02e72d4e4dd135794bece3203037251a.ppt
- Количество слайдов: 99

ACTELION Ltd TALEB Nadia WILLAUME Leslie COUTURIER Sandrine DESQUEMACK Thibaut 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 1

Safe Harbor This is an independent study performed by students from the Faculté des Sciences Pharmaceutiques de Lille The opinions expressed are our own and not necessarily those of Actelion 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 2
SUMMARY 1. 2. 3. 4. 5. 6. 7. 8. 8/02/07 Introduction Bosentan TRACLEER® Miglustat ZAVESCA® Life cycle New drugs on development New concurrents Strategic principle Conclusion S. Couturier; T. Desquemack; N. Taleb; L. Willaume 3
1. Introduction n Founded in 1997 by J-P Clozel and his team research in Allwisch (Switzerland) n Lead product: Bosentan TRACLEER® n Discovery, development, and commercialisation of synthetic small-molecule drugs as innovative treatments to serve High Unmet Medical Needs. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 4
J-P Clozel A. Mueller Widmann 8/02/07 W. Fischli M. Clozel T. S. Couturier; T. Desquemack; N. Taleb; L. Willaume 5
A)Actelion’s genesis: F. Hoffmann-La Roche n Mid-1980: -researches on a substance secreted from the endothelium n 1988: -the substance is identified as « endothelin » 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 6
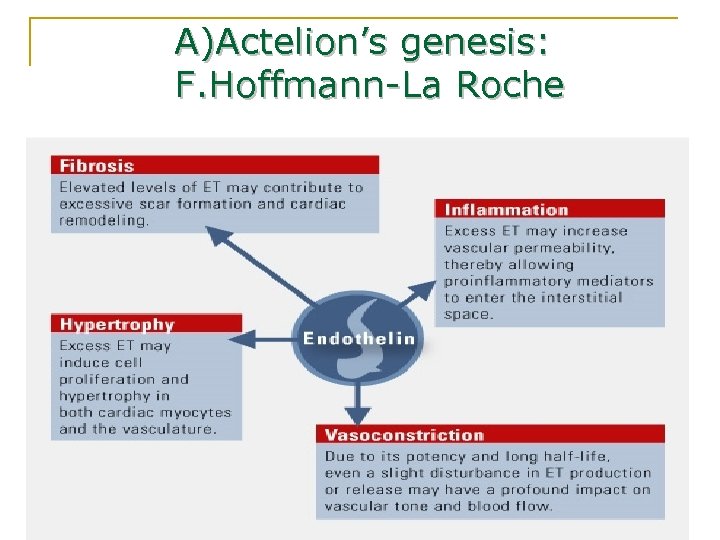
A)Actelion’s genesis: F. Hoffmann-La Roche 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 7
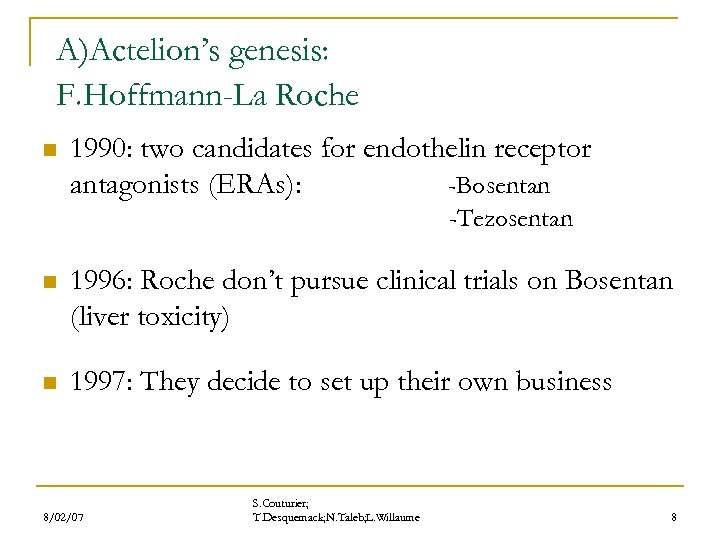
A)Actelion’s genesis: F. Hoffmann-La Roche n 1990: two candidates for endothelin receptor antagonists (ERAs): -Bosentan -Tezosentan n 1996: Roche don’t pursue clinical trials on Bosentan (liver toxicity) n 1997: They decide to set up their own business 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 8

A)Actelion’s genesis: F. Hoffmann-La Roche n 1998: The Group enter into license agreements with F. Hoffmann-La Roche for tezosentan & bosentan… 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 9

