318c6af55751cff512c0d76a16c0c98e.ppt
- Количество слайдов: 75
 Acid Deposition Locations and Effects Fall 2012, Lecture 10 1
Acid Deposition Locations and Effects Fall 2012, Lecture 10 1
 What is Acid Deposition? • Acid deposition consists of delivery of acidic substances, mainly sulfur and nitrogen oxides, acids and salts, through the atmosphere to the earth's surface 2
What is Acid Deposition? • Acid deposition consists of delivery of acidic substances, mainly sulfur and nitrogen oxides, acids and salts, through the atmosphere to the earth's surface 2
 Acid Rain Graphic 3
Acid Rain Graphic 3
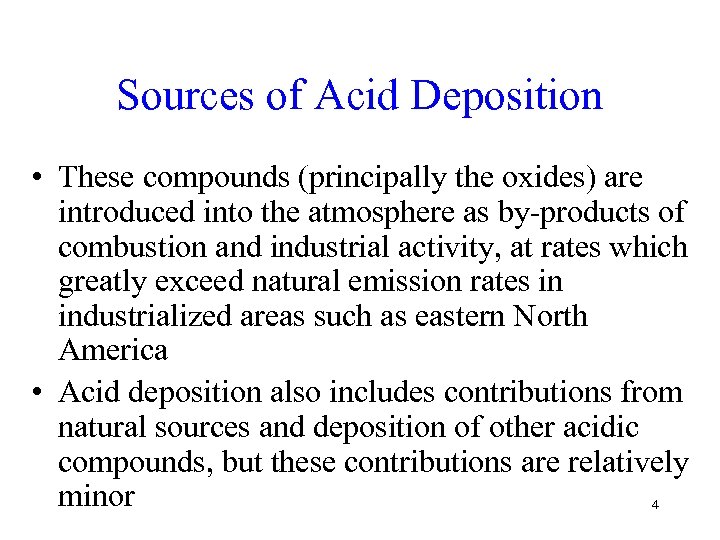 Sources of Acid Deposition • These compounds (principally the oxides) are introduced into the atmosphere as by-products of combustion and industrial activity, at rates which greatly exceed natural emission rates in industrialized areas such as eastern North America • Acid deposition also includes contributions from natural sources and deposition of other acidic compounds, but these contributions are relatively minor 4
Sources of Acid Deposition • These compounds (principally the oxides) are introduced into the atmosphere as by-products of combustion and industrial activity, at rates which greatly exceed natural emission rates in industrialized areas such as eastern North America • Acid deposition also includes contributions from natural sources and deposition of other acidic compounds, but these contributions are relatively minor 4
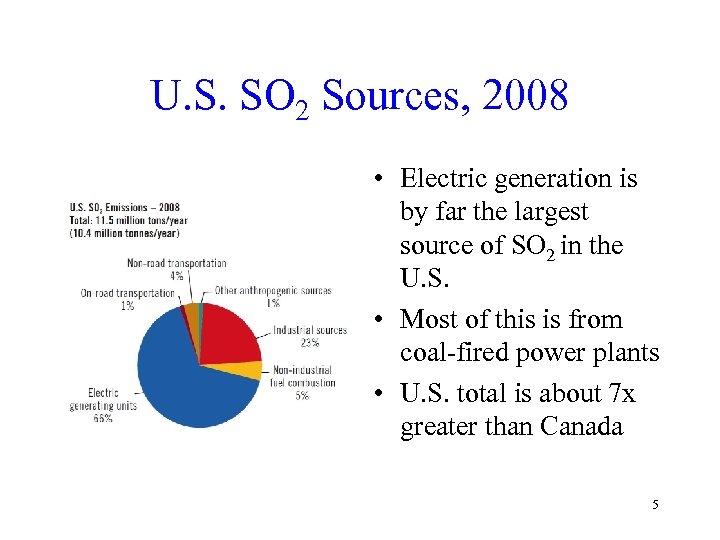 U. S. SO 2 Sources, 2008 • Electric generation is by far the largest source of SO 2 in the U. S. • Most of this is from coal-fired power plants • U. S. total is about 7 x greater than Canada 5
U. S. SO 2 Sources, 2008 • Electric generation is by far the largest source of SO 2 in the U. S. • Most of this is from coal-fired power plants • U. S. total is about 7 x greater than Canada 5
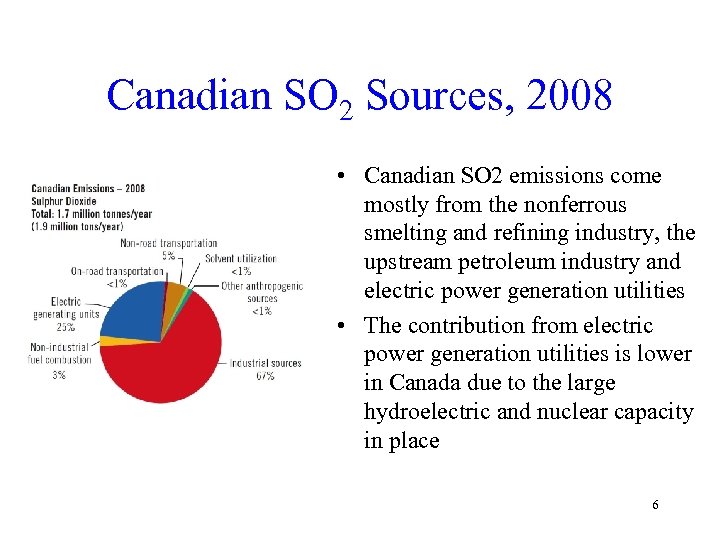 Canadian SO 2 Sources, 2008 • Canadian SO 2 emissions come mostly from the nonferrous smelting and refining industry, the upstream petroleum industry and electric power generation utilities • The contribution from electric power generation utilities is lower in Canada due to the large hydroelectric and nuclear capacity in place 6
Canadian SO 2 Sources, 2008 • Canadian SO 2 emissions come mostly from the nonferrous smelting and refining industry, the upstream petroleum industry and electric power generation utilities • The contribution from electric power generation utilities is lower in Canada due to the large hydroelectric and nuclear capacity in place 6
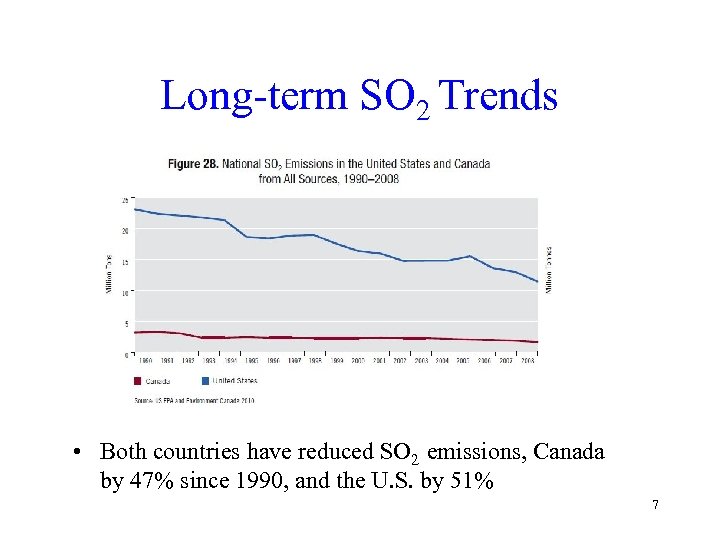 Long-term SO 2 Trends • Both countries have reduced SO 2 emissions, Canada by 47% since 1990, and the U. S. by 51% 7
Long-term SO 2 Trends • Both countries have reduced SO 2 emissions, Canada by 47% since 1990, and the U. S. by 51% 7
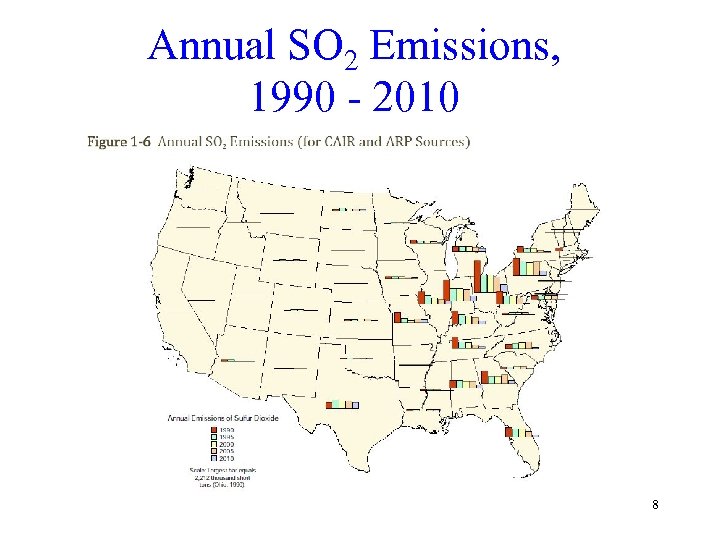 Annual SO 2 Emissions, 1990 - 2010 8
Annual SO 2 Emissions, 1990 - 2010 8
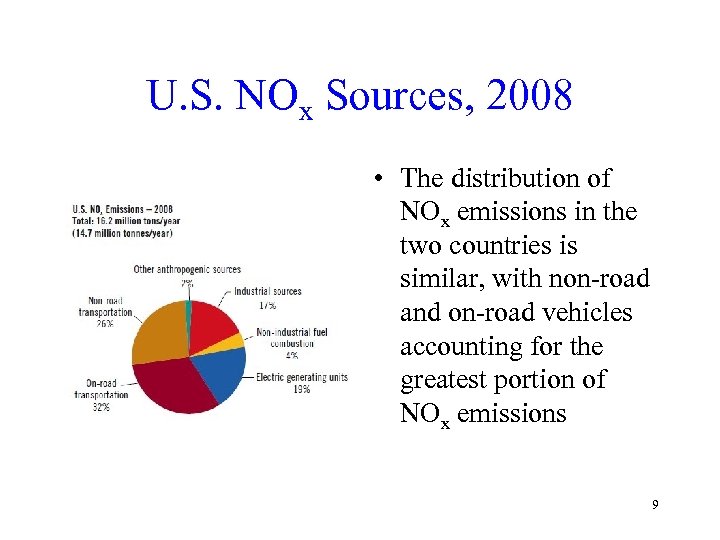 U. S. NOx Sources, 2008 • The distribution of NOx emissions in the two countries is similar, with non-road and on-road vehicles accounting for the greatest portion of NOx emissions 9
U. S. NOx Sources, 2008 • The distribution of NOx emissions in the two countries is similar, with non-road and on-road vehicles accounting for the greatest portion of NOx emissions 9
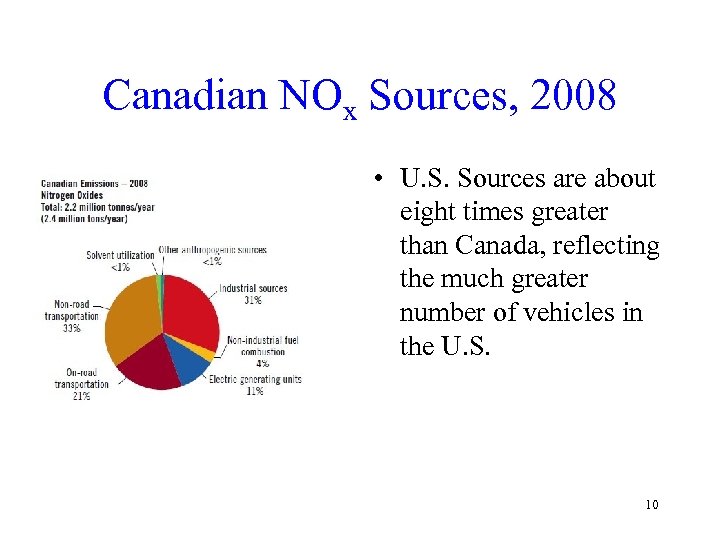 Canadian NOx Sources, 2008 • U. S. Sources are about eight times greater than Canada, reflecting the much greater number of vehicles in the U. S. 10
Canadian NOx Sources, 2008 • U. S. Sources are about eight times greater than Canada, reflecting the much greater number of vehicles in the U. S. 10
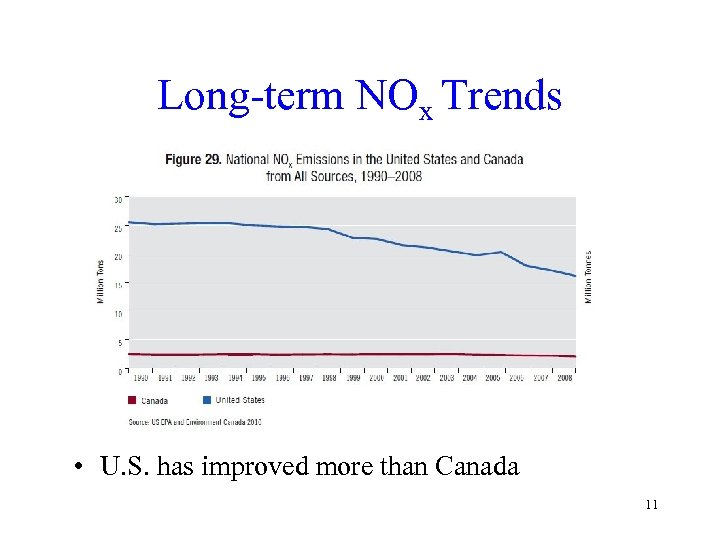 Long-term NOx Trends • U. S. has improved more than Canada 11
Long-term NOx Trends • U. S. has improved more than Canada 11
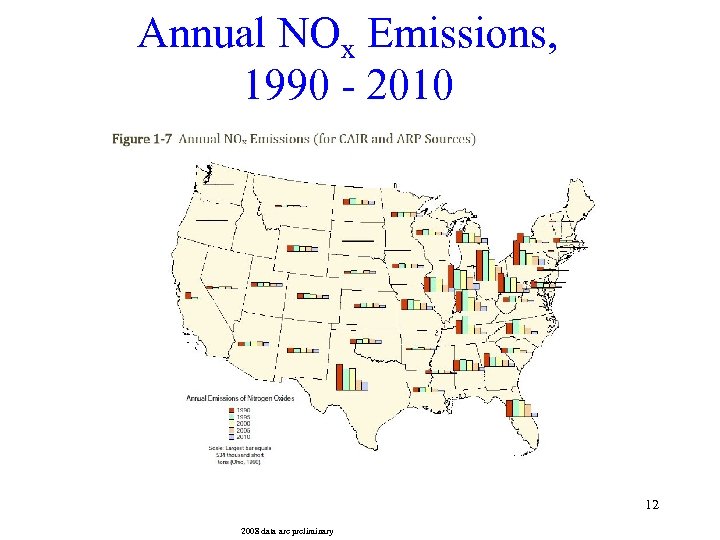 Annual NOx Emissions, 1990 - 2010 12 2008 data are preliminary
Annual NOx Emissions, 1990 - 2010 12 2008 data are preliminary
 International Cooperation • Both Canada and the United States committed to reduce the impact of transboundary air pollution through the 1991 Canada–United States Air Quality Agreement (AQA) • The Acid Rain Annex, negotiated with the original 1991 agreement, committed both Canada and the United States to reducing acid rain-causing emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) 13
