Болезни ускоренного старения-2017.ppt
- Количество слайдов: 100
 Accelerated aging diseases and their genetic causes Complex pattern of aging markers in primary human fibroblasts
Accelerated aging diseases and their genetic causes Complex pattern of aging markers in primary human fibroblasts
 • Primary non-transformed cell cultures tend to change with every passage. Population doubling time increases, cell morphology alteres, larger rotund cells appear, which are regarded as older, in comparison with smaller oblong ones (Мikhelson, 1984; Lorenzini et al. , 2005). Biochemical markers appear simultaneously with morphological alterations. They characterize aging cells in culture, defined as “aging markers”, which include changes in chromatine, nucleus and cytoplasmatic skeleton, high level of non-repaired DNA damages, etc. (Campisi, 2005); as well as in cell instability towards action of damaging agents, primarily hydrogen peroxide (Chen et al. , 1998, Ryter et al. , 2007).
• Primary non-transformed cell cultures tend to change with every passage. Population doubling time increases, cell morphology alteres, larger rotund cells appear, which are regarded as older, in comparison with smaller oblong ones (Мikhelson, 1984; Lorenzini et al. , 2005). Biochemical markers appear simultaneously with morphological alterations. They characterize aging cells in culture, defined as “aging markers”, which include changes in chromatine, nucleus and cytoplasmatic skeleton, high level of non-repaired DNA damages, etc. (Campisi, 2005); as well as in cell instability towards action of damaging agents, primarily hydrogen peroxide (Chen et al. , 1998, Ryter et al. , 2007).
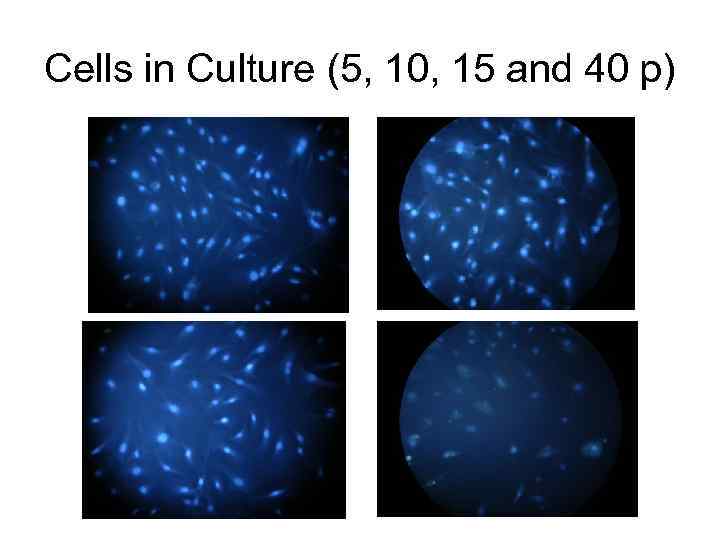 Cells in Culture (5, 10, 15 and 40 p)
Cells in Culture (5, 10, 15 and 40 p)
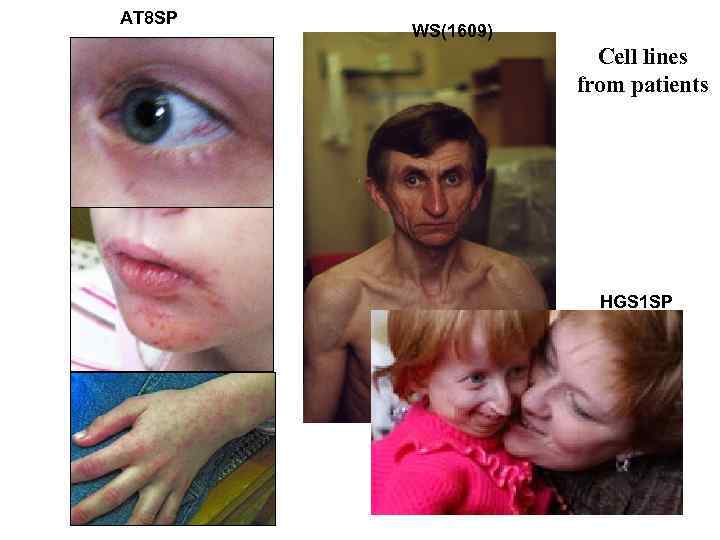 AT 8 SP WS(1609) Cell lines from patients HGS 1 SP
AT 8 SP WS(1609) Cell lines from patients HGS 1 SP
 • The most frequently used marker of aging is lysosomal β-galactosidase (SA-β-gal) associated with aging, its activity dramatically increasing in old cells (Dimri et al. , 1995; Lee et al. , 2006)
• The most frequently used marker of aging is lysosomal β-galactosidase (SA-β-gal) associated with aging, its activity dramatically increasing in old cells (Dimri et al. , 1995; Lee et al. , 2006)
 • Primary fibroblasts of skin from donors of varios ages, and patients with premature ageing with Hutchinson-Gilford syndrome (children’s progeria), and the Werner one (adult progeria), normally serve as model for the detection of ageing markers • Fibroblasts taken from old donors, and from the progeria patients, are currently regarded as containing more ageing markers, than those taken from young healthy donors (Scafidi, Misteli, 2006, Sedelnikova et al. , 2008). • Fibroblasts of skin of other mammals (mice) may also be applied for the detection of ageing markers (taking into account the Hyflick limit)
• Primary fibroblasts of skin from donors of varios ages, and patients with premature ageing with Hutchinson-Gilford syndrome (children’s progeria), and the Werner one (adult progeria), normally serve as model for the detection of ageing markers • Fibroblasts taken from old donors, and from the progeria patients, are currently regarded as containing more ageing markers, than those taken from young healthy donors (Scafidi, Misteli, 2006, Sedelnikova et al. , 2008). • Fibroblasts of skin of other mammals (mice) may also be applied for the detection of ageing markers (taking into account the Hyflick limit)
 Hutchinson-Gilford syndrome • • • • Autosomal dominant, emerging “de novo”, getting, probably, from the father site 15 (21) LMNA 1 q 21. 2 FACE-1/ZMPSTE 24 1 q 34 Skin atrophy. Bird’s face Loss of subcutaneous fat Hair loss. Arteriosclerosis Increased metabolic rate Hypogonadism Grow hormone insensitivity? Slow growth. Reduced replicative life span of cultured cells Shot telomeres DNA repair defects? Silent mutation in (1 -4)-βGalactosyl-transferase gene (594 C>T)
Hutchinson-Gilford syndrome • • • • Autosomal dominant, emerging “de novo”, getting, probably, from the father site 15 (21) LMNA 1 q 21. 2 FACE-1/ZMPSTE 24 1 q 34 Skin atrophy. Bird’s face Loss of subcutaneous fat Hair loss. Arteriosclerosis Increased metabolic rate Hypogonadism Grow hormone insensitivity? Slow growth. Reduced replicative life span of cultured cells Shot telomeres DNA repair defects? Silent mutation in (1 -4)-βGalactosyl-transferase gene (594 C>T)
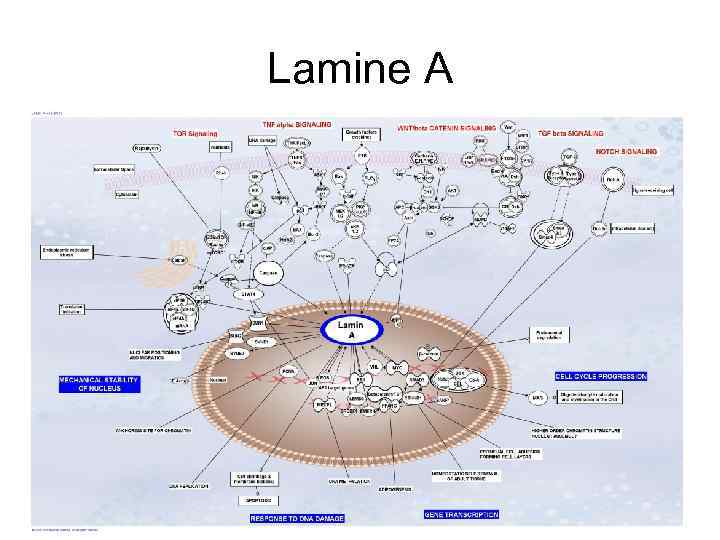 Lamine A
Lamine A
 Nuclear lamina • Structural transformations of the nuclear lamina, occurring as a result of the accumulation of aberrant product of the LMNA gene, progerin, were demonstrated in the course of the study of cells of patients with Hutchinson-Gilford syndrome, as well as of cell cultures, acquired from aged donors (Scaffidi, Misteli, 2006). Results acquired by these authors, taken in complex with the present-day data concerning participation of components of nuclear lamina, including lamin А in the wide range of cell processes. Allow to regard structural alterations of nuclear lamina as the basic process for induction of markers of ageing, associated with various levels of cell regulation.
Nuclear lamina • Structural transformations of the nuclear lamina, occurring as a result of the accumulation of aberrant product of the LMNA gene, progerin, were demonstrated in the course of the study of cells of patients with Hutchinson-Gilford syndrome, as well as of cell cultures, acquired from aged donors (Scaffidi, Misteli, 2006). Results acquired by these authors, taken in complex with the present-day data concerning participation of components of nuclear lamina, including lamin А in the wide range of cell processes. Allow to regard structural alterations of nuclear lamina as the basic process for induction of markers of ageing, associated with various levels of cell regulation.
