d00c1aa86386b61435e9fe781f417da3.ppt
- Количество слайдов: 74
Acid-Base Balance Interactive Tutorial Emily Phillips MSN 621 Spring 2009 E-mail: emmalemma. RN@hotmail. com All images imported from Microsoft Clipart & Yahoo Image gallery

How to navigate this tutorial: o To advance to the next slide click on the box o To return to the previous slide click on the box o To return to the Main Menu: click the box o Hover underlined text for a definition/explanation o To return to the last slide viewed click on the button o Click the for additional information

Objectives: o Define acid base balance/imbalance o Explain the pathophysiology of organs involved in acid base balance/imbalance o Identify normal/abnormal and compensated/uncompensated lab values o Explain symptoms related to acid base imbalances and compensated vs. uncompensated o Appropriate interventions and expected outcomes

Main Menu: Acid-Base Pretest The Buffer Systems Acid-Base Review test Metabolic Distubances Respiratory Disturbances Acid-Base Compensation Diagnostic Lab Values ABG Interpretation & Case Studies
Acid-Base Pretest: o What is the normal range for arterial blood p. H? 7. 38 – 7. 46 7. 40 – 7. 52 7. 35 – 7. 45

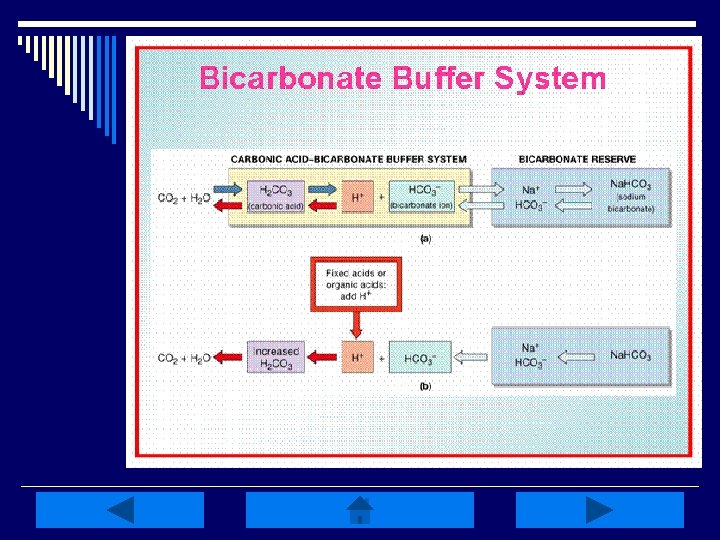
Acid-Base Pretest: o What 2 extracellular substances work together to regulate p. H? Sodium bicarbonate & carbonic acid Carbonic acid & bicarbonate Acetic acid & carbonic acid

Acid-Base Pretest: o Characterize an acid & a based on the choices below. Acids release hydrogen (H+) ions & bases accept H+ ions. Acids accept H+ ions & bases release H+ ions Both acids & bases can release & accept H+ ions

Acid-Base Pretest: o Buffering is a normal body mechanism that occurs rapidly in response to acidbase disturbances in order to prevent changes in what? HCO 3 - H 2 CO 3 H+
Acid-Base Pretest: o What are the two systems in the body that work to regulate p. H in acid-base balance & which one works fastest? The Respiratory & Renal systems Renal The Respiratory & Renal systems Respiratory The Renal & GI systems Renal
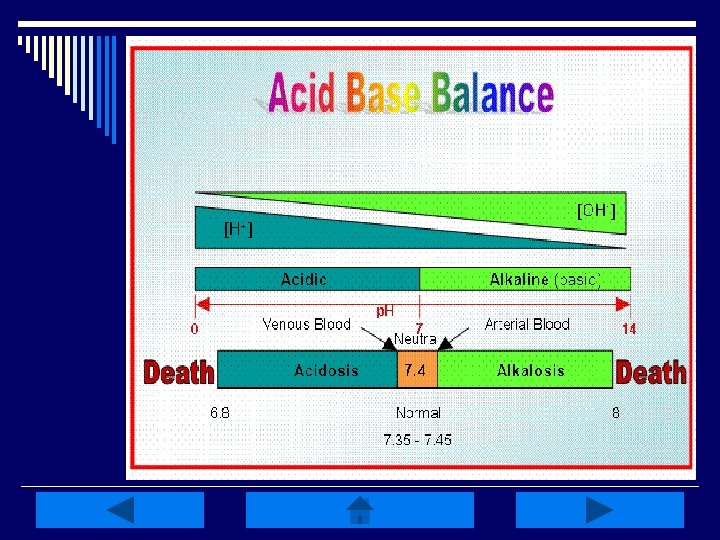
Acid-Base Balance: o Homeostasis of bodily fluids at a normal arterial blood p. H o p. H is regulated by extracellular carbonic acid (H 2 CO 3) and bicarbonate (HCO 3 -) o Acids are molecules that release hydrogen ions (H+) o A base is a molecule that accepts or combines with H+ ions


Acids and Bases can be strong or weak: o A strong acid or base is one that dissociates completely in a solution - HCl, Na. OH, and H 2 SO 4 o A weak acid or base is one that dissociates partially in a solution -H 2 CO 3, C 3 H 6 O 3, and CH 2 O

The Body and p. H: o Homeostasis of p. H is controlled through Protein Buffer system HCO 3 Buffer system K+ - H + Exchange extracellular & intracellular buffering systems o Respiratory: eliminate CO 2 o Renal: conserve HCO 3 - and eliminate H+ ions o Electrolytes: composition of extracellular (ECF) & intracellular fluids (ICF) - ECF is maintained at 7. 40

