6bcde298ec65074f49e02b01577a7cb4.ppt
- Количество слайдов: 43

ACIDS AND BASES

. . . STATE COMPETENCIES • Recognize acids and bases in terms of the presence of hydronium and hydroxide ions and relate their concentration to the p. H scale. • Compare and contrast the nature, behavior, concentration, and strength of acids and bases • Name and write the formulas for binary and oxyacids
• Distinguish between strong and weak acids and bases • Calculate the p. H and p. OH using the hydronium or hydroxide ion concentration • Explain how indicators are used in titrations • Perform an acid/base titaration

GENERAL INFORMATION • Acids and bases are present in the soil, the foods we eat, the products you buy. • Amino acids are the fabric of every organ in your body and crucial to your existence

PROPERTIES OF ACIDS • • • Sour taste – many are poisonous Reacts with metals and carbonated Contains H Conducts electricity Some are oily ( not like oil) Will ionize in water to produce the hydronium ion

PROPERTIES OF BASES • • • Bitter taste Fell slippery Conducts electricity Bases breaks down fats and oils Bases ionize to produce hydroxide ions in a water solution

TERMS • Acidic solution – contains more hydrogen ions than hydroxide ions • Basic solution – contains more hydroxide ions than hydrogen ions • Neutral solution – contains equal concentration of both. • Ionization of water

NAMING ACIDS • Handout • Binary acid – acid that contains hydrogen and one other element • Oxyanion – is a polyatomic ion that contains oxygen • Oxyacid – any acid that contains a hydrogen and an oxyanion

MONOPROTIC AND POLYPROTIC ACIDS • Monoprotic acid – acid that can donate 1 hydrogen ion per molecule – HCl • Polyprotic acid – any acid that has more than ionizable hydrogen atom – Ionize in steps – H 2 SO 4

Sample problem • Show the complete ionization of the polyprotic acid, hydrogen phospate.
BRONSTED – LOWRY MODEL • Acid – hydrogen – ion donor • Base is a hydrogen-ion acceptor • Conjugate acid – species produced when a base accepts a hydrogen ion from an acid • Conjugate base – results when an acid donates a hydrogen ion to a base • Conjugate acid-base pair – refers to the 2 substances

Sample problem • Identify the conjugate acid-base pair when ammonia dissolves in water.

STRENGTH OF ACIDS AND BASES • Acids and bases conduct electricity in water. • The strength depends on the degree of ionization • Strong acids/bases – completely ionize • Weak acids/bases – partially ionize • Sample problems

