850925565f005b63415ac2e39e3282f6.ppt
- Количество слайдов: 40

Access to Controlled Medicines Technical Briefing Seminar November 2009 Geneva, Switzerland Willem Scholten, Team Leader, Access to Controlled Medicines, Department of Essential Medicines and Pharmaceutical Policies

Overview of the presentation Part I: International drug control – – – Illicit drug market International drug conventions UN agencies involved in prevention of drug abuse Part II: Improving access to controlled medicines – – Medical uses Access to Controlled Medications Programme

Part I International drug control

Illicit drug use Worldwide, 2008: Problem drug users (severely dependent on drugs of abuse): 26 million 1 Injecting drug users: 16 million 2 à Protection of populations against abuse and dependence is necessary World illicit drug market ● Over $ 332 billion 3 or $ 45 - 280 billion 4 1. 2. 3. 1. UNODC, World Drug Report, 2008 2. Bradley, Global epidemiology of Injecting Drug Use and HIV, Lancet, 2008 3. UNODC, World drug Report, 2005 4. Peter Reuter, unpublished.

International Drug Control Conventions ● ● ● Single Convention on Narcotic Drugs (1961) United Nations Convention on Psychotropic Substances (1971) United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances (1988)

Conventions' Objectives 1961 and 1971 Conventions: Two goals: 1. Prevention of harm from drug dependence 2. Availability for rational medical use Public health interests are best served if all control measures aim at the optimum between medical availability and prevention of abuse

Convention principles 1961 and 1971 Conventions: ● ● Both have 4 lists of substances "schedules" Each schedule is related to a set of control measures
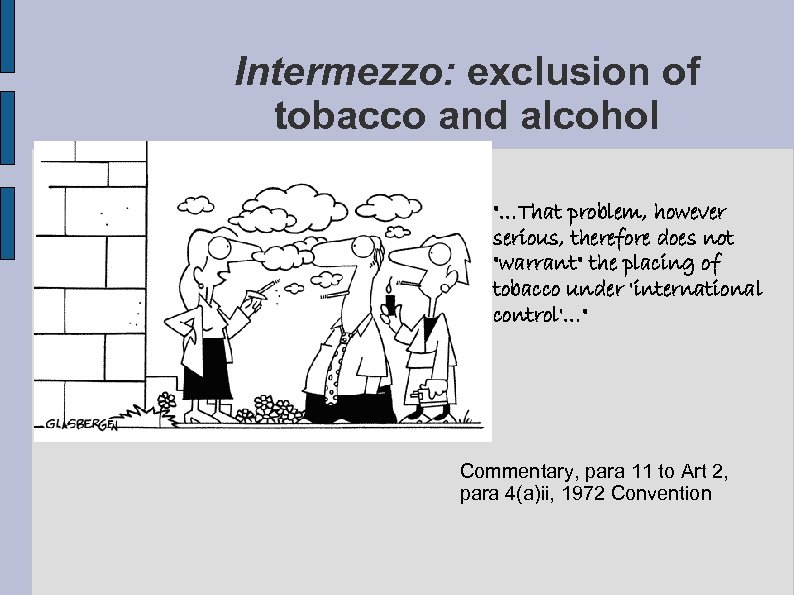
Intermezzo: exclusion of tobacco and alcohol "…That problem, however serious, therefore does not "warrant" the placing of tobacco under 'international control'…" Commentary, para 11 to Art 2, para 4(a)ii, 1972 Convention

UN agencies involved in the drug conventions Commission on Narcotic Drugs (CND) Assembly of the countries that are party to the conventions World Health Organization (WHO) Medical and scientific functions International Narcotics Control Board (INCB) Control body monitoring implementation of the conventions UN Office of Drugs and Crime (UNODC) Research, prevention and treatment of drug abuse

Role of WHO ● ● Nominates 3 out of 13 candidates to the INCB Recommends on the composition of the schedules (lists) with substances in the conventions WHO Expert Committee on Drug Dependence (ECDD; since 1949) ● Promoting access for medical use