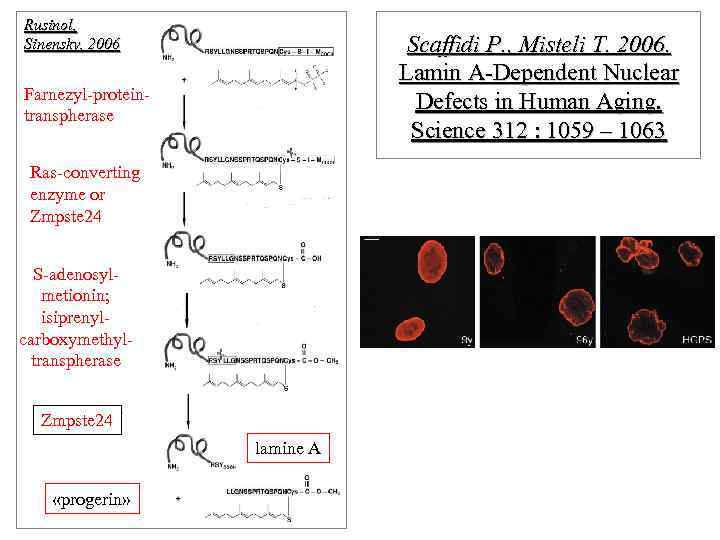 Rusinol, Sinensky, 2006 Scaffidi P. , Misteli T. 2006. Lamin A-Dependent Nuclear Defects in Human Aging. Science 312 : 1059 – 1063 Farnezyl-proteintranspherase Ras-converting enzyme or Zmpste 24 S-adenosylmetionin; isiprenylcarboxymethyltranspherase Zmpste 24 lamine А «progerin»
Rusinol, Sinensky, 2006 Scaffidi P. , Misteli T. 2006. Lamin A-Dependent Nuclear Defects in Human Aging. Science 312 : 1059 – 1063 Farnezyl-proteintranspherase Ras-converting enzyme or Zmpste 24 S-adenosylmetionin; isiprenylcarboxymethyltranspherase Zmpste 24 lamine А «progerin»
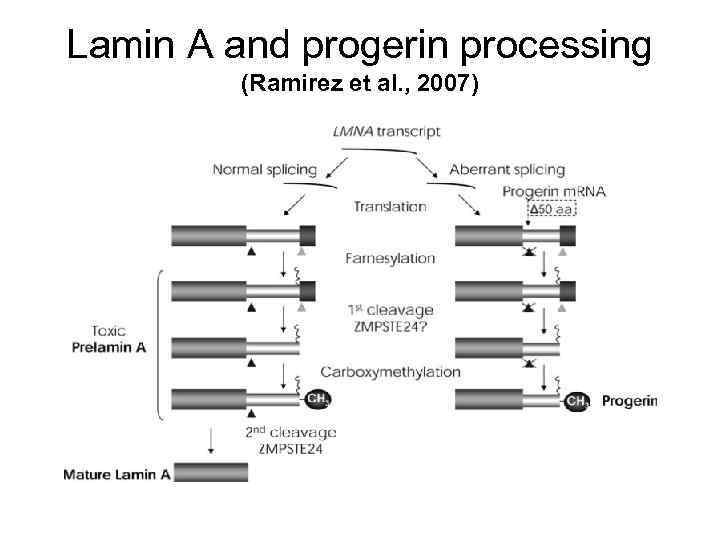 Lamin A and progerin processing (Ramirez et al. , 2007)
Lamin A and progerin processing (Ramirez et al. , 2007)
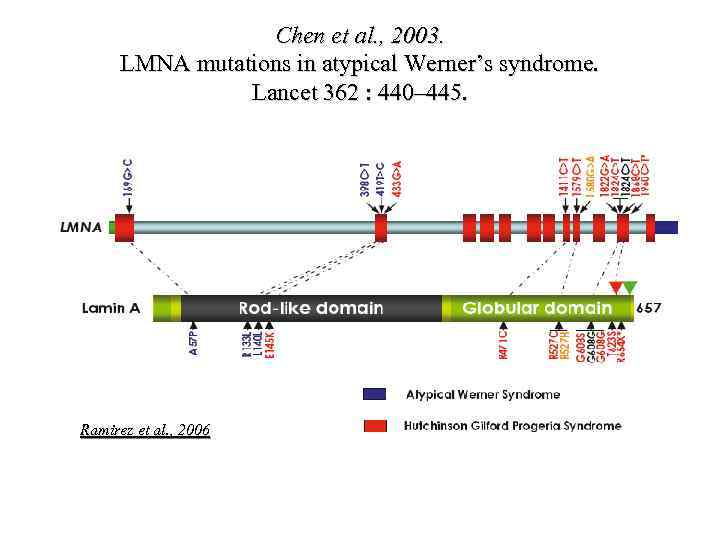 Chen et al. , 2003. LMNA mutations in atypical Werner’s syndrome. Lancet 362 : 440– 445. Ramirez et al. , 2006
Chen et al. , 2003. LMNA mutations in atypical Werner’s syndrome. Lancet 362 : 440– 445. Ramirez et al. , 2006
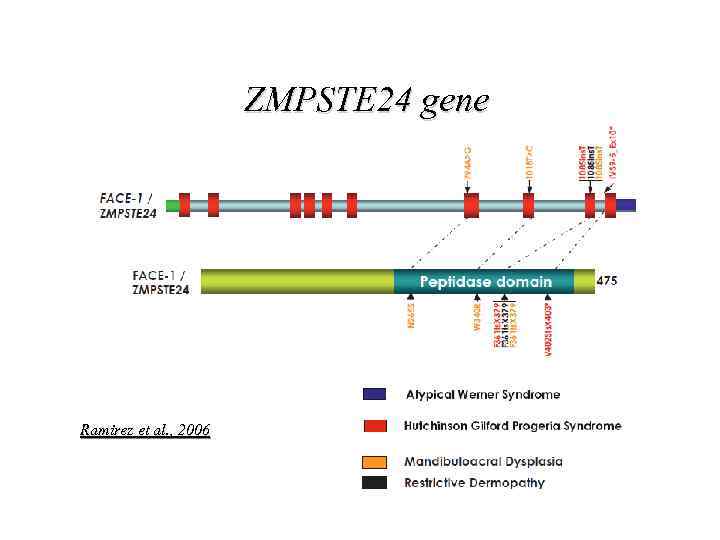 ZMPSTE 24 gene Ramirez et al. , 2006
ZMPSTE 24 gene Ramirez et al. , 2006
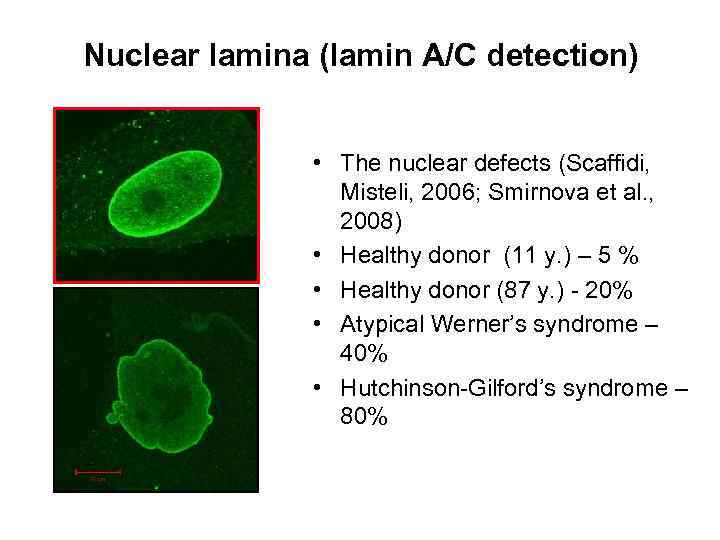 Nuclear lamina (lamin A/C detection) • The nuclear defects (Scaffidi, Misteli, 2006; Smirnova et al. , 2008) • Healthy donor (11 у. ) – 5 % • Healthy donor (87 y. ) - 20% • Atypical Werner’s syndrome – 40% • Hutchinson-Gilford’s syndrome – 80%
Nuclear lamina (lamin A/C detection) • The nuclear defects (Scaffidi, Misteli, 2006; Smirnova et al. , 2008) • Healthy donor (11 у. ) – 5 % • Healthy donor (87 y. ) - 20% • Atypical Werner’s syndrome – 40% • Hutchinson-Gilford’s syndrome – 80%
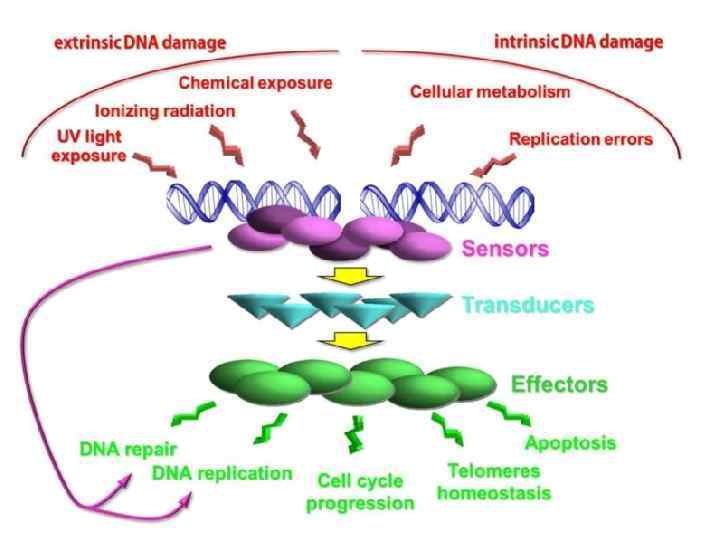
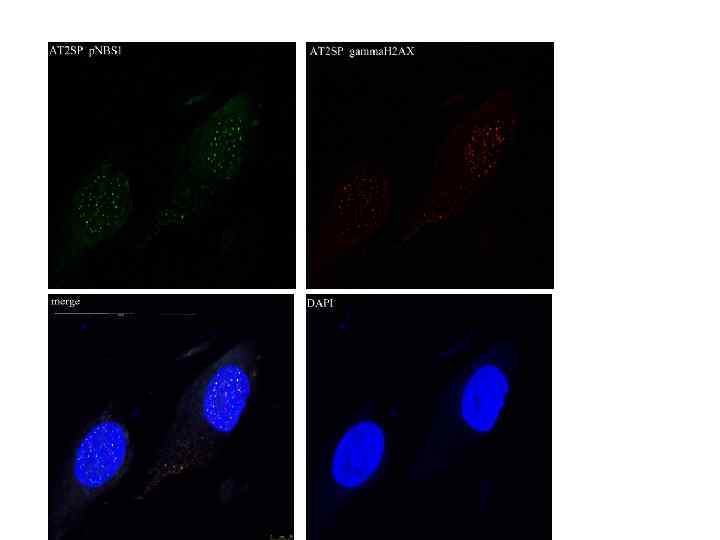
 • Accumulation of foci of γ-Н 2 АХ is observed in nuclei of aging cells in culture, cells from old donors, and from Progeria patients (Sedelnikova et al. , 2004, 2008). • This phenomenon may be linked to both accumulation of either non-repaired DSBs or, modifications of chromatine, apprehended as DSBs by protein kinases АТМ and DNA-PK after DNA damage (Stiff et al. , 2004), or else, ATR kinase during replication halt (Takahashi, Ohnishi, 2005); as well as by appearance of uncapping telomeres (Hao et al. , 2004). • As a result, accumulation in cell population of cells with foci of γ-Н 2 АХ occurs, which forms an objective criterion of ageing on cell level.
• Accumulation of foci of γ-Н 2 АХ is observed in nuclei of aging cells in culture, cells from old donors, and from Progeria patients (Sedelnikova et al. , 2004, 2008). • This phenomenon may be linked to both accumulation of either non-repaired DSBs or, modifications of chromatine, apprehended as DSBs by protein kinases АТМ and DNA-PK after DNA damage (Stiff et al. , 2004), or else, ATR kinase during replication halt (Takahashi, Ohnishi, 2005); as well as by appearance of uncapping telomeres (Hao et al. , 2004). • As a result, accumulation in cell population of cells with foci of γ-Н 2 АХ occurs, which forms an objective criterion of ageing on cell level.