2. Bosentan TRACLEER 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 10
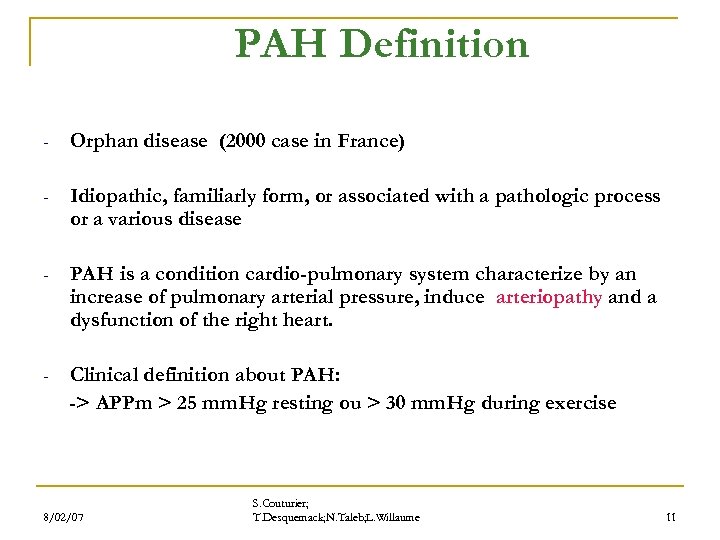
PAH Definition - Orphan disease (2000 case in France) - Idiopathic, familiarly form, or associated with a pathologic process or a various disease - PAH is a condition cardio-pulmonary system characterize by an increase of pulmonary arterial pressure, induce arteriopathy and a dysfunction of the right heart. - Clinical definition about PAH: -> APPm > 25 mm. Hg resting ou > 30 mm. Hg during exercise 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 11
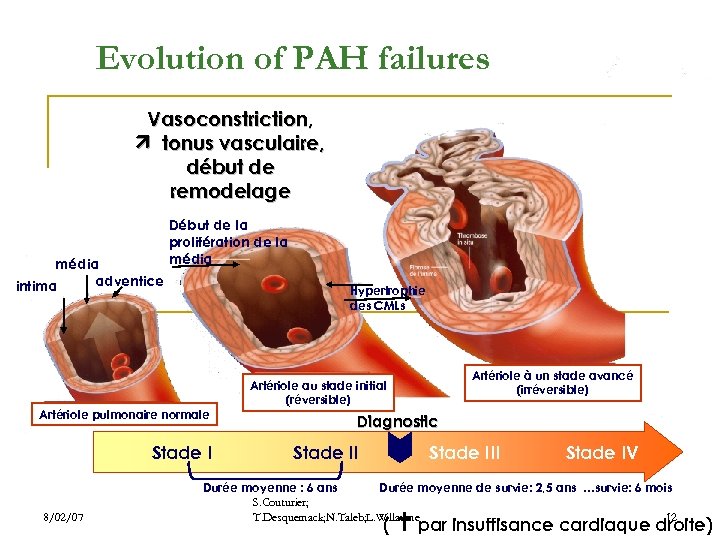
Evolution of PAH failures Vasoconstriction, tonus vasculaire, début de remodelage média adventice intima Début de la prolifération de la média Hypertrophie des CMLs Artériole pulmonaire normale Stade I 8/02/07 Artériole à un stade avancé (irréversible) Artériole au stade initial (réversible) Diagnostic Stade III Stade IV Durée moyenne : 6 ans Durée moyenne de survie: 2, 5 ans …survie: 6 mois S. Couturier; T. Desquemack; N. Taleb; L. Willaume 12 ( par insuffisance cardiaque droite)
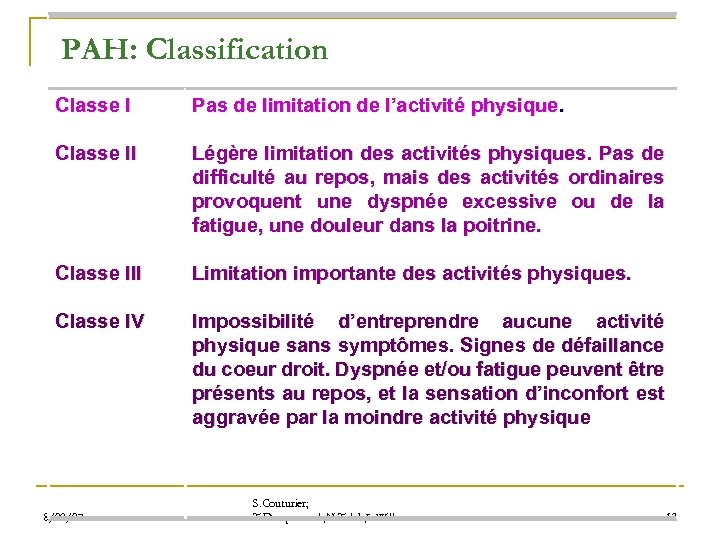
PAH: Classification Classe I Pas de limitation de l’activité physique. Classe II Légère limitation des activités physiques. Pas de difficulté au repos, mais des activités ordinaires provoquent une dyspnée excessive ou de la fatigue, une douleur dans la poitrine. Classe III Limitation importante des activités physiques. Classe IV Impossibilité d’entreprendre aucune activité physique sans symptômes. Signes de défaillance du coeur droit. Dyspnée et/ou fatigue peuvent être présents au repos, et la sensation d’inconfort est aggravée par la moindre activité physique 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 13
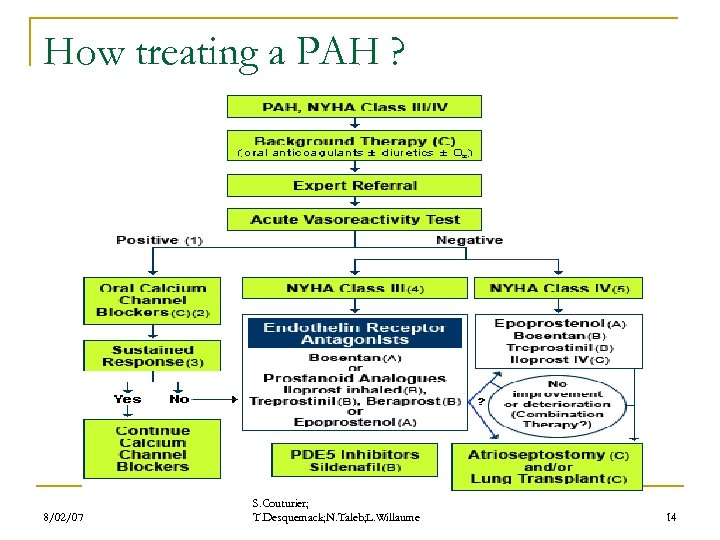
How treating a PAH ? 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 14
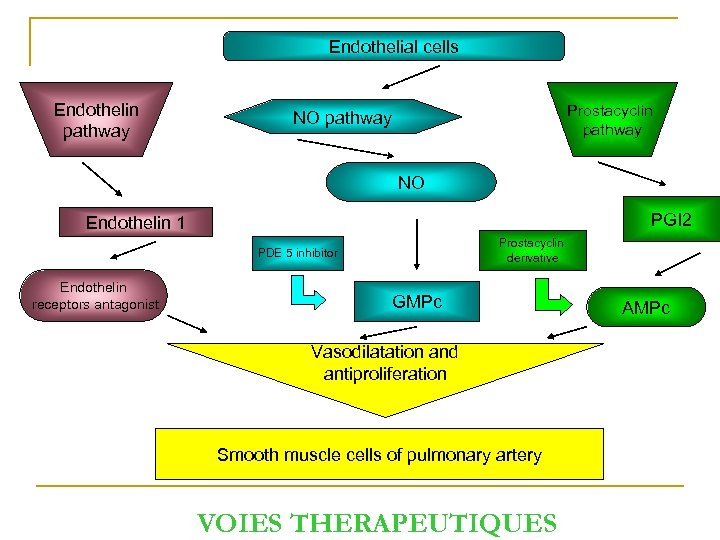
Endothelial cells Endothelin pathway Prostacyclin pathway NO PGI 2 Endothelin 1 Prostacyclin derivative PDE 5 inhibitor Endothelin receptors antagonist GMPc Vasodilatation and antiproliferation Smooth muscle cells of pulmonary artery VOIES THERAPEUTIQUES AMPc
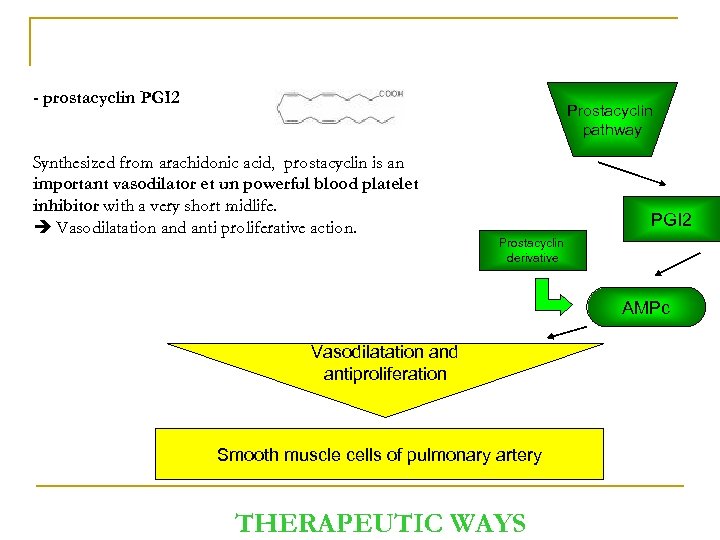
- prostacyclin PGI 2 Prostacyclin pathway Synthesized from arachidonic acid, prostacyclin is an important vasodilator et un powerful blood platelet inhibitor with a very short midlife. Vasodilatation and anti proliferative action. PGI 2 Prostacyclin derivative AMPc Vasodilatation and antiproliferation Smooth muscle cells of pulmonary artery THERAPEUTIC WAYS
epoprostenol FLOLAN® n Intraveinous prostacyclin correspondant n Old PAH , used in intraveinous perfusion SMR: Important. 1 st or 2 nd intention ASMR: Level II Low impact on quality of life and mortality Cost 75 000 $/ year n n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 17

8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 18

8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 19

8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 20

8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 21

8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 22
treponistil REMODULIN® n n n Subcutaneous prostacyclin correspondant. Indicated PAH III SMR: gentle, 1 st ou 2 nd intention. ASMR: Level II Cost: 45 000 $ an 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 23
iloprost VENTAVIS® n n n n Inhaled prostacyclin correspondant HAP class III SMR: Gentle. Symtomatic treatment. ASMR: Level II Posology: 6 à 9 nébulisations/day Cost 3100€/year in France( drug only) January 2007: Actelion bought Cotherix, producer an manufacturer of l’iloprost in US 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 24

