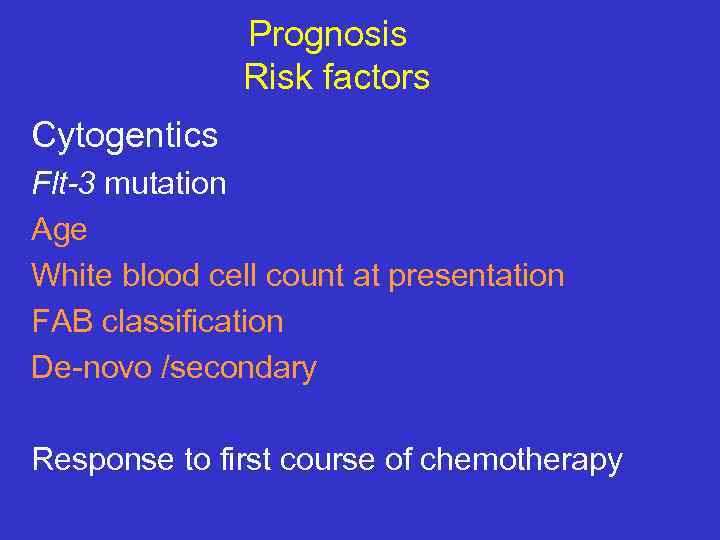 Prognosis Risk factors Cytogentics Flt-3 mutation Age White blood cell count at presentation FAB classification De-novo /secondary Response to first course of chemotherapy
Prognosis Risk factors Cytogentics Flt-3 mutation Age White blood cell count at presentation FAB classification De-novo /secondary Response to first course of chemotherapy
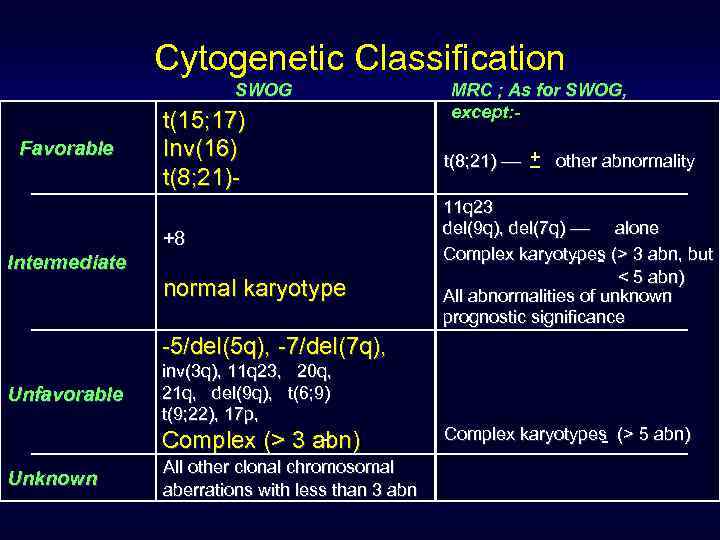 Cytogenetic Classification SWOG Favorable t(15; 17) Inv(16) t(8; 21)+8 Intermediate normal karyotype MRC ; As for SWOG, except: _ t(8; 21) –– + other abnormality 11 q 23 del(9 q), del(7 q) –– alone Complex karyotypes (> 3 abn, but < 5 abn) All abnormalities of unknown prognostic significance -5/del(5 q), -7/del(7 q), Unfavorable inv(3 q), 11 q 23, 20 q, 21 q, del(9 q), t(6; 9) t(9; 22), 17 p, Complex (> 3 abn) Unknown All other clonal chromosomal aberrations with less than 3 abn Complex karyotypes (> 5 abn)
Cytogenetic Classification SWOG Favorable t(15; 17) Inv(16) t(8; 21)+8 Intermediate normal karyotype MRC ; As for SWOG, except: _ t(8; 21) –– + other abnormality 11 q 23 del(9 q), del(7 q) –– alone Complex karyotypes (> 3 abn, but < 5 abn) All abnormalities of unknown prognostic significance -5/del(5 q), -7/del(7 q), Unfavorable inv(3 q), 11 q 23, 20 q, 21 q, del(9 q), t(6; 9) t(9; 22), 17 p, Complex (> 3 abn) Unknown All other clonal chromosomal aberrations with less than 3 abn Complex karyotypes (> 5 abn)