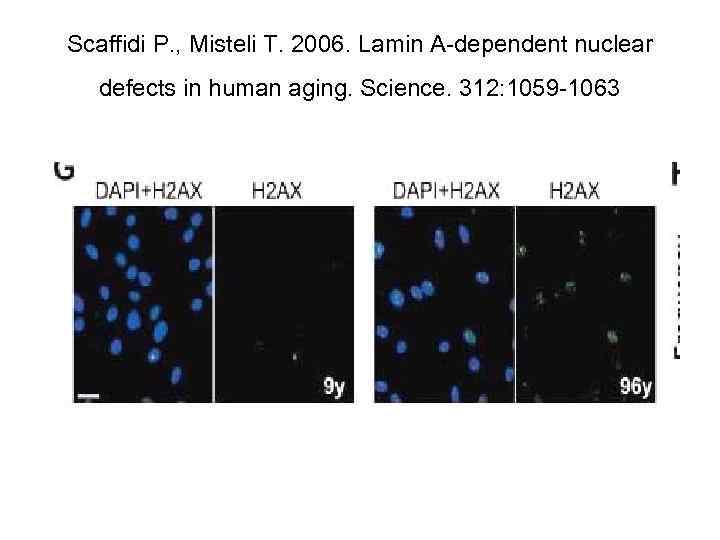 Scaffidi P. , Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312: 1059 -1063
Scaffidi P. , Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312: 1059 -1063
 • Cells change their epigenetic status with age. Heritable changes in gene regulation with no changes in the DNA sequence itself are considered as epigenetic determinants. In recent years, the role of epigenetic mechanisms in the process of carcinogenesis (Jones, Baylin, 2002), as well as in cellular and organismal aging (Wilson, Jones, 1983; Issa, 2003; Fraga et al. , 2005) was demonstrated.
• Cells change their epigenetic status with age. Heritable changes in gene regulation with no changes in the DNA sequence itself are considered as epigenetic determinants. In recent years, the role of epigenetic mechanisms in the process of carcinogenesis (Jones, Baylin, 2002), as well as in cellular and organismal aging (Wilson, Jones, 1983; Issa, 2003; Fraga et al. , 2005) was demonstrated.
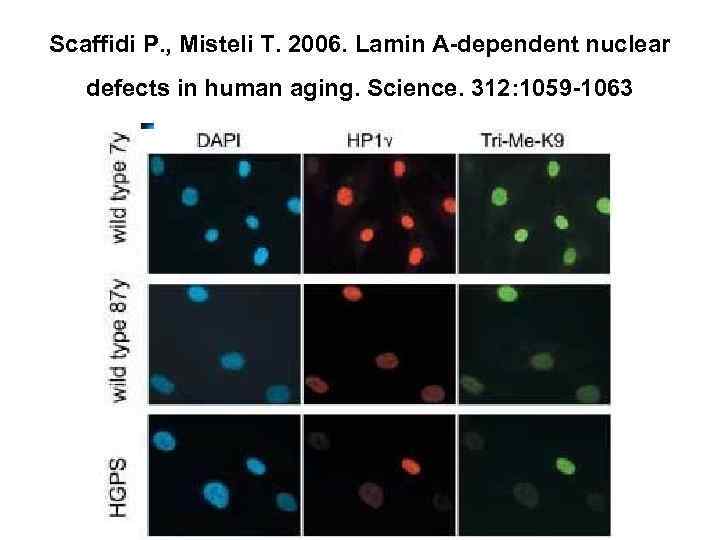 Scaffidi P. , Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312: 1059 -1063
Scaffidi P. , Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312: 1059 -1063
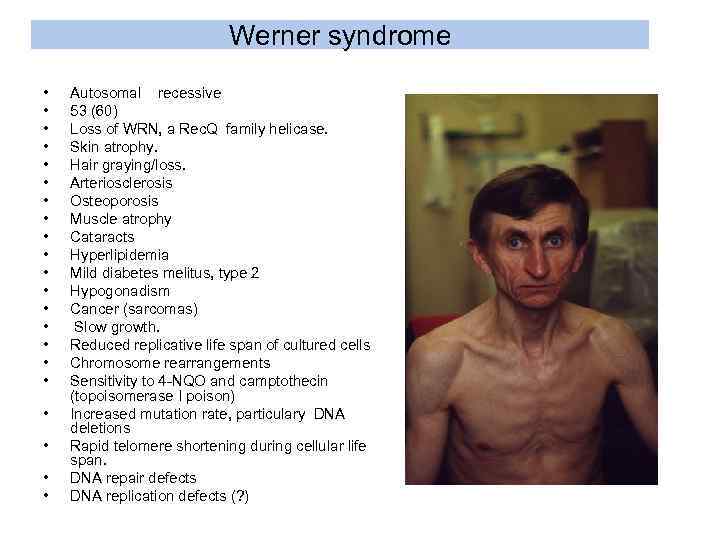 Werner syndrome • • • • • • Autosomal recessive 53 (60) Loss of WRN, a Rec. Q family helicase. Skin atrophy. Hair graying/loss. Arteriosclerosis Osteoporosis Muscle atrophy Cataracts Hyperlipidemia Mild diabetes melitus, type 2 Hypogonadism Cancer (sarcomas) Slow growth. Reduced replicative life span of cultured cells Chromosome rearrangements Sensitivity to 4 -NQO and camptothecin (topoisomerase I poison) Increased mutation rate, particulary DNA deletions Rapid telomere shortening during cellular life span. DNA repair defects DNA replication defects (? )
Werner syndrome • • • • • • Autosomal recessive 53 (60) Loss of WRN, a Rec. Q family helicase. Skin atrophy. Hair graying/loss. Arteriosclerosis Osteoporosis Muscle atrophy Cataracts Hyperlipidemia Mild diabetes melitus, type 2 Hypogonadism Cancer (sarcomas) Slow growth. Reduced replicative life span of cultured cells Chromosome rearrangements Sensitivity to 4 -NQO and camptothecin (topoisomerase I poison) Increased mutation rate, particulary DNA deletions Rapid telomere shortening during cellular life span. DNA repair defects DNA replication defects (? )
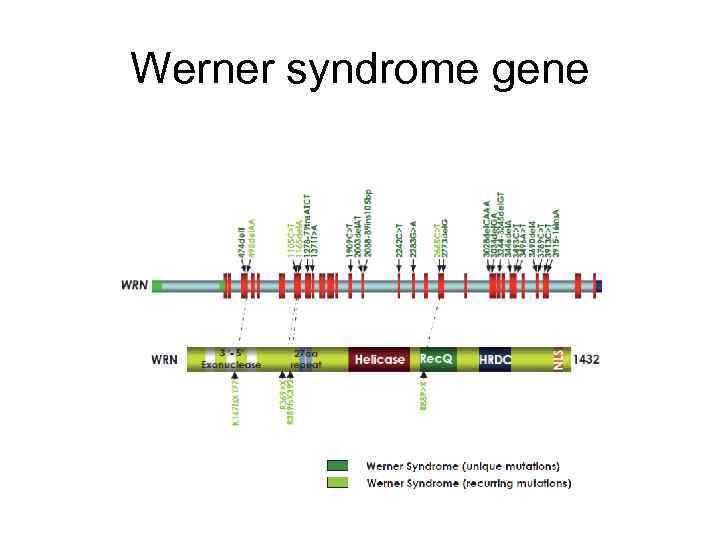 Werner syndrome gene
Werner syndrome gene
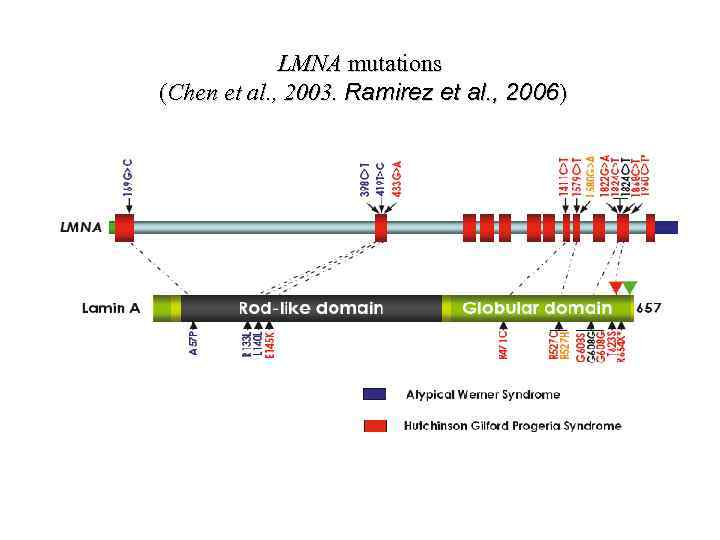 LMNA mutations (Chen et al. , 2003. Ramirez et al. , 2006)
LMNA mutations (Chen et al. , 2003. Ramirez et al. , 2006)
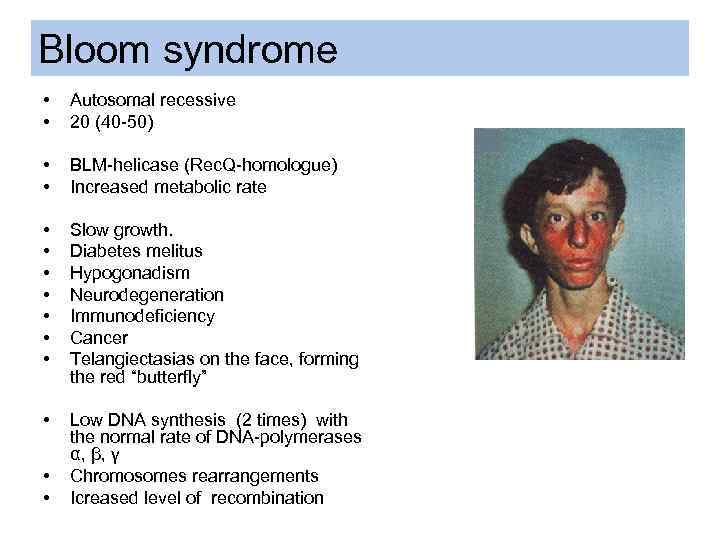 Bloom syndrome • • Autosomal recessive 20 (40 -50) • • BLM-helicase (Rec. Q-homologue) Increased metabolic rate • • Slow growth. Diabetes melitus Hypogonadism Neurodegeneration Immunodeficiency Cancer Telangiectasias on the face, forming the red “butterfly” • Low DNA synthesis (2 times) with the normal rate of DNA-polymerases α, β, γ Chromosomes rearrangements Icreased level of recombination • •