The Prodose AAD® System 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 25
Bosentan (Tracleer®) 1 st antagonist mixt of endothelin receptors A and B - AMM : the 15 th of May, 2002 (for 2 dosages) - - - Orally treatment for PAH, ASMR level I Improve efforts tolerance and symptom's patient Side effects: = Hépatotoxicity = Hematologic toxicity = teratogenic - Cost: 33000 euros / year 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 26
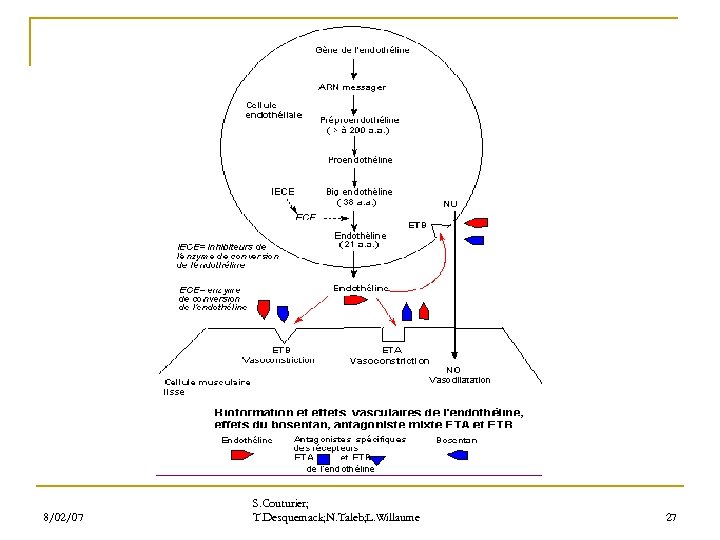
8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 27
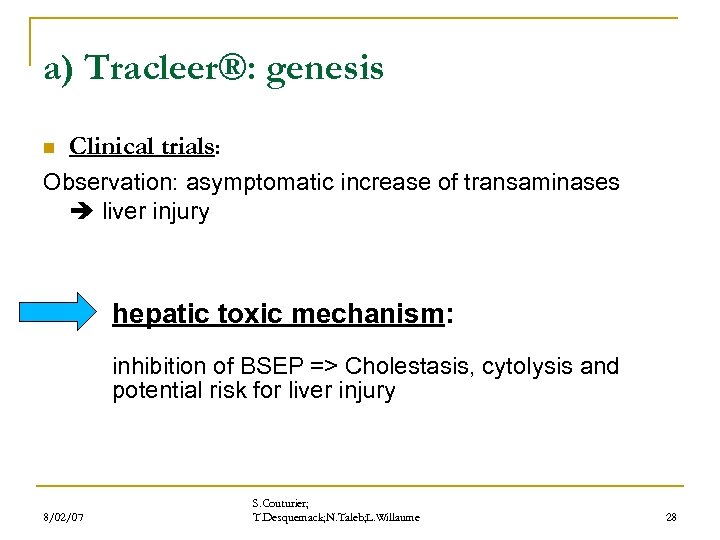
a) Tracleer®: genesis n Clinical trials: Observation: asymptomatic increase of transaminases liver injury hepatic toxic mechanism: inhibition of BSEP => Cholestasis, cytolysis and potential risk for liver injury 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 28
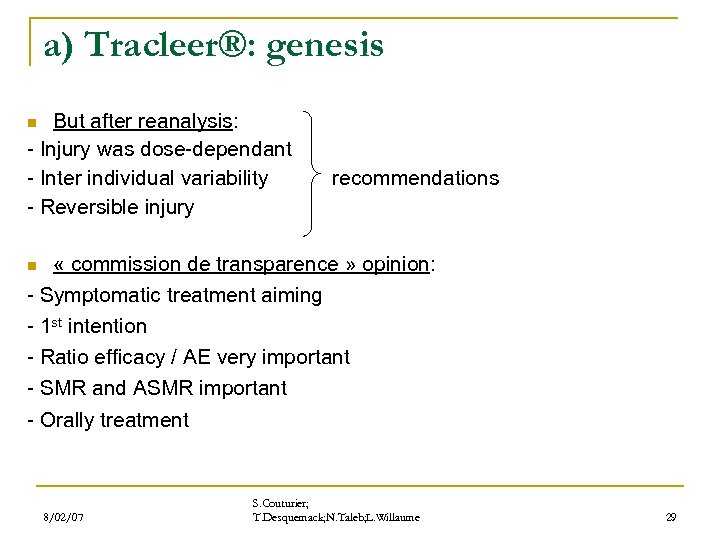
a) Tracleer®: genesis But after reanalysis: - Injury was dose-dependant - Inter individual variability recommendations - Reversible injury n « commission de transparence » opinion: - Symptomatic treatment aiming - 1 st intention - Ratio efficacy / AE very important - SMR and ASMR important - Orally treatment n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 29

a)Tracleer®: strategy n 3 endpoints for the marketing strategy: - First and only orally active drug approved for PAH - Established as the cornerstone therapy of PAH with both symptomatic benefit and long term experience - Efficacy and well documented Tracleer® efficiency and safety profile with more than 4 years experience on the market 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 30

a)Tracleer®: strategy n Continue to gain market share in PAH - Maintain the existing customer base against new competitors - Extend the overall market by finding untreated patient and investigating new indications n Marketing initiatives - Upstream approach of targeted prescriptors - Development of clinical trials with competitor’s drugs 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 31
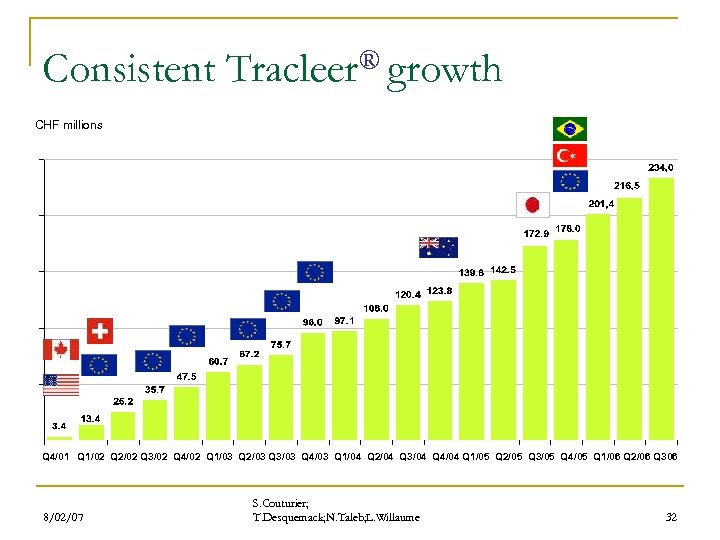
Consistent Tracleer® growth CHF millions Q 4/01 Q 1/02 Q 2/02 Q 3/02 Q 4/02 Q 1/03 Q 2/03 Q 3/03 Q 4/03 Q 1/04 Q 2/04 Q 3/04 Q 4/04 Q 1/05 Q 2/05 Q 3/05 Q 4/05 Q 1/06 Q 2/06 Q 306 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 32