Quick Review: Click the Boxes A donator of H+ ions An Acid is: w/ p. H <7. 0 An acceptor of H+ A Base is: ions w/ p. H >7. 0 Regulated by EC p. H is: H 2 CO 3 & HCO 3 Controlled by EC p. H is: & IC buffer systems Eliminates CO 2 Conserves HCO 3 Renal System: Eliminates H+ ions Respiratory System:
Respiratory Control Mechanisms: o Works within minutes to control p. H; maximal in o o 12 -24 hours Only about 50 -75% effective in returning p. H to normal Excess CO 2 & H+ in the blood act directly on respiratory centers in the brain CO 2 readily crosses blood-brain barrier reacting w/ H 2 O to form H 2 CO 3 splits into H+ & HCO 3 - & the H+ stimulates an increase or decrease in respirations

Renal Control Mechanisms: o Don’t work as fast as the respiratory system; function for days to restore p. H to, or close to, normal o Regulate p. H through excreting acidic or alkaline urine; excreting excess H+ & regenerating or reabsorbing HCO 3 o Excreting acidic urine decreases acid in the EC fluid & excreting alkaline urine removes base H+ elimination & HCO 3 conservation
Mechanisms of Acid-Base Balance: o The ratio of HCO 3 - base to the volatile H 2 CO 3 Phosphate Buffer system Ammonia Buffer system determines p. H o Concentrations of volatile H 2 CO 3 are regulated by changing the rate & depth of respiration o Plasma concentration of HCO 3 - is regulated by the kidneys via 2 processes: reabsorption of filtered HCO 3 - & generation of new HCO 3 -, or elimination of H+ buffered by tubular systems to maintain a luminal p. H of at least 4. 5

Acid-Base Balance Review test: o The kidneys regulate p. H by excreting HCO 3 - and retaining or regenerating H+ TRUE FALSE

Acid-Base Review test: o H 2 CO 3 splits into HCO 3 - & H+ & it is the H+ that stimulates either an increase or decrease in the rate & depth of respirations. TRUE FALSE

Acid-Base Review test: o Plasma concentration of HCO 3 - is controlled by the kidneys through reabsorption/regeneration of HCO 3 -, or elimination of buffered H+ via the tubular systems. TRUE FALSE
Acid-Base Review test: o The ratio of H+ to HCO 3 - determines p. H. TRUE FALSE

Acid-Base Review test: o Secreted H+ couples with filtered HCO 3 - & CO 2 & H 2 O result. TRUE FALSE
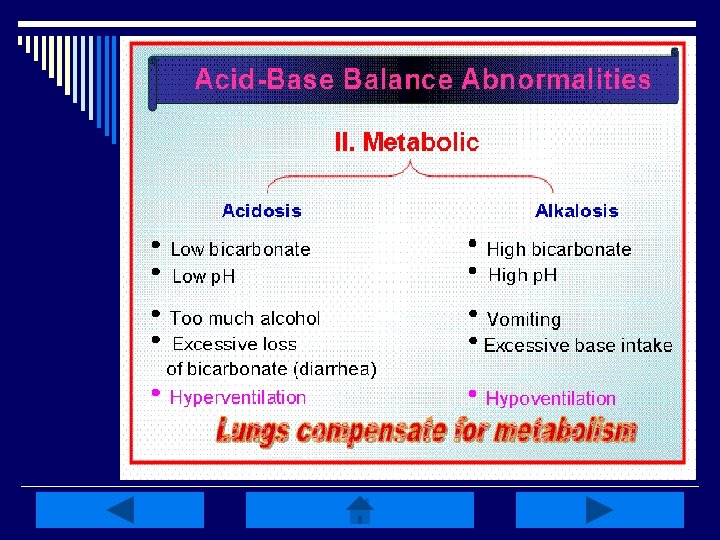
Metabolic Disturbances: o Alkalosis: elevated HCO 3 - (>26 m. Eq/L) n Causes include: Cl- depletion (vomiting, prolonged nasogastric suctioning), Cushing’s syndrome, K+ deficiency, massive blood transfusions, ingestion of antacids, etc. o Acidosis: decreased HCO 3 - (<22 m. Eq/L) n Causes include: DKA, shock, sepsis, renal failure, diarrhea, salicylates (aspirin), etc. o Compensation is respiratory-related

Metabolic Alkalosis: o Caused by an increase in p. H (>7. 45) related to an excess in plasma HCO 3 n Caused by a loss of H+ ions, net gain in HCO 3 - , or loss of Cl- ions in excess of HCO 3 - o Most HCO 3 - comes from CO 2 produced during metabolic processes, reabsorption of filtered HCO 3 -, or generation of new HCO 3 - by the kidneys o Proximal tubule reabsorbs 99. 9% of filtered HCO 3 -; excess is excreted in urine

Metabolic Alkalosis Manifestations: o Signs & symptoms (s/sx) of volume depletion or hypokalemia o Compensatory hypoventilation, hypoxemia & respiratory acidosis o Neurological s/sx may include mental confusion, hyperactive reflexes, tetany and carpopedal spasm o Severe alkalosis (>7. 55) causes respiratory failure, dysrhthmias, seizures & coma

Treatment of Metabolic Alkalosis: o Correct the cause of the imbalance n May include KCl supplementation for K+/Cldeficits o Fluid replacement with 0. 9 normal saline or 0. 45 normal saline for s/sx of volume depletion o Intubation & mechanical ventilation may be required in the presence of respiratory failure

