01923b773111ea9c7268b690c774c8ef.ppt
- Количество слайдов: 18
 Access to ARV : Non Rational. . . Approach to Inappropriate Treatment Institute of Health Research Chulalongkorn University This is a part of the project to study The Information Needed to PHA for Decision Making about their Treatments
Access to ARV : Non Rational. . . Approach to Inappropriate Treatment Institute of Health Research Chulalongkorn University This is a part of the project to study The Information Needed to PHA for Decision Making about their Treatments
 Abstract: Access to Anti-Retroviral Drugs: PHA Non-Rational Approach to Inappropriate Treatment Authors : Palanuvej C. and Chaisorn W. Problem Statement: Since the emergence of the HIV/AIDS epidemic in Thailand in the 1990 s, anti-retroviral drugs (ARVa) have been promoted as the best alternative for treatment by health professionals, although access to ARVs was restricted to a few major hospitals in highly urbanized areas without subsidy for the high cost of the drugs. The belief in their life-saving efficacy and the economic stress of people having HIV/AIDS (PHA) generated non-rational approaches to ARV access and inappropriate treatment outcomes. In view of the current public health policy shift to subsidize ARV treatment and the introduction of locally produced low cost ARVs, the prevailing undercurrent of non-rational approaches to ARV treatment for PHA poses a real threat for the future of the PHA population. Objectives: To demonstrate the pattern of non-rational approaches to ARV treatment of PHA and the evidence- based public health policy implications for future benefits. Design: Key informant qualitative method. Setting and Population: Collection of data and information from key informants recruited through the network of those with PHA who are in contact with the HIV self help groups/organization, providing service on access to ARV treatment. The interviews were performed with the subject’s consent under a guarantee of anonymity. Results: The primary strategy for managing PHA was to be in HIV/AIDS drug projects of governmental, private or non profit organizations because of the medicinal subsidy. However, most projects were short term clinical trials of specific regimens, but those with PHA knew there were life-long treatment needs. Therefore, as the only way of complying with ARV regimens, they falsify the history of their illness / treatment in order to meet project inclusion criteria. Their therapies were then determined by seeking projects, year by year, and adjusting regimen dosages themselves to prolong their supplies of drugs during gaps in projects. Some skipped from a 2 drug to a 3 drugs project because they believed that more drugs were better. Peer supporters among those with PHA provided not only knowledge, hope and care, but also the medicines. Some stopped their ARV treatment or reduced their drug intake by themselves and sold the rest of their drugs to their peers or to others unable to enroll in the projects. Some sold the expensive drugs they got for free and used the cheaper ones instead (e. g. Efavirenz vs. Nevirapine). Overseas procurement markets for medicines including ARVs expanded PHA opportunities for access. They could purchase the drugs, via peer-to-peer brokers, from abroad, for example China or India where the drugs were cheaper. There were non-systematic controls for the drug procurement by those with PHA. Moreover, little knowledge about the ARVs and the dosage affected PHA decision making to non -rational access to and use of the ARV. Conclusions: Individual ability to improve access to ARVs increases the risk of non-rational use of drugs. Supply, education, health provider and especially systematic management must be improved expeditiously and dynamically to overcome the nationwide use of non-prescription ARVs.