ACIDITY OF A SOLUTION • Pure water at 25°C will ionize to form equal concentration of H+ and OH- ions. • Equation • Ion concentration is 1 x 10 -7 for both H+ and OH- ions • Kw is the ion product constant for pure water at 25°Celsius (298 K)
![CALCULATE [H+] AND [OH-] • Formula • Kw = 1. 0 x 10 -14 CALCULATE [H+] AND [OH-] • Formula • Kw = 1. 0 x 10 -14](https://present5.com/presentation/6bcde298ec65074f49e02b01577a7cb4/image-15.jpg)
CALCULATE [H+] AND [OH-] • Formula • Kw = 1. 0 x 10 -14 (pure water at 298 K) • Compare the H+ ion concentration and the OH- ion concentration to determine is it is an acid or base • .
Sample problem • At 298 K, the H+ ion concentration of an aqueous solution is 1. 0 x 10 -5 M. What is the OH-ion concentration in the solution. Is the solution acidic, basic, or neutral?
p. H SCALE • The p. H of a solution shows its acidity in terms of its hydronium concentration – Easier way to express H+ ions instead of using exponents. • Neutral solution has a p. H of 7. – Ex. Pure water • Reference p. H scale in text
p. H INDICATORS • Indicators are usually organic compounds that are different colors in acidic or basic solutions – Litmus paper • Blue litmus paper turns red in an acidic solution • Red litmus paper turns blue in a basic solution – Phenolphthalein, Methyl orange, red cabbage juice
• p. H hydrion papers – shows approximate p. H range • p. H meter – determines p. H by measuring the electrical potential difference between the electrodes that are placed in solution
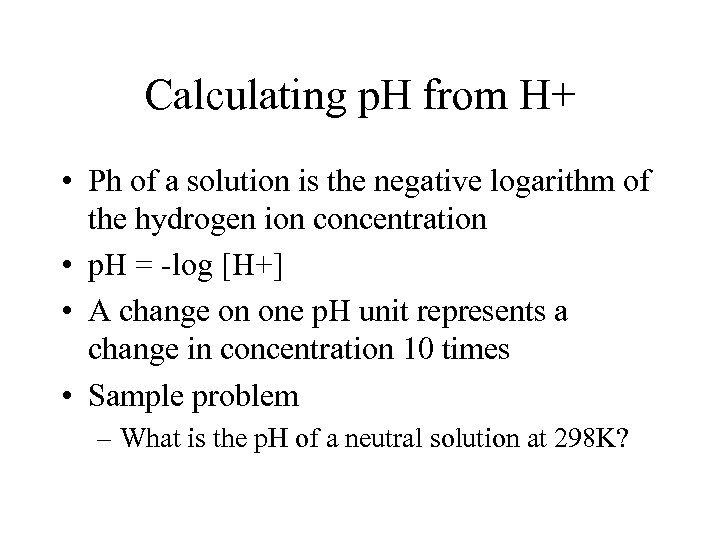
Calculating p. H from H+ • Ph of a solution is the negative logarithm of the hydrogen ion concentration • p. H = -log [H+] • A change on one p. H unit represents a change in concentration 10 times • Sample problem – What is the p. H of a neutral solution at 298 K?
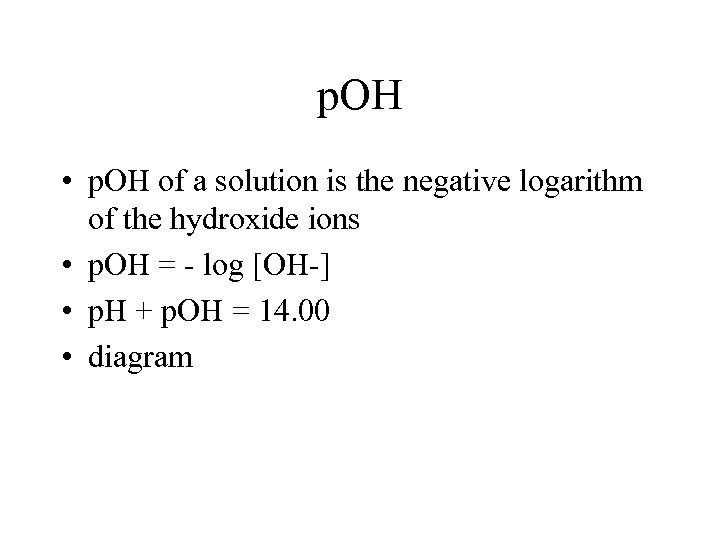
p. OH • p. OH of a solution is the negative logarithm of the hydroxide ions • p. OH = - log [OH-] • p. H + p. OH = 14. 00 • diagram
Calculating p. H and p. OH • Sample problem – An ordinary household ammonia cleaner is an aqueous solution of ammonia gas with hydroxide-ion concentration of 4. 0 x 10 -3 M. Calculate the p. OH and p. H of a typical cleaner at 298 K
![Calculating [H+] AND [OH-] from p. H • What are the [H+] AND [OH-] Calculating [H+] AND [OH-] from p. H • What are the [H+] AND [OH-]](https://present5.com/presentation/6bcde298ec65074f49e02b01577a7cb4/image-23.jpg)
Calculating [H+] AND [OH-] from p. H • What are the [H+] AND [OH-] in a healthy person’s blood that has a p. H of 7. 4? Assume that the temperature is 298 K.

