ad144306cf9daf9c0413016efe4dc7fb.ppt
- Количество слайдов: 18
 ACCOAST A Comparison of prasugrel at the time of percutaneous C C oronary intervention Or as pretreatment At the time O A ST of diagnosis in patients with non-ST-elevation MI G Montalescot, L Bolognese, D Dudek, P Goldstein, C Hamm, JF Tanguay, JM ten Berg, DL Miller, TM Costigan, J Goedicke, J Silvain, P Angioli, J Legutko, M Niethammer, Z Motovska, JA Jakubowski, G Cayla, LO Visconti, E Vicaut, P Widimsky for the ACCOAST investigators COI DISCLOSURE FOR DR. MONTALESCOT are availalble @ http: //www. action-coeur. org
ACCOAST A Comparison of prasugrel at the time of percutaneous C C oronary intervention Or as pretreatment At the time O A ST of diagnosis in patients with non-ST-elevation MI G Montalescot, L Bolognese, D Dudek, P Goldstein, C Hamm, JF Tanguay, JM ten Berg, DL Miller, TM Costigan, J Goedicke, J Silvain, P Angioli, J Legutko, M Niethammer, Z Motovska, JA Jakubowski, G Cayla, LO Visconti, E Vicaut, P Widimsky for the ACCOAST investigators COI DISCLOSURE FOR DR. MONTALESCOT are availalble @ http: //www. action-coeur. org
 Trial conduct - Coordinating Center: ACTION Study Group - Institute of Cardiology – Pitié-Salpêtrière University Hospital, Paris, France - Sponsors: Daiichi-Sankyo Company, Ltd. and Eli Lilly and Company - Global Trial Operations: ICON Clinical Research and Quintiles Trial Operations: (site management), Inventiv (data management and statistical services), Tata Consultancy Services (statistical programming) - External Academic Statistical Center : ACTION Study Group Study • Executive Committee: G Montalescot (Chairman), L Bolognese, D Dudek, Executive Committee: P Goldstein, C Hamm, JF Tanguay, J ten Berg, P Widimsky • Endpoint Adjudication Committee: M Flather (Chairman), A Bardají, Endpoint Adjudication Committee: A Baumbach, M Dalby, A Kapur, F Philippe, P Sabouret, AF van den Heuvel, A Zaman • Data Safety Monitoring Board: M Bertrand (Chairman), C Di Mario, E Vicaut Data Safety Monitoring Board:
Trial conduct - Coordinating Center: ACTION Study Group - Institute of Cardiology – Pitié-Salpêtrière University Hospital, Paris, France - Sponsors: Daiichi-Sankyo Company, Ltd. and Eli Lilly and Company - Global Trial Operations: ICON Clinical Research and Quintiles Trial Operations: (site management), Inventiv (data management and statistical services), Tata Consultancy Services (statistical programming) - External Academic Statistical Center : ACTION Study Group Study • Executive Committee: G Montalescot (Chairman), L Bolognese, D Dudek, Executive Committee: P Goldstein, C Hamm, JF Tanguay, J ten Berg, P Widimsky • Endpoint Adjudication Committee: M Flather (Chairman), A Bardají, Endpoint Adjudication Committee: A Baumbach, M Dalby, A Kapur, F Philippe, P Sabouret, AF van den Heuvel, A Zaman • Data Safety Monitoring Board: M Bertrand (Chairman), C Di Mario, E Vicaut Data Safety Monitoring Board:
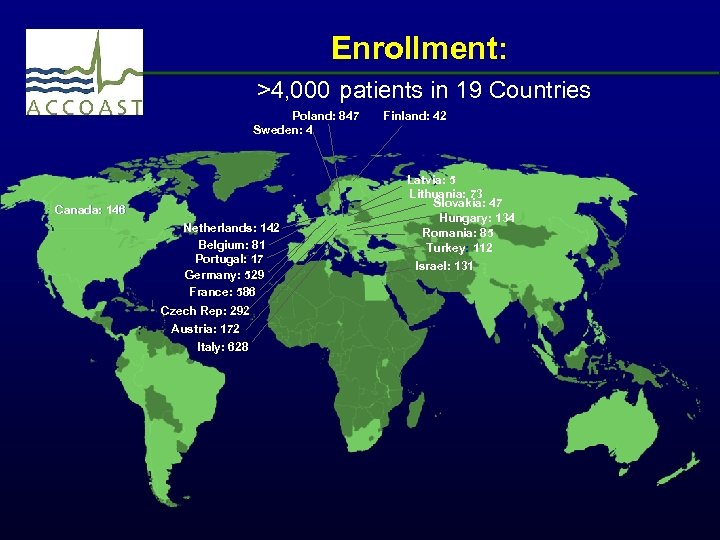 Enrollment: >4, 000 patients in 19 Countries Poland: 847 Sweden: 4 Canada: 146 Netherlands: 142 Belgium: 81 Portugal: 17 Germany: 529 France: 586 Czech Rep: 292 Austria: 172 Italy: 628 Finland: 42 Latvia: 5 Lithuania: 73 Slovakia: 47 Hungary: 134 Romania: 85 Turkey: 112 Israel: 131
Enrollment: >4, 000 patients in 19 Countries Poland: 847 Sweden: 4 Canada: 146 Netherlands: 142 Belgium: 81 Portugal: 17 Germany: 529 France: 586 Czech Rep: 292 Austria: 172 Italy: 628 Finland: 42 Latvia: 5 Lithuania: 73 Slovakia: 47 Hungary: 134 Romania: 85 Turkey: 112 Israel: 131