Metabolic Acidosis: o Primary deficit in base HCO 3 - (<22 m. Eq/L) and p. H (<7. 35) o Caused by 1 of 4 mechanisms n Increase in nonvolatile metabolic acids, decreased acid secretion by kidneys, excessive loss of HCO 3 -, or an increase in Cl- o Metabolic acids increase w/ an accumulation of lactic acid, overproduction of ketoacids, or drug/chemical anion ingestion
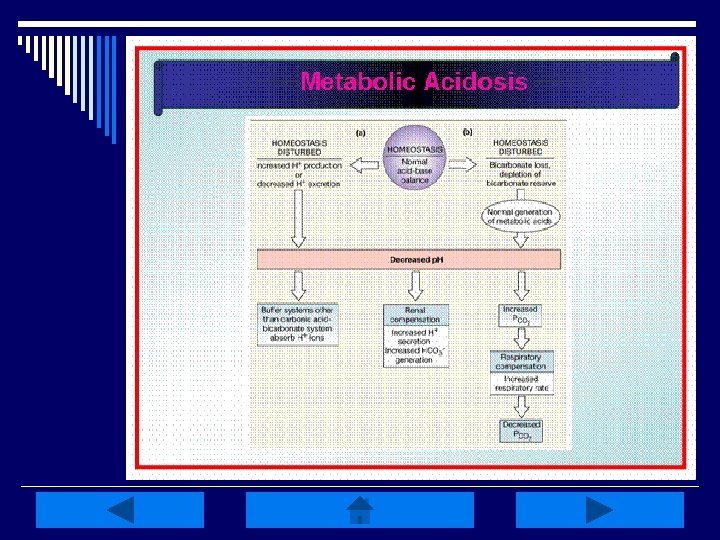

Metabolic Acidosis Manifestations: o Hyperventialtion (to reduce CO 2 levels), & dyspnea o Complaints of weakness, fatigue, general malaise, or a dull headache o Pt’s may also have anorexia, N/V, & abdominal pain o If the acidosis progresses, stupor, coma & LOC may decline o Skin is often warm & flush related to sympathetic stimulation

Treatment of Metabolic Acidosis: o Treat the condition that first caused the imbalance o Na. HCO 3 infusion for HCO 3 - <22 m. Eq/L o Restoration of fluids and treatment of electrolyte imbalances o Administration of supplemental O 2 or mechanical ventilation should the respiratory system begin to fail

Quick Metabolic Review: o Metabolic disturbances indicate an excess/deficit in HCO 3 - (<22 m. Eq/L or >26 m. Eq/L o Reabsorption of filtered HCO 3 - & generation of new HCO 3 - occurs in the kidneys o Respiratory system is the compensatory mechanism o ALWAYS treat the primary disturbance

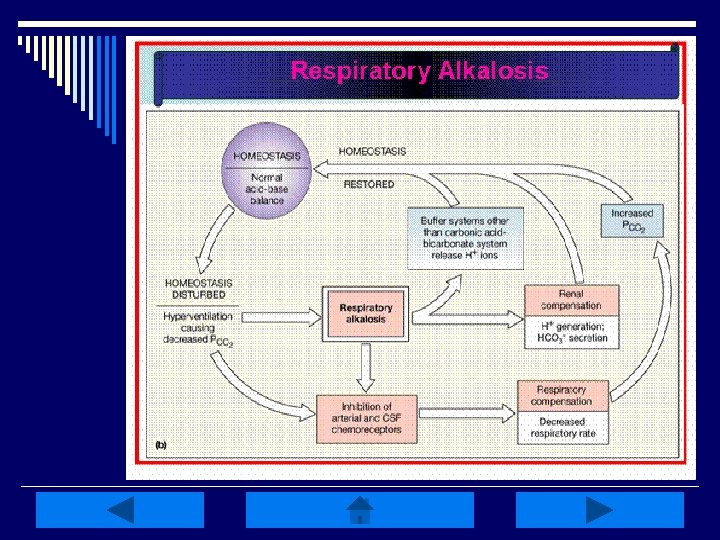
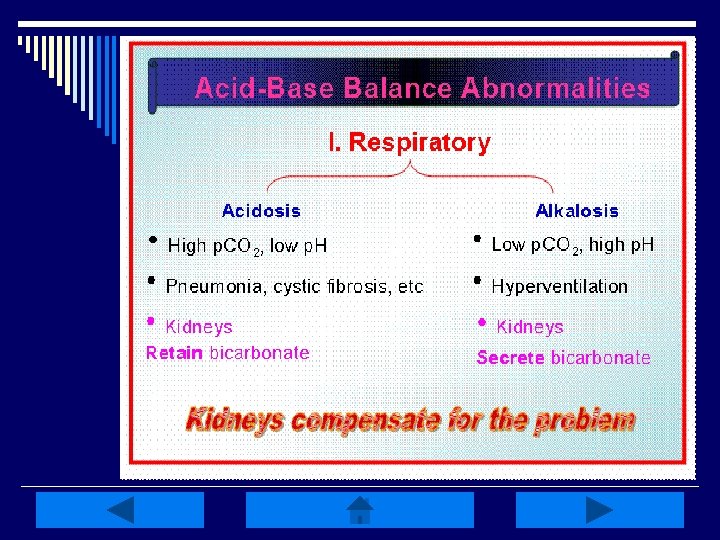
Respiratory Disturbances: o Alkalosis: low Pa. CO 2 (<35 mm. Hg) n Caused by HYPERventilation of any etiology (hypoxemia, anxiety, PE, pulmonary edema, pregnancy, excessive ventilation w/ mechanical ventilator, etc. ) o Acidosis: elevated Pa. CO 2 (>45 mm. Hg) n Caused by HYPOventilation of any etiology (sleep apnea, oversedation, head trauma, drug overdose, pneumothorax, etc. ) o Compensation is metabolic-related
Respiratory Alkalosis: o Characterized by an initial decrease in plasma Pa. CO 2 (<35 mm. Hg) or hypocapnia o Produces elevation of p. H (>7. 45) w/ a subsequent decrease in HCO 3 - (<22 m. Eq/L) o Caused by hyperventilation or RR in excess of what is necessary to maintain normal Pa. CO 2 levels
Respiratory Alkalosis Manifestations: o S/sx are associated w/ hyperexcitiability of the nervous system & decreases in cerebral blood flow o Increases protein binding of EC Ca+, reducing ionized Ca+ levels causing neuromuscular excitability o Lightheadedness, dizziness, tingling, numbness of fingers & toes, dyspnea, air hunger, palpitations & panic may result

Treatment of Respiratory Alkalosis: o Always treat the underlying/initial cause o Supplemental O 2 or mechanical ventilation may be required o Pt’s may require reassurance, rebreathing into a paper bag (for hyperventilation) during symptomatic attacks, & attention/treatment of psychological stresses.