3. Miglustat ZAVESCA® 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 33
Products & Company Strategy ZAVESCA® -Type 1 Gaucher n Miglustat n 2 nd marketed product (2003) n 2002: licensed from Oxford Glyco. Sciences n Treatment of mild to moderate type 1 Gaucher patients for whom ERT is unsuitable 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 34
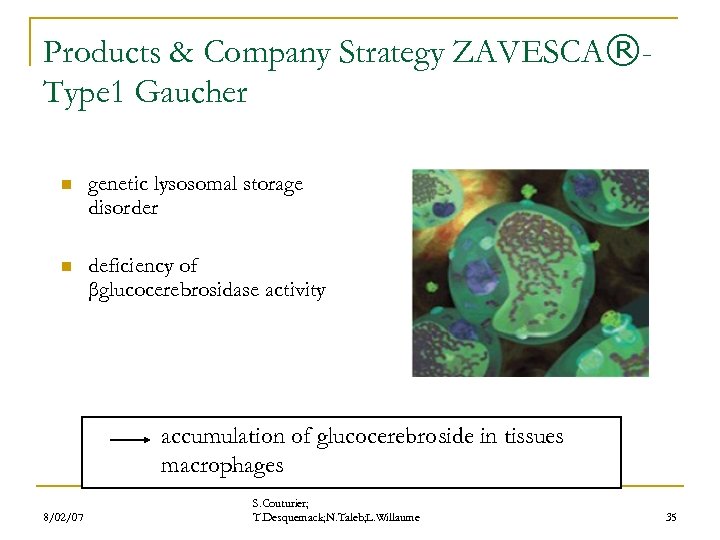
Products & Company Strategy ZAVESCA®Type 1 Gaucher n genetic lysosomal storage disorder n deficiency of βglucocerebrosidase activity accumulation of glucocerebroside in tissues macrophages 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 35
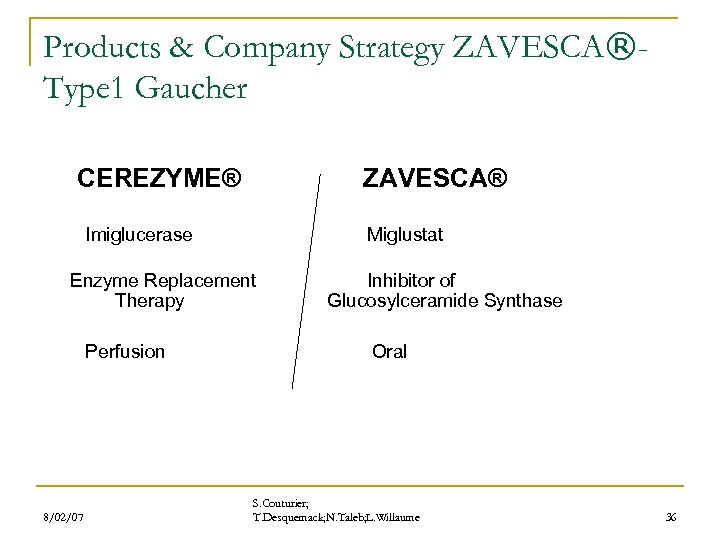
Products & Company Strategy ZAVESCA®Type 1 Gaucher CEREZYME® ZAVESCA® Imiglucerase Miglustat Enzyme Replacement Inhibitor of Therapy Glucosylceramide Synthase Perfusion Oral 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 36
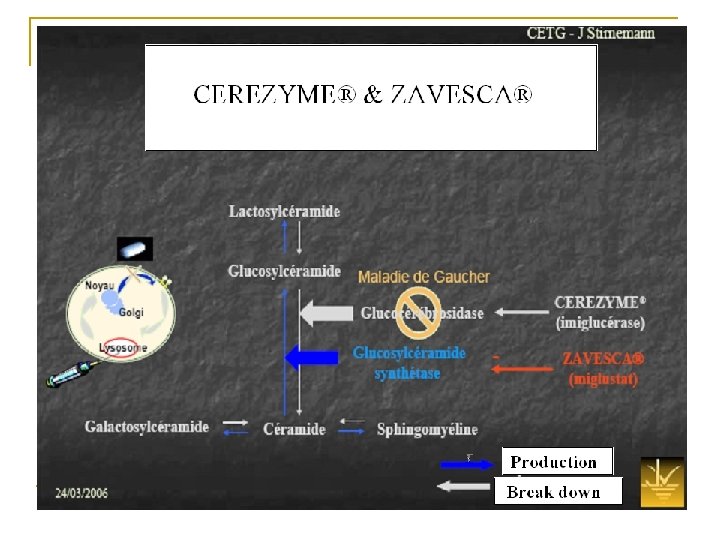
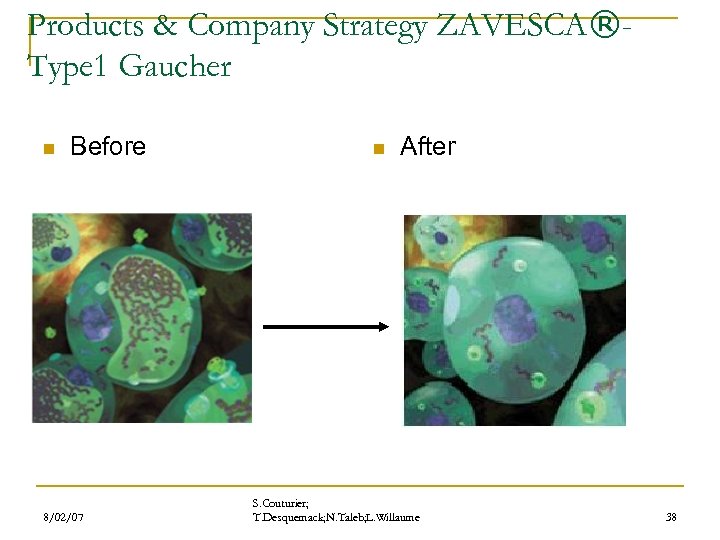
Products & Company Strategy ZAVESCA®Type 1 Gaucher n Before 8/02/07 n After S. Couturier; T. Desquemack; N. Taleb; L. Willaume 38
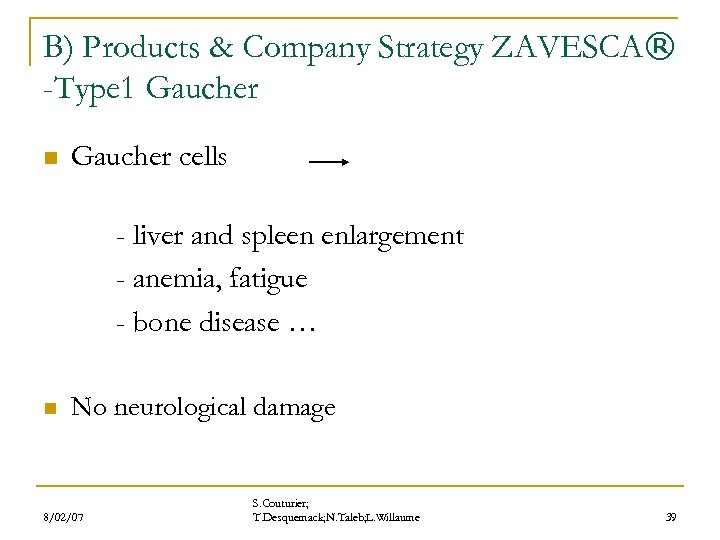
B) Products & Company Strategy ZAVESCA® -Type 1 Gaucher n Gaucher cells - liver and spleen enlargement - anemia, fatigue - bone disease … n No neurological damage 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 39

B) Products & Company Strategy ZAVESCA® -Type 1 Gaucher n Ethnical predilection: Ashkenazi Jewish people n Incidence: 1/40 000 Ashkenazi Jewish : 1/450 -1/2 500 (US NY: Manhattan Beach , Israël, Occidental Europe) n Prevalence: 1/106 000 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 40

B) Products & Company Strategy ZAVESCA® -Strategy n The First & Only Oral Substrate Reduction Therapy 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 41

B) Products & Company Strategy ZAVESCA® -Strategy n Important SMR n Patients not willing or able to undergo ERT Increased market shares in type 1 Gaucher disease 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 42
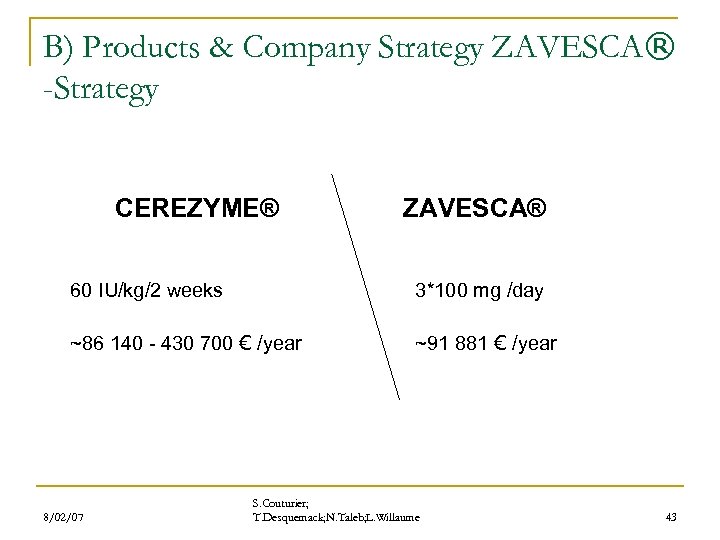
B) Products & Company Strategy ZAVESCA® -Strategy CEREZYME® ZAVESCA® 60 IU/kg/2 weeks ~86 140 - 430 700 € /year 8/02/07 3*100 mg /day ~91 881 € /year S. Couturier; T. Desquemack; N. Taleb; L. Willaume 43

B) Products & Company Strategy ZAVESCA® -Strategy n Extension of the license agreement Cell Tech / Actelion n Full responsability for the management of all clinical trials n License granted to TEVA for Israël 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 44

B) Products & Company Strategy ZAVESCA® -Strategy n Geographical expansion (36 countries) Proven benefits that continue to build through at least 2 years of therapy n Growth potential: n (cross the Blood-Brain Barrier) -Type 3 Gaucher disease Niemann-Pick type C Late Onset Tay-Sachs disease n Phase 3 MAINTENANCE study in type 1 Gaucher disease - 45

Research and developement 4. Life cycle 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 46
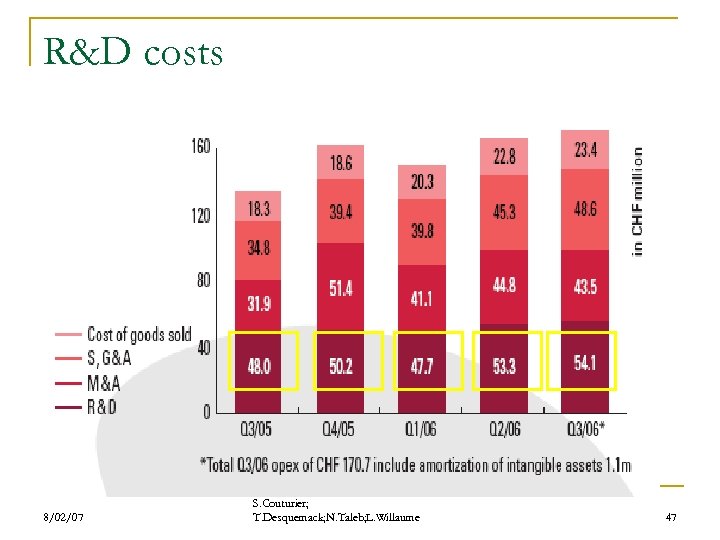
R&D costs 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 47
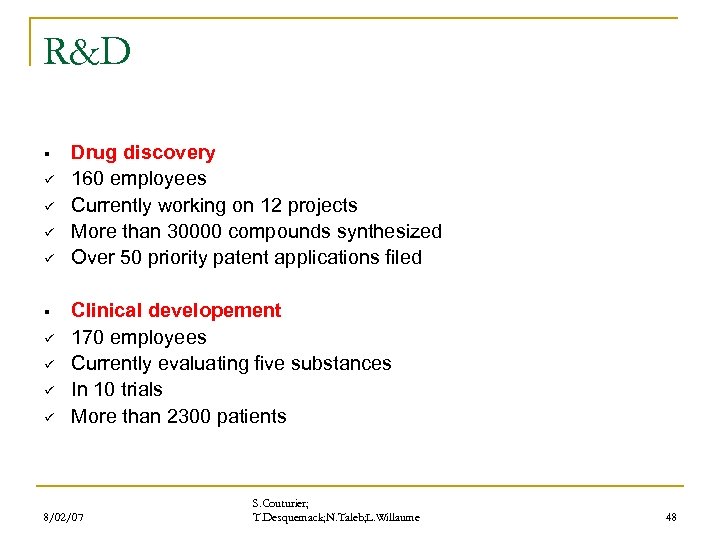
R&D § ü ü ü ü Drug discovery 160 employees Currently working on 12 projects More than 30000 compounds synthesized Over 50 priority patent applications filed Clinical developement 170 employees Currently evaluating five substances In 10 trials More than 2300 patients 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 48

