9a666e109711466c704459ec346ba426.ppt
- Количество слайдов: 13
 ACOSOG Sarcoma Committee Chair: Peter W. T. Pisters, MD Vice Chairs: Edward Cheng, MD (Orthopedic Oncology) Robert Maki, MD, Ph. D (Medical Oncology) Brian O’Sullivan, MD (Radiation Oncology) Brian Rubin, MD, Ph. D (Pathology, Correlative Science) ACOSOG
ACOSOG Sarcoma Committee Chair: Peter W. T. Pisters, MD Vice Chairs: Edward Cheng, MD (Orthopedic Oncology) Robert Maki, MD, Ph. D (Medical Oncology) Brian O’Sullivan, MD (Radiation Oncology) Brian Rubin, MD, Ph. D (Pathology, Correlative Science) ACOSOG
 Research Goals and Themes • Clinical trials evaluating combined modality management of patients with localized soft tissue sarcomas (STS) • Neoadjuvant trials for patients with STS where surgeons are the portal of entry into the cancer care system • International network of investigators • Correlative science studies facilitated by surgeons as an intrinsic part of protocol design • Collaboration with ACRIN, SWOG, RTOG, ICAS and other cooperative groups ACOSOG
Research Goals and Themes • Clinical trials evaluating combined modality management of patients with localized soft tissue sarcomas (STS) • Neoadjuvant trials for patients with STS where surgeons are the portal of entry into the cancer care system • International network of investigators • Correlative science studies facilitated by surgeons as an intrinsic part of protocol design • Collaboration with ACRIN, SWOG, RTOG, ICAS and other cooperative groups ACOSOG
 Trial Summary Completed Accrual: • Z 9000 Phase II imatinib in localized GIST Open: • Z 9001 • Z 9031 - Phase III imatinib versus placebo in localized GIST Phase III surgery versus pre-op XRT plus surgery in retroperitoneal soft tissue sarcoma • S 0344/Z 9041 chondrosarcoma Phase II intralesional treatment of Developing: • ACOSOG/ACRIN – Phase II PET – response assessment ACOSOG
Trial Summary Completed Accrual: • Z 9000 Phase II imatinib in localized GIST Open: • Z 9001 • Z 9031 - Phase III imatinib versus placebo in localized GIST Phase III surgery versus pre-op XRT plus surgery in retroperitoneal soft tissue sarcoma • S 0344/Z 9041 chondrosarcoma Phase II intralesional treatment of Developing: • ACOSOG/ACRIN – Phase II PET – response assessment ACOSOG
 Completed Trial Z 9000 Phase II study of adjuvant Imatinib in patients following completely resected high-risk GIST. Ronald De. Matteo, MD High-risk GIST = Complete Gross Resection Tumor KIT + Imatinib x 1 yr ACOSOG Ruptured > 10 cm Multifocal
Completed Trial Z 9000 Phase II study of adjuvant Imatinib in patients following completely resected high-risk GIST. Ronald De. Matteo, MD High-risk GIST = Complete Gross Resection Tumor KIT + Imatinib x 1 yr ACOSOG Ruptured > 10 cm Multifocal
 Completed Trial Rationale • Surgery alone – standard of care • Imatinib prolongs PFS in Stage IV GIST Primary & Secondary Objectives • Overall survival • Recurrence • Toxicity Correlative Science • Mutational analysis (R 01 and R 21 funded) Z 9000 Accrual • Activation: 6/1/2001 • Completed: 9/30/2003 • Total Accrual: 110 • ACOSOG Sites: 31 • CTSU Sites: 29 Plans • Final toxicity data: 11/2004 • Primary endpoint analysis: 2006 ACOSOG