CALCULATING p. H of STRONG ACID/BASE SOLUTIONS Calculate the p. H of a 7. 5 x 10 -5 M solution of calcium hydroxide.
Ka • Ka – acid ionization constant • Indicates whether reactants or products are favored in equilibrium • Weak acids have small Ka values – Their solutions have the highest concentrations of unionized acid molecules • expression

Calculating Ka from p. H • The p. H of a 1. 100 M solution of formic acid is 2. 38 What is Ka for HCOOH? • Remember – Polyprotic acids ionize in steps – We will look only at the initial step

NEUTRALIZATION REACTIONS • • Double displacement Reaction between acid and base Salt and water form Salt – is an ionic compound made up of a cation from a base and an anion from an acid. • Write the equation for the reaction of magnesium hydroxide and hydrochloric acid

ACID/BASE TITRATION • Method for determining the concentration of a solution by reacting a known volume of a solution with a solution of know concentration • Provides a sensitive means of determining the chemically equivalent volumes of acidic and basic solutions • Standard solution – titrating solution of known concentration

Titration • Is the controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentration.
• Equivalence point – point at which moles of H+ = moles of OH– Stoichiometric point – p. H meter or p. H indicaitor detects • End point – the point at which the indictor used in the titration changes color
Indictors • Must match indicator to type titration – Indicators that change color at p. H 7 are used to determine the equivalence point of strong acid/strong base titration – Indicators that change color at p. H lower than 7 are useful in determining the equivalence point of strong acid/weak base titrations

STOICHIOMETRY
Titration graphs • http: //www. bcpl. net/~kdrews/titration/titrati oncurve. html

• Titration curves generally follow a basic form. The example shown here is a typical curve obtained by titrating an acid with a base.
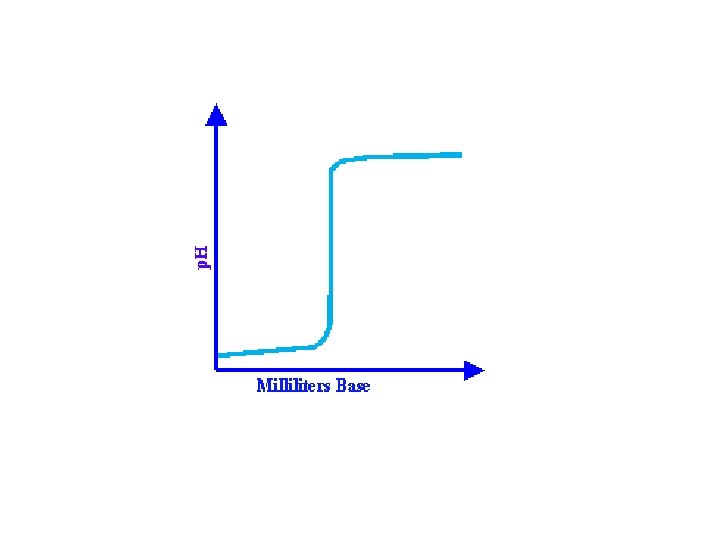