R&D strategy n n n Maximize TRACLEER and ZAVESCA indications. Trying to develop new drugs. Partnership with Big Pharma to develop potential blockbusters. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 49
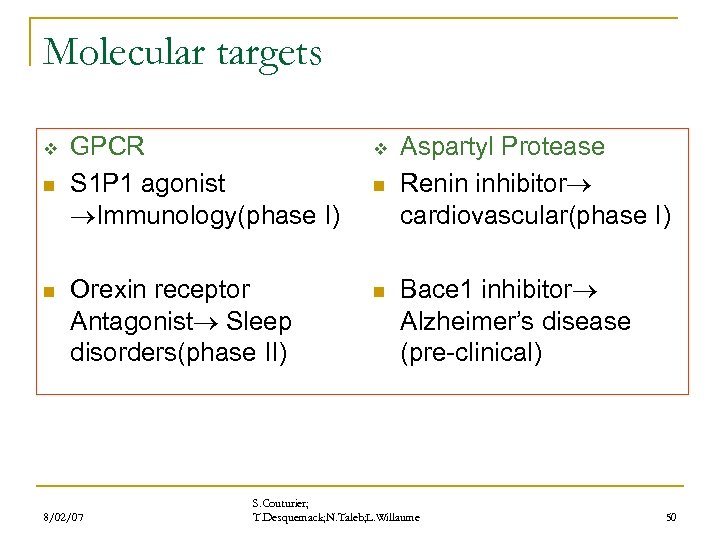
Molecular targets v n n GPCR S 1 P 1 agonist Immunology(phase I) v Orexin receptor Antagonist Sleep disorders(phase II) n 8/02/07 n Aspartyl Protease Renin inhibitor cardiovascular(phase I) Bace 1 inhibitor Alzheimer’s disease (pre-clinical) S. Couturier; T. Desquemack; N. Taleb; L. Willaume 50
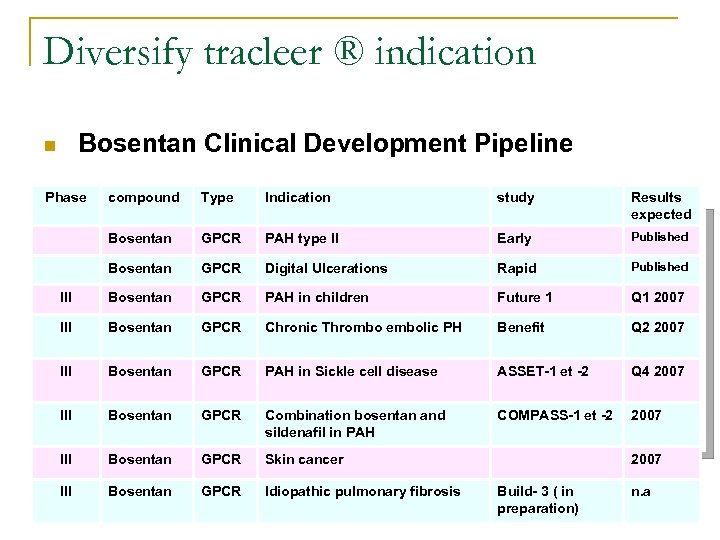
Diversify tracleer ® indication Bosentan Clinical Development Pipeline n Phase compound Type Indication study Results expected Bosentan GPCR PAH type II Early Published Bosentan GPCR Digital Ulcerations Rapid Published III Bosentan GPCR PAH in children Future 1 Q 1 2007 III Bosentan GPCR Chronic Thrombo embolic PH Benefit Q 2 2007 III Bosentan GPCR PAH in Sickle cell disease ASSET-1 et -2 Q 4 2007 III Bosentan GPCR Combination bosentan and sildenafil in PAH COMPASS-1 et -2 2007 III Bosentan GPCR Skin cancer III Bosentan GPCR 8/02/07 Idiopathic pulmonary fibrosis S. Couturier; T. Desquemack; N. Taleb; L. Willaume 2007 Build- 3 ( in preparation) n. a 51
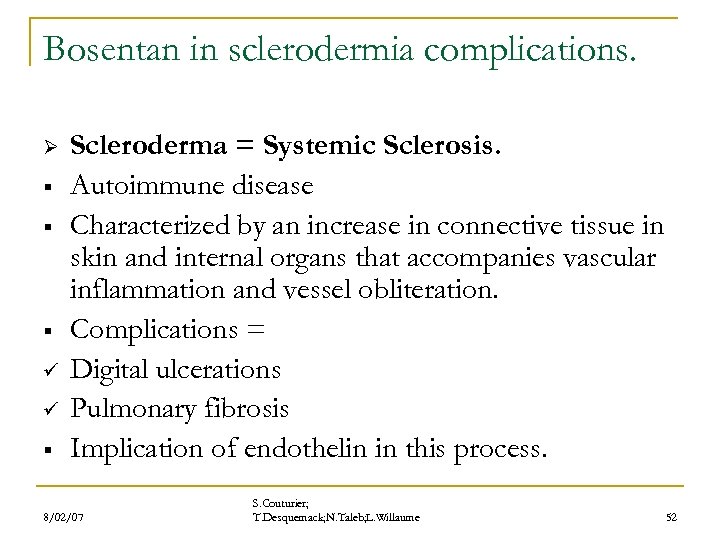
Bosentan in sclerodermia complications. Ø § § § ü ü § Scleroderma = Systemic Sclerosis. Autoimmune disease Characterized by an increase in connective tissue in skin and internal organs that accompanies vascular inflammation and vessel obliteration. Complications = Digital ulcerations Pulmonary fibrosis Implication of endothelin in this process. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 52
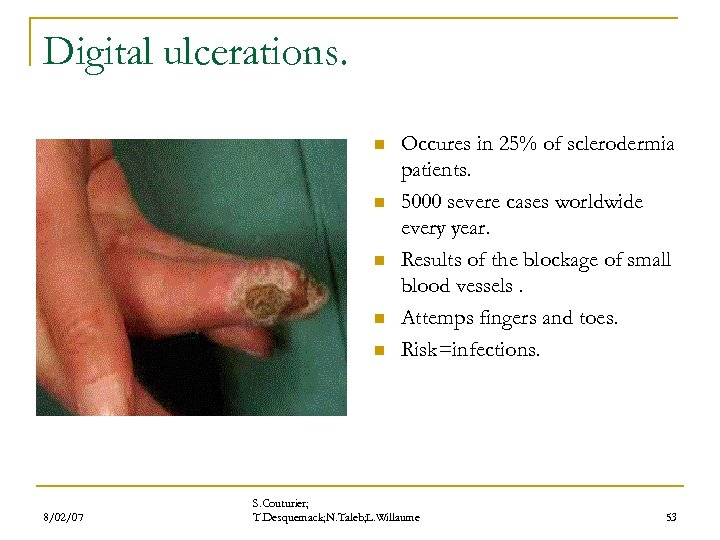
Digital ulcerations. n n n 8/02/07 Occures in 25% of sclerodermia patients. 5000 severe cases worldwide every year. Results of the blockage of small blood vessels. Attemps fingers and toes. Risk=infections. S. Couturier; T. Desquemack; N. Taleb; L. Willaume 53
Clinical developpement of bosentan in digital ulceration. 2002: RAPIDS 1=first study with bosentan in patients with digital ulcerations(DUs). Results: Bosentan prevented the occurrence of new Dus in patients with scleroderma. Safety and well tolerability of Bosentan. Ø 8/02/07 2003: RAPIDS 2 = Examined the effects of bosentan on the prevention and healing of Dus in patients with active DUs. Results : Significant reduction in the occurrence of new DUs during the 6 months treatment period. Ø S. Couturier; T. Desquemack; N. Taleb; L. Willaume 54
Bosentan and skin cancer. n § § Several pre clinical experiments have shown that endothelin plays a role in proliferation and growth of cancerous cells, particulary melanocytes. IN vitro & in vivo pre-clinical findings: findings UVB- induced melanin synthesis is ET-mediated Presence of ET receptors on many tumor cells, especially melanoma ET-1 is a growth factor for human melanocytes ETB activation(in vitro) promotes proliferation and inhibits differenciation of melanoma cells Effects of bosentan in melanoma? 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 55
Bosentan in melanoma n 2003: Actelion initiated a study with bosentan in patients with late stage metastatic melanoma evaluating tumor response and survival. n Safety analysis bosentan is well tolerated even at doses four times higher than what is prescribed today in PAH. Results of phase II expected in 2007. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 56
Bosentan in sickle cell disease(SCD). Inherited disease of red blood cells. n Characterized by: pain episodes, anemia, serious infections and damage to vital organs. n Cause = abnormal hemoglobin forming stiff. n Complication=PAH. n Clinical trial=ASSET 1 -2. evaluation of safety and efficacy of Tracleer® in PAH secondary to SCD. Exploration of Tracleer®’s potential to reduce the frequency of sickle crises for which endothelin is believed to play a central role. n Results expected in 2007. n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 57
SCD 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 58
Tracleer® and type II PAH. n Etudes Early To demonstrate that bosentan improves cardiac hemodynamics and, as subordinate, exercise capacity in mildly symptomatic PAH patients( NYHA II). Significant reduction in pulmonary vascular resistance Strong trend towards improvement in 6 MWT Significant delay in Time to Clinical Worsening. n Regulatory submissions for label expansion n n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 59