Completed Trial Rationale • Surgery alone – standard of care • Imatinib prolongs PFS in Stage IV GIST Primary & Secondary Objectives • Overall survival • Recurrence • Toxicity Correlative Science • Mutational analysis (R 01 and R 21 funded) Z 9000 Accrual • Activation: 6/1/2001 • Completed: 9/30/2003 • Total Accrual: 110 • ACOSOG Sites: 31 • CTSU Sites: 29 Plans • Final toxicity data: 11/2004 • Primary endpoint analysis: 2006 ACOSOG
 Active Trial Z 9001 Phase III randomized double-blind study of adjuvant Imatinib versus placebo in patients following resection of primary GIST. Ronald De. Matteo, MD Primary GIST > 3 cm Complete Gross Resection Tumor KIT + Randomize Placebo x 1 yr Imatinib x 1 yr ACOSOG
Active Trial Z 9001 Phase III randomized double-blind study of adjuvant Imatinib versus placebo in patients following resection of primary GIST. Ronald De. Matteo, MD Primary GIST > 3 cm Complete Gross Resection Tumor KIT + Randomize Placebo x 1 yr Imatinib x 1 yr ACOSOG
 Active Trial Z 9001 Rationale • Surgery alone – standard of care • Imatinib prolongs PFS in Stage IV GIST • Need for randomized, placebocontrolled trial Primary & Secondary Objectives • Overall survival • Recurrence • Toxicity Correlative Science (RO 1 and R 21 funded) • Mutational analysis • Mechanisms of imatinib resistance Accrual • Activation: 6/1/2002 • First Registration: 7/31/2002 • Total Accrual: 281 • Annual Accrual: 2002: 19 2003: 99 2004: estimated 200 • Goal: 380, 3. 8 years • ACOSOG Sites: 51 • CTSU Sites: 71 ACOSOG
Active Trial Z 9001 Rationale • Surgery alone – standard of care • Imatinib prolongs PFS in Stage IV GIST • Need for randomized, placebocontrolled trial Primary & Secondary Objectives • Overall survival • Recurrence • Toxicity Correlative Science (RO 1 and R 21 funded) • Mutational analysis • Mechanisms of imatinib resistance Accrual • Activation: 6/1/2002 • First Registration: 7/31/2002 • Total Accrual: 281 • Annual Accrual: 2002: 19 2003: 99 2004: estimated 200 • Goal: 380, 3. 8 years • ACOSOG Sites: 51 • CTSU Sites: 71 ACOSOG
 Z 9031 Phase III Trial - Background • Concept from 2002 Sarcoma State of Science meeting • Evolved through RTOG following Intergroup discussion • Transitioned to ACOSOG – Feasibility (RTOG S 0124 Phase II trial closed for poor accrual) – Primary involvement of surgeons critical for success ACOSOG
Z 9031 Phase III Trial - Background • Concept from 2002 Sarcoma State of Science meeting • Evolved through RTOG following Intergroup discussion • Transitioned to ACOSOG – Feasibility (RTOG S 0124 Phase II trial closed for poor accrual) – Primary involvement of surgeons critical for success ACOSOG
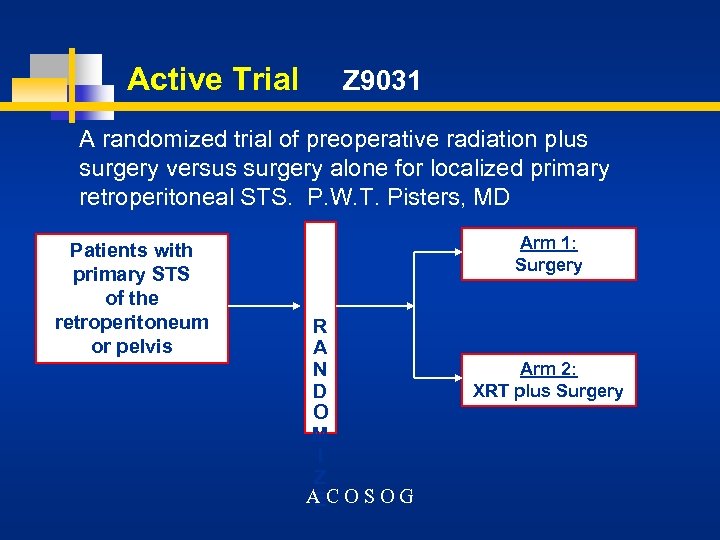 Active Trial Z 9031 A randomized trial of preoperative radiation plus surgery versus surgery alone for localized primary retroperitoneal STS. P. W. T. Pisters, MD Patients with primary STS of the retroperitoneum or pelvis Arm 1: Surgery R A N D O M I Z ACOSOG E Arm 2: XRT plus Surgery
Active Trial Z 9031 A randomized trial of preoperative radiation plus surgery versus surgery alone for localized primary retroperitoneal STS. P. W. T. Pisters, MD Patients with primary STS of the retroperitoneum or pelvis Arm 1: Surgery R A N D O M I Z ACOSOG E Arm 2: XRT plus Surgery