Abstract: Access to Anti-Retroviral Drugs: PHA Non-Rational Approach to Inappropriate Treatment Authors : Palanuvej C. and Chaisorn W. Problem Statement: Since the emergence of the HIV/AIDS epidemic in Thailand in the 1990 s, anti-retroviral drugs (ARVa) have been promoted as the best alternative for treatment by health professionals, although access to ARVs was restricted to a few major hospitals in highly urbanized areas without subsidy for the high cost of the drugs. The belief in their life-saving efficacy and the economic stress of people having HIV/AIDS (PHA) generated non-rational approaches to ARV access and inappropriate treatment outcomes. In view of the current public health policy shift to subsidize ARV treatment and the introduction of locally produced low cost ARVs, the prevailing undercurrent of non-rational approaches to ARV treatment for PHA poses a real threat for the future of the PHA population. Objectives: To demonstrate the pattern of non-rational approaches to ARV treatment of PHA and the evidence- based public health policy implications for future benefits. Design: Key informant qualitative method. Setting and Population: Collection of data and information from key informants recruited through the network of those with PHA who are in contact with the HIV self help groups/organization, providing service on access to ARV treatment. The interviews were performed with the subject’s consent under a guarantee of anonymity. Results: The primary strategy for managing PHA was to be in HIV/AIDS drug projects of governmental, private or non profit organizations because of the medicinal subsidy. However, most projects were short term clinical trials of specific regimens, but those with PHA knew there were life-long treatment needs. Therefore, as the only way of complying with ARV regimens, they falsify the history of their illness / treatment in order to meet project inclusion criteria. Their therapies were then determined by seeking projects, year by year, and adjusting regimen dosages themselves to prolong their supplies of drugs during gaps in projects. Some skipped from a 2 drug to a 3 drugs project because they believed that more drugs were better. Peer supporters among those with PHA provided not only knowledge, hope and care, but also the medicines. Some stopped their ARV treatment or reduced their drug intake by themselves and sold the rest of their drugs to their peers or to others unable to enroll in the projects. Some sold the expensive drugs they got for free and used the cheaper ones instead (e. g. Efavirenz vs. Nevirapine). Overseas procurement markets for medicines including ARVs expanded PHA opportunities for access. They could purchase the drugs, via peer-to-peer brokers, from abroad, for example China or India where the drugs were cheaper. There were non-systematic controls for the drug procurement by those with PHA. Moreover, little knowledge about the ARVs and the dosage affected PHA decision making to non -rational access to and use of the ARV. Conclusions: Individual ability to improve access to ARVs increases the risk of non-rational use of drugs. Supply, education, health provider and especially systematic management must be improved expeditiously and dynamically to overcome the nationwide use of non-prescription ARVs.
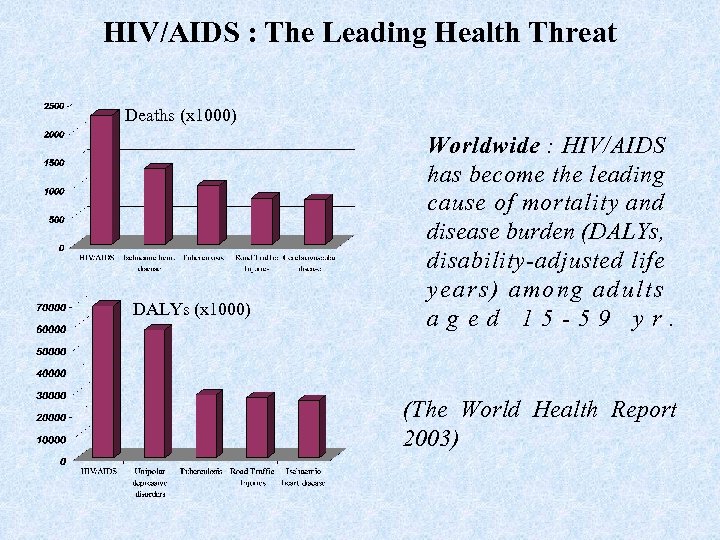 HIV/AIDS : The Leading Health Threat Deaths (x 1000) DALYs (x 1000) Worldwide : HIV/AIDS has become the leading cause of mortality and disease burden (DALYs, disability-adjusted life years) among adults aged 15 -59 yr. (The World Health Report 2003)