Substance Review ● ● ● Pre-review, then critical review Recommendation by ECDD Note Verbale from Director-General WHO to Secretary-General UN Note Verbale from Secretary-General UN to Member States Decision by Commission on Narcotic Drugs (CND) – on adding, changing of schedule/convention, removing a substance

Critical Review ● ● ● Critical Review Report prepared by WHO Secretariat Questionnaire to Member States Report on questionnaire outcome Peer review by two experts Discussion in expert meeting Recommendation(s) and Expert Committee Report

On the WHO website: Guidelines for the WHO review of psychoactive substances for international control ECDD reports 1949 – 2006

Part II Improving access to controlled medicines

Millions have a drug problem photo: WHO/Marko Kokic They can't get any
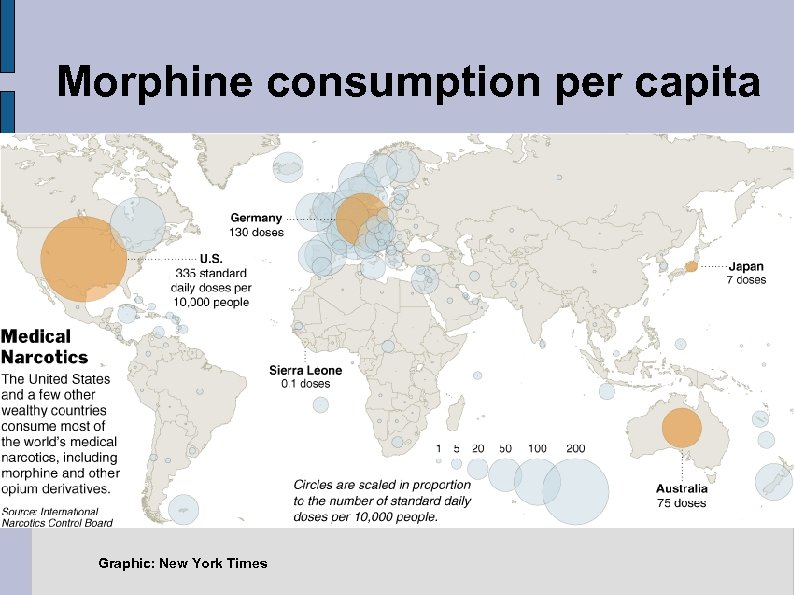
Morphine consumption per capita Graphic: New York Times
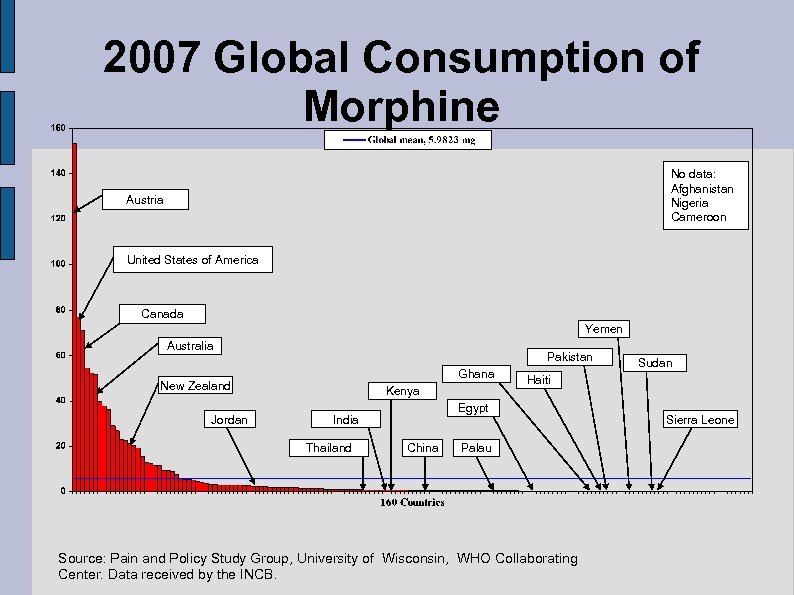
2007 Global Consumption of Morphine No data: Afghanistan Nigeria Cameroon Austria United States of America Canada Yemen Australia Pakistan Ghana New Zealand Jordan Kenya Thailand Haiti Egypt India China Sudan Palau Source: Pain and Policy Study Group, University of Wisconsin, WHO Collaborating Center. Data received by the INCB. Sierra Leone