All curves start out with a very slow, or moderate, change in p. H while the base is being added to the acid. As the titration continues and the endpoint is approached, the p. H of the solution will start to change more dramatically. At the endpoint, the line changes most dramatically. Once the endpoint has been passed, the rate of p. H change diminishes again. It will resemble the first part of the graph except at a higher p. H value.
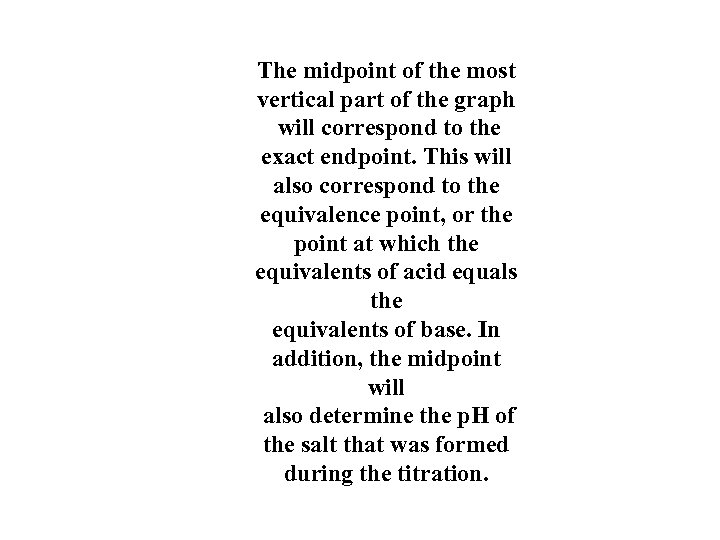
The midpoint of the most vertical part of the graph will correspond to the exact endpoint. This will also correspond to the equivalence point, or the point at which the equivalents of acid equals the equivalents of base. In addition, the midpoint will also determine the p. H of the salt that was formed during the titration.
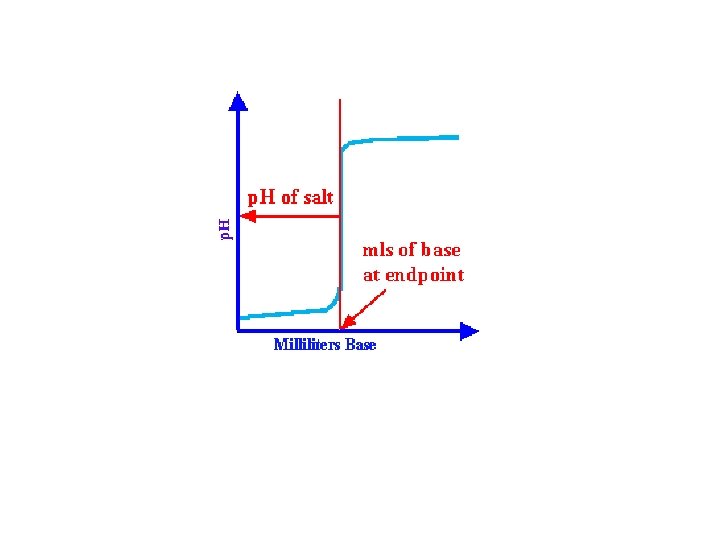

Not all titration curves are exactly the same. The graphs will differ somewhat in shape, depending upon whether the acid that is being titrated is a strong acid, weak acid, monoprotic, or polyprotic
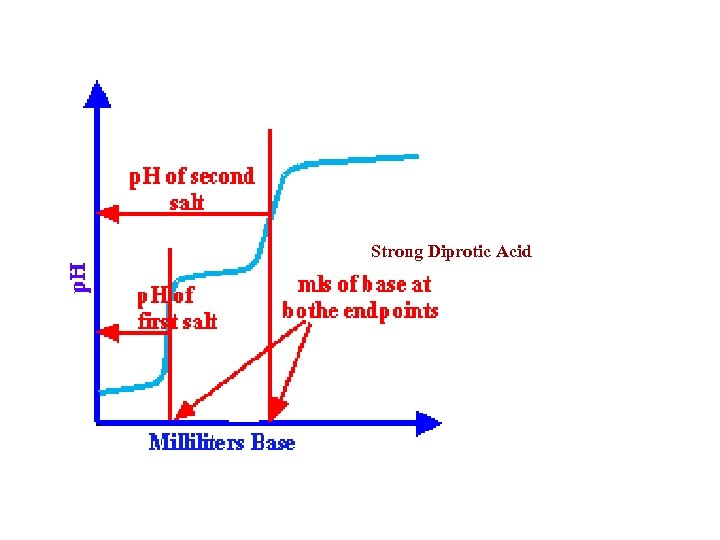
Strong Diprotic Acid
Molarity and Titration • Standard solution – the solution that contains the precisely known concentration of a solute is known as a standard solution. – Also “known solution” • Primary standard – highly purified solid compound used to check the concentration of the known solution in a titration

Sample problem • Ina titration, 27. 4 ml of 0. 014 M barium hudroxide is added to a 20. 0 ml sample of hydrochloric acid solution of unknown concentration. What is the molarity of the acid solution?

Steps 1. 2. 3. 4. Complete and balance equation Determined mole of known solution Determine moles of unknown solution Determine molarity of unknown solution.
6bcde298ec65074f49e02b01577a7cb4.ppt