 Early Enrollment Completion • The DSMB after the last scheduled meeting (Nov 2012) recommended to stop enrollment, pretreatment being associated with an increased risk of major bleeding with no reduction in CV events (but no between-group imbalance in mortality) • Investigators and Regulatory agencies were immediately notified the enrollment to the ACCOAST study was stopped. • Follow-up of patients continued. The PI and ACCOAST study team remained blinded until the datalock. • Sample size calculation: 400 patients with a 1° EP event (approximately 4100 patients needed) End of study: 398 patients had an event (4033 patients enrolled)
Early Enrollment Completion • The DSMB after the last scheduled meeting (Nov 2012) recommended to stop enrollment, pretreatment being associated with an increased risk of major bleeding with no reduction in CV events (but no between-group imbalance in mortality) • Investigators and Regulatory agencies were immediately notified the enrollment to the ACCOAST study was stopped. • Follow-up of patients continued. The PI and ACCOAST study team remained blinded until the datalock. • Sample size calculation: 400 patients with a 1° EP event (approximately 4100 patients needed) End of study: 398 patients had an event (4033 patients enrolled)
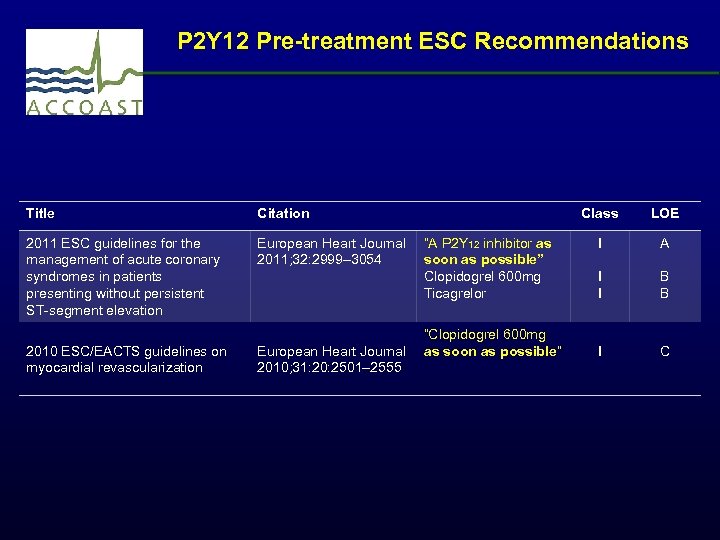 P 2 Y 12 Pre-treatment ESC Recommendations Title Citation 2011 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation European Heart Journal “A P 2 Y 12 inhibitor as 2011; 32: 2999– 3054 soon as possible” Clopidogrel 600 mg Ticagrelor 2010 ESC/EACTS guidelines on myocardial revascularization “Clopidogrel 600 mg European Heart Journal as soon as possible” 2010; 31: 20: 2501– 2555 Class LOE I A I I B B I C
P 2 Y 12 Pre-treatment ESC Recommendations Title Citation 2011 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation European Heart Journal “A P 2 Y 12 inhibitor as 2011; 32: 2999– 3054 soon as possible” Clopidogrel 600 mg Ticagrelor 2010 ESC/EACTS guidelines on myocardial revascularization “Clopidogrel 600 mg European Heart Journal as soon as possible” 2010; 31: 20: 2501– 2555 Class LOE I A I I B B I C