Controlled medicines on the WHO EML – Opioid analgesics: Morphine moderate to severe pain – Long-acting opioid agonists: methadone, buprenorphine treatment of opioid dependence – Ergometrine and ephedrine emergency obstetrics – Benzodiazepines anxiolytics, hypnotics, antiepileptics – Phenobarbital antiepileptic
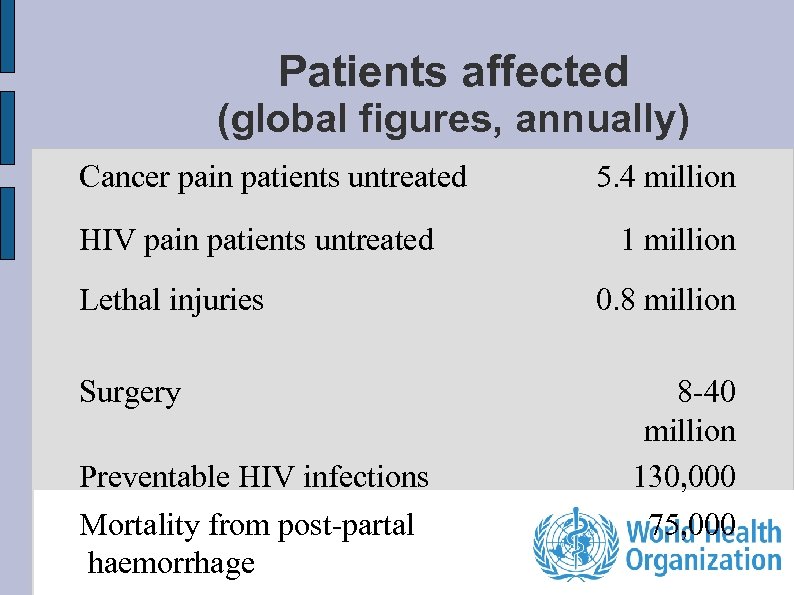
Patients affected (global figures, annually) Cancer pain patients untreated HIV pain patients untreated Lethal injuries Surgery Preventable HIV infections Mortality from post-partal haemorrhage 5. 4 million 1 million 0. 8 million 8 -40 million 130, 000 75, 000

Drug conventions Recognizing that the medical use of narcotic drugs continues to be indispensable for the relief of pain and suffering and that adequate provision must be made to ensure the availability of narcotic drugs for such purposes … (Preamble Single Conv. on Narcotic Drugs)

Reasons for low access to controlled medicines ● Excessive fear for dependence ● Excessive fear for diversion ● Neglected medical needs

Treatment with opioids a. Pain

Three Step Ladder WHO Three step ladder on cancer pain (1986) 1. Non-opioid + adjuvant e. g. paracetamol If pain persisting/increasing: 2. Weak acting opioid (e. g. codeine, tramadol) If pain persisting/increasing: 3. Strong acting opioid (e. g. morphine, methadone) Increase dosage until freedom from pain There is no maximum dose: the right dose is the dose that works
Opioid analgesics Used for all moderate to severe pain due to: • Cancer • AIDS/HIV • Chronic pain – Some exceptions • • Traffic and other accidents Myocardial infarction Sickle cell anaemia Surgery
A myth: Dependence from opioid analgesics ● Very low incidence – ● ● Many doctors claim dependence from treatment is non-existent Withdrawal is unequal to dependence Pain population very different from heroin user populations

Treatment with opioids b. Opioid Dependence

Long-Acting Opioid Agonist Therapy ● Methadone Maintenance Therapy (MMT) – – – ● Supervised administration of Methadone oral solution Dosage level high enough to stop heroin use Continuously Other modalities (e. g. buprenorphine: BMT)