International Cooperation • Both Canada and the United States committed to reduce the impact of transboundary air pollution through the 1991 Canada–United States Air Quality Agreement (AQA) • The Acid Rain Annex, negotiated with the original 1991 agreement, committed both Canada and the United States to reducing acid rain-causing emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) 13
 AQA Addendum • The Ozone Annex, added to the Agreement in 2000, committed both countries to reducing emissions of NOx and volatile organic compounds (VOCs), the precursors to groundlevel ozone, a key component of smog • Between 2000 and 2008, the United States has reduced NOx emissions by 33% in the transboundary ozone region while Canada’s total NOx emissions decreased by 32% in the region. 14
AQA Addendum • The Ozone Annex, added to the Agreement in 2000, committed both countries to reducing emissions of NOx and volatile organic compounds (VOCs), the precursors to groundlevel ozone, a key component of smog • Between 2000 and 2008, the United States has reduced NOx emissions by 33% in the transboundary ozone region while Canada’s total NOx emissions decreased by 32% in the region. 14
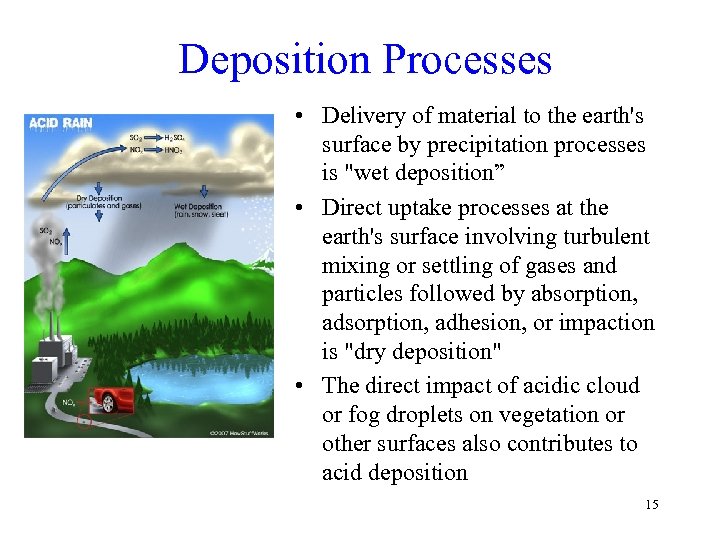 Deposition Processes • Delivery of material to the earth's surface by precipitation processes is "wet deposition” • Direct uptake processes at the earth's surface involving turbulent mixing or settling of gases and particles followed by absorption, adsorption, adhesion, or impaction is "dry deposition" • The direct impact of acidic cloud or fog droplets on vegetation or other surfaces also contributes to acid deposition 15
Deposition Processes • Delivery of material to the earth's surface by precipitation processes is "wet deposition” • Direct uptake processes at the earth's surface involving turbulent mixing or settling of gases and particles followed by absorption, adsorption, adhesion, or impaction is "dry deposition" • The direct impact of acidic cloud or fog droplets on vegetation or other surfaces also contributes to acid deposition 15
 Systems Affected by Acid Deposition • • • Aquatic systems (lakes, rivers) Vegetation Human health Human economic livelihood Inanimate objects built by humans 16
Systems Affected by Acid Deposition • • • Aquatic systems (lakes, rivers) Vegetation Human health Human economic livelihood Inanimate objects built by humans 16
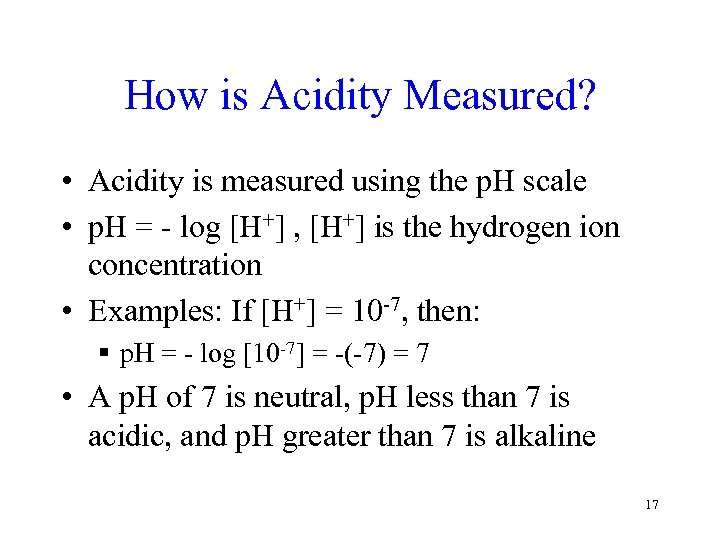 How is Acidity Measured? • Acidity is measured using the p. H scale • p. H = - log [H+] , [H+] is the hydrogen ion concentration • Examples: If [H+] = 10 -7, then: § p. H = - log [10 -7] = -(-7) = 7 • A p. H of 7 is neutral, p. H less than 7 is acidic, and p. H greater than 7 is alkaline 17
How is Acidity Measured? • Acidity is measured using the p. H scale • p. H = - log [H+] , [H+] is the hydrogen ion concentration • Examples: If [H+] = 10 -7, then: § p. H = - log [10 -7] = -(-7) = 7 • A p. H of 7 is neutral, p. H less than 7 is acidic, and p. H greater than 7 is alkaline 17
 Why p. H? • The term p. H comes from the French, “pouvoir Hydrogene”, which means power of hydrogen 18
Why p. H? • The term p. H comes from the French, “pouvoir Hydrogene”, which means power of hydrogen 18
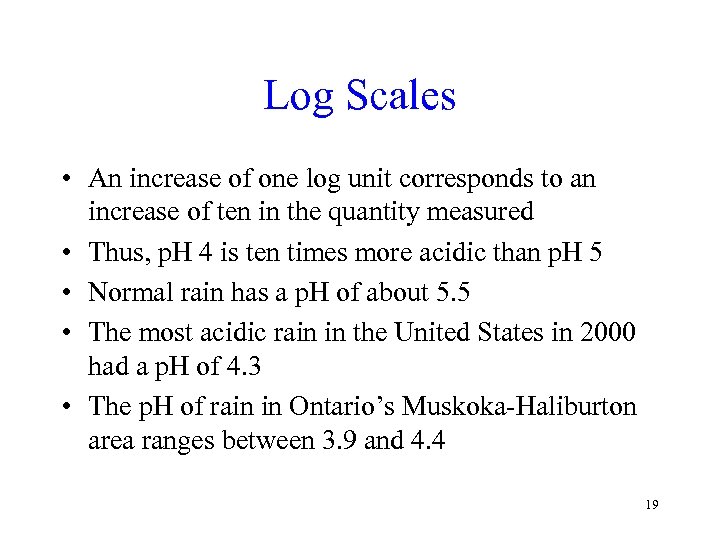 Log Scales • An increase of one log unit corresponds to an increase of ten in the quantity measured • Thus, p. H 4 is ten times more acidic than p. H 5 • Normal rain has a p. H of about 5. 5 • The most acidic rain in the United States in 2000 had a p. H of 4. 3 • The p. H of rain in Ontario’s Muskoka-Haliburton area ranges between 3. 9 and 4. 4 19
Log Scales • An increase of one log unit corresponds to an increase of ten in the quantity measured • Thus, p. H 4 is ten times more acidic than p. H 5 • Normal rain has a p. H of about 5. 5 • The most acidic rain in the United States in 2000 had a p. H of 4. 3 • The p. H of rain in Ontario’s Muskoka-Haliburton area ranges between 3. 9 and 4. 4 19
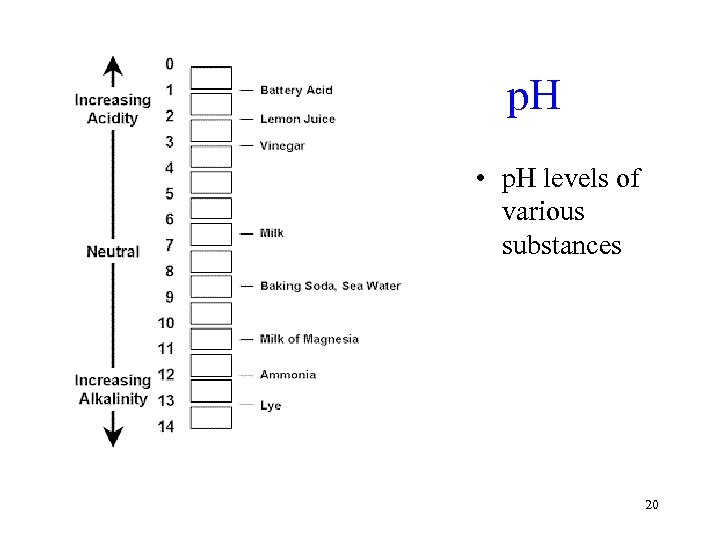 p. H • p. H levels of various substances 20
p. H • p. H levels of various substances 20
 Aquatic Systems • Acid deposition can lower p. H • Bedrock of stream or lake and the surrounding watershed determines the susceptibility to acid deposition • Susceptible systems show a decline in fish populations 21
Aquatic Systems • Acid deposition can lower p. H • Bedrock of stream or lake and the surrounding watershed determines the susceptibility to acid deposition • Susceptible systems show a decline in fish populations 21