 ● Pre-treatment with aspirin and a P 2 Y 12 antagonist has been a class I recommendation and common practice for the treatment of NSTE-ACS ● However, no trial has ever randomized patients presenting with NSTE-ACS, invasively managed, to pre-treatment with clopidogrel, prasugrel or ticagrelor vs. no pre-treatment.
● Pre-treatment with aspirin and a P 2 Y 12 antagonist has been a class I recommendation and common practice for the treatment of NSTE-ACS ● However, no trial has ever randomized patients presenting with NSTE-ACS, invasively managed, to pre-treatment with clopidogrel, prasugrel or ticagrelor vs. no pre-treatment.
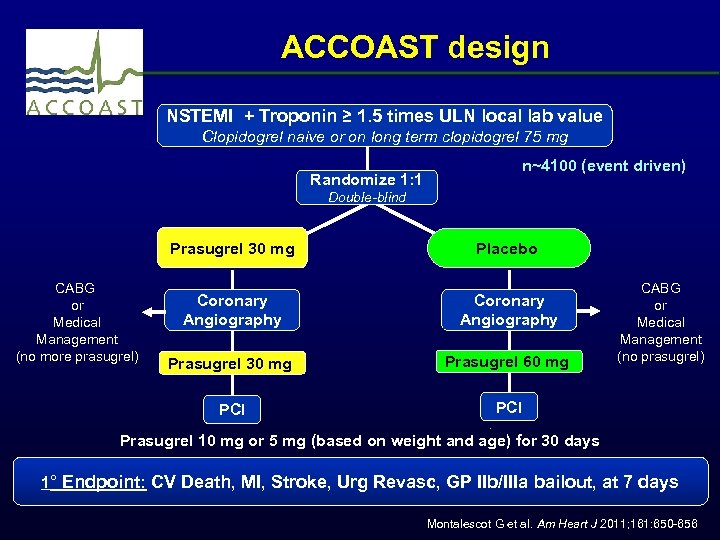 ACCOAST design NSTEMI + Troponin ≥ 1. 5 times ULN local lab value Clopidogrel naive or on long term clopidogrel 75 mg Randomize 1: 1 n~4100 (event driven) Double-blind Prasugrel 30 mg Coronary Angiography Prasugrel 30 mg Prasugrel 60 mg PCI CABG or Medical Management (no more prasugrel) Placebo PCI CABG or Medical Management (no prasugrel) Prasugrel 10 mg or 5 mg (based on weight and age) for 30 days 1° Endpoint: CV Death, MI, Stroke, Urg Revasc, GP IIb/IIIa bailout, at 7 days Montalescot G et al. Am Heart J 2011; 161: 650 -656
ACCOAST design NSTEMI + Troponin ≥ 1. 5 times ULN local lab value Clopidogrel naive or on long term clopidogrel 75 mg Randomize 1: 1 n~4100 (event driven) Double-blind Prasugrel 30 mg Coronary Angiography Prasugrel 30 mg Prasugrel 60 mg PCI CABG or Medical Management (no more prasugrel) Placebo PCI CABG or Medical Management (no prasugrel) Prasugrel 10 mg or 5 mg (based on weight and age) for 30 days 1° Endpoint: CV Death, MI, Stroke, Urg Revasc, GP IIb/IIIa bailout, at 7 days Montalescot G et al. Am Heart J 2011; 161: 650 -656