ZAVESCA in development n Based on its unique mode of action Zavesca®, approved for type 1 Gaucher disease, could potentially benefit patients with other lipid storage diseases. n Three clinical trials were initiated, evaluating Zavesca in: ü Type 3 Gaucher disease Niemann-Pick Type C disease Late Onset Tay Sachs disease ü ü =lysosomal storage disorders with predominant neurological manifestations Actually in phase III clinical trial. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 60
Niemann-Pick disease. n n n 8/02/07 Caused by mutations in the gene encoding the NPC 1 protein. results from a defect in cholesterol trafficking Characterized by accumulated glycolipids in the brain and raised level of cholesterol in the liver and spleen. S. Couturier; T. Desquemack; N. Taleb; L. Willaume 61
Tay-Sachs disease. n n n Inherited disorder in which nerve cells deteriorate and eventually die. Caused by the malfunction of a protein called hexosaminidase A (HEXA). Normally HEXA is found in the lysosomes of cells, specifically nerve cells. HEXA: breaks down GM 2 ganglioside needed for making nerve cell membran TS disease GM 2 ganglioside molecules can’t be broken down and they accumulate in lysosome. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 62
Research an development 5. Discover new drugs Innovation. Four field of research: • Immunology • Cardiovascular • CNS • Antibacterials 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 63
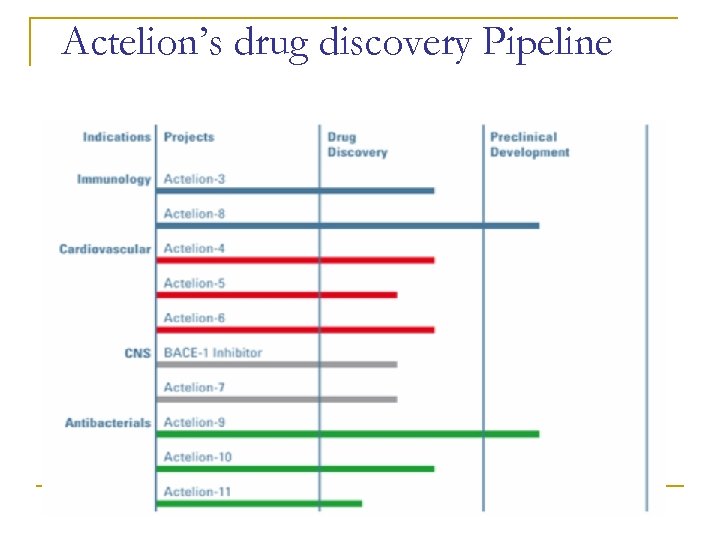
Actelion’s drug discovery Pipeline
Partnership in R&D. Renin inhibitor n Potential breakthrough in cardiovascular care Selective S 1 P 1 agonist n Potential breakthrough in autoimmune disorders 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 65
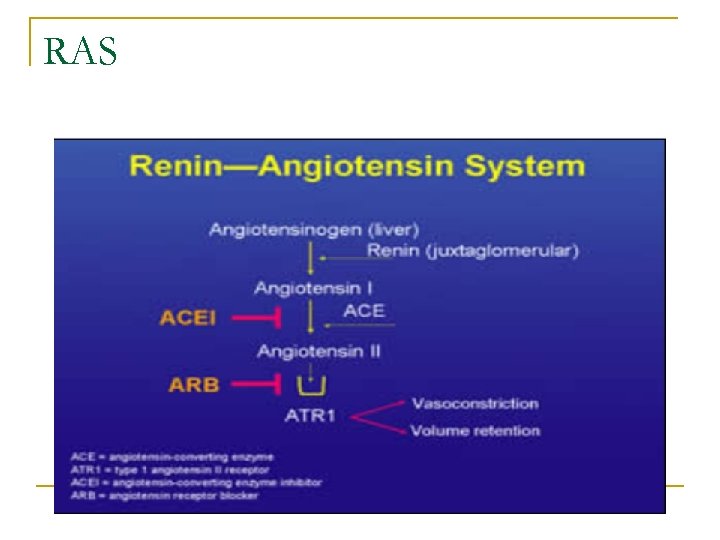
RAS
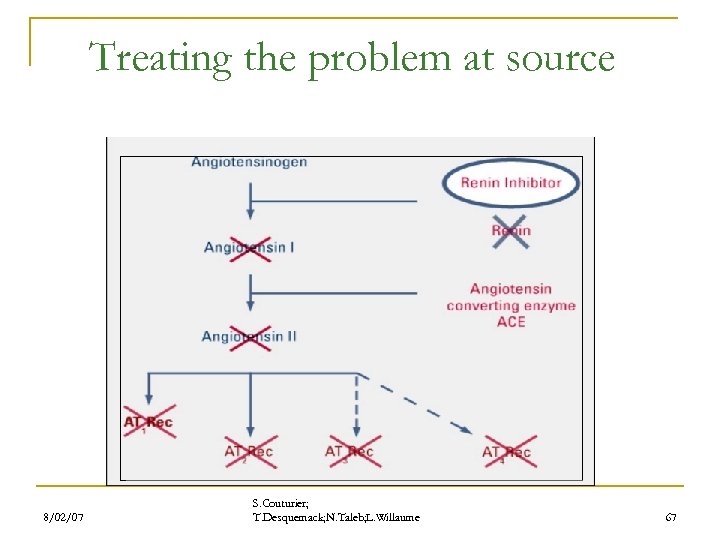
Treating the problem at source 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 67