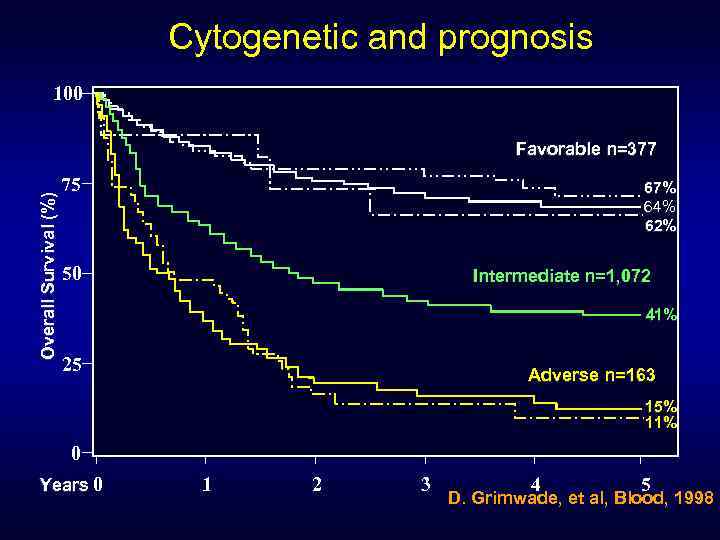 Cytogenetic and prognosis 100 Overall Survival (%) Favorable n=377 75 67% 64% 62% 50 Intermediate n=1, 072 41% 25 Adverse n=163 15% 11% 0 Years 0 1 2 3 4 5 D. Grimwade, et al, Blood, 1998
Cytogenetic and prognosis 100 Overall Survival (%) Favorable n=377 75 67% 64% 62% 50 Intermediate n=1, 072 41% 25 Adverse n=163 15% 11% 0 Years 0 1 2 3 4 5 D. Grimwade, et al, Blood, 1998
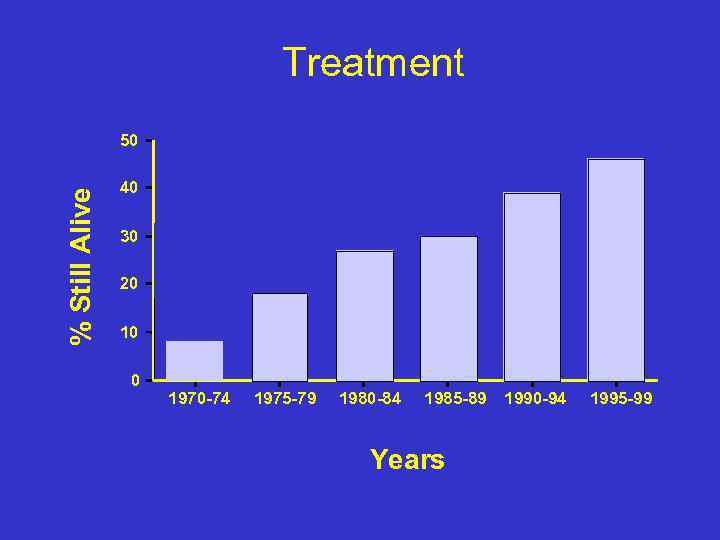 Treatment % Still Alive 50 40 30 20 10 0 1970 -74 1975 -79 1980 -84 1985 -89 Years 1990 -94 1995 -99
Treatment % Still Alive 50 40 30 20 10 0 1970 -74 1975 -79 1980 -84 1985 -89 Years 1990 -94 1995 -99
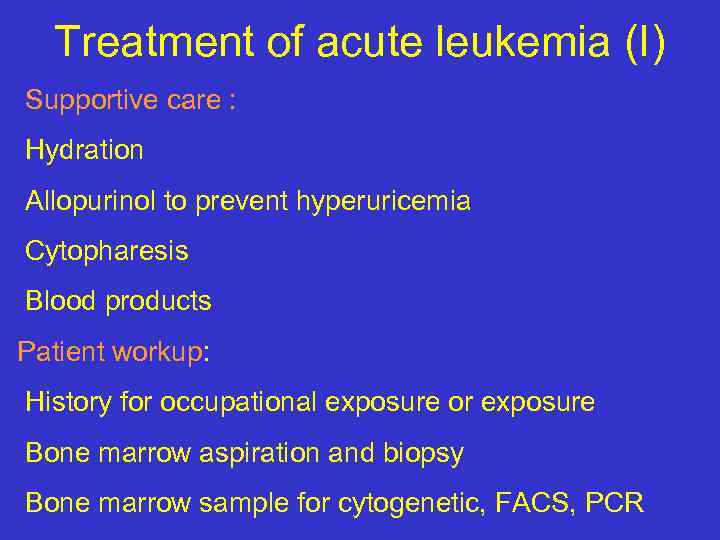 Treatment of acute leukemia (I) Supportive care : Hydration Allopurinol to prevent hyperuricemia Cytopharesis Blood products Patient workup: History for occupational exposure or exposure Bone marrow aspiration and biopsy Bone marrow sample for cytogenetic, FACS, PCR
Treatment of acute leukemia (I) Supportive care : Hydration Allopurinol to prevent hyperuricemia Cytopharesis Blood products Patient workup: History for occupational exposure or exposure Bone marrow aspiration and biopsy Bone marrow sample for cytogenetic, FACS, PCR
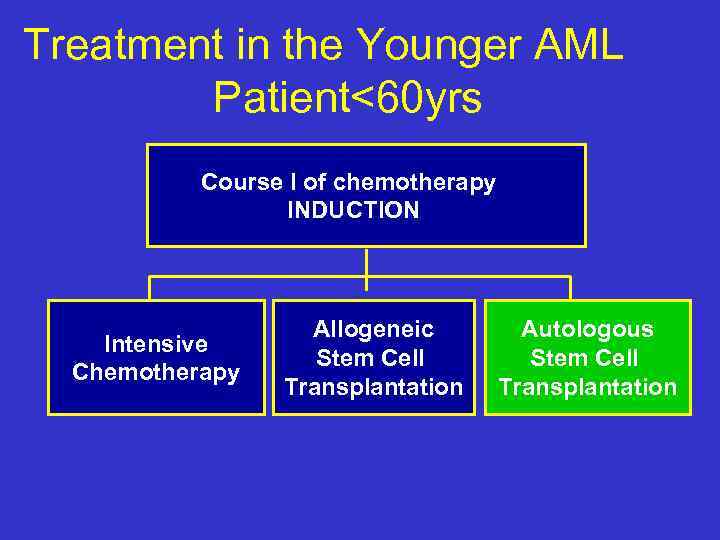 Treatment in the Younger AML Patient<60 yrs Course I of chemotherapy INDUCTION Intensive Chemotherapy Allogeneic Stem Cell Transplantation Autologous Stem Cell Transplantation
Treatment in the Younger AML Patient<60 yrs Course I of chemotherapy INDUCTION Intensive Chemotherapy Allogeneic Stem Cell Transplantation Autologous Stem Cell Transplantation