HIV/AIDS : The Leading Health Threat Deaths (x 1000) DALYs (x 1000) Worldwide : HIV/AIDS has become the leading cause of mortality and disease burden (DALYs, disability-adjusted life years) among adults aged 15 -59 yr. (The World Health Report 2003)
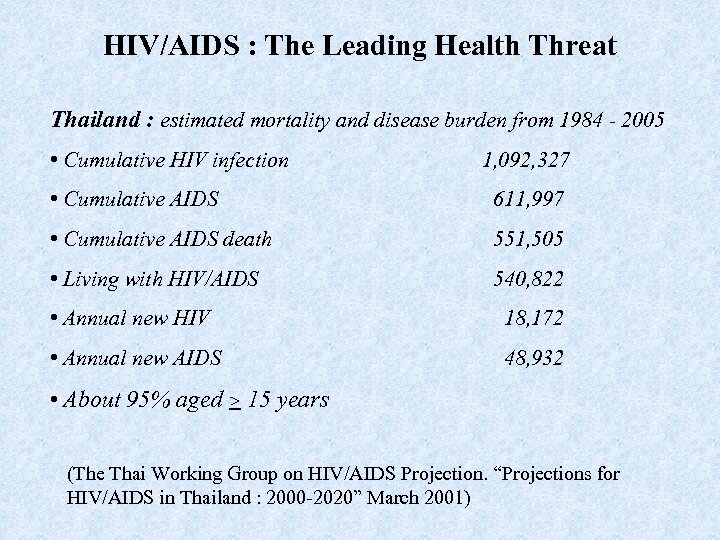 HIV/AIDS : The Leading Health Threat Thailand : estimated mortality and disease burden from 1984 - 2005 • Cumulative HIV infection 1, 092, 327 • Cumulative AIDS 611, 997 • Cumulative AIDS death 551, 505 • Living with HIV/AIDS 540, 822 • Annual new HIV 18, 172 • Annual new AIDS 48, 932 • About 95% aged > 15 years (The Thai Working Group on HIV/AIDS Projection. “Projections for HIV/AIDS in Thailand : 2000 -2020” March 2001)
HIV/AIDS : The Leading Health Threat Thailand : estimated mortality and disease burden from 1984 - 2005 • Cumulative HIV infection 1, 092, 327 • Cumulative AIDS 611, 997 • Cumulative AIDS death 551, 505 • Living with HIV/AIDS 540, 822 • Annual new HIV 18, 172 • Annual new AIDS 48, 932 • About 95% aged > 15 years (The Thai Working Group on HIV/AIDS Projection. “Projections for HIV/AIDS in Thailand : 2000 -2020” March 2001)
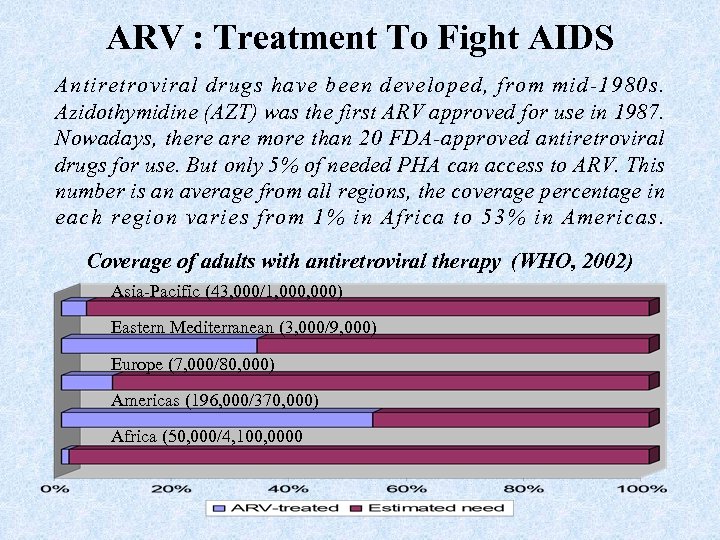 ARV : Treatment To Fight AIDS Antiretroviral drugs have been developed, from mid-1980 s. Azidothymidine (AZT) was the first ARV approved for use in 1987. Nowadays, there are more than 20 FDA-approved antiretroviral drugs for use. But only 5% of needed PHA can access to ARV. This number is an average from all regions, the coverage percentage in each region varies from 1% in Africa to 53% in Americas. Coverage of adults with antiretroviral therapy (WHO, 2002) Asia-Pacific (43, 000/1, 000) Eastern Mediterranean (3, 000/9, 000) Europe (7, 000/80, 000) Americas (196, 000/370, 000) Africa (50, 000/4, 100, 0000
ARV : Treatment To Fight AIDS Antiretroviral drugs have been developed, from mid-1980 s. Azidothymidine (AZT) was the first ARV approved for use in 1987. Nowadays, there are more than 20 FDA-approved antiretroviral drugs for use. But only 5% of needed PHA can access to ARV. This number is an average from all regions, the coverage percentage in each region varies from 1% in Africa to 53% in Americas. Coverage of adults with antiretroviral therapy (WHO, 2002) Asia-Pacific (43, 000/1, 000) Eastern Mediterranean (3, 000/9, 000) Europe (7, 000/80, 000) Americas (196, 000/370, 000) Africa (50, 000/4, 100, 0000
 ARV : Treatment To Fight AIDS WHO declares ARV treatment gap a global health emergency and aims to narrow the gap by providing ARV therapy for 3, 000 people living with HIV/AIDS (PHA) in developing countries by the end of 2005. The strategy includes scaling up access to treatment and also increasing procurement volumes by lessen expensive generic medicines for low-income countries Worldwide : WHO backs “ 3 by 5” target of ARV treatment for 3 million people by 2005
ARV : Treatment To Fight AIDS WHO declares ARV treatment gap a global health emergency and aims to narrow the gap by providing ARV therapy for 3, 000 people living with HIV/AIDS (PHA) in developing countries by the end of 2005. The strategy includes scaling up access to treatment and also increasing procurement volumes by lessen expensive generic medicines for low-income countries Worldwide : WHO backs “ 3 by 5” target of ARV treatment for 3 million people by 2005
 Thailand : Access To ARV Ministry of Public Health subsidized ARV for PHA from 1993. There about 10, 000 PHA access to ARV treatment in 2003 and 50, 000 PHA are goal-directed toll by the end of 2004 under National Access to Anti-retroviral Programs for PHA (NAPHA).