Renin inhibitor in development. Major pharmaceutical companies have succeeded in finding prototypical renin inhibitors. problem=poor oral bioavailability. in the mid 1990 s, efforts of research was abandoned. Þ December 2003 : Actelion and Merck formed an exclusive worldwide alliance n n To discover To develop To market new classes of orally available renin inhibitors. n 8/02/07 Actually no renin inhibitor are marketed. S. Couturier; T. Desquemack; N. Taleb; L. Willaume 68
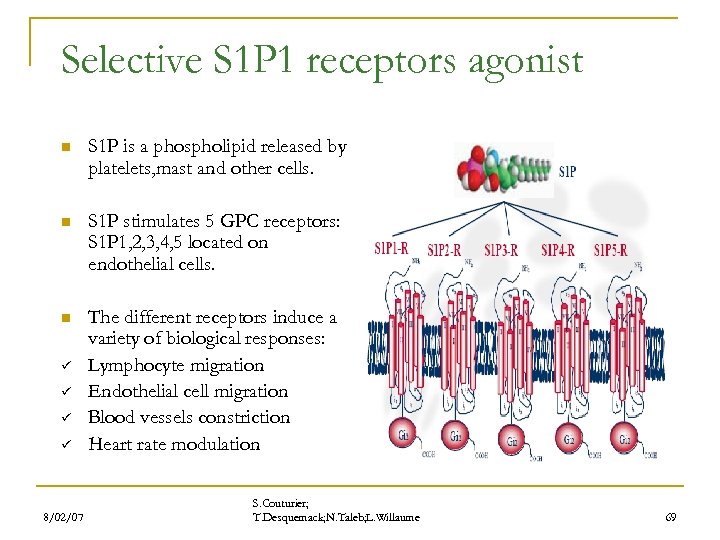
Selective S 1 P 1 receptors agonist n S 1 P is a phospholipid released by platelets, mast and other cells. n S 1 P stimulates 5 GPC receptors: S 1 P 1, 2, 3, 4, 5 located on endothelial cells. n The different receptors induce a variety of biological responses: Lymphocyte migration Endothelial cell migration Blood vessels constriction Heart rate modulation ü ü 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 69
Selective S 1 P 1 receptors agonist n S 1 P receptor modulators inhibit the egress and recirculation of lymphocytes from lymphe nodes. New therapeutic strategy for autoimmune disorders such as: ü Psoriasis ü Rheumatoid arthritis ü Rejection of transplantated organs 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 70
S 1 P 1 agonist in developement. Actelion started in 1999 to work on S 1 P 1 because of its localization on the endothelium. n 2005: selection of current S 1 P 1 agonist for full preclinical developement: Selective S 1 P 1 agonist: ü Is effective for reducing lymphocyte trafficking. ü Is efficacious in autoimmune disease model. ü Have a potential for rapid onset of action and rapid reversibility. n 2006: entry into man Phase I ongoing. n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 71
6. New competitors 6. 1 phosphodiesterase inhibitors 6. 2 Sentans in development 6. 3 Pipeline competition 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 72
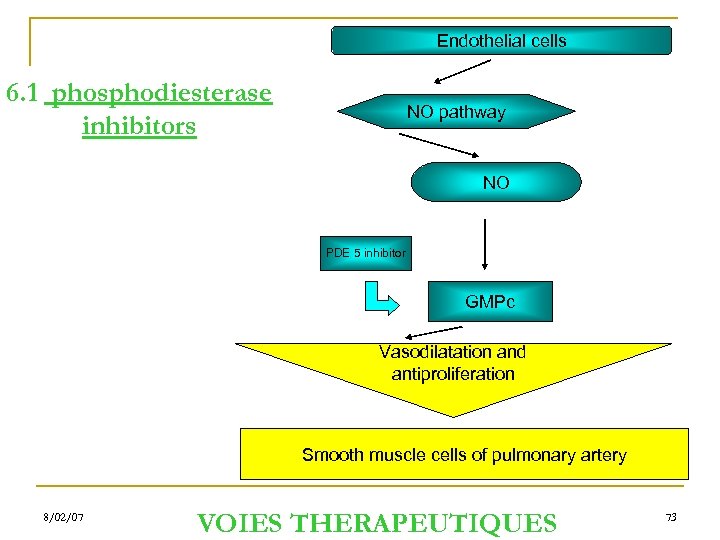
Endothelial cells 6. 1 phosphodiesterase inhibitors NO pathway NO PDE 5 inhibitor GMPc Vasodilatation and antiproliferation Smooth muscle cells of pulmonary artery 8/02/07 VOIES THERAPEUTIQUES 73
6. 1. 1 Action mode n n 11 ilk of PDE 5 localised from cavernous tissue, vascular muscle cells and blood platelet. Leader of line: Sildenafil VIAGRA® erectile dysfonction mountain sickness (off label) REVATIO® HAP 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 74
6. 1. 2 Sildenafil REVATIO® n n n PDE 5 inhibitor per os HAP level III SMR: Important. ASMR: level I Used in 2 nd intention after failure of TRACLEER in association with bosentan and prostacycline for severe form( European Respiratory Society) Cost US 10 000 $ year 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 75
6. 1. 2 Sildenafil REVATIO® n n Comparaison of Sildenafil, Vardenafil, Tadalafil on PAH Vardenafilthe most rapid but lack of sensivity : Tadalafilthe most long-lasting but lack of sensivity : Only sildenafil improve significaly the arterial oxygenation 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 76
6. 1. 2 Sildenafil REVATIO® n n Bosentan and sildenafil combination: 2 phase III studies. Compass 1: will evaluate the hemodynamic effects of sildenafil versus placebo in PAH patients treated with Tracleer®. Compass 2: will examine the morbidity/mortality outcome of the combination Tracleer® and sildenafil versus sildenafil in monotherapy Results expected for 2007 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 77

6. New competitor 6. 2: SENTANS IN DEVELOPMENT. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 78
6. 2. 1 sitaxentan THELIN® n n n Encysive pharmaceuticals Selective inhibitor type A endothelin receptor. (only on muscle cell of vascular wall) Once a day Even efficacy and less hépatotoxic than TRACLEER ( STRIDE- 2 study) more practice UK marketing autorisation for the treatment of patients with pulmonary arterial hypertension classified 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 79
6. 2. 2 ambrisentan n n MYOGEN laboratory( buyed buy GILEAD) Inhibitor of endothelin type A receptor 2 study in phase III(ARIES 1 and ARIES 2: ambrisentan Vs placebo) Only abstract available: « ambrisentan improved exercise capacity, delayed clinical worsening and imroved symptoms of patient with PAH. Ambrisentan was well tolerated and was not associated with any clinically significant serum aminotransferase abnormalities. » Deposed Market authorization on FDA (19/12/2006) 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 80
6 New competitor 6. 3 Pipeline competition 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 81
6. 3 pipeline competition: Renin inhibitors. n n n Actelion/ merck : Renin alliance develop a new renin inhibitor No data available. Novartis aliskiren( TEKTURNA® ) Groups who take d’aliskiren a day, with a losartan shown a decrease TA. (p<0, 05). Marketing authorization was demanded on FDA and EMEA at the end of 2006 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 82

7. The strategy of the company 7. 1/ Follow innovation where it leads 7. 2/ Retain the value of innovation 7. 3/ Excel in sales & marketing 7. 4/ Financial results 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 83
7. 1 Follow innovation where it leads n Identify and balance -Promising and innovative scientific concepts -Unmet medical needs -Appropriate indications -Commercial potential 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 84
7. 2 Retain the value of innovation • Develop projects as far as possible itself -design and execute its own clinical trials -optimize the life-cycles of existing products as well as to manage the portfolio of new compounds reaching development 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 85
7. 2 Retain the value of innovation n Evaluate external opportunities (partnerships, acquisitions, …) To maximize the value of its products 2003: -Alliance with Merck in renin inhibitors -Acquisition of Axovan 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 86
7. 2 Retain the value of innovation 2006: -Alliance with Roche in S 1 P 1 receptors agonists -Acquisition of US-based Co. Therix, Inc Strategic or financial benefit Complement in-house R&D (+ 2004: Sale of Hesperion) 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 87

7. 3 Excel in sales & marketing n Strategic priorities: - To grow and defend current top line revenues - To bring one or two innovative new products to the market in the short term - To continue to innovate through substantial R&D 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 88
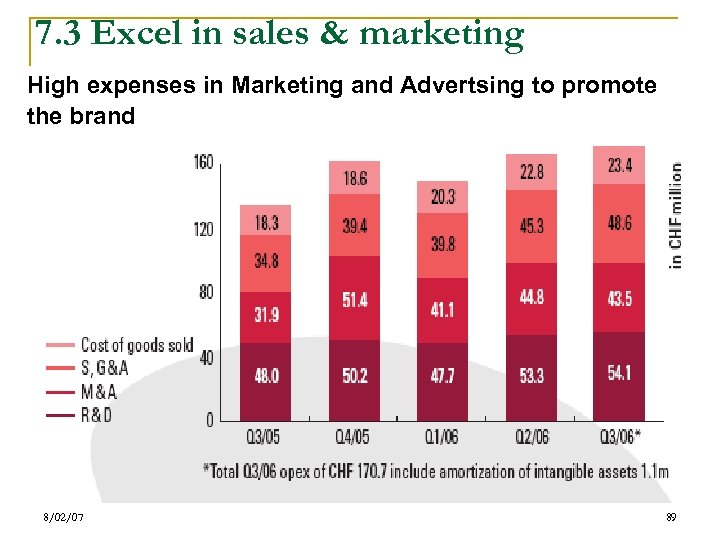
7. 3 Excel in sales & marketing High expenses in Marketing and Advertsing to promote the brand 8/02/07 89
7. 3 Excel in sales & marketing • An international presence. . . 21 countries Opening new markets local market condition q q q • . . . supported by an efficient commercial team -585 professionals 45% -Expert in market -Expert in medical market -A life cycle team New decision can be implemented rapidly and effectively on local markets 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 90