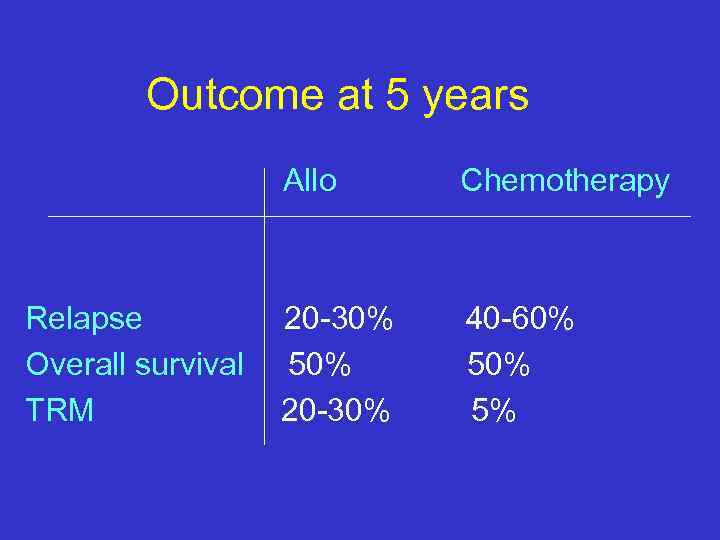 Outcome at 5 years Allo Relapse Overall survival TRM Chemotherapy 20 -30% 50% 20 -30% 40 -60% 5%
Outcome at 5 years Allo Relapse Overall survival TRM Chemotherapy 20 -30% 50% 20 -30% 40 -60% 5%
 So how to choose which therapy to a specific patient? use the prognostic factors to estimate relapse rate and survival
So how to choose which therapy to a specific patient? use the prognostic factors to estimate relapse rate and survival
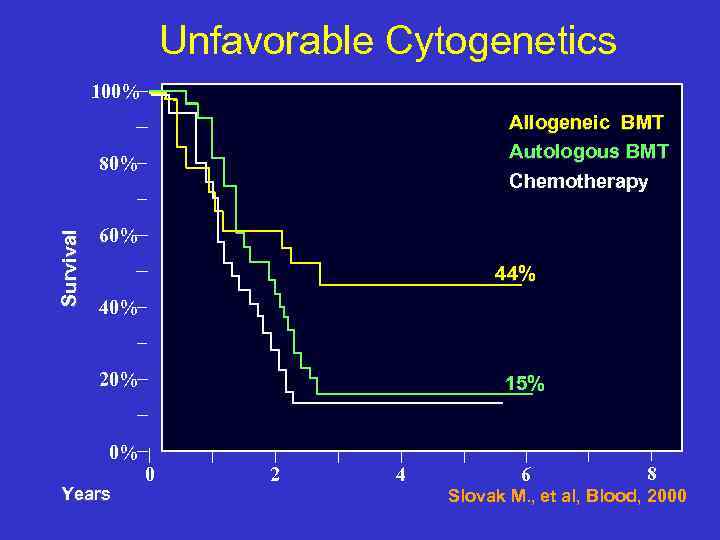 Unfavorable Cytogenetics 100% Allogeneic BMT Autologous BMT Chemotherapy Survival 80% 60% 44% 40% 20% 0% Years 15% 0 2 4 6 8 Slovak M. , et al, Blood, 2000
Unfavorable Cytogenetics 100% Allogeneic BMT Autologous BMT Chemotherapy Survival 80% 60% 44% 40% 20% 0% Years 15% 0 2 4 6 8 Slovak M. , et al, Blood, 2000
 What is the best treatment? Who should have a Patients with poor risk matched related Allo and standard risk younger than SCT ? 35/40 years in CR 1 Patients in CR 2 or beyond Who should have an Favourable/standard risk patients who relapsed, Auto SCT? responded again to chemotherapy and have no matched donor Patients in CR 1 ?
What is the best treatment? Who should have a Patients with poor risk matched related Allo and standard risk younger than SCT ? 35/40 years in CR 1 Patients in CR 2 or beyond Who should have an Favourable/standard risk patients who relapsed, Auto SCT? responded again to chemotherapy and have no matched donor Patients in CR 1 ?