Respiratory Acidosis: o Occurs w/ impairment in alveolar ventilation causing increased Pa. CO 2 (>45 mm. Hg), or hypercapnia, along w/ decreased p. H (<7. 35) o Associated w/ rapid rise in arterial Pa. CO 2 w/ minimal increase in HCO 3 - & large decreases in p. H o Causes include decreased respiratory drive, lung disease, or disorders of CW/respiratory muscles
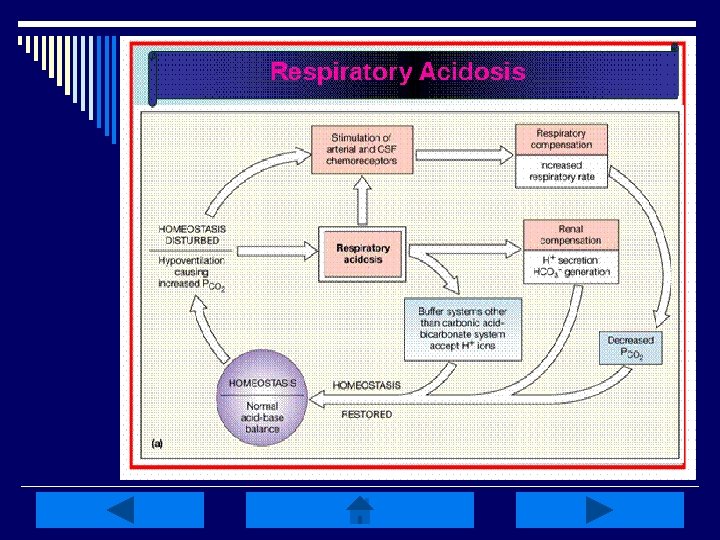

Respiratory Acidosis Manifestations: o Elevated CO 2 levels cause cerebral vasodilation resulting in HA, blurred vision, irritability, muscle twitching & psychological disturbances o If acidosis is prolonged & severe, increased CSF pressure & papilledema may result o Impaired LOC, lethargy/coma, paralysis of extremities, warm/flushed skin, weakness & tachycardia may also result

Treatment of Respiratory Acidosis: o Treatment is directed toward improving ventilation; mechanical ventilation may be necessary o Treat the underlying cause n Drug OD, lung disease, chest trauma/injury, weakness of respiratory muscles, airway obstruction, etc. o Eliminate excess CO 2

Quick Respiratory Review: o Caused by either low or elevated Pa. CO 2 levels (<35 or >45 mm. Hg) o Watch for HYPOventilation or HYPERventilation; mechanical ventilation may be required o Kidneys will compensate by conserving HCO 3 - & H+ o REMEMBER to treat the primary disturbance/underlying cause of the imbalance

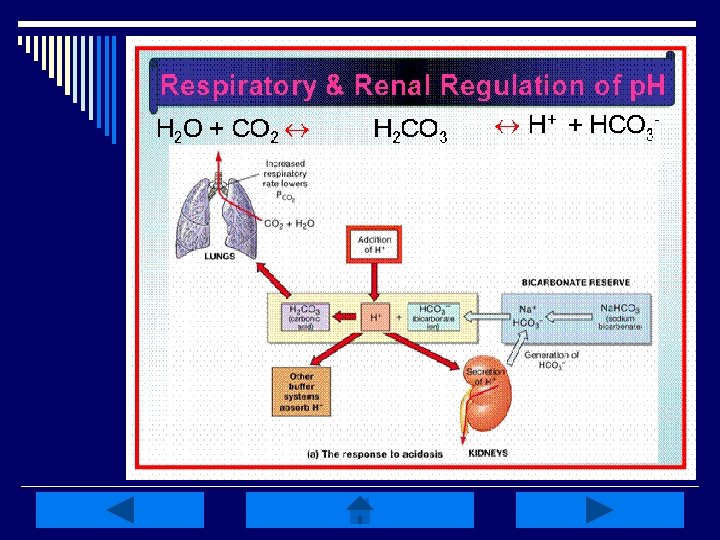
Compensatory Mechanisms: o Adjust the p. H toward a more normal level w/ out correcting the underlying cause o Respiratory compensation by increasing/decreasing ventilation is rapid, but the stimulus is lost as p. H returns toward normal o Kidney compensation by conservation of HCO 3 - & H+ is more efficient, but takes longer to recruit

Metabolic Compensation: o Results in pulmonary compensation beginning rapidly but taking time to become maximal o Compensation for Metabolic Alkalosis: n HYPOventilation (limited by degree of rise in Pa. CO 2) o Compensation for Metabolic Acidosis: n HYPERventilation to decrease Pa. CO 2 Begins in 1 -2 hrs, maximal in 12 -24 hrs

Respiratory Compensation: o Results in renal compensation which takes days to become maximal o Compensation for Respiratory Alkalosis: n Kidneys excrete HCO 3 - o Compensation for Respiratory Acidosis: n n Kidneys excrete more acid Kidneys increase HCO 3 - reabsorption