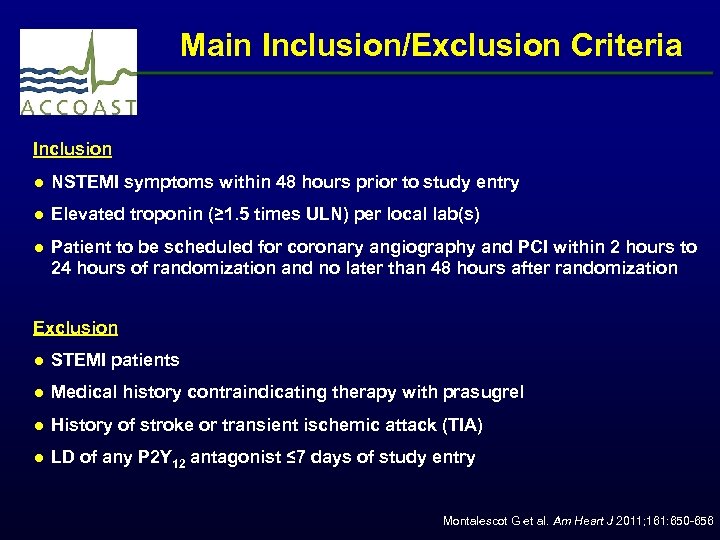 Main Inclusion/Exclusion Criteria Inclusion ● NSTEMI symptoms within 48 hours prior to study entry ● Elevated troponin (≥ 1. 5 times ULN) per local lab(s) ● Patient to be scheduled for coronary angiography and PCI within 2 hours to 24 hours of randomization and no later than 48 hours after randomization Exclusion ● STEMI patients ● Medical history contraindicating therapy with prasugrel ● History of stroke or transient ischemic attack (TIA) ● LD of any P 2 Y 12 antagonist ≤ 7 days of study entry Montalescot G et al. Am Heart J 2011; 161: 650 -656
Main Inclusion/Exclusion Criteria Inclusion ● NSTEMI symptoms within 48 hours prior to study entry ● Elevated troponin (≥ 1. 5 times ULN) per local lab(s) ● Patient to be scheduled for coronary angiography and PCI within 2 hours to 24 hours of randomization and no later than 48 hours after randomization Exclusion ● STEMI patients ● Medical history contraindicating therapy with prasugrel ● History of stroke or transient ischemic attack (TIA) ● LD of any P 2 Y 12 antagonist ≤ 7 days of study entry Montalescot G et al. Am Heart J 2011; 161: 650 -656
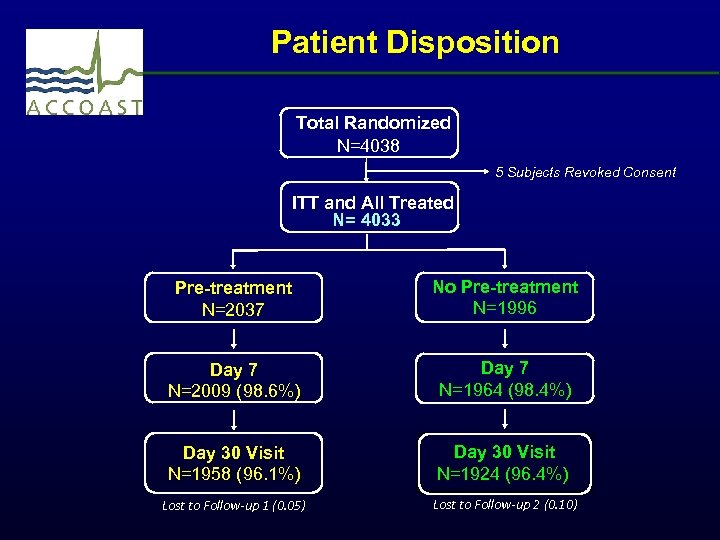 Patient Disposition Total Randomized N=4038 5 Subjects Revoked Consent ITT and All Treated N= 4033 Pre-treatment N=2037 No Pre-treatment N=1996 Day 7 N=2009 (98. 6%) Day 7 N=1964 (98. 4%) Day 30 Visit N=1958 (96. 1%) Day 30 Visit N=1924 (96. 4%) Lost to Follow-up 1 (0. 05) Lost to Follow-up 2 (0. 10)
Patient Disposition Total Randomized N=4038 5 Subjects Revoked Consent ITT and All Treated N= 4033 Pre-treatment N=2037 No Pre-treatment N=1996 Day 7 N=2009 (98. 6%) Day 7 N=1964 (98. 4%) Day 30 Visit N=1958 (96. 1%) Day 30 Visit N=1924 (96. 4%) Lost to Follow-up 1 (0. 05) Lost to Follow-up 2 (0. 10)
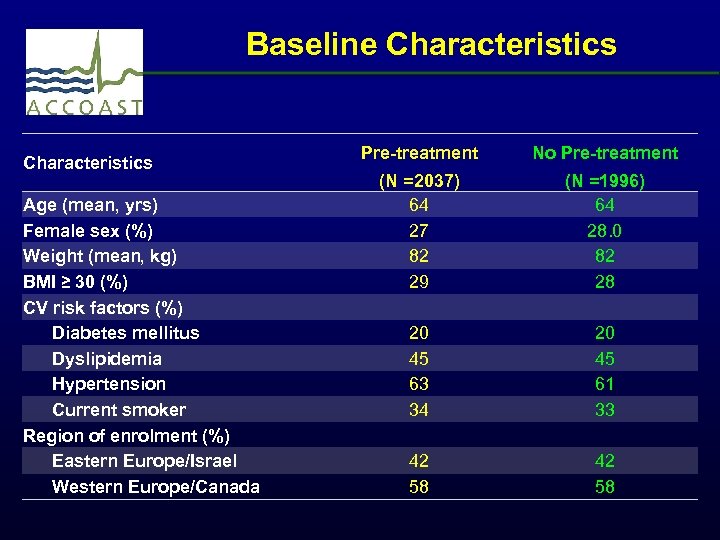 Baseline Characteristics Age (mean, yrs) Female sex (%) Weight (mean, kg) BMI ≥ 30 (%) CV risk factors (%) Diabetes mellitus Dyslipidemia Hypertension Current smoker Region of enrolment (%) Eastern Europe/Israel Western Europe/Canada Pre-treatment No Pre-treatment (N =2037) 64 27 82 29 20 45 63 34 42 58 (N =1996) 64 28. 0 82 28 20 45 61 33 42 58