 Acidified Lakes • Contain high concentrations of toxic heavy metals like mercury, aluminum, and cadmium • Soil and bedrock surrounding the water body is the source of the toxic metals 22
Acidified Lakes • Contain high concentrations of toxic heavy metals like mercury, aluminum, and cadmium • Soil and bedrock surrounding the water body is the source of the toxic metals 22
 Acid Shock • Occurs in mid-latitudes where snow accumulates in winter • Acidic deposits can build-up in the snowpack • When spring arrives, snowpack begins to melt quickly • Acids are released over a short period of time at concentrations 5 to 10 times more acidic than rainfall 23
Acid Shock • Occurs in mid-latitudes where snow accumulates in winter • Acidic deposits can build-up in the snowpack • When spring arrives, snowpack begins to melt quickly • Acids are released over a short period of time at concentrations 5 to 10 times more acidic than rainfall 23
 Gradual Acidification • Gradual decline in p. H – no acid shock • Prolonged acidity interferes with fish reproduction and spawning • Over time, a decrease in fish population density and a shift in the size and age of the population to older and larger fish occurs 24
Gradual Acidification • Gradual decline in p. H – no acid shock • Prolonged acidity interferes with fish reproduction and spawning • Over time, a decrease in fish population density and a shift in the size and age of the population to older and larger fish occurs 24
 Effects on Aquatic Plants • Alterations in the composition and structure of the aquatic plant communities • In experimental studies, differences in nutrient level(phosphorous and nitrogen) appeared to be the limiting factor • Reductions in biodiversity 25
Effects on Aquatic Plants • Alterations in the composition and structure of the aquatic plant communities • In experimental studies, differences in nutrient level(phosphorous and nitrogen) appeared to be the limiting factor • Reductions in biodiversity 25
 Aquatic Microorganisms • Acidification reduces microbiological activity § Reduces rates of decomposition and the accumulation of organic matter in aquatic ecosystems • Reduces nutrient recycling • Reduces energy available to biologic systems 26
Aquatic Microorganisms • Acidification reduces microbiological activity § Reduces rates of decomposition and the accumulation of organic matter in aquatic ecosystems • Reduces nutrient recycling • Reduces energy available to biologic systems 26
 Effects on Vegetation • Severity of acid deposition on vegetation is greatly dependent on the type of soil the plants grow in • Many soils have a natural buffering capacity and are able to neutralize acid inputs • Limy soils are better at neutralizing acids than those that are made up of siliceous sand or weathered acidic bedrock 27
Effects on Vegetation • Severity of acid deposition on vegetation is greatly dependent on the type of soil the plants grow in • Many soils have a natural buffering capacity and are able to neutralize acid inputs • Limy soils are better at neutralizing acids than those that are made up of siliceous sand or weathered acidic bedrock 27
 Mt. Mitchell State Park, North Carolina 28
Mt. Mitchell State Park, North Carolina 28
 Acid Rain Video 29
Acid Rain Video 29
 Lime is a Buffer • Lime is calcium carbonate • Carbonates react with acids, neutralizing them • As long as lime is available, the soil, and water in contact with it, cannot become overly acidic 30
Lime is a Buffer • Lime is calcium carbonate • Carbonates react with acids, neutralizing them • As long as lime is available, the soil, and water in contact with it, cannot become overly acidic 30
 Why Acid Affects Unbuffered Soils • • • Leaching of plants nutrients Declining growth rates Aluminum mobilization Inhibition of seed germination Inhibition of seedling growth 31
Why Acid Affects Unbuffered Soils • • • Leaching of plants nutrients Declining growth rates Aluminum mobilization Inhibition of seed germination Inhibition of seedling growth 31
 Why Acid Affects Unbuffered Soils • Soil organism mortality § Inhibits decomposition and nutrient recycling • • Nitrogen Saturation Acid rain damages foliage Dry deposition affects water retention Acid leaches nutrients from plant tissues 32
Why Acid Affects Unbuffered Soils • Soil organism mortality § Inhibits decomposition and nutrient recycling • • Nitrogen Saturation Acid rain damages foliage Dry deposition affects water retention Acid leaches nutrients from plant tissues 32