Bloom syndrome • • Autosomal recessive 20 (40 -50) • • BLM-helicase (Rec. Q-homologue) Increased metabolic rate • • Slow growth. Diabetes melitus Hypogonadism Neurodegeneration Immunodeficiency Cancer Telangiectasias on the face, forming the red “butterfly” • Low DNA synthesis (2 times) with the normal rate of DNA-polymerases α, β, γ Chromosomes rearrangements Icreased level of recombination • •
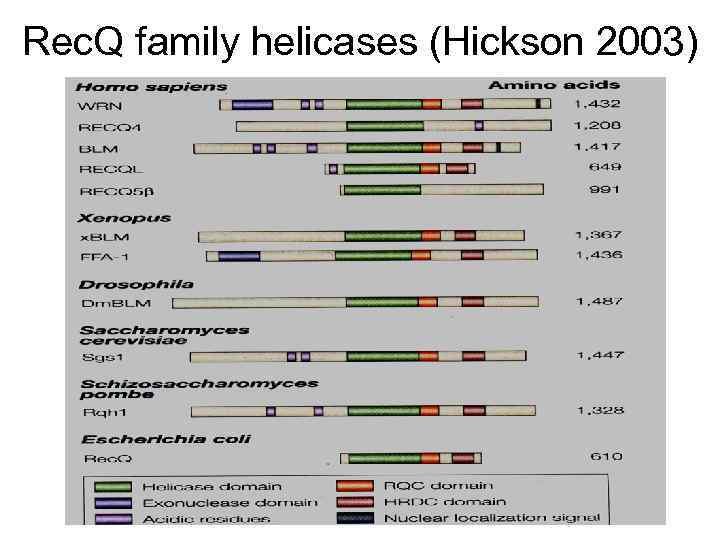 Rec. Q family helicases (Hickson 2003)
Rec. Q family helicases (Hickson 2003)
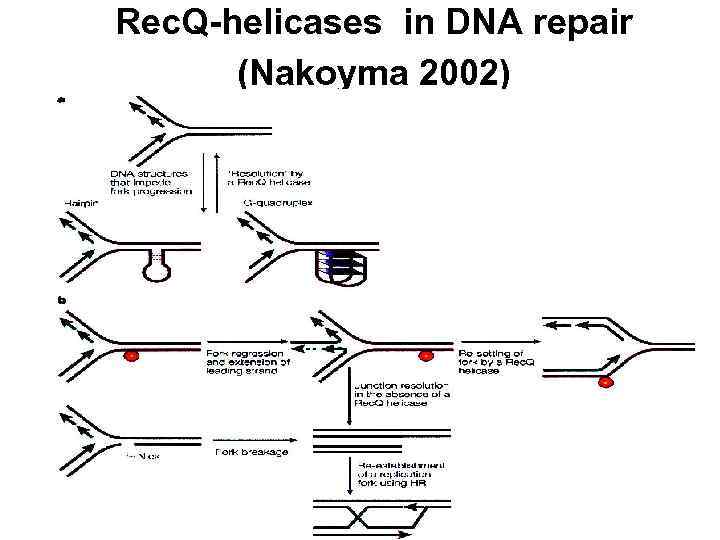 Rec. Q-helicases in DNA repair (Nakoyma 2002)
Rec. Q-helicases in DNA repair (Nakoyma 2002)
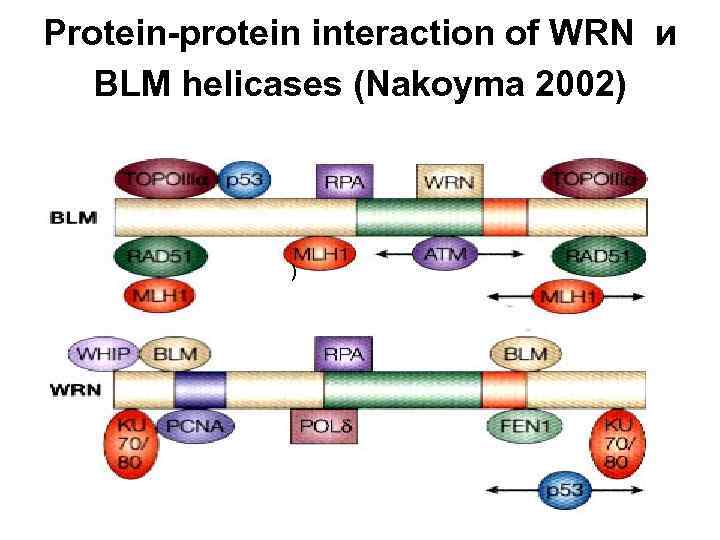 Protein-protein interaction of WRN и BLM helicases (Nakoyma 2002) )
Protein-protein interaction of WRN и BLM helicases (Nakoyma 2002) )
 • In the study of cells from a patient with Hutchinson-Gilford syndrome, besides the identification of all markers of aging described by Scaffidi P. , Misteli T (2006), we observed a significant discrepancy between these cells and cells from old donors. The level of stable chromosomal aberrations in the investigated cells was not elevated and, by this the marker, normally showing the "real" biological age, our patient was consistent with her 9 years.
• In the study of cells from a patient with Hutchinson-Gilford syndrome, besides the identification of all markers of aging described by Scaffidi P. , Misteli T (2006), we observed a significant discrepancy between these cells and cells from old donors. The level of stable chromosomal aberrations in the investigated cells was not elevated and, by this the marker, normally showing the "real" biological age, our patient was consistent with her 9 years.
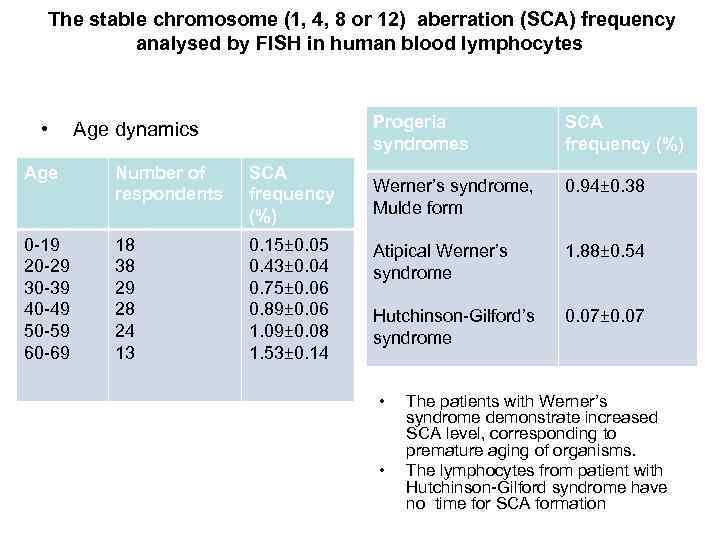 The stable chromosome (1, 4, 8 or 12) aberration (SCA) frequency analysed by FISH in human blood lymphocytes Progeria syndromes • Age dynamics Age Number of respondents SCA frequency (%) 0 -19 20 -29 30 -39 40 -49 50 -59 60 -69 18 38 29 28 24 13 0. 15± 0. 05 0. 43± 0. 04 0. 75± 0. 06 0. 89± 0. 06 1. 09± 0. 08 1. 53± 0. 14 SCA frequency (%) Werner’s syndrome, Mulde form 0. 94± 0. 38 Atipical Werner’s syndrome 1. 88± 0. 54 Hutchinson-Gilford’s syndrome 0. 07± 0. 07 • • The patients with Werner’s syndrome demonstrate increased SCA level, corresponding to premature aging of organisms. The lymphocytes from patient with Hutchinson-Gilford syndrome have no time for SCA formation
The stable chromosome (1, 4, 8 or 12) aberration (SCA) frequency analysed by FISH in human blood lymphocytes Progeria syndromes • Age dynamics Age Number of respondents SCA frequency (%) 0 -19 20 -29 30 -39 40 -49 50 -59 60 -69 18 38 29 28 24 13 0. 15± 0. 05 0. 43± 0. 04 0. 75± 0. 06 0. 89± 0. 06 1. 09± 0. 08 1. 53± 0. 14 SCA frequency (%) Werner’s syndrome, Mulde form 0. 94± 0. 38 Atipical Werner’s syndrome 1. 88± 0. 54 Hutchinson-Gilford’s syndrome 0. 07± 0. 07 • • The patients with Werner’s syndrome demonstrate increased SCA level, corresponding to premature aging of organisms. The lymphocytes from patient with Hutchinson-Gilford syndrome have no time for SCA formation
 Cutix laxa • Autosomal recessive • (40)-50 • Skin atrophy • DNA repair defects
Cutix laxa • Autosomal recessive • (40)-50 • Skin atrophy • DNA repair defects
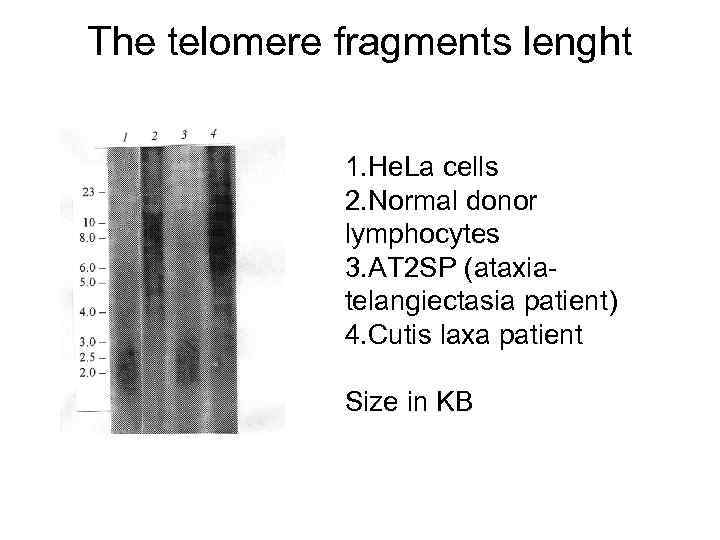 The telomere fragments lenght 1. He. La cells 2. Normal donor lymphocytes 3. AT 2 SP (ataxiatelangiectasia patient) 4. Cutis laxa patient Size in KB