Thailand : Access To ARV Ministry of Public Health subsidized ARV for PHA from 1993. There about 10, 000 PHA access to ARV treatment in 2003 and 50, 000 PHA are goal-directed toll by the end of 2004 under National Access to Anti-retroviral Programs for PHA (NAPHA).
 Thailand : Access To ARV AZT(Antivir®), dd. I (Divir®), d 4 T (Stavir®), 3 TC (Lamvir®), NVP (Neravir®), AZT+3 TC (Zilarvir®) and d 4 T+3 TC+NVP (GPO-vir®) are generic HIV/AIDS drugs manufactured by The Government Pharmaceutical Organization (GPO) of Thailand (Tradename’s shown in the parenthesis). GPOvir, the one pill of d 4 T, 3 TC and NVP, is the first choice of regimen for naive under NAPHA.
Thailand : Access To ARV AZT(Antivir®), dd. I (Divir®), d 4 T (Stavir®), 3 TC (Lamvir®), NVP (Neravir®), AZT+3 TC (Zilarvir®) and d 4 T+3 TC+NVP (GPO-vir®) are generic HIV/AIDS drugs manufactured by The Government Pharmaceutical Organization (GPO) of Thailand (Tradename’s shown in the parenthesis). GPOvir, the one pill of d 4 T, 3 TC and NVP, is the first choice of regimen for naive under NAPHA.
 Thailand : Access To ARV National Program • from Access To Care (ATC) to National Access to Antiretroviral Programs for PHA (NAPHA) • Free of charge / co-payment • naive / experienced PHA
Thailand : Access To ARV National Program • from Access To Care (ATC) to National Access to Antiretroviral Programs for PHA (NAPHA) • Free of charge / co-payment • naive / experienced PHA
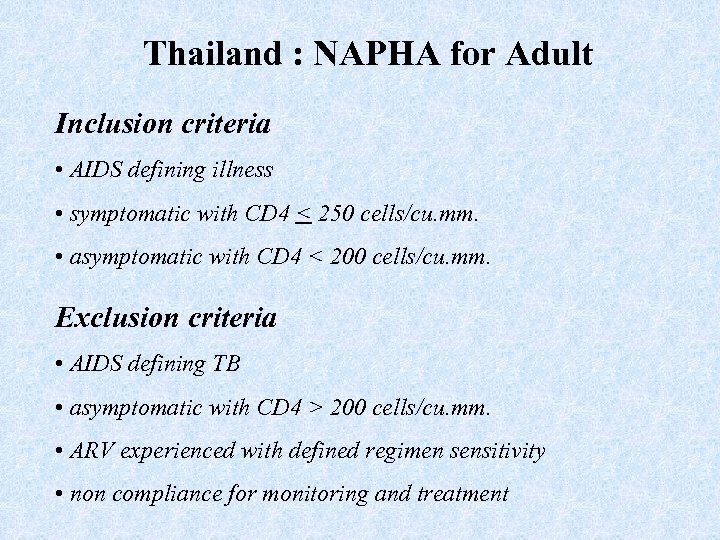 Thailand : NAPHA for Adult Inclusion criteria • AIDS defining illness • symptomatic with CD 4 < 250 cells/cu. mm. • asymptomatic with CD 4 < 200 cells/cu. mm. Exclusion criteria • AIDS defining TB • asymptomatic with CD 4 > 200 cells/cu. mm. • ARV experienced with defined regimen sensitivity • non compliance for monitoring and treatment
Thailand : NAPHA for Adult Inclusion criteria • AIDS defining illness • symptomatic with CD 4 < 250 cells/cu. mm. • asymptomatic with CD 4 < 200 cells/cu. mm. Exclusion criteria • AIDS defining TB • asymptomatic with CD 4 > 200 cells/cu. mm. • ARV experienced with defined regimen sensitivity • non compliance for monitoring and treatment
 Thailand PHA: Access to ARV Subsidized (Free/Co-payment) • National Program – ATC/NAPHA • Clinical trial – International/ National/ Medical School Projects Non-Subsidized • Hospitals– Governmental/Private/ Medical School PHA can purchase ARV at many medical sectors with prescription
Thailand PHA: Access to ARV Subsidized (Free/Co-payment) • National Program – ATC/NAPHA • Clinical trial – International/ National/ Medical School Projects Non-Subsidized • Hospitals– Governmental/Private/ Medical School PHA can purchase ARV at many medical sectors with prescription
 Health Self Help Groups for HIV/AIDS • There about 600 HIV self-help groups in Thailand • About 50% are non-governmental organization • About 30% are in Bangkok, 70% are in other provinces • Some groups coordinate their activities with medical sectors and subsidize some basic health examination and opportunistic prophylactic treatment • Mostly, PHA share their knowledge and treatment experiences which include ARV and also traditional treatment