 Overall Effects on Vegetation • Combination of these effects can lead to plants that have reduced growth rates, flowering ability and yields • Makes plants more vulnerable to diseases, insects, droughts and frosts 33
Overall Effects on Vegetation • Combination of these effects can lead to plants that have reduced growth rates, flowering ability and yields • Makes plants more vulnerable to diseases, insects, droughts and frosts 33
 Effects on Humans • Release of toxic metals • Respiratory illness • Increased likelihood of chest colds, allergies, coughs, asthma 34
Effects on Humans • Release of toxic metals • Respiratory illness • Increased likelihood of chest colds, allergies, coughs, asthma 34
 Economic Impacts • Decline in fishing § Commercial § Sport • Damage to forests 35
Economic Impacts • Decline in fishing § Commercial § Sport • Damage to forests 35
 Effects on Construction • Buildings, headstones, etc. constructed of limestone or marble may be dissolved • Paint, especially automotive paint, may be damaged • European churches and cathedrals have been damaged 36
Effects on Construction • Buildings, headstones, etc. constructed of limestone or marble may be dissolved • Paint, especially automotive paint, may be damaged • European churches and cathedrals have been damaged 36
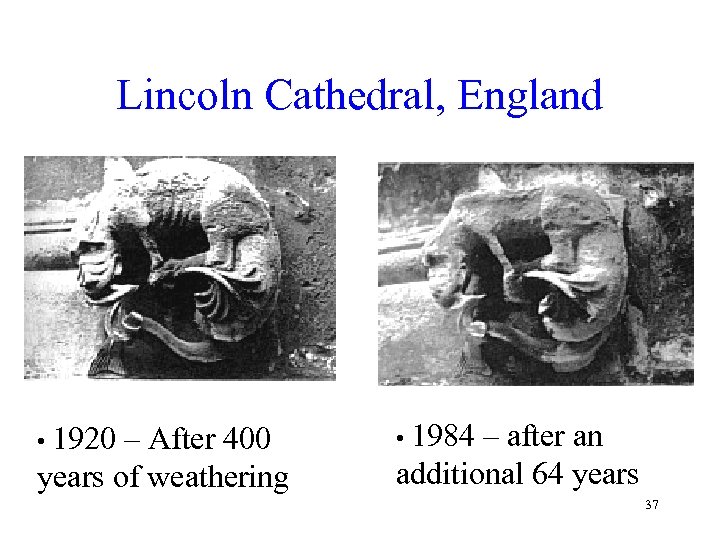 Lincoln Cathedral, England • 1920 – After 400 • 1984 – after an years of weathering additional 64 years 37
Lincoln Cathedral, England • 1920 – After 400 • 1984 – after an years of weathering additional 64 years 37
 Jefferson Memorial • Marble surfaces exposed to rain develop a rough "sugary" texture because the calcite grains are loosened as the edges dissolve in the rain water • Column capital volute, Jefferson Memorial, Washington, D. C. 38
Jefferson Memorial • Marble surfaces exposed to rain develop a rough "sugary" texture because the calcite grains are loosened as the edges dissolve in the rain water • Column capital volute, Jefferson Memorial, Washington, D. C. 38
 Acid Rain Video • Discovery Acid Rain Presentation 39
Acid Rain Video • Discovery Acid Rain Presentation 39
 Sunland Park Mall El Paso, Texas • Sunland Park Mall was built in 1989 of polished pink marble • Much of the marble looks just as fresh today as on the day it was installed • In some areas, however, the marble has badly deteriorated 40
Sunland Park Mall El Paso, Texas • Sunland Park Mall was built in 1989 of polished pink marble • Much of the marble looks just as fresh today as on the day it was installed • In some areas, however, the marble has badly deteriorated 40
 Sunland Mall Marble Closeup • Deterioration occurs where sprinkler system repeatedly wets the marble • Water dissolves the mineral calcite, the main mineral in marble • Marble far above ground level still looks fresh • Intensity of chemical weathering increases down toward the level of the bushes 41
Sunland Mall Marble Closeup • Deterioration occurs where sprinkler system repeatedly wets the marble • Water dissolves the mineral calcite, the main mineral in marble • Marble far above ground level still looks fresh • Intensity of chemical weathering increases down toward the level of the bushes 41
 Sunland Mall Marble Closeup • Weathering rate may be increased by fertilizers or other substances sprayed on the bushes • Acidic fertilizers promote dissolution of marble 42
Sunland Mall Marble Closeup • Weathering rate may be increased by fertilizers or other substances sprayed on the bushes • Acidic fertilizers promote dissolution of marble 42
 U. S. Acid Rain Program • Title IV (the Acid Rain Program or ARP) of the Clean Air Act Amendments of 1990 requires major reductions of SO 2 and NOx emissions from the electric power sector, the highest SO 2 emitting sector • Under the ARP, the SO 2 program set a permanent cap on the total amount of SO 2 that may be emitted by electric generation units in the contiguous United States starting in 1995 • The reductions are phased in over time, with the final 2010 SO 2 cap set at 8. 95 million tons 43
U. S. Acid Rain Program • Title IV (the Acid Rain Program or ARP) of the Clean Air Act Amendments of 1990 requires major reductions of SO 2 and NOx emissions from the electric power sector, the highest SO 2 emitting sector • Under the ARP, the SO 2 program set a permanent cap on the total amount of SO 2 that may be emitted by electric generation units in the contiguous United States starting in 1995 • The reductions are phased in over time, with the final 2010 SO 2 cap set at 8. 95 million tons 43