The telomere fragments lenght 1. He. La cells 2. Normal donor lymphocytes 3. AT 2 SP (ataxiatelangiectasia patient) 4. Cutis laxa patient Size in KB
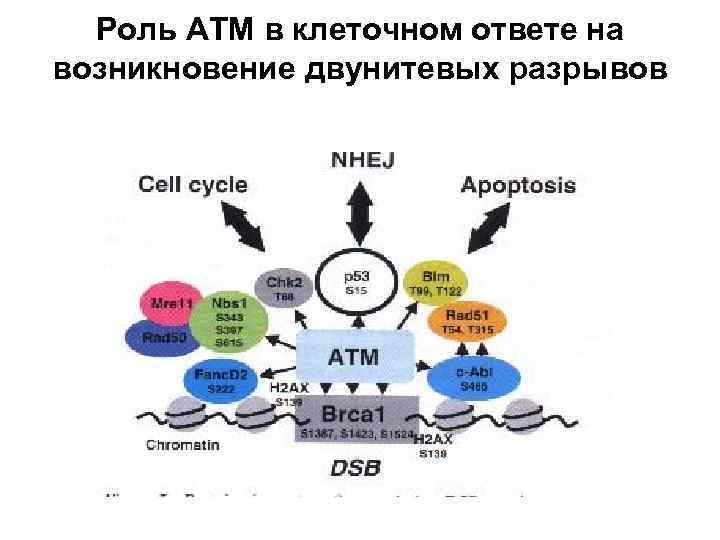 Роль АТМ в клеточном ответе на возникновение двунитевых разрывов
Роль АТМ в клеточном ответе на возникновение двунитевых разрывов
 Ataxia-telangiectasia (Luis-Bar syndrome) • • • Autosomal recessive 20 (40 -50) ATM, a protein kinase of the PI-3 kinase family • • • Neurodegeneration Immunodeficiency Cancer (leukemias and lymphomas) Occulocutaneous telangiectasias Progeroid skin and hear changes Hypogonadism Defectif cell cycle chekpoint arrest Reduced replicative life span Chromosomal rearrangements Sensitivity to ionizing radiation Inoppropriate apoptosis • • Shot telomeres Delayed/absence P 53 induction and accumulation after DNA damage Defects in repair of DNA double-strand breaks Defects in V(D)J recombination • •
Ataxia-telangiectasia (Luis-Bar syndrome) • • • Autosomal recessive 20 (40 -50) ATM, a protein kinase of the PI-3 kinase family • • • Neurodegeneration Immunodeficiency Cancer (leukemias and lymphomas) Occulocutaneous telangiectasias Progeroid skin and hear changes Hypogonadism Defectif cell cycle chekpoint arrest Reduced replicative life span Chromosomal rearrangements Sensitivity to ionizing radiation Inoppropriate apoptosis • • Shot telomeres Delayed/absence P 53 induction and accumulation after DNA damage Defects in repair of DNA double-strand breaks Defects in V(D)J recombination • •
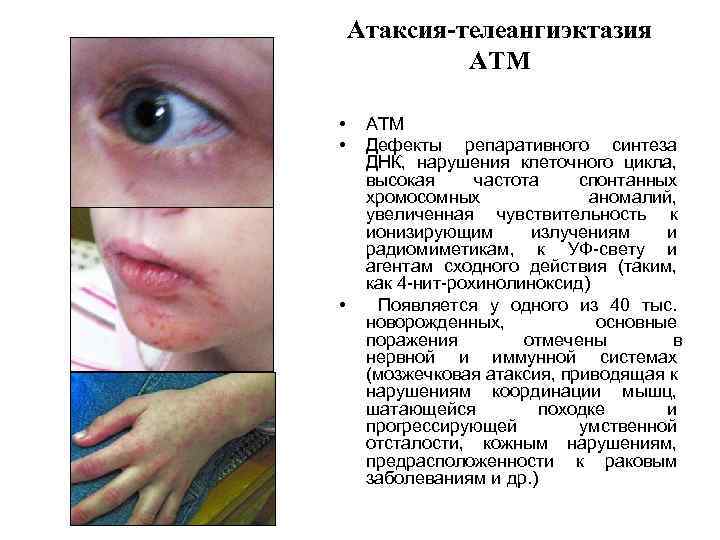 Атаксия-телеангиэктазия АТМ • • • АТМ Дефекты репаративного синтеза ДНК, нарушения клеточного цикла, высокая частота спонтанных хромосомных аномалий, увеличенная чувствительность к ионизирующим излучениям и радиомиметикам, к УФ-свету и агентам сходного действия (таким, как 4 -нит-рохинолиноксид) Появляется у одного из 40 тыс. новорожденных, основные поражения отмечены в нервной и иммунной системах (мозжечковая атаксия, приводящая к нарушениям координации мышц, шатающейся походке и прогрессирующей умственной отсталости, кожным нарушениям, предрасположенности к раковым заболеваниям и др. )
Атаксия-телеангиэктазия АТМ • • • АТМ Дефекты репаративного синтеза ДНК, нарушения клеточного цикла, высокая частота спонтанных хромосомных аномалий, увеличенная чувствительность к ионизирующим излучениям и радиомиметикам, к УФ-свету и агентам сходного действия (таким, как 4 -нит-рохинолиноксид) Появляется у одного из 40 тыс. новорожденных, основные поражения отмечены в нервной и иммунной системах (мозжечковая атаксия, приводящая к нарушениям координации мышц, шатающейся походке и прогрессирующей умственной отсталости, кожным нарушениям, предрасположенности к раковым заболеваниям и др. )
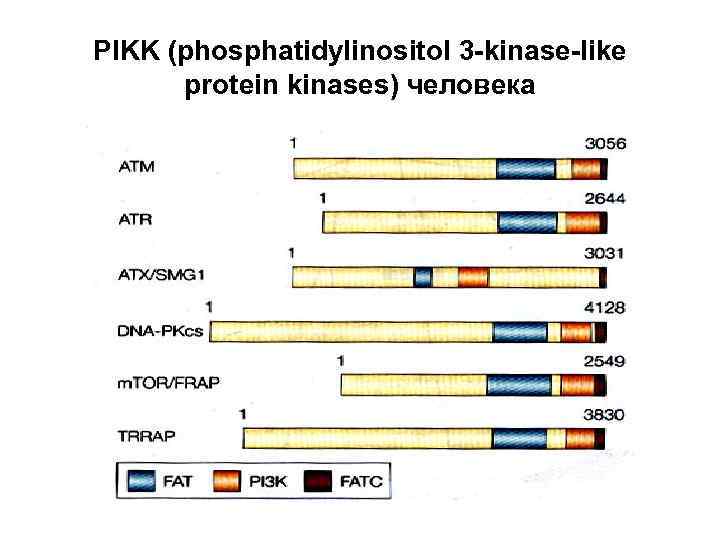 PIKK (phosphatidylinositol 3 -kinase-like protein kinases) человека
PIKK (phosphatidylinositol 3 -kinase-like protein kinases) человека
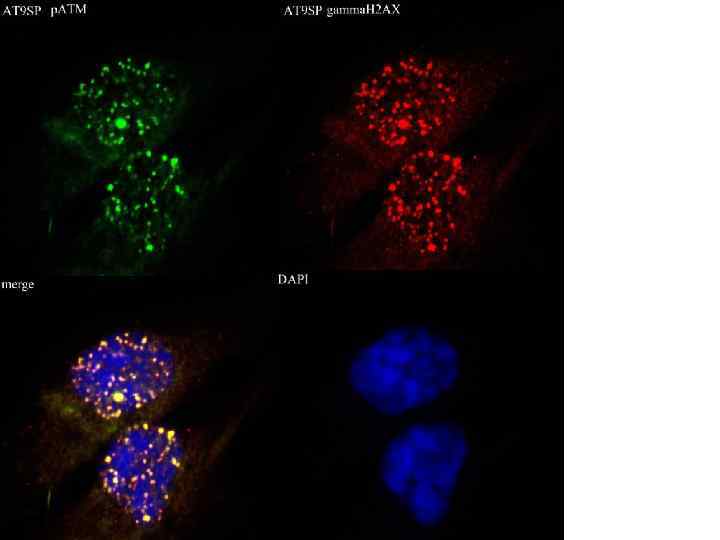
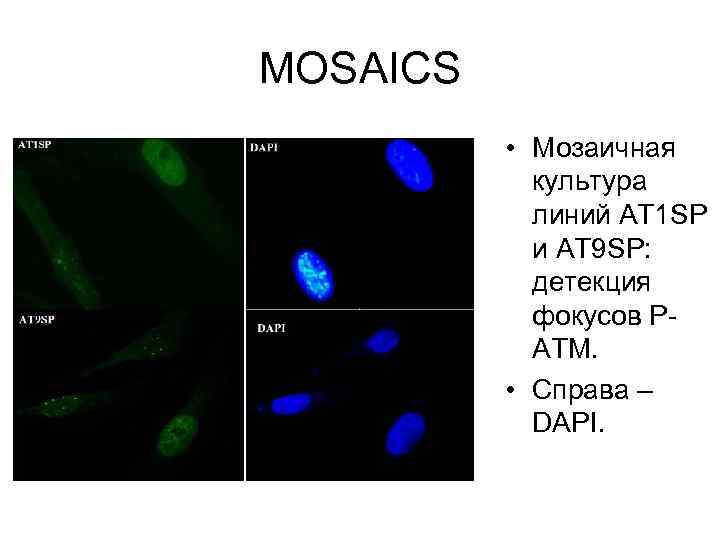 MOSAICS • Мозаичная культура линий AT 1 SP и AT 9 SP: детекция фокусов РАТМ. • Справа – DAPI.
MOSAICS • Мозаичная культура линий AT 1 SP и AT 9 SP: детекция фокусов РАТМ. • Справа – DAPI.