Baseline Characteristics Age (mean, yrs) Female sex (%) Weight (mean, kg) BMI ≥ 30 (%) CV risk factors (%) Diabetes mellitus Dyslipidemia Hypertension Current smoker Region of enrolment (%) Eastern Europe/Israel Western Europe/Canada Pre-treatment No Pre-treatment (N =2037) 64 27 82 29 20 45 63 34 42 58 (N =1996) 64 28. 0 82 28 20 45 61 33 42 58
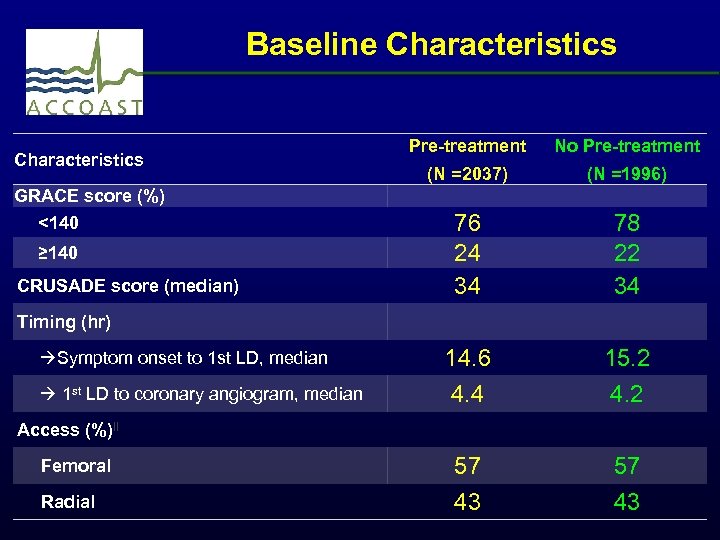 Baseline Characteristics GRACE score (%) <140 ≥ 140 CRUSADE score (median) Timing (hr) Symptom onset to 1 st LD, median 1 st LD to coronary angiogram, median Access (%)‖ Femoral Radial Pre-treatment No Pre-treatment (N =2037) (N =1996) 76 24 34 14. 6 4. 4 57 43 78 22 34 15. 2 4. 2 57 43
Baseline Characteristics GRACE score (%) <140 ≥ 140 CRUSADE score (median) Timing (hr) Symptom onset to 1 st LD, median 1 st LD to coronary angiogram, median Access (%)‖ Femoral Radial Pre-treatment No Pre-treatment (N =2037) (N =1996) 76 24 34 14. 6 4. 4 57 43 78 22 34 15. 2 4. 2 57 43
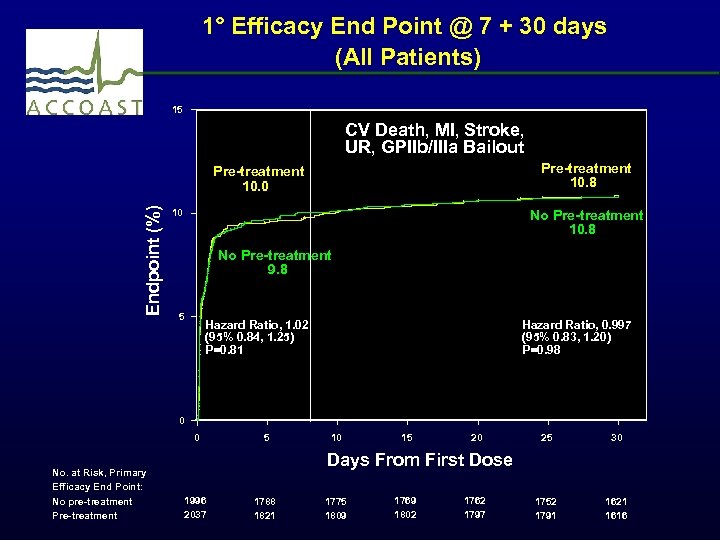 1° Efficacy End Point @ 7 + 30 days (All Patients) 15 CV Death, MI, Stroke, UR, GPIIb/IIIa Bailout Pre-treatment 10. 8 Endpoint (%) Pre-treatment 10. 0 10 No Pre-treatment 10. 8 No Pre-treatment 9. 8 5 Hazard Ratio, 1. 02 (95% 0. 84, 1. 25) P=0. 81 Hazard Ratio, 0. 997 (95% 0. 83, 1. 20) P=0. 98 0 0 No. at Risk, Primary Efficacy End Point: No pre-treatment Pre-treatment 5 10 15 20 25 30 1752 1791 1621 1616 Days From First Dose 1996 2037 1788 1821 1775 1809 1769 1802 1762 1797
1° Efficacy End Point @ 7 + 30 days (All Patients) 15 CV Death, MI, Stroke, UR, GPIIb/IIIa Bailout Pre-treatment 10. 8 Endpoint (%) Pre-treatment 10. 0 10 No Pre-treatment 10. 8 No Pre-treatment 9. 8 5 Hazard Ratio, 1. 02 (95% 0. 84, 1. 25) P=0. 81 Hazard Ratio, 0. 997 (95% 0. 83, 1. 20) P=0. 98 0 0 No. at Risk, Primary Efficacy End Point: No pre-treatment Pre-treatment 5 10 15 20 25 30 1752 1791 1621 1616 Days From First Dose 1996 2037 1788 1821 1775 1809 1769 1802 1762 1797