 U. S. Acid Rain Program Target • Cut SO 2 emissions by 50% from 1980 levels by 2010 • Improve visibility in eastern U. S. by 30% • Increase “value” by more than $1 billion dollars annually by 2010 44
U. S. Acid Rain Program Target • Cut SO 2 emissions by 50% from 1980 levels by 2010 • Improve visibility in eastern U. S. by 30% • Increase “value” by more than $1 billion dollars annually by 2010 44
 U. S. Acid Rain Program Achievement • The United States succeeded in meeting its commitment to reduce annual SO 2 emissions by 10 million tons from 1980 levels by 2000. • Additionally, in 2007, emissions of SO 2 from the electric power sector in the United States fell below the 2010 national emission cap of 8. 95 million tons for the first time, achieving the U. S. commitment three years early 45
U. S. Acid Rain Program Achievement • The United States succeeded in meeting its commitment to reduce annual SO 2 emissions by 10 million tons from 1980 levels by 2000. • Additionally, in 2007, emissions of SO 2 from the electric power sector in the United States fell below the 2010 national emission cap of 8. 95 million tons for the first time, achieving the U. S. commitment three years early 45
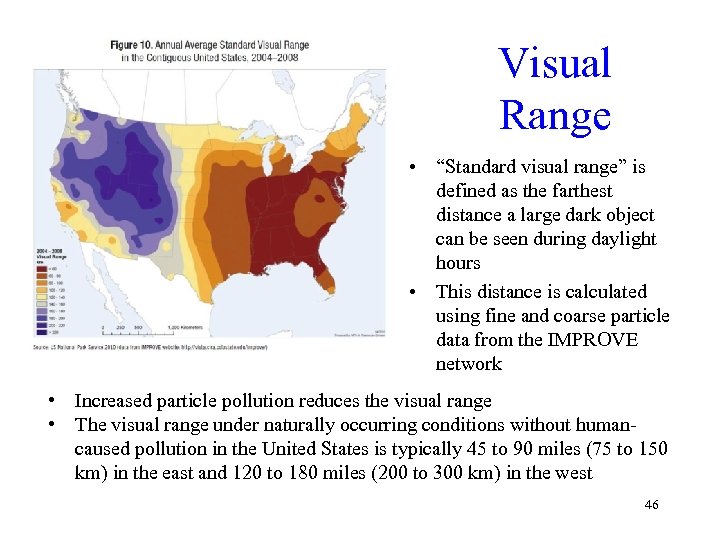 Visual Range • “Standard visual range” is defined as the farthest distance a large dark object can be seen during daylight hours • This distance is calculated using fine and coarse particle data from the IMPROVE network • Increased particle pollution reduces the visual range • The visual range under naturally occurring conditions without humancaused pollution in the United States is typically 45 to 90 miles (75 to 150 km) in the east and 120 to 180 miles (200 to 300 km) in the west 46
Visual Range • “Standard visual range” is defined as the farthest distance a large dark object can be seen during daylight hours • This distance is calculated using fine and coarse particle data from the IMPROVE network • Increased particle pollution reduces the visual range • The visual range under naturally occurring conditions without humancaused pollution in the United States is typically 45 to 90 miles (75 to 150 km) in the east and 120 to 180 miles (200 to 300 km) in the west 46
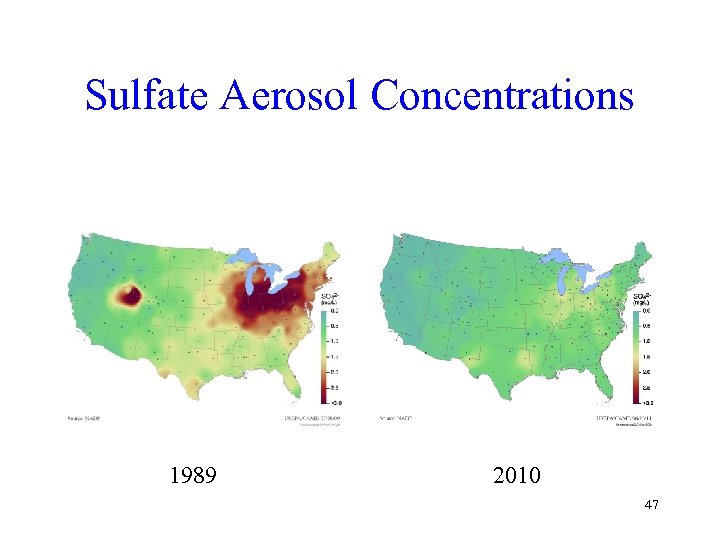 Sulfate Aerosol Concentrations 1989 2010 47
Sulfate Aerosol Concentrations 1989 2010 47
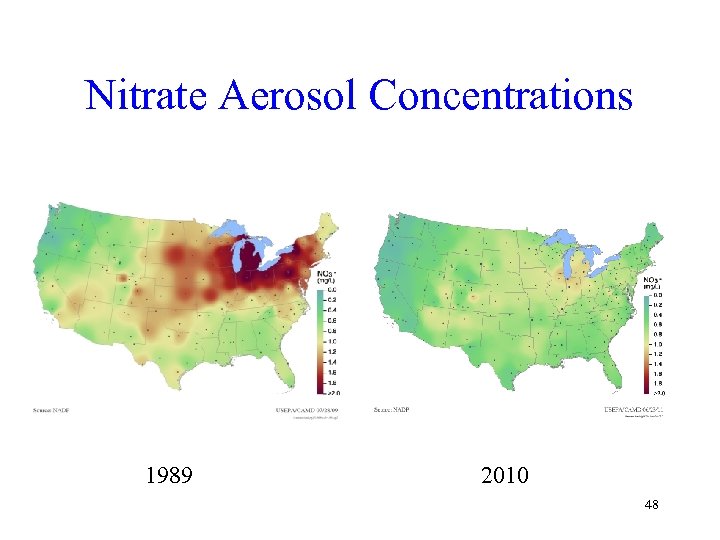 Nitrate Aerosol Concentrations 1989 2010 48
Nitrate Aerosol Concentrations 1989 2010 48
 Clean Air Act Difference • Examination of the last three slides shows large improvements in both sulfate and nitrate from 1989 (just before the new Clean Air Act programs) to 2010 (latest available data) • The Clean Air Act has clearly made a huge difference 49
Clean Air Act Difference • Examination of the last three slides shows large improvements in both sulfate and nitrate from 1989 (just before the new Clean Air Act programs) to 2010 (latest available data) • The Clean Air Act has clearly made a huge difference 49
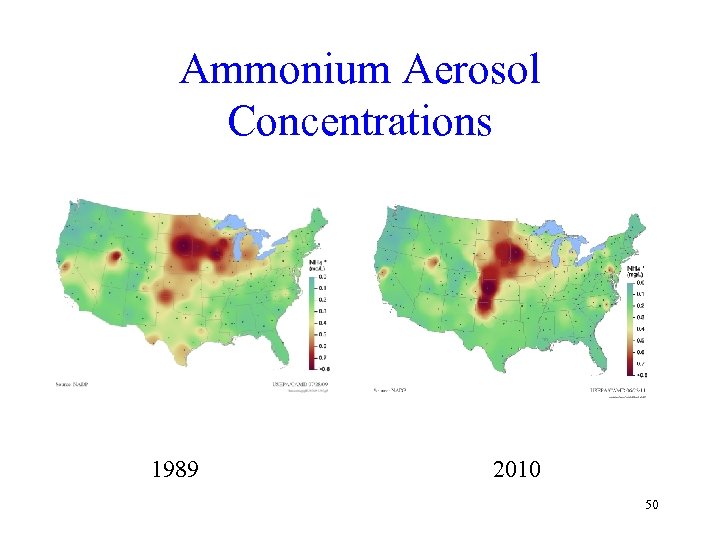 Ammonium Aerosol Concentrations 1989 2010 50
Ammonium Aerosol Concentrations 1989 2010 50
 Agricultural Air Pollution • Ammonium comes primarily from agricultural application of fertilizer • This is not regulated by the Clean Air Act • In the center of the country, ammonium concentrations have risen • In the corn belt, the new agricultural practices are having an effect (Wisconsin, Michigan, Indiana and parts of Minnesota and Iowa) 51
Agricultural Air Pollution • Ammonium comes primarily from agricultural application of fertilizer • This is not regulated by the Clean Air Act • In the center of the country, ammonium concentrations have risen • In the corn belt, the new agricultural practices are having an effect (Wisconsin, Michigan, Indiana and parts of Minnesota and Iowa) 51
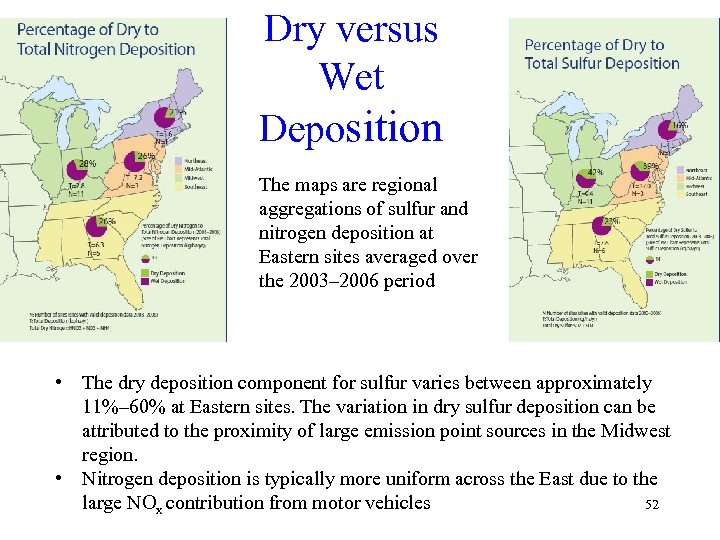 Dry versus Wet Deposition The maps are regional aggregations of sulfur and nitrogen deposition at Eastern sites averaged over the 2003– 2006 period • The dry deposition component for sulfur varies between approximately 11%– 60% at Eastern sites. The variation in dry sulfur deposition can be attributed to the proximity of large emission point sources in the Midwest region. • Nitrogen deposition is typically more uniform across the East due to the large NOx contribution from motor vehicles 52