 Active Trial Z 9031 Rationale • Local recurrence is primary pattern of failure for RP STS • Phase III data support use of XRT for extremity STS Primary & Secondary Objectives • Progression-free survival • Combined modality toxicity • Complete resection rate (R 0) • Overall survival Correlative Science • Comparative analysis of proteomic and DNA methylation patterns for treatment effect and prediction of clinical outcomes Accrual • Activation: 8/23/04 • First Registration: 9/24/04 • Goal: 370 patients in 4. 5 years Plans • International participation • Investigator meetings at ASTRO, ACOSOG
Active Trial Z 9031 Rationale • Local recurrence is primary pattern of failure for RP STS • Phase III data support use of XRT for extremity STS Primary & Secondary Objectives • Progression-free survival • Combined modality toxicity • Complete resection rate (R 0) • Overall survival Correlative Science • Comparative analysis of proteomic and DNA methylation patterns for treatment effect and prediction of clinical outcomes Accrual • Activation: 8/23/04 • First Registration: 9/24/04 • Goal: 370 patients in 4. 5 years Plans • International participation • Investigator meetings at ASTRO, ACOSOG
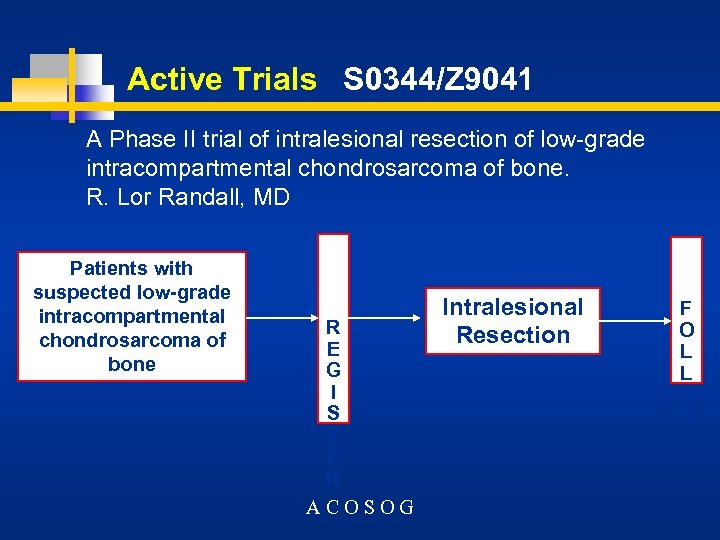 Active Trials S 0344/Z 9041 A Phase II trial of intralesional resection of low-grade intracompartmental chondrosarcoma of bone. R. Lor Randall, MD Patients with suspected low-grade intracompartmental chondrosarcoma of bone R E G I S T E R ACOSOG Intralesional Resection F O L L O W
Active Trials S 0344/Z 9041 A Phase II trial of intralesional resection of low-grade intracompartmental chondrosarcoma of bone. R. Lor Randall, MD Patients with suspected low-grade intracompartmental chondrosarcoma of bone R E G I S T E R ACOSOG Intralesional Resection F O L L O W
 Active Trials S 0344/Z 9041 Rationale • Intralesional resection with margin expansion has excellent local and systemic control rates Primary & Secondary Objectives • Local complications • Local recurrence • Functional status Correlative Science • Correlation of telomerase activity with chondrosarcoma tumor grade • c. DNA microarray hierarchical cluster analysis Accrual • Goal: 60 patients in 4 years Plans • Develop orthopedic oncology network ACOSOG
Active Trials S 0344/Z 9041 Rationale • Intralesional resection with margin expansion has excellent local and systemic control rates Primary & Secondary Objectives • Local complications • Local recurrence • Functional status Correlative Science • Correlation of telomerase activity with chondrosarcoma tumor grade • c. DNA microarray hierarchical cluster analysis Accrual • Goal: 60 patients in 4 years Plans • Develop orthopedic oncology network ACOSOG
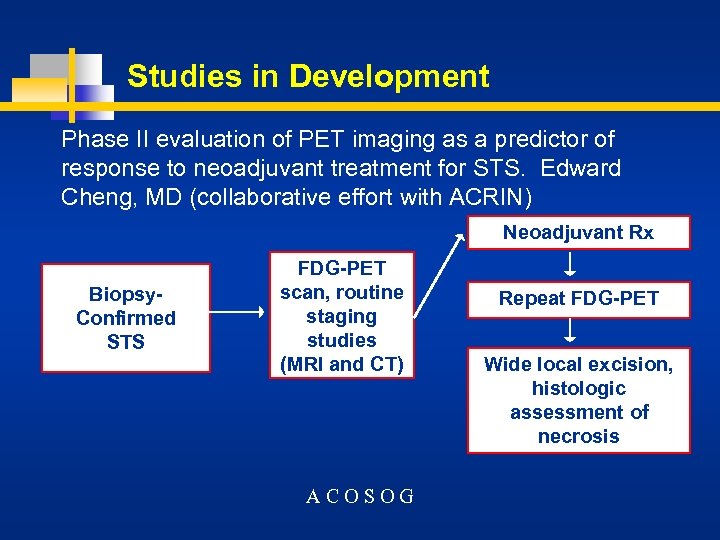 Studies in Development Phase II evaluation of PET imaging as a predictor of response to neoadjuvant treatment for STS. Edward Cheng, MD (collaborative effort with ACRIN) Neoadjuvant Rx Biopsy. Confirmed STS FDG-PET scan, routine staging studies (MRI and CT) ACOSOG Repeat FDG-PET Wide local excision, histologic assessment of necrosis
Studies in Development Phase II evaluation of PET imaging as a predictor of response to neoadjuvant treatment for STS. Edward Cheng, MD (collaborative effort with ACRIN) Neoadjuvant Rx Biopsy. Confirmed STS FDG-PET scan, routine staging studies (MRI and CT) ACOSOG Repeat FDG-PET Wide local excision, histologic assessment of necrosis