 AML in Elderly patients(>60 years) The majority of the patients are older than 60 Lower remission rate Higher treatment –related morbidity & mortality Very poor outcome higher frequency of poor risk cytogenetics & resistance to chemotherapy
AML in Elderly patients(>60 years) The majority of the patients are older than 60 Lower remission rate Higher treatment –related morbidity & mortality Very poor outcome higher frequency of poor risk cytogenetics & resistance to chemotherapy
 Future directions Identify new prognostic factors New therapies : Modulation of drug resistance Biological, specific treatments: Monoclonal antibodies ATRA in APL, t (15; 17)
Future directions Identify new prognostic factors New therapies : Modulation of drug resistance Biological, specific treatments: Monoclonal antibodies ATRA in APL, t (15; 17)
 Summary The majority of patients still die of their disease (significantly poor outcome in elderly patients) Further improvement is needed: Better ability to predict patients outcome Tailoring treatment to patient’s risk factors Improving therapy & supportive care New strategies for elderly patients
Summary The majority of patients still die of their disease (significantly poor outcome in elderly patients) Further improvement is needed: Better ability to predict patients outcome Tailoring treatment to patient’s risk factors Improving therapy & supportive care New strategies for elderly patients
 Suggested Reading Hoffbrand Hematology Williams Hematology Harrison’s Text book of Internal Medicine תודה
Suggested Reading Hoffbrand Hematology Williams Hematology Harrison’s Text book of Internal Medicine תודה