Dry versus Wet Deposition The maps are regional aggregations of sulfur and nitrogen deposition at Eastern sites averaged over the 2003– 2006 period • The dry deposition component for sulfur varies between approximately 11%– 60% at Eastern sites. The variation in dry sulfur deposition can be attributed to the proximity of large emission point sources in the Midwest region. • Nitrogen deposition is typically more uniform across the East due to the large NOx contribution from motor vehicles 52
 Clean Air Status and Trends Network(CASTNET) 53
Clean Air Status and Trends Network(CASTNET) 53
 CASTNET Monitoring Procedures • At each site, there is a temperature-controlled shelter which houses a computer, a data logger, and a continuous ozone monitor § Weekly samples of particulate matter and select gasses are collected using a 3 -stage filter pack with a controlled flow rate located atop a 10 m tower § For quality assurance, site audits are performed once every six months and biennially by the CASTNET contractor and a third party auditor, respectively 54
CASTNET Monitoring Procedures • At each site, there is a temperature-controlled shelter which houses a computer, a data logger, and a continuous ozone monitor § Weekly samples of particulate matter and select gasses are collected using a 3 -stage filter pack with a controlled flow rate located atop a 10 m tower § For quality assurance, site audits are performed once every six months and biennially by the CASTNET contractor and a third party auditor, respectively 54
 CSAPR replaces CAIR and ARP • The Clean Air Interstate Rule (CAIR) and the Acid Rain Program (ARP) are both cap and trade programs designed to reduce emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) from power plants • On July 6, 2011, EPA finalized the Cross-State Air Pollution Rule (CSAPR), which will replace CAIR starting in 2012 55
CSAPR replaces CAIR and ARP • The Clean Air Interstate Rule (CAIR) and the Acid Rain Program (ARP) are both cap and trade programs designed to reduce emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) from power plants • On July 6, 2011, EPA finalized the Cross-State Air Pollution Rule (CSAPR), which will replace CAIR starting in 2012 55
 Clean Air Interstate Rule • The CSAPR will require 27 states in the eastern half of the U. S. to significantly improve air quality by reducing power plant emissions of SO 2 and NOx that cross state lines and contribute to smog (ground-level ozone) and soot (fine particle pollution) in other states 56
Clean Air Interstate Rule • The CSAPR will require 27 states in the eastern half of the U. S. to significantly improve air quality by reducing power plant emissions of SO 2 and NOx that cross state lines and contribute to smog (ground-level ozone) and soot (fine particle pollution) in other states 56
 CSAPR Timetable • The first phase of compliance begins January 1, 2012 for SO 2 and annual NOx reductions and May 1, 2012 for ozone season NOx reductions • Additional SO 2 reductions are required by sixteen Group 1 states in 2014 to eliminate their contribution to downwind air quality problems 57
CSAPR Timetable • The first phase of compliance begins January 1, 2012 for SO 2 and annual NOx reductions and May 1, 2012 for ozone season NOx reductions • Additional SO 2 reductions are required by sixteen Group 1 states in 2014 to eliminate their contribution to downwind air quality problems 57
 Acid Rain Program in Canada • In 2008, Canada’s total SO 2 emissions were 1. 7 million tons, or about 47% below the national cap of 3. 2 million tons • This represents more than a 63% reduction from Canada’s total SO 2 emissions in 1980 and a 46% decrease from the 1990 emission level • This overall reduction in national SO 2 emission levels can be attributed to the SO 2 emission reductions undertaken by the four eastern provinces (New Brunswick, Nova Scotia, Quebec and Ontario) targeted by the Acid Rain Strategy 58
Acid Rain Program in Canada • In 2008, Canada’s total SO 2 emissions were 1. 7 million tons, or about 47% below the national cap of 3. 2 million tons • This represents more than a 63% reduction from Canada’s total SO 2 emissions in 1980 and a 46% decrease from the 1990 emission level • This overall reduction in national SO 2 emission levels can be attributed to the SO 2 emission reductions undertaken by the four eastern provinces (New Brunswick, Nova Scotia, Quebec and Ontario) targeted by the Acid Rain Strategy 58