Long-Acting Opioid Agonist Therapy ● ● To treat opioid dependence (which is a disease) Methadone less reinforcing then heroin Normalization of body responses and social life Interruption of transmission of – – – HIV Hepatitis C Virus (HCV) Other blood borne disease

WHO Treatment Guidelines ● ● WHO, 2009 www. who. int: > Programmes and projects > Substance abuse > Treatment of opioid dependence
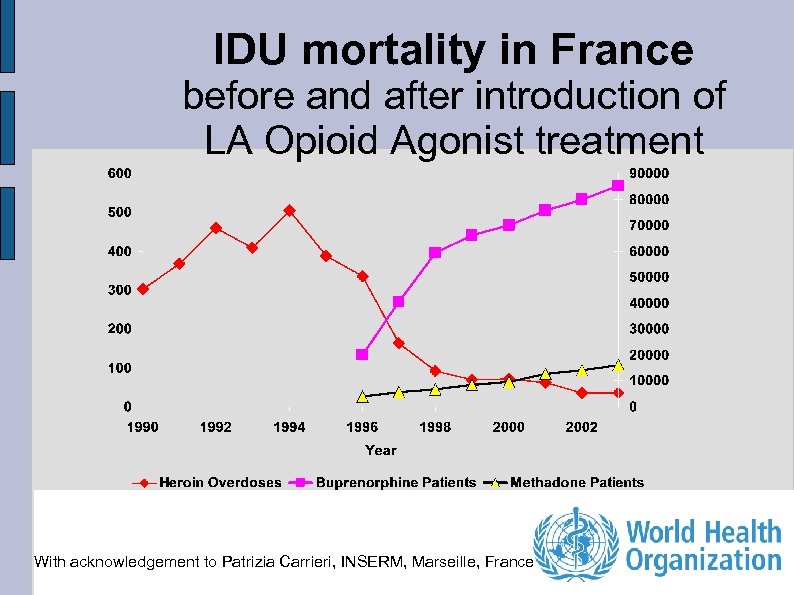
IDU mortality in France before and after introduction of LA Opioid Agonist treatment With acknowledgement to Patrizia Carrieri, INSERM, Marseille, France

Access to Controlled Medications Programme (ACMP)

Access to Controlled Medications Programme ● ● ● Response to Resolutions ECOSOC 2005/25 and WHA 58. 22 WHO Programme to improve access to controlled medicines Launched in 2007 by WHO and the INCB

Access to Controlled Medications Programme ● ● ● Addresses all medicines controlled under the international drug conventions Essential Medicines in particular Problems and solutions supposed to be very similar, giving opportunities – for finding allies – to prevent double work

ACMP Activities Normative work ● Guidelines ● Technical standards etcetera Country support mainly developing countries

Normative work ● ● ● Pain guidelines (all pain) WHO/INCB Manual for estimates Update of WHO Policy guidelines "Achieving Balance in Nat. Opioid Control Policies" Model legislation Guidelines treatment opioid dependence

Country support ● ● Situational analysis and drafting a plan Introduction of balanced policy – optimum for accessibility for medical use and prevention of dependence and abuse Model plan drafted with involvement of Mo. H Ghana, APCA and health care workers can easily be adapted to local needs elsewhere

Example: analysing barriers ● ● Policy barriers Regulatory and administrative barriers Attitudinal and educational barriers Supply barriers

Country support • Update of national essential medicines list • Oral morphine • Oral methadone • Update of National Medicines Policy Plan • Training of civil servants • Estimates/statistics • Support to health education institutions

Other tools – International Opioid Consumption Database • International Observatory End of Life Care, Lancaster, UK and WHO ACMP – Collecting global figures on actual needs and adequacy of opioid consumption • Article on figures for 2006 submitted • Working on 2007 and first trend analysis

Access to Controlled Medicines Willem Scholten, Pharm. D. , MPA Team Leader, Access to Controlled Medicines Essential Medicines and Pharmaceutical Policies World Health Organization Geneva, Switzerland scholtenw@who. int +41 22 79 15540
850925565f005b63415ac2e39e3282f6.ppt