DIAGNOSTIC LAB VALUES & INTERPRETATION
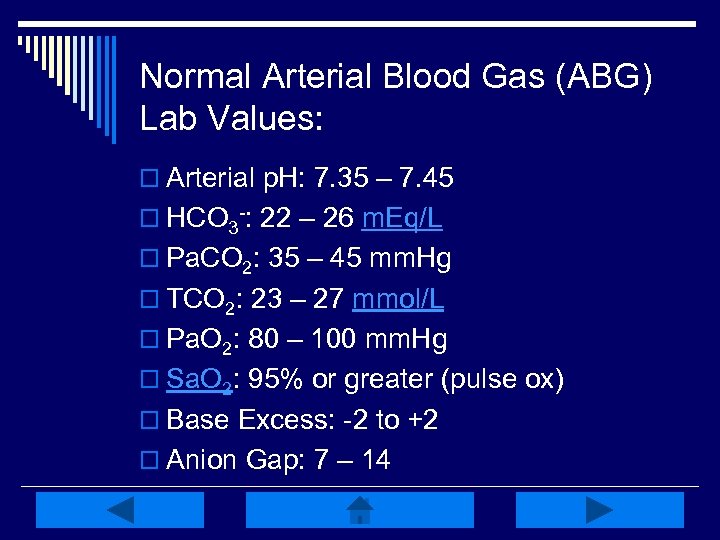
Normal Arterial Blood Gas (ABG) Lab Values: o Arterial p. H: 7. 35 – 7. 45 o HCO 3 -: 22 – 26 m. Eq/L o Pa. CO 2: 35 – 45 mm. Hg o TCO 2: 23 – 27 mmol/L o Pa. O 2: 80 – 100 mm. Hg o Sa. O 2: 95% or greater (pulse ox) o Base Excess: -2 to +2 o Anion Gap: 7 – 14
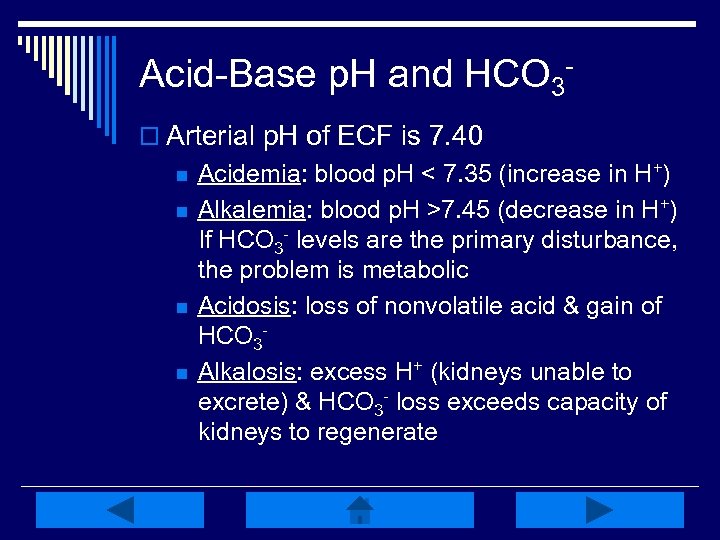
Acid-Base p. H and HCO 3 o Arterial p. H of ECF is 7. 40 n n Acidemia: blood p. H < 7. 35 (increase in H+) Alkalemia: blood p. H >7. 45 (decrease in H+) If HCO 3 - levels are the primary disturbance, the problem is metabolic Acidosis: loss of nonvolatile acid & gain of HCO 3 Alkalosis: excess H+ (kidneys unable to excrete) & HCO 3 - loss exceeds capacity of kidneys to regenerate
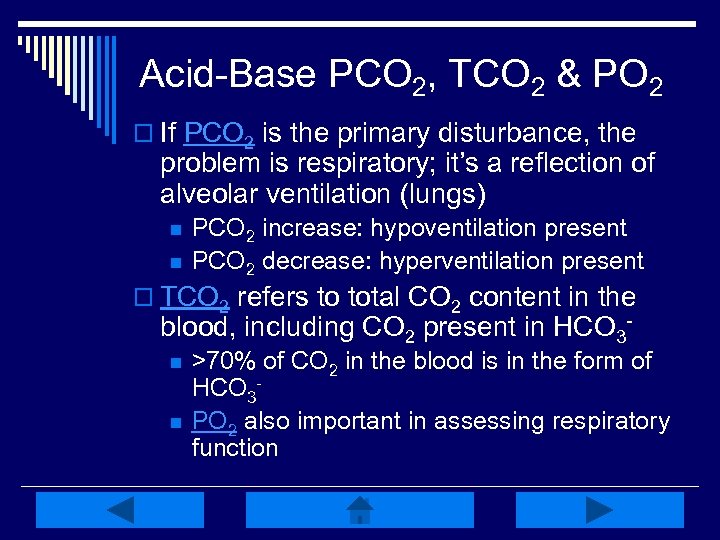
Acid-Base PCO 2, TCO 2 & PO 2 o If PCO 2 is the primary disturbance, the problem is respiratory; it’s a reflection of alveolar ventilation (lungs) n n PCO 2 increase: hypoventilation present PCO 2 decrease: hyperventilation present o TCO 2 refers to total CO 2 content in the blood, including CO 2 present in HCO 3 n n >70% of CO 2 in the blood is in the form of HCO 3 PO 2 also important in assessing respiratory function
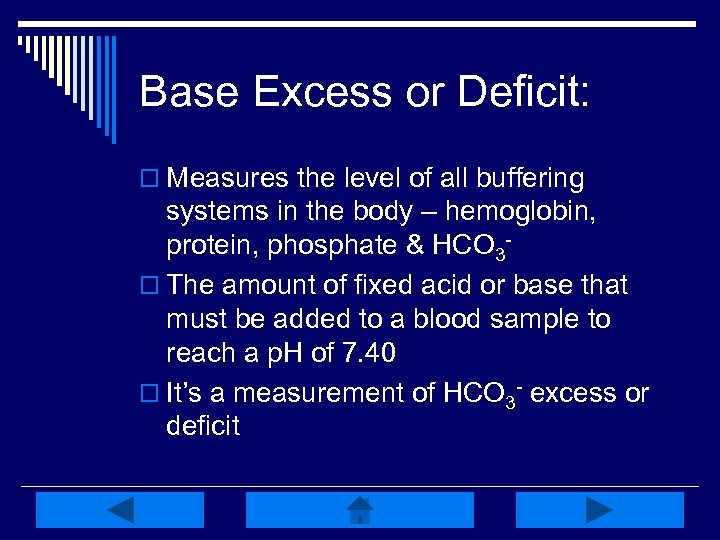
Base Excess or Deficit: o Measures the level of all buffering systems in the body – hemoglobin, protein, phosphate & HCO 3 o The amount of fixed acid or base that must be added to a blood sample to reach a p. H of 7. 40 o It’s a measurement of HCO 3 - excess or deficit