Health Self Help Groups for HIV/AIDS • There about 600 HIV self-help groups in Thailand • About 50% are non-governmental organization • About 30% are in Bangkok, 70% are in other provinces • Some groups coordinate their activities with medical sectors and subsidize some basic health examination and opportunistic prophylactic treatment • Mostly, PHA share their knowledge and treatment experiences which include ARV and also traditional treatment
 PHA - Access to ARV • Male, aged 40 • Jan 2000 – May 2002 : Participated in the project (AZT, dd. I) • July – Dec 2002 : Paid by himself for GPOvir (3 TC, d 4 T, NVP) 1, 320. baht/mo. • Dec 2002 – Jan 2003 : Stopped ARV for TB treatment • Feb 2003 – present : Participated in national ATC program (GPOvir) • Female, aged 47 • Dec 1997 - Jan 2002 : Participated in the project (AZT) • Feb 2002 - present : Paid by herself for GPOvir • She borrowed or bought some pills from her friend sometimes when she was short of money • Nowadays, she still seeks for ARV subsidized project/program
PHA - Access to ARV • Male, aged 40 • Jan 2000 – May 2002 : Participated in the project (AZT, dd. I) • July – Dec 2002 : Paid by himself for GPOvir (3 TC, d 4 T, NVP) 1, 320. baht/mo. • Dec 2002 – Jan 2003 : Stopped ARV for TB treatment • Feb 2003 – present : Participated in national ATC program (GPOvir) • Female, aged 47 • Dec 1997 - Jan 2002 : Participated in the project (AZT) • Feb 2002 - present : Paid by herself for GPOvir • She borrowed or bought some pills from her friend sometimes when she was short of money • Nowadays, she still seeks for ARV subsidized project/program
 PHA - Access to ARV • Male, aged 39 • Still healthy • PHA peer encouraged the benefit of ARV treatment and the subsidy of ARV and health care from the 5 -year project • Sep 2001 – present : Participated in the project (d 4 T, dd. I) • He received only 2 ARV because his CD 4 was over 250 • Female, aged 28 • Still healthy • PHA peer encouraged the benefit of ARV treatment, the subsidy of ARV from the project and especially the benefit of access to ARV when immunity was not deficient • Sep 2003 – present : Participated in the project (Saquinavir, Norvia, Combid) • Sep 2003 – CD 4 = 316, Dec 2003 – CD 4 = 396
PHA - Access to ARV • Male, aged 39 • Still healthy • PHA peer encouraged the benefit of ARV treatment and the subsidy of ARV and health care from the 5 -year project • Sep 2001 – present : Participated in the project (d 4 T, dd. I) • He received only 2 ARV because his CD 4 was over 250 • Female, aged 28 • Still healthy • PHA peer encouraged the benefit of ARV treatment, the subsidy of ARV from the project and especially the benefit of access to ARV when immunity was not deficient • Sep 2003 – present : Participated in the project (Saquinavir, Norvia, Combid) • Sep 2003 – CD 4 = 316, Dec 2003 – CD 4 = 396
 PHA - Access to ARV • Male, aged 38 • Aug 1999 – Mar 2002 : Participated in the project (d 4 T, dd. I) • He planned to stop drug occasionally to reserve drug after the end of the project • The second project he approached was 3 drugs therapy but he was excluded because the project was for naive • So, when he approached to the third project (3 TC, d 4 T, NVP), he did not tell about his drug experience • Male, aged 43 • He was not able to receive ARV subsidy because his CD 4 was over than the inclusion criteria of the project, but he wanted ARV treatment • Jan 2003 : He paid by himself for GPOvir