 SA-beta-GAL in AT-cells
SA-beta-GAL in AT-cells
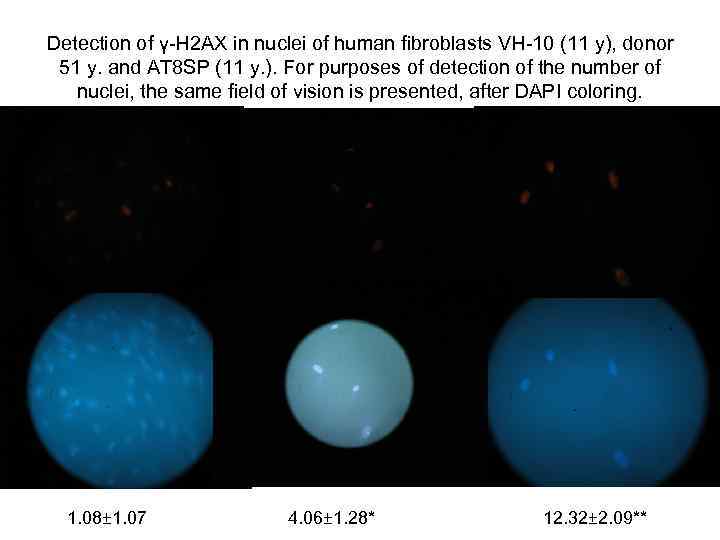 Detection of γ-Н 2 АХ in nuclei of human fibroblasts VH-10 (11 y), donor 51 y. and АТ 8 SP (11 y. ). For purposes of detection of the number of nuclei, the same field of vision is presented, after DAPI coloring. 1. 08± 1. 07 4. 06± 1. 28* 12. 32± 2. 09**
Detection of γ-Н 2 АХ in nuclei of human fibroblasts VH-10 (11 y), donor 51 y. and АТ 8 SP (11 y. ). For purposes of detection of the number of nuclei, the same field of vision is presented, after DAPI coloring. 1. 08± 1. 07 4. 06± 1. 28* 12. 32± 2. 09**
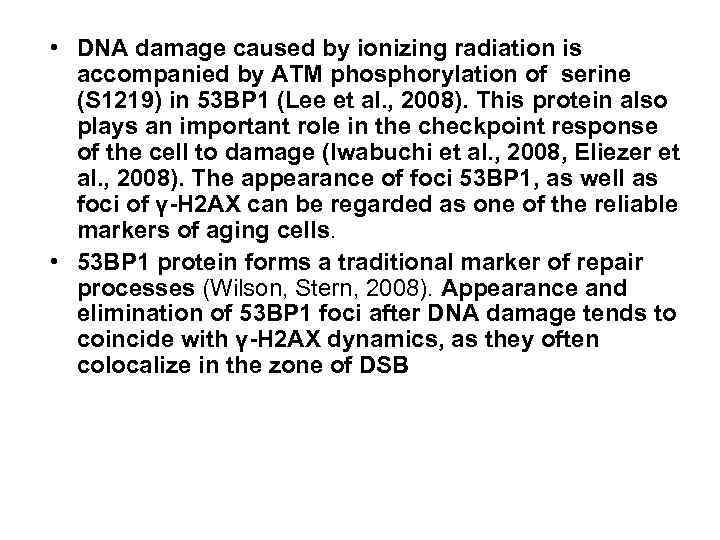 • DNA damage caused by ionizing radiation is accompanied by ATM phosphorylation of serine (S 1219) in 53 BP 1 (Lee et al. , 2008). This protein also plays an important role in the checkpoint response of the cell to damage (Iwabuchi et al. , 2008, Eliezer et al. , 2008). The appearance of foci 53 ВР 1, as well as foci of γ-Н 2 AХ can be regarded as one of the reliable markers of aging cells. • 53 ВР 1 protein forms a traditional marker of repair processes (Wilson, Stern, 2008). Appearance and elimination of 53 ВР 1 foci after DNA damage tends to coincide with γ-Н 2 АХ dynamics, as they often colocalize in the zone of DSB
• DNA damage caused by ionizing radiation is accompanied by ATM phosphorylation of serine (S 1219) in 53 BP 1 (Lee et al. , 2008). This protein also plays an important role in the checkpoint response of the cell to damage (Iwabuchi et al. , 2008, Eliezer et al. , 2008). The appearance of foci 53 ВР 1, as well as foci of γ-Н 2 AХ can be regarded as one of the reliable markers of aging cells. • 53 ВР 1 protein forms a traditional marker of repair processes (Wilson, Stern, 2008). Appearance and elimination of 53 ВР 1 foci after DNA damage tends to coincide with γ-Н 2 АХ dynamics, as they often colocalize in the zone of DSB
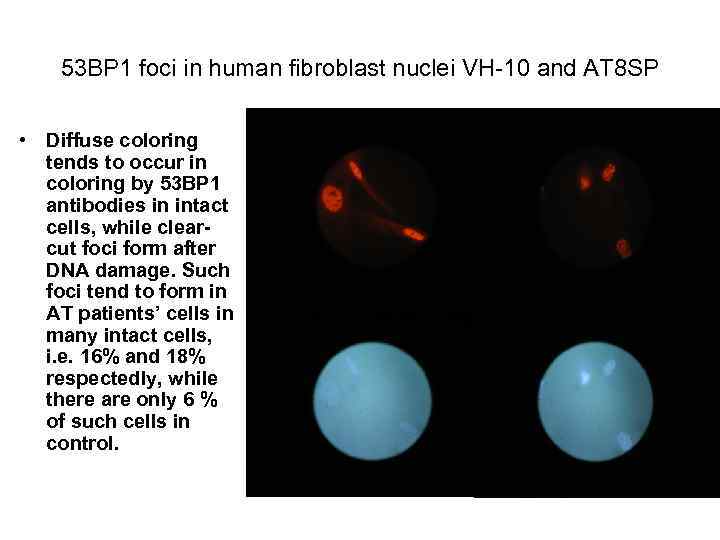 53 ВР 1 foci in human fibroblast nuclei VH-10 and АТ 8 SP • Diffuse coloring tends to occur in coloring by 53 ВР 1 antibodies in intact cells, while clearcut foci form after DNA damage. Such foci tend to form in AT patients’ cells in many intact cells, i. e. 16% and 18% respectedly, while there are only 6 % of such cells in control.
53 ВР 1 foci in human fibroblast nuclei VH-10 and АТ 8 SP • Diffuse coloring tends to occur in coloring by 53 ВР 1 antibodies in intact cells, while clearcut foci form after DNA damage. Such foci tend to form in AT patients’ cells in many intact cells, i. e. 16% and 18% respectedly, while there are only 6 % of such cells in control.
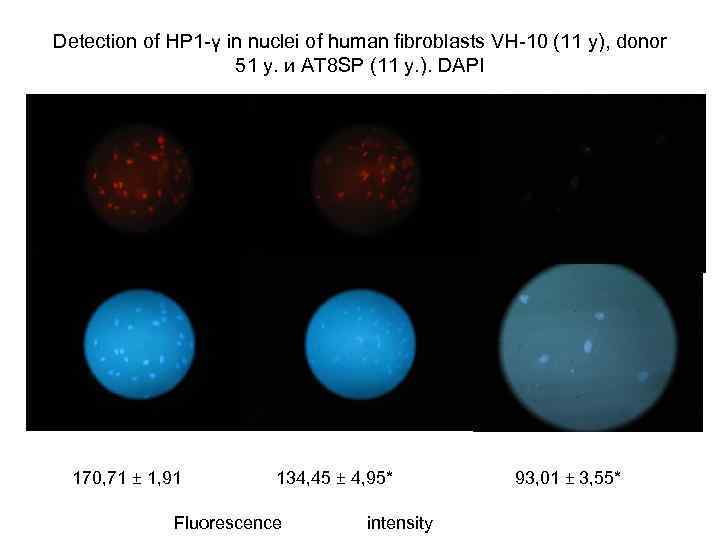 Detection of НР 1 -γ in nuclei of human fibroblasts VH-10 (11 y), donor 51 y. и АТ 8 SP (11 y. ). DAPI 170, 71 ± 1, 91 134, 45 ± 4, 95* Fluorescence intensity 93, 01 ± 3, 55*
Detection of НР 1 -γ in nuclei of human fibroblasts VH-10 (11 y), donor 51 y. и АТ 8 SP (11 y. ). DAPI 170, 71 ± 1, 91 134, 45 ± 4, 95* Fluorescence intensity 93, 01 ± 3, 55*
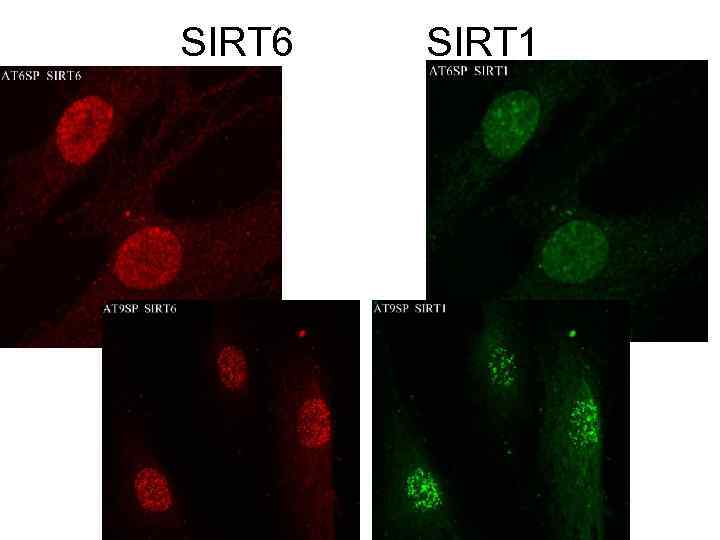 SIRT 6 SIRT 1
SIRT 6 SIRT 1
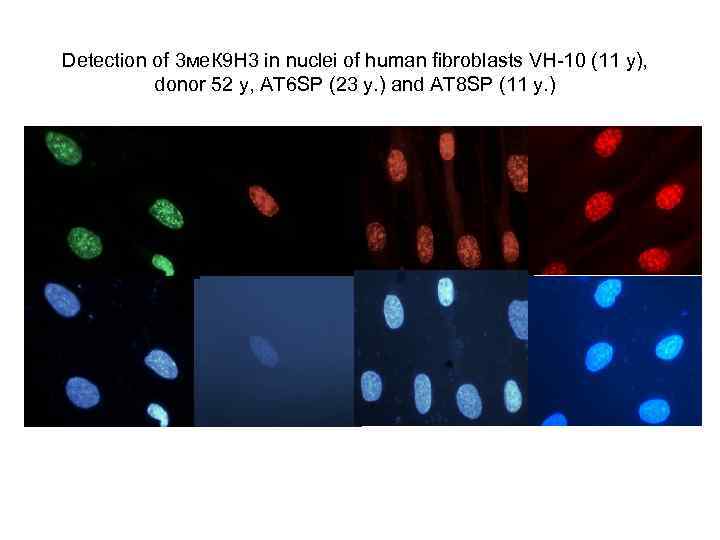 Detection of 3 ме. К 9 Н 3 in nuclei of human fibroblasts VH-10 (11 y), donor 52 y, AT 6 SP (23 y. ) and АТ 8 SP (11 y. )
Detection of 3 ме. К 9 Н 3 in nuclei of human fibroblasts VH-10 (11 y), donor 52 y, AT 6 SP (23 y. ) and АТ 8 SP (11 y. )