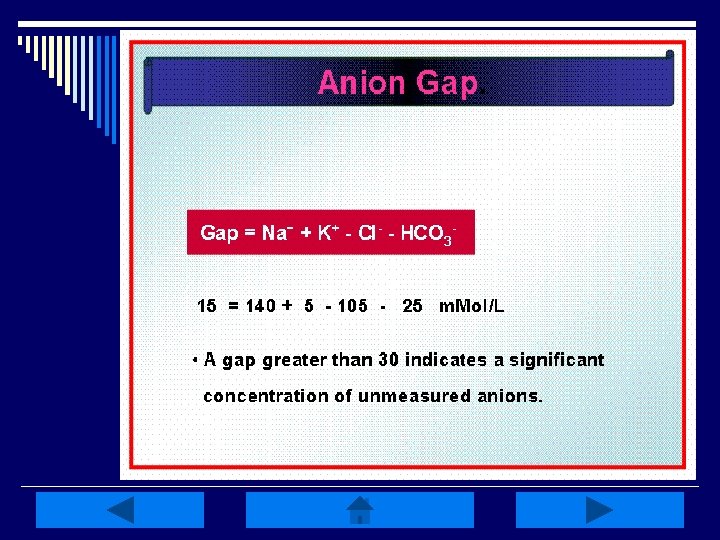
Anion Gap: o The difference between plasma concentration of Na+ & the sum of measured anions (Cl- & HCO 3 -) o Representative of the concentration of unmeasured anions (phosphates, sulfates, organic acids & proteins) o Anion gap of urine can also be measured via the cations Na+ & K+, & the anion Clto give an estimate of NH 4+ excretion

Anion Gap o The anion gap is increased in conditions such as lactic acidosis, and DKA that result from elevated levels of metabolic acids (metabolic acidosis) n n A low anion gap occurs in conditions that cause a fall in unmeasured anions (primarily albumin) OR a rise in unmeasured cations A rise in unmeasured cations is seen in hyperkalemia, hypercalcemia, hypermagnesemia, lithium intoxication or multiple myeloma
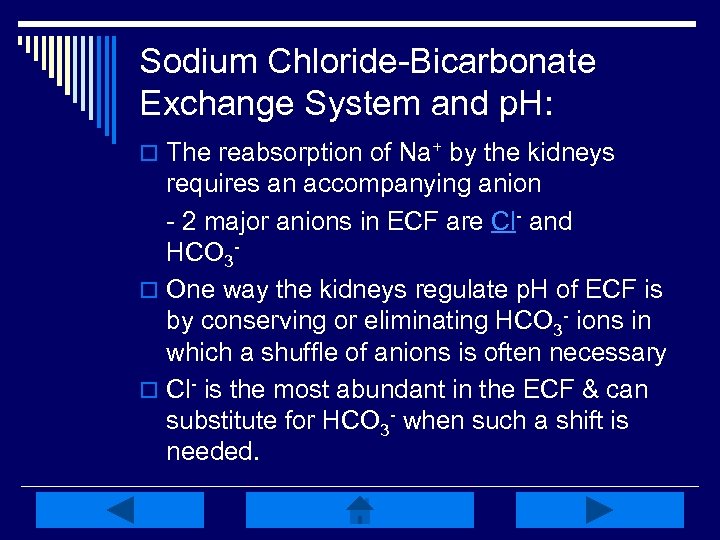
Sodium Chloride-Bicarbonate Exchange System and p. H: o The reabsorption of Na+ by the kidneys requires an accompanying anion - 2 major anions in ECF are Cl- and HCO 3 o One way the kidneys regulate p. H of ECF is by conserving or eliminating HCO 3 - ions in which a shuffle of anions is often necessary o Cl- is the most abundant in the ECF & can substitute for HCO 3 - when such a shift is needed.

Acid-Base Interpretation Practice: o Please use the following key to interpret the following ABG readings. o Click on the blue boxes to reveal the answers o Use the button to return to the key at any time o Or use the “Back to Key” button at the bottom left of the screen
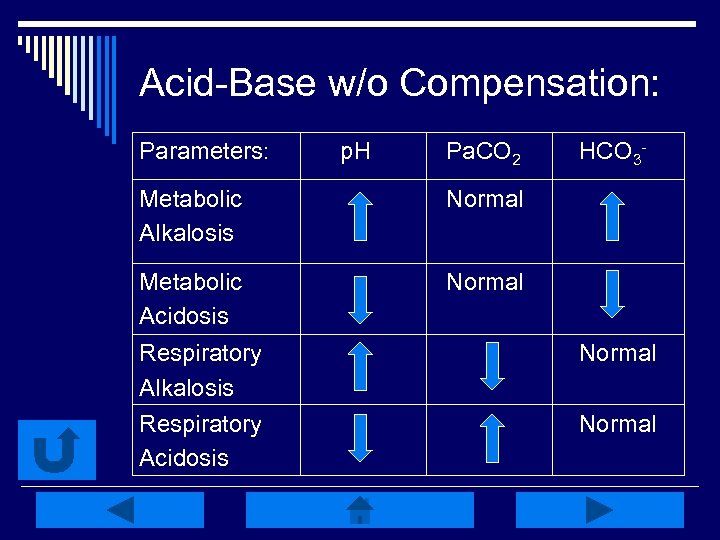
Acid-Base w/o Compensation: Parameters: p. H Pa. CO 2 Metabolic Alkalosis Normal Metabolic Acidosis HCO 3 - Normal Respiratory Alkalosis Respiratory Acidosis Normal