PHA - Access to ARV • Male, aged 38 • Aug 1999 – Mar 2002 : Participated in the project (d 4 T, dd. I) • He planned to stop drug occasionally to reserve drug after the end of the project • The second project he approached was 3 drugs therapy but he was excluded because the project was for naive • So, when he approached to the third project (3 TC, d 4 T, NVP), he did not tell about his drug experience • Male, aged 43 • He was not able to receive ARV subsidy because his CD 4 was over than the inclusion criteria of the project, but he wanted ARV treatment • Jan 2003 : He paid by himself for GPOvir
 PHA - Access to ARV • Male, aged 30 • Jun – Sep 2001 : Participated in the project (d 4 T, dd. I) • Sep 2001 – present : Changed regimen to 3 TC, AZT, EFV • The practitioner gave him over EFV pills but less 3 TC pills, he did not go back to the project • Alternatively PHA peer introduced him to buy 3 TC at the private clinic • There were the private clinics or drug stores that PHA could buy ARV at cheaper price without prescription • PHA network helped themselves for cheaper ARV • Efavirenz was 3 -4 times cheaper in India or China than in Thailand, so PHA network set their broker to buy lots of drug from abroad
PHA - Access to ARV • Male, aged 30 • Jun – Sep 2001 : Participated in the project (d 4 T, dd. I) • Sep 2001 – present : Changed regimen to 3 TC, AZT, EFV • The practitioner gave him over EFV pills but less 3 TC pills, he did not go back to the project • Alternatively PHA peer introduced him to buy 3 TC at the private clinic • There were the private clinics or drug stores that PHA could buy ARV at cheaper price without prescription • PHA network helped themselves for cheaper ARV • Efavirenz was 3 -4 times cheaper in India or China than in Thailand, so PHA network set their broker to buy lots of drug from abroad
 PHA Non-Rational Access to ARV • Falsify history of illness/treatment to the project criteria • Adjust drug dosage to prolong drug supply during projects gap • Adjust regimen by seeking new project because of the belief in many drugs benefit • drug procurement among PHA • drug procurement from private clinic without prescription • drug procurement from abroad e. g. India, China
PHA Non-Rational Access to ARV • Falsify history of illness/treatment to the project criteria • Adjust drug dosage to prolong drug supply during projects gap • Adjust regimen by seeking new project because of the belief in many drugs benefit • drug procurement among PHA • drug procurement from private clinic without prescription • drug procurement from abroad e. g. India, China
 PHA Non-Rational Access to ARV • Individual ability to improve access to ARV increases the risk of non-rational drug use Prevention • Increasing the volume of drug supply for various stages of PHA • Decreasing drug cost • Education of drug pro’s and con’s and drug adherence via HIV Self Help Group • Management of ARV treatment among projects, programs and private sectors should be systematic monitoring and control
PHA Non-Rational Access to ARV • Individual ability to improve access to ARV increases the risk of non-rational drug use Prevention • Increasing the volume of drug supply for various stages of PHA • Decreasing drug cost • Education of drug pro’s and con’s and drug adherence via HIV Self Help Group • Management of ARV treatment among projects, programs and private sectors should be systematic monitoring and control