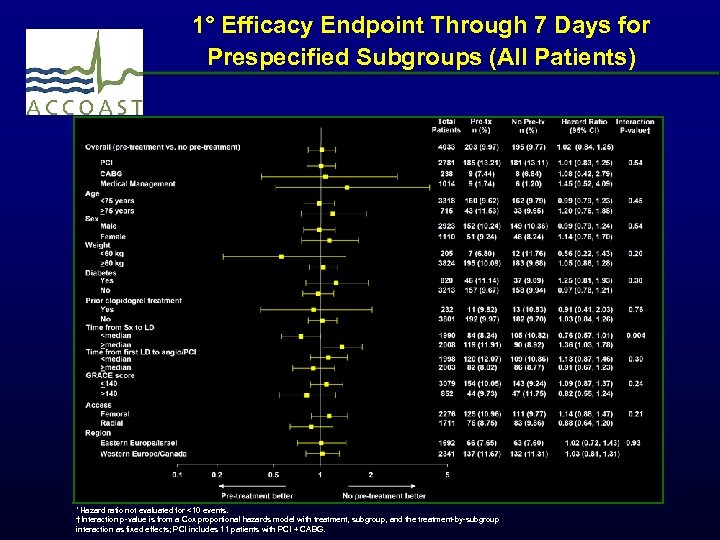 1° Efficacy Endpoint Through 7 Days for Prespecified Subgroups (All Patients) *Hazard ratio not evaluated for <10 events. †Interaction p-value is from a Cox proportional hazards model with treatment, subgroup, and the treatment-by-subgroup interaction as fixed effects; PCI includes 11 patients with PCI + CABG.
1° Efficacy Endpoint Through 7 Days for Prespecified Subgroups (All Patients) *Hazard ratio not evaluated for <10 events. †Interaction p-value is from a Cox proportional hazards model with treatment, subgroup, and the treatment-by-subgroup interaction as fixed effects; PCI includes 11 patients with PCI + CABG.
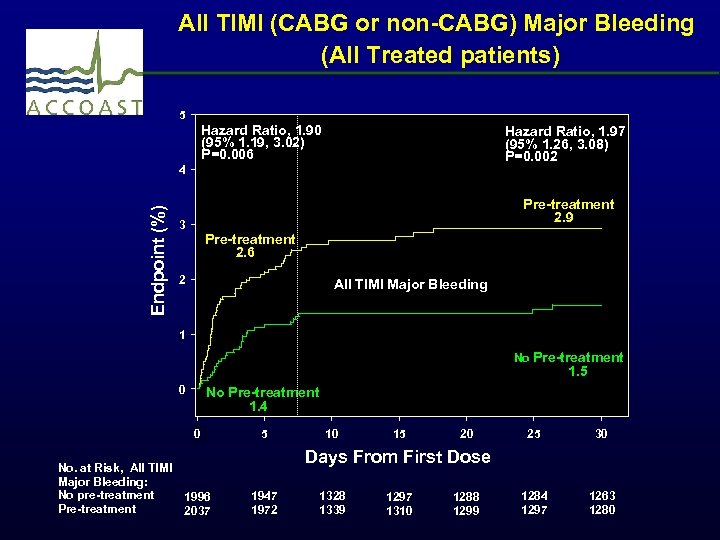 All TIMI (CABG or non-CABG) Major Bleeding (All Treated patients) 5 Hazard Ratio, 1. 90 (95% 1. 19, 3. 02) P=0. 006 Hazard Ratio, 1. 97 (95% 1. 26, 3. 08) P=0. 002 Endpoint (%) 4 Pre-treatment 2. 9 3 Pre-treatment 2. 6 2 All TIMI Major Bleeding 1 No Pre-treatment 1. 5 0 No Pre-treatment 1. 4 0 No. at Risk, All TIMI Major Bleeding: No pre-treatment 1996 Pre-treatment 2037 5 10 15 20 25 30 1284 1297 1263 1280 Days From First Dose 1947 1972 1328 1339 1297 1310 1288 1299
All TIMI (CABG or non-CABG) Major Bleeding (All Treated patients) 5 Hazard Ratio, 1. 90 (95% 1. 19, 3. 02) P=0. 006 Hazard Ratio, 1. 97 (95% 1. 26, 3. 08) P=0. 002 Endpoint (%) 4 Pre-treatment 2. 9 3 Pre-treatment 2. 6 2 All TIMI Major Bleeding 1 No Pre-treatment 1. 5 0 No Pre-treatment 1. 4 0 No. at Risk, All TIMI Major Bleeding: No pre-treatment 1996 Pre-treatment 2037 5 10 15 20 25 30 1284 1297 1263 1280 Days From First Dose 1947 1972 1328 1339 1297 1310 1288 1299