 Effect of SO 2 Reduction on Climate • The IPCC February, 2000 report increased the estimate of maximum temperature increase by 2100 to 5. 8 degrees Celsius • Maximum temperature increase due to reduction in SO 2 haze, which reflects sunlight and cools the surface 59
Effect of SO 2 Reduction on Climate • The IPCC February, 2000 report increased the estimate of maximum temperature increase by 2100 to 5. 8 degrees Celsius • Maximum temperature increase due to reduction in SO 2 haze, which reflects sunlight and cools the surface 59
 Ocean Acidification • Another aspect of acid deposition is the acidification of the oceans • Since man first began using fossil fuels, we have added carbon dioxide (CO 2) to the atmosphere • Atmosphere-ocean exchange transfers some of the CO 2 to the ocean 60
Ocean Acidification • Another aspect of acid deposition is the acidification of the oceans • Since man first began using fossil fuels, we have added carbon dioxide (CO 2) to the atmosphere • Atmosphere-ocean exchange transfers some of the CO 2 to the ocean 60
 The Other Carbon Problem • Many people are familiar with the idea that CO 2 is a greenhouse gas, responsible for a significant increase in temperature • The acidification of the oceans, due to the addition of CO 2 from the atmosphere, is an equally significant, but much less well known, problem • It is often called “The Other Carbon Problem” 61
The Other Carbon Problem • Many people are familiar with the idea that CO 2 is a greenhouse gas, responsible for a significant increase in temperature • The acidification of the oceans, due to the addition of CO 2 from the atmosphere, is an equally significant, but much less well known, problem • It is often called “The Other Carbon Problem” 61
 Chemistry • The basic chemistry of CO 2 addition is: • CO 2 + H 2 O = H 2 CO 3 • Carbon dioxide + water = carbonic acid 62
Chemistry • The basic chemistry of CO 2 addition is: • CO 2 + H 2 O = H 2 CO 3 • Carbon dioxide + water = carbonic acid 62
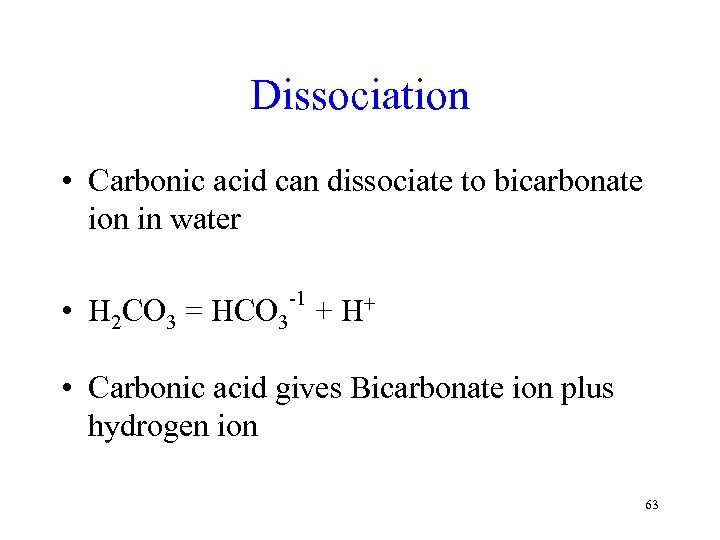 Dissociation • Carbonic acid can dissociate to bicarbonate ion in water • -1 H 2 CO 3 = HCO 3 + H+ • Carbonic acid gives Bicarbonate ion plus hydrogen ion 63
Dissociation • Carbonic acid can dissociate to bicarbonate ion in water • -1 H 2 CO 3 = HCO 3 + H+ • Carbonic acid gives Bicarbonate ion plus hydrogen ion 63
 Further Dissociation • Bicarbonate ion further dissociates • -1 -2 HCO 3 = CO 3 + H+ • Bicarbonate ion gives carbonate ion plus hydrogen ion 64
Further Dissociation • Bicarbonate ion further dissociates • -1 -2 HCO 3 = CO 3 + H+ • Bicarbonate ion gives carbonate ion plus hydrogen ion 64
 Chemical Adjustments • In the oceans, carbonic acid, bicarbonate ion, and carbonate ion are all present • In most cases, they achieve chemical equilibrium • This means the amount of each substance adjusts as conditions, such as temperature and p. H, change 65
Chemical Adjustments • In the oceans, carbonic acid, bicarbonate ion, and carbonate ion are all present • In most cases, they achieve chemical equilibrium • This means the amount of each substance adjusts as conditions, such as temperature and p. H, change 65
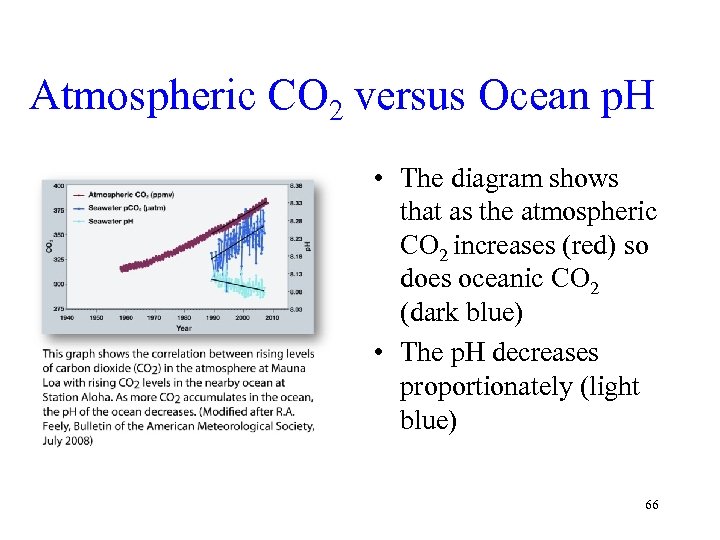 Atmospheric CO 2 versus Ocean p. H • The diagram shows that as the atmospheric CO 2 increases (red) so does oceanic CO 2 (dark blue) • The p. H decreases proportionately (light blue) 66
Atmospheric CO 2 versus Ocean p. H • The diagram shows that as the atmospheric CO 2 increases (red) so does oceanic CO 2 (dark blue) • The p. H decreases proportionately (light blue) 66
 Computer Animation of Acidification • The movie (next slide) shows a computer recreation of surface ocean p. H from 1895 to the present, and it forecasts how ocean p. H will drop even more between now and 2094 • Dark gray dots show cold-water coral reefs • Medium gray dots show warm-water coral reefs 67
Computer Animation of Acidification • The movie (next slide) shows a computer recreation of surface ocean p. H from 1895 to the present, and it forecasts how ocean p. H will drop even more between now and 2094 • Dark gray dots show cold-water coral reefs • Medium gray dots show warm-water coral reefs 67
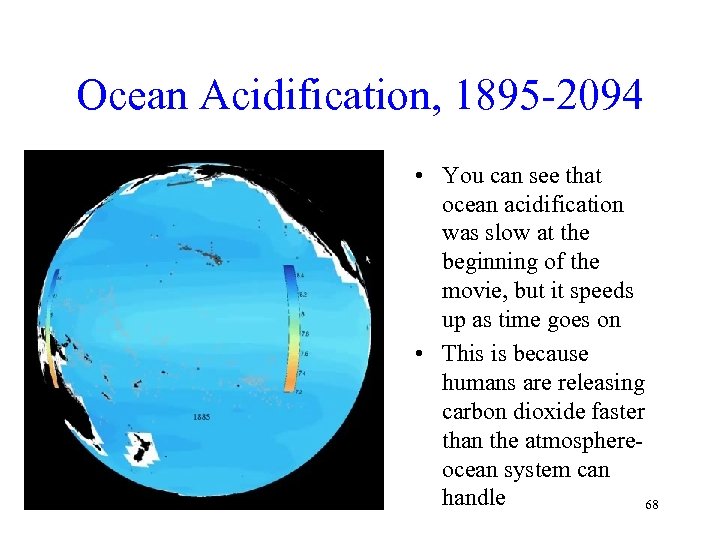 Ocean Acidification, 1895 -2094 • You can see that ocean acidification was slow at the beginning of the movie, but it speeds up as time goes on • This is because humans are releasing carbon dioxide faster than the atmosphereocean system can handle 68