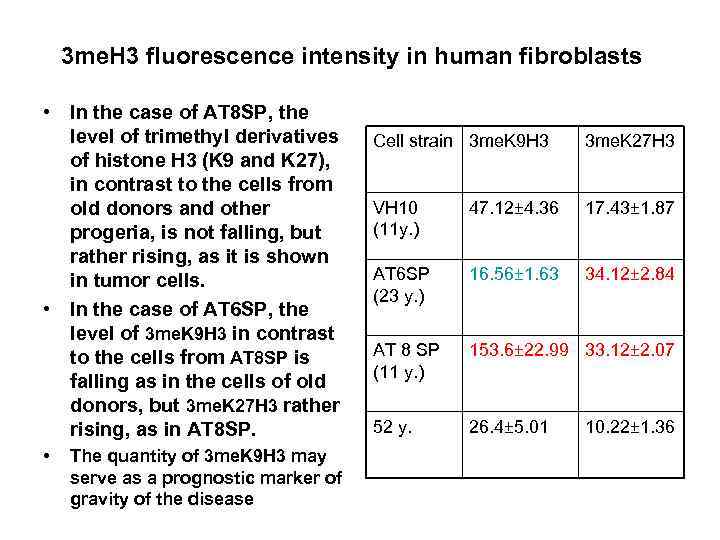 3 me. H 3 fluorescence intensity in human fibroblasts • In the case of AT 8 SP, the level of trimethyl derivatives of histone H 3 (K 9 and K 27), in contrast to the cells from old donors and other progeria, is not falling, but rather rising, as it is shown in tumor cells. • In the case of AT 6 SP, the level of 3 me. K 9 H 3 in contrast to the cells from AT 8 SP is falling as in the cells of old donors, but 3 me. K 27 H 3 rather rising, as in AT 8 SP. • Cell strain 3 me. K 9 H 3 3 me. K 27 H 3 VH 10 (11 y. ) 47. 12± 4. 36 17. 43± 1. 87 AT 6 SP (23 y. ) 16. 56± 1. 63 34. 12± 2. 84 AT 8 SP (11 y. ) 153. 6± 22. 99 33. 12± 2. 07 52 y. 26. 4± 5. 01 10. 22± 1. 36 The quantity of 3 me. K 9 H 3 may serve as a prognostic marker of gravity of the disease
3 me. H 3 fluorescence intensity in human fibroblasts • In the case of AT 8 SP, the level of trimethyl derivatives of histone H 3 (K 9 and K 27), in contrast to the cells from old donors and other progeria, is not falling, but rather rising, as it is shown in tumor cells. • In the case of AT 6 SP, the level of 3 me. K 9 H 3 in contrast to the cells from AT 8 SP is falling as in the cells of old donors, but 3 me. K 27 H 3 rather rising, as in AT 8 SP. • Cell strain 3 me. K 9 H 3 3 me. K 27 H 3 VH 10 (11 y. ) 47. 12± 4. 36 17. 43± 1. 87 AT 6 SP (23 y. ) 16. 56± 1. 63 34. 12± 2. 84 AT 8 SP (11 y. ) 153. 6± 22. 99 33. 12± 2. 07 52 y. 26. 4± 5. 01 10. 22± 1. 36 The quantity of 3 me. K 9 H 3 may serve as a prognostic marker of gravity of the disease
 Seckel syndrome (atr) • O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad 3 related protein (ATR) results in Seckel syndrome. Nat Genet. , 33: 497 -501
Seckel syndrome (atr) • O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad 3 related protein (ATR) results in Seckel syndrome. Nat Genet. , 33: 497 -501
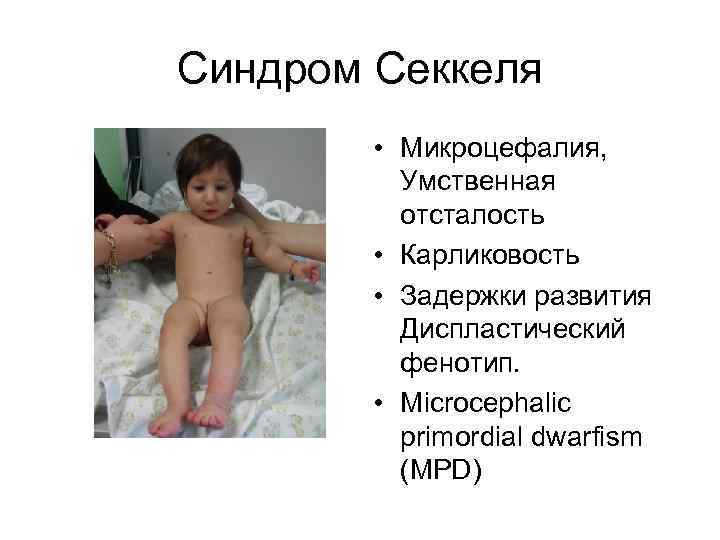 Синдром Секкеля • Микроцефалия, Умственная отсталость • Карликовость • Задержки развития Диспластический фенотип. • Microcephalic primordial dwarfism (MPD)
Синдром Секкеля • Микроцефалия, Умственная отсталость • Карликовость • Задержки развития Диспластический фенотип. • Microcephalic primordial dwarfism (MPD)
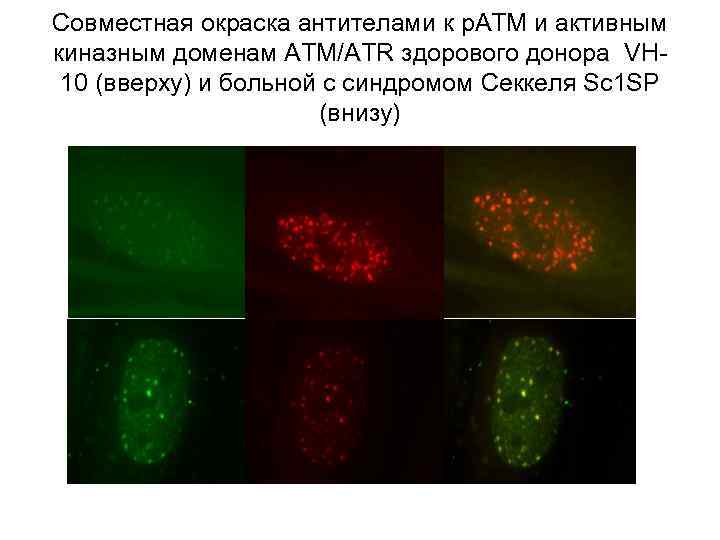 Совместная окраска антителами к р. АТМ и активным киназным доменам АТМ/АTR здорового донора VH 10 (вверху) и больной с синдромом Секкеля Sc 1 SP (внизу)
Совместная окраска антителами к р. АТМ и активным киназным доменам АТМ/АTR здорового донора VH 10 (вверху) и больной с синдромом Секкеля Sc 1 SP (внизу)
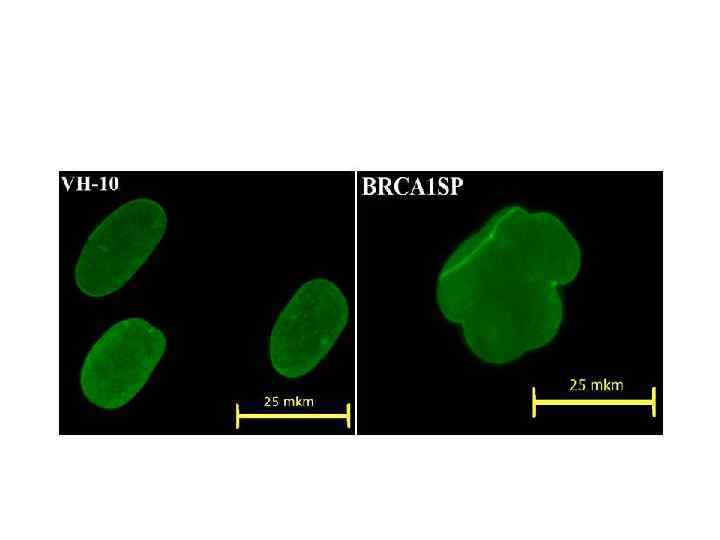
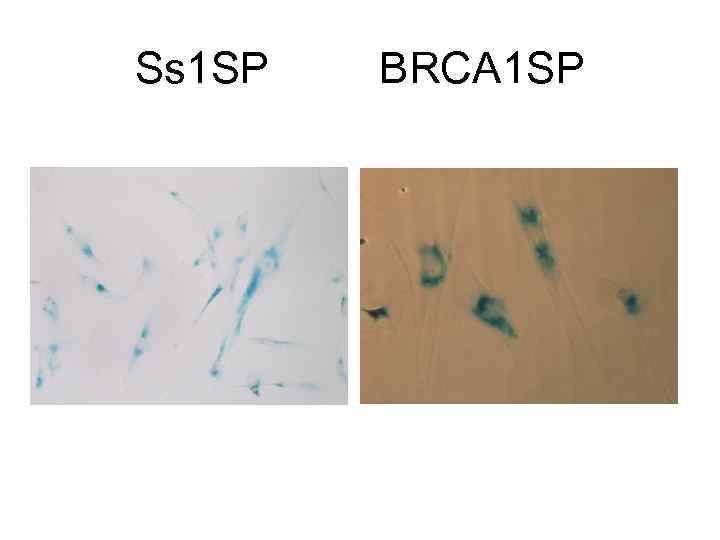 Ss 1 SP BRCA 1 SP
Ss 1 SP BRCA 1 SP
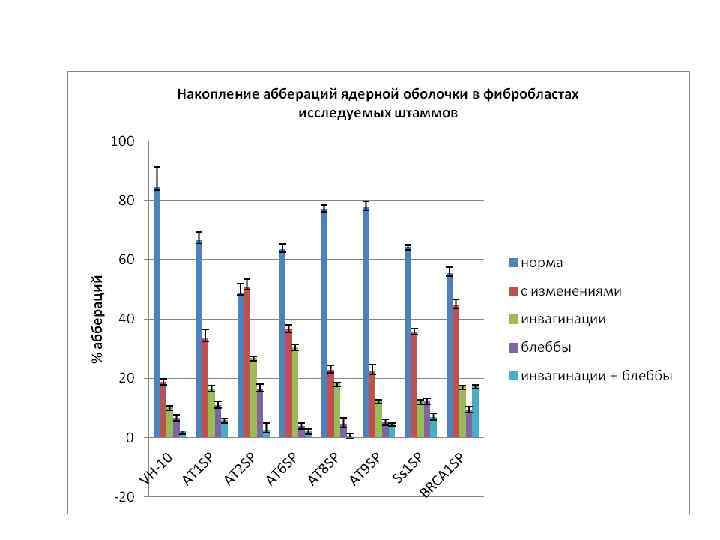
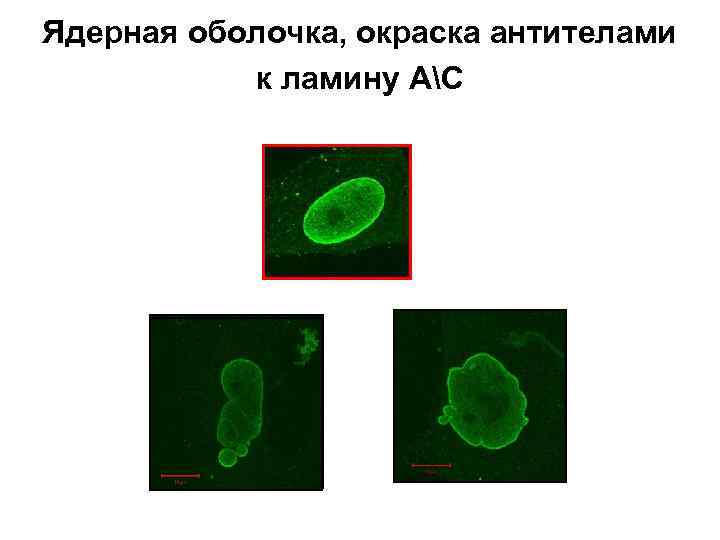 Ядерная оболочка, окраска антителами к ламину АС
Ядерная оболочка, окраска антителами к ламину АС
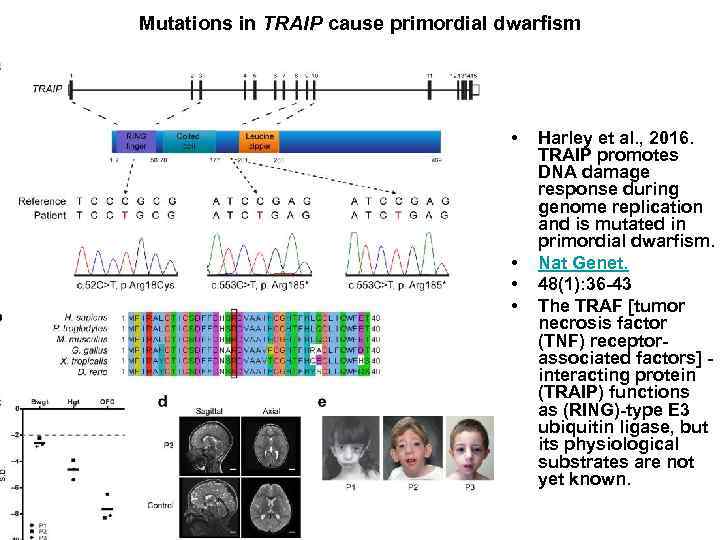 Mutations in TRAIP cause primordial dwarfism • • Harley et al. , 2016. TRAIP promotes DNA damage response during genome replication and is mutated in primordial dwarfism. Nat Genet. 48(1): 36 -43 The TRAF [tumor necrosis factor (TNF) receptorassociated factors] interacting protein (TRAIP) functions as (RING)-type E 3 ubiquitin ligase, but its physiological substrates are not yet known.