Interpretation Practice: o p. H: 7. 31 Resp. Acidosis Right! o Pa. CO 2: 48 Resp. Alkalosis Try Again o HCO 3 -: 24 Try Again Metabolic Acidosis o p. H: 7. 47 Resp. Alkalosis Try Again Metabolic Alkalosis Right! Metabolic Acidosis Try Again o Pa. CO 2 : 45 o HCO 3 - : 33 Back to Key

Interpretation Practice: o p. H: 7. 20 o HCO 3 -: 14 Try Again Metabolic Alkalosis Try Again Resp. Acidosis Metabolic Acidosis Right! o p. H: 7. 50 Try Again Metabolic Alkalosis o Pa. CO 2: 36 o Pa. CO 2 : 29 Right! Resp. Alkalosis o HCO 3 - -: 22 Resp. Acidosis Try Again Back to Key
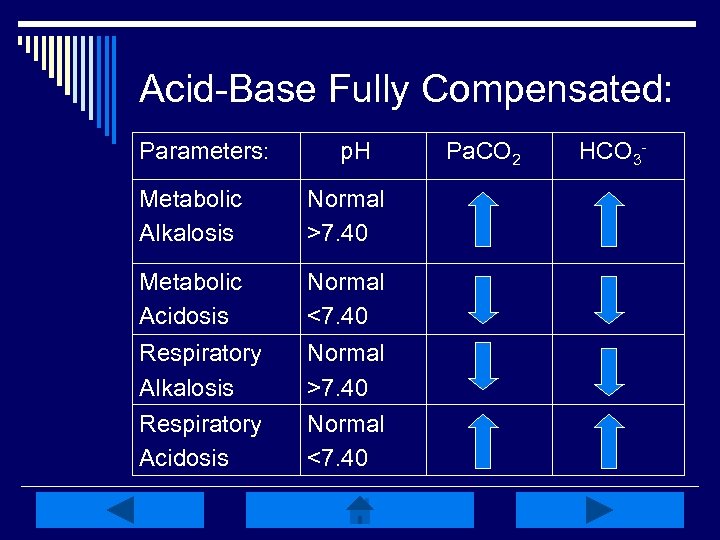
Acid-Base Fully Compensated: Parameters: p. H Metabolic Alkalosis Normal >7. 40 Metabolic Acidosis Normal <7. 40 Respiratory Alkalosis Respiratory Acidosis Normal >7. 40 Normal <7. 40 Pa. CO 2 HCO 3 -

Interpretation Practice: o p. H: 7. 36 o Pa. CO 2: 56 Compensated Resp. Alkalosis Try Again Compensated Again Acidosis Try Metabolic o HCO 3 -: 31. 4 Right! Compensated Resp. Acidosis o p. H: 7. 43 Compensated Resp. Alkalosis Right! o Pa. CO 2 : 32 Compensated Again Alkalosis Try Metabolic o HCO 3: 21 Try Metabolic Compensated Again Acidosis Back to Key
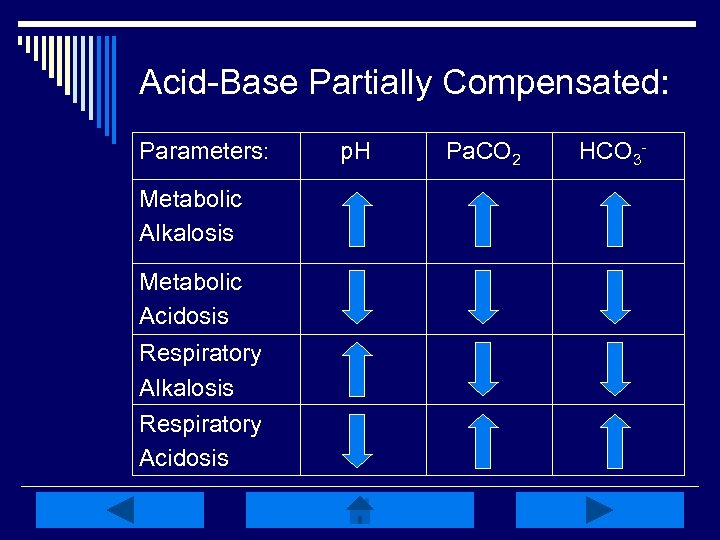
Acid-Base Partially Compensated: Parameters: Metabolic Alkalosis Metabolic Acidosis Respiratory Alkalosis Respiratory Acidosis p. H Pa. CO 2 HCO 3 -
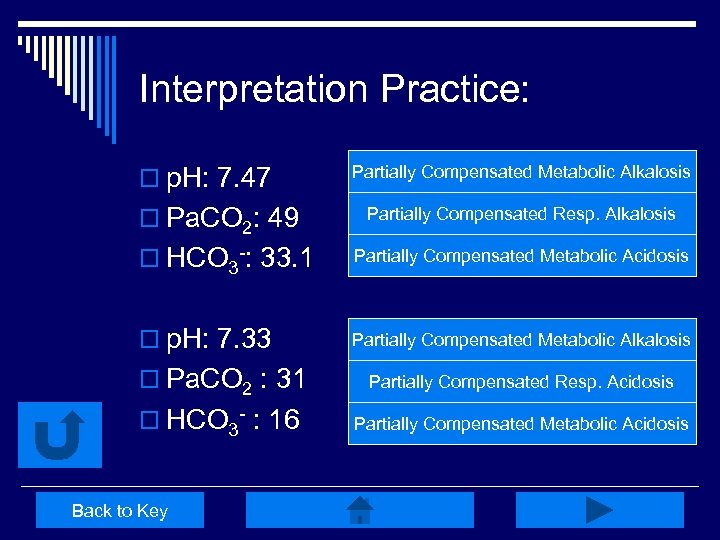
Interpretation Practice: o p. H: 7. 47 Partially Compensated Metabolic Alkalosis o HCO 3 -: 33. 1 Right! Partially Compensated Resp. Alkalosis Try Again Partially Compensated Metabolic Acidosis Try Again o p. H: 7. 33 Partially Compensated Metabolic Alkalosis Try Again o Pa. CO 2: 49 o Pa. CO 2 : 31 Partially Compensated Resp. Acidosis Try Again o HCO 3 - : 16 Right! Partially Compensated Metabolic Acidosis Back to Key
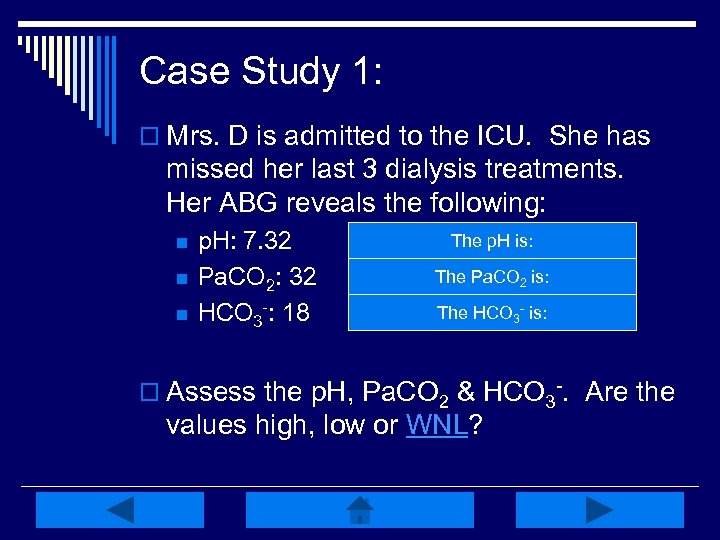
Case Study 1: o Mrs. D is admitted to the ICU. She has missed her last 3 dialysis treatments. Her ABG reveals the following: n n n p. H: 7. 32 Pa. CO 2: 32 HCO 3 -: 18 The 7. 35 -7. 45 Low, WNL =p. H is: The = 35 -45 mm. Hg Low, WNLPa. CO 2 is: The = 22 -26 m. Eq/L Low, WNL HCO 3 - is: o Assess the p. H, Pa. CO 2 & HCO 3 -. Are the values high, low or WNL?