Ocean Acidification, 1895 -2094 • You can see that ocean acidification was slow at the beginning of the movie, but it speeds up as time goes on • This is because humans are releasing carbon dioxide faster than the atmosphereocean system can handle 68
 Omega • Ocean acidification also decreases the number of carbonate ions in seawater • Scientists often track ocean acidification by measuring “omega” • This is the saturation state of calcium carbonate minerals, which is shown in the movie (next slide) and is important because carbonate ions are the building blocks that marine animals like corals, clams, and some algae use to make hard shells and skeletons 69
Omega • Ocean acidification also decreases the number of carbonate ions in seawater • Scientists often track ocean acidification by measuring “omega” • This is the saturation state of calcium carbonate minerals, which is shown in the movie (next slide) and is important because carbonate ions are the building blocks that marine animals like corals, clams, and some algae use to make hard shells and skeletons 69
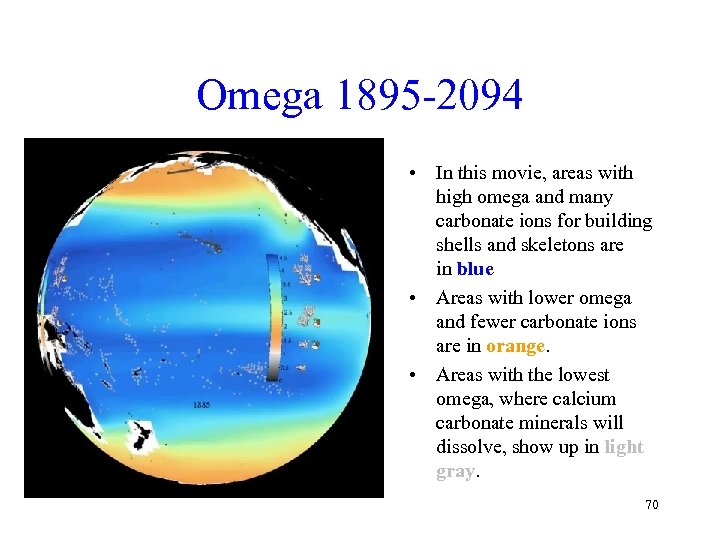 Omega 1895 -2094 • In this movie, areas with high omega and many carbonate ions for building shells and skeletons are in blue • Areas with lower omega and fewer carbonate ions are in orange. • Areas with the lowest omega, where calcium carbonate minerals will dissolve, show up in light gray. 70
Omega 1895 -2094 • In this movie, areas with high omega and many carbonate ions for building shells and skeletons are in blue • Areas with lower omega and fewer carbonate ions are in orange. • Areas with the lowest omega, where calcium carbonate minerals will dissolve, show up in light gray. 70
 Effect on Coral Reefs • Australian scientists discuss ocean acidification’s effects 71
Effect on Coral Reefs • Australian scientists discuss ocean acidification’s effects 71
 Google Earth Tour: Ocean Acidification • “This Google Earth Tour, narrated by Dan Laffoley from the International Union for Conservation of Nature (IUCN), who is Chair of Europe's Ocean Acidification Reference User Group, takes us on a global journey to understand what impact carbon dioxide has on ocean chemistry. It explores the phenomenon of ocean acidification and explains why even small changes to ocean chemistry could have profound implications for marine life and future economic activities. ” 72
Google Earth Tour: Ocean Acidification • “This Google Earth Tour, narrated by Dan Laffoley from the International Union for Conservation of Nature (IUCN), who is Chair of Europe's Ocean Acidification Reference User Group, takes us on a global journey to understand what impact carbon dioxide has on ocean chemistry. It explores the phenomenon of ocean acidification and explains why even small changes to ocean chemistry could have profound implications for marine life and future economic activities. ” 72
 Google Earth Tour cont. • “… first presented at the 3 rd Symposium on the Ocean in a High CO 2 World in Monterey in September 2012 and was prepared in partnership with Jenifer Austin Foulkes (Google), with script by Owen Gaffney (International Geosphere and Biosphere Programme) and Dan Laffoley. The animated sequence of ocean acidification through to the year 2300 was created using data provided by the Max Planck Institute for Meteorology www. mpimet. mpg. de (courtesy Dr. Tatiana Ilyina) and the visualization tools of the German Climate Computing Center www. dkrz. de (courtesy Dr. Michael Böttinger). ” 73
Google Earth Tour cont. • “… first presented at the 3 rd Symposium on the Ocean in a High CO 2 World in Monterey in September 2012 and was prepared in partnership with Jenifer Austin Foulkes (Google), with script by Owen Gaffney (International Geosphere and Biosphere Programme) and Dan Laffoley. The animated sequence of ocean acidification through to the year 2300 was created using data provided by the Max Planck Institute for Meteorology www. mpimet. mpg. de (courtesy Dr. Tatiana Ilyina) and the visualization tools of the German Climate Computing Center www. dkrz. de (courtesy Dr. Michael Böttinger). ” 73
 Google Earth Tour Video 74
Google Earth Tour Video 74
 Acid Test: The Global Challenge of Ocean Acidification • NRDC video featuring Sigourney Weaver, originally aired on Discovery Planet Green, copyright 2009 75
Acid Test: The Global Challenge of Ocean Acidification • NRDC video featuring Sigourney Weaver, originally aired on Discovery Planet Green, copyright 2009 75