Mutations in TRAIP cause primordial dwarfism • • Harley et al. , 2016. TRAIP promotes DNA damage response during genome replication and is mutated in primordial dwarfism. Nat Genet. 48(1): 36 -43 The TRAF [tumor necrosis factor (TNF) receptorassociated factors] interacting protein (TRAIP) functions as (RING)-type E 3 ubiquitin ligase, but its physiological substrates are not yet known.
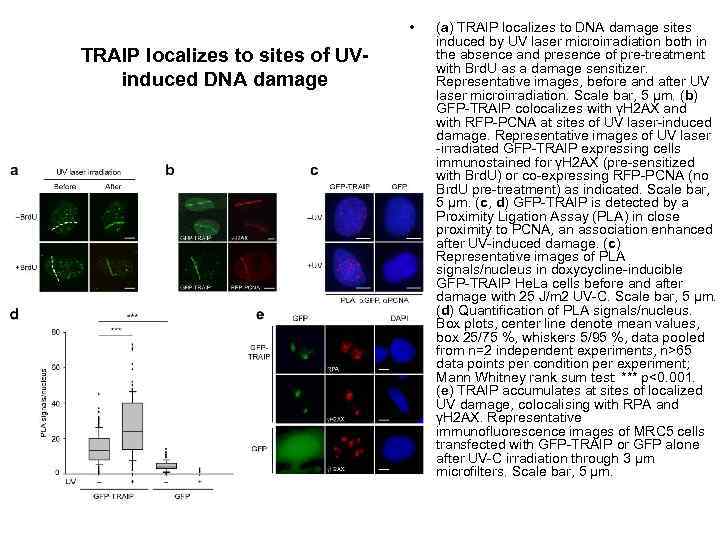 • TRAIP localizes to sites of UVinduced DNA damage (a) TRAIP localizes to DNA damage sites induced by UV laser microirradiation both in the absence and presence of pre-treatment with Brd. U as a damage sensitizer. Representative images, before and after UV laser microirradiation. Scale bar, 5 μm. (b) GFP-TRAIP colocalizes with γH 2 AX and with RFP-PCNA at sites of UV laser-induced damage. Representative images of UV laser -irradiated GFP-TRAIP expressing cells immunostained for γH 2 AX (pre-sensitized with Brd. U) or co-expressing RFP-PCNA (no Brd. U pre-treatment) as indicated. Scale bar, 5 μm. (c, d) GFP-TRAIP is detected by a Proximity Ligation Assay (PLA) in close proximity to PCNA, an association enhanced after UV-induced damage. (c) Representative images of PLA signals/nucleus in doxycycline-inducible GFP-TRAIP He. La cells before and after damage with 25 J/m 2 UV-C. Scale bar, 5 μm. (d) Quantification of PLA signals/nucleus. Box plots, center line denote mean values, box 25/75 %, whiskers 5/95 %, data pooled from n=2 independent experiments, n>65 data points per condition per experiment; Mann Whitney rank sum test: *** p<0. 001. (e) TRAIP accumulates at sites of localized UV damage, colocalising with RPA and γH 2 AX. Representative immunofluorescence images of MRC 5 cells transfected with GFP-TRAIP or GFP alone after UV-C irradiation through 3 μm microfilters. Scale bar, 5 μm.
• TRAIP localizes to sites of UVinduced DNA damage (a) TRAIP localizes to DNA damage sites induced by UV laser microirradiation both in the absence and presence of pre-treatment with Brd. U as a damage sensitizer. Representative images, before and after UV laser microirradiation. Scale bar, 5 μm. (b) GFP-TRAIP colocalizes with γH 2 AX and with RFP-PCNA at sites of UV laser-induced damage. Representative images of UV laser -irradiated GFP-TRAIP expressing cells immunostained for γH 2 AX (pre-sensitized with Brd. U) or co-expressing RFP-PCNA (no Brd. U pre-treatment) as indicated. Scale bar, 5 μm. (c, d) GFP-TRAIP is detected by a Proximity Ligation Assay (PLA) in close proximity to PCNA, an association enhanced after UV-induced damage. (c) Representative images of PLA signals/nucleus in doxycycline-inducible GFP-TRAIP He. La cells before and after damage with 25 J/m 2 UV-C. Scale bar, 5 μm. (d) Quantification of PLA signals/nucleus. Box plots, center line denote mean values, box 25/75 %, whiskers 5/95 %, data pooled from n=2 independent experiments, n>65 data points per condition per experiment; Mann Whitney rank sum test: *** p<0. 001. (e) TRAIP accumulates at sites of localized UV damage, colocalising with RPA and γH 2 AX. Representative immunofluorescence images of MRC 5 cells transfected with GFP-TRAIP or GFP alone after UV-C irradiation through 3 μm microfilters. Scale bar, 5 μm.
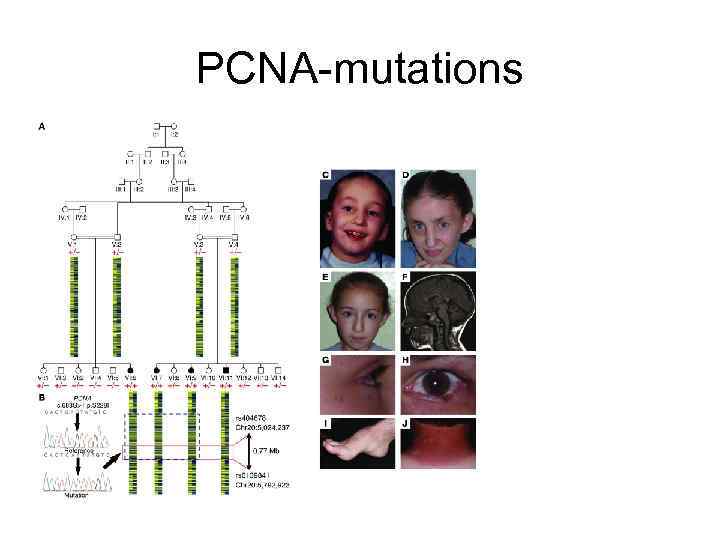 PCNA-mutations
PCNA-mutations
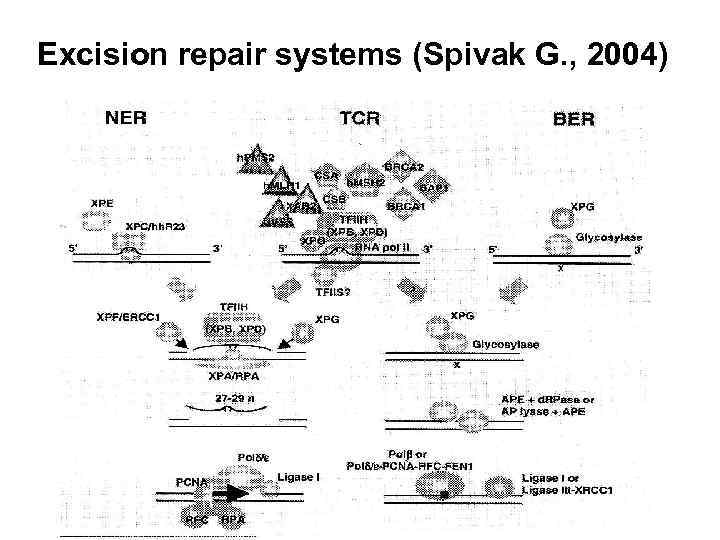 Excision repair systems (Spivak G. , 2004)
Excision repair systems (Spivak G. , 2004)
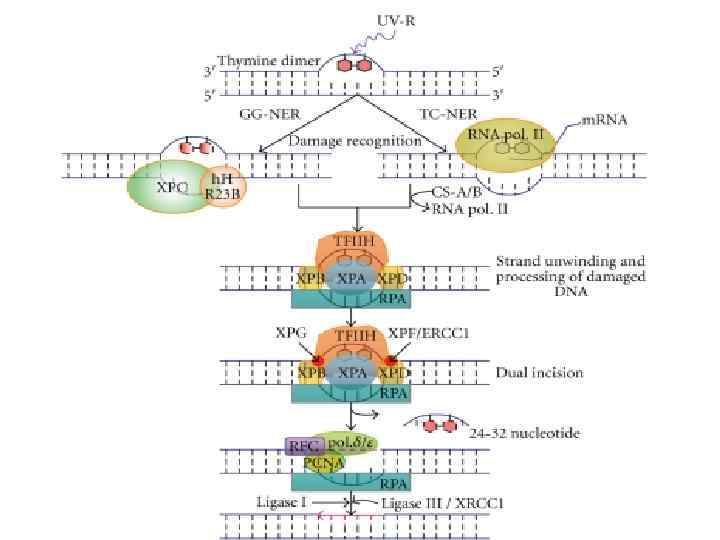
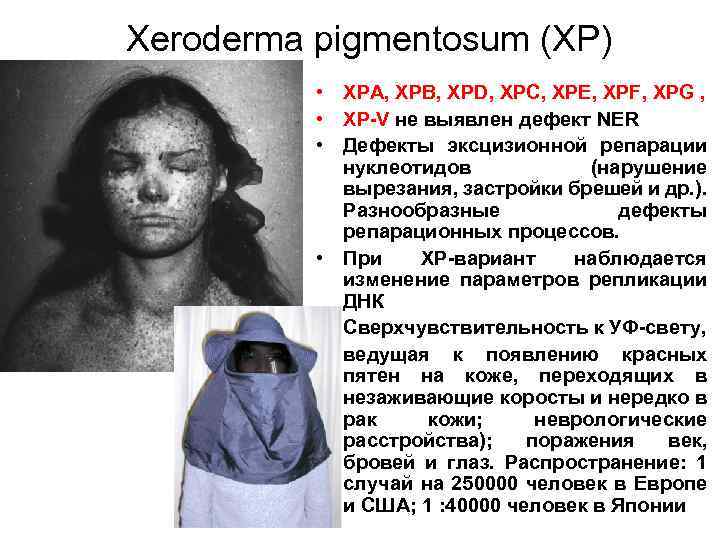 Xeroderma pigmentosum (XP) • XPA, XPB, XPD, XPC, XPE, XPF, XPG , • XP-V не выявлен дефект NER • Дефекты эксцизионной репарации нуклеотидов (нарушение вырезания, застройки брешей и др. ). Разнообразные дефекты репарационных процессов. • При XP-