Case Study 1 Continued: o What is Mrs. D’s acid-base imbalance? Partially Compensated Metabolic Acidosis Right! Try Again Fully Compensated Resp. Acidosis o Remember the difference between full & partial compensation. Go back & use the appropriate key if necessary.

Case Study 2: o Mr. M is a pt w/ chronic COPD. He is admitted to your unit pre-operatively. His admission lab work is as follows: n n n p. H: 7. 35 Pa. CO 2: 52 HCO 3 -: 50 The p. H is: WNL = 7. 35 -7. 45 The = 35 -45 mm. Hg High, WNLPa. CO 2 is: The = 22 -26 m. Eq/L High, WNL HCO 3 - is: o Assess the above labs. Are they abnormal or WNL?

Case Study 2 Continued: o What is Mr. M’s acid-base disturbance? Fully Compensated Metabolic Acidosis Try Again Fully Compensated Resp. Acidosis Right! o Think about appropriate interventions- if the problem is metabolic, the respiratory system compensates & vice versa

Case Study 3: o Miss L is a 32 year old female admitted w/ decreased LOC after c/o the “worst HA of her life. ” She is lethargic, but arouseable; diagnosed w/ a SAH. Her ABG reads: n n n p. H: 7. 48 Pa. CO 2: 32 HCO 3 -: 25 The 7. 35 -7. 45 High; WNL =p. H is: The = 35 -45 mm. Hg Low; WNL Pa. CO 2 is: The = 22 -26 m. Eq/L High; WNL HCO 3 - is: o What is the significance of her ABG values?

Case Study 3 Continued: o What is Miss L’s imbalance? Resp. Alkalosis Right! Try Again Metabolic Alkalosis o Great Job! You’ve reached the end of the tutorial & I hope you found it helpful. Thank you!

REFERENCES: http: //www. healthline. com/galecontent/acid-basebalance? utm_medium=ask&utm_source=smart&utm_campaign=article &utm_term=Acid+Base+Equilibrium&ask_return=Acid-Base+Balance. Retrieved 3/5/09. Porth, C. M. (2005). Pathophysiology Concepts of Altered Health States (7 th ed. ). Philadelphia: Lippincott Williams & Wilkins. http: //en. wikipedia. org/wiki/Dissociation_(chemistry). Retrieved 3/6/09. http: //www. clt. astate. edu/mgilmore/pathophysiology/Acid and Base. ppt#1. Retrieved 3/6/09. http: //www. uhmc. sunysb. edu/internalmed/nephro/webpages/Part_E. htm. Retrieved 3/6/09. http: //medical-dictionary. thefreedictionary. com/Volatile+acid. Retrieved 3/6/09.

REFERENCES http: //wiki. answers. com/Q/How_does_the_phosphate_buffer_system_help_ in_maintaining_the_ph_of_our_body. Retrieved 3/10/09. Alspach, J. G. (1998). American Association of Critical-Care Nurses Core Curriculum for Critical Care Nursing (5 th ed. ). Philadelphia: Saunders. http: //medical-dictionary. thefreedictionary. com. Retrieved 4/14/09. Acid-Base Balance & Oxygenation Power Point. (2007). Milwaukee: Froedtert Lutheran Memorial Hospital Critical Care Class.
d00c1aa86386b61435e9fe781f417da3.ppt