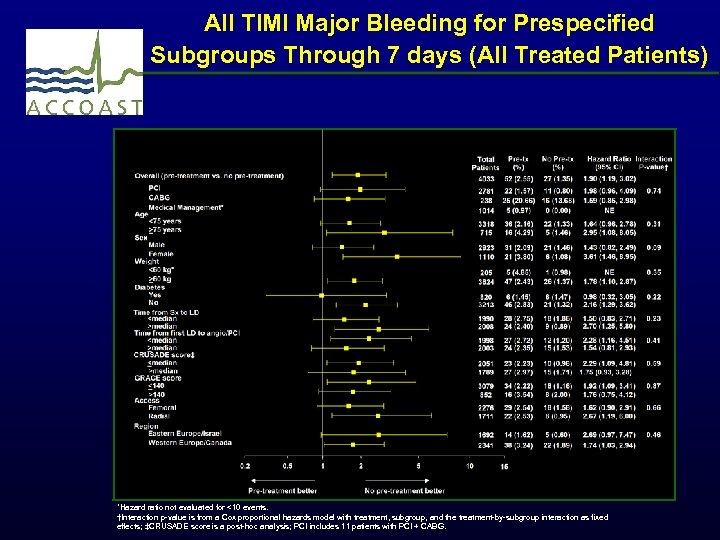 All TIMI Major Bleeding for Prespecified Subgroups Through 7 days (All Treated Patients) *Hazard ratio not evaluated for <10 events. †Interaction p-value is from a Cox proportional hazards model with treatment, subgroup, and the treatment-by-subgroup interaction as fixed effects; ‡CRUSADE score is a post-hoc analysis; PCI includes 11 patients with PCI + CABG.
All TIMI Major Bleeding for Prespecified Subgroups Through 7 days (All Treated Patients) *Hazard ratio not evaluated for <10 events. †Interaction p-value is from a Cox proportional hazards model with treatment, subgroup, and the treatment-by-subgroup interaction as fixed effects; ‡CRUSADE score is a post-hoc analysis; PCI includes 11 patients with PCI + CABG.
 Conclusions ● In NSTE-ACS patients managed invasively within 48 hours of admission, pre-treatment with prasugrel does not reduce major ischemic events through 30 days but increases major bleeding complications. ● The results are consistent among patients undergoing PCI supporting treatment with prasugrel once the coronary anatomy has been defined. ● No subgroup appears to have a favorable risk/benefit ratio of pre-treatment. ● Reappraisal of routine pre-treatment strategies in NSTEACS is needed.
Conclusions ● In NSTE-ACS patients managed invasively within 48 hours of admission, pre-treatment with prasugrel does not reduce major ischemic events through 30 days but increases major bleeding complications. ● The results are consistent among patients undergoing PCI supporting treatment with prasugrel once the coronary anatomy has been defined. ● No subgroup appears to have a favorable risk/benefit ratio of pre-treatment. ● Reappraisal of routine pre-treatment strategies in NSTEACS is needed.
 Thank you to all investigators in 19 countries, at 171 Centers
Thank you to all investigators in 19 countries, at 171 Centers