7. 3 Excel in sales & marketing n Opened up new business opportunities in a niche unoccupied by « Big Pharma » - with orphan drugs lot of advantages justify the investment n Continue it innovate, through substantial R&D - New mechanism for treating Alzheimer disease • Evaluate external opportunities - S 1 P 1 Rennin inhibitors - 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 91
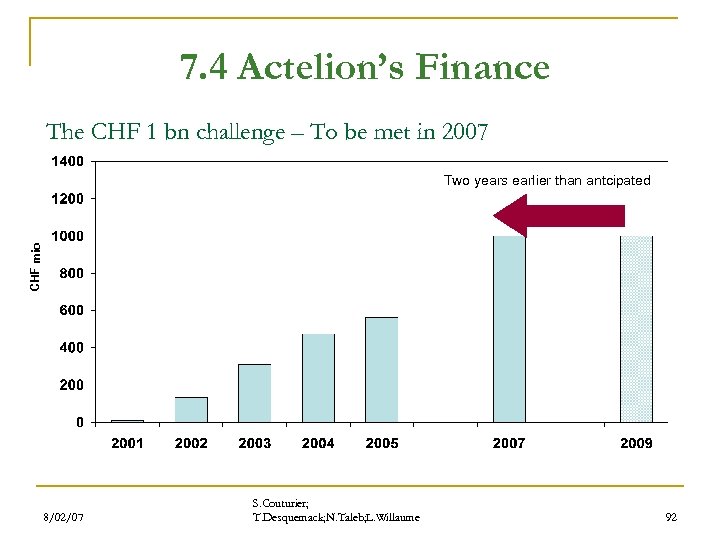
7. 4 Actelion’s Finance The CHF 1 bn challenge – To be met in 2007 CHF mio Two years earlier than antcipated 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 92
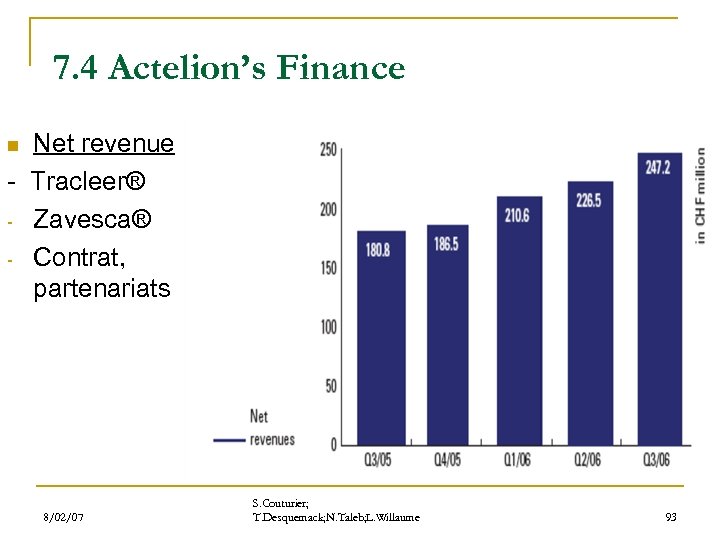
7. 4 Actelion’s Finance Net revenue - Tracleer® - Zavesca® - Contrat, partenariats n 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 93
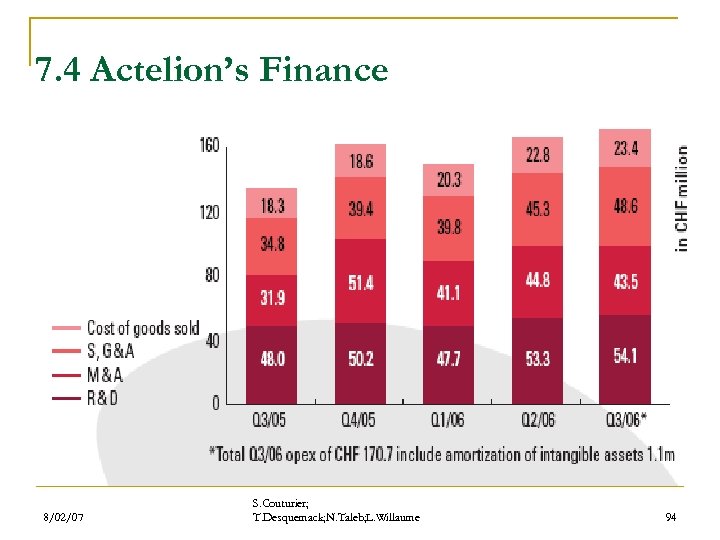
7. 4 Actelion’s Finance 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 94
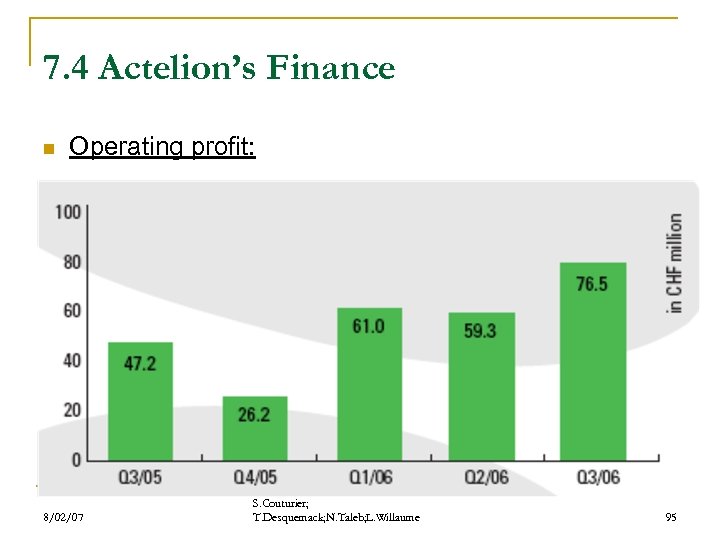
7. 4 Actelion’s Finance n Operating profit: 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 95
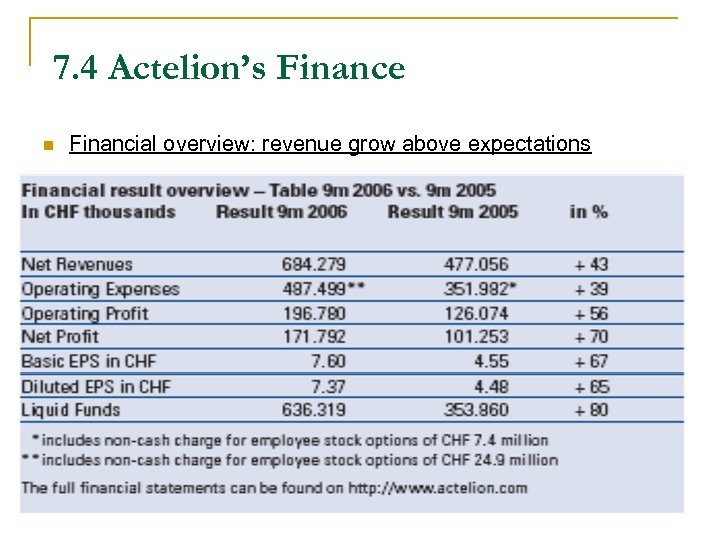
7. 4 Actelion’s Finance n Financial overview: revenue grow above expectations
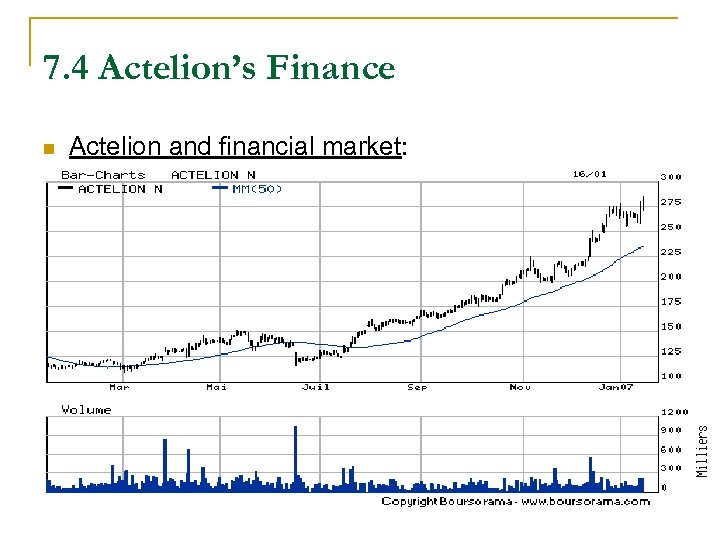
7. 4 Actelion’s Finance n Actelion and financial market:

8. Conclusion n n Actelion is in good health New drugs indicated in PAH will come on the market. Actelion short term future pass by an expension of indication of TRACLEER® On the long range, actelion future pass by a success of his pipeline 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 98

Thanks for your attention. 8/02/07 S. Couturier; T. Desquemack; N. Taleb; L. Willaume 99
02e72d4e4dd135794bece3203037251a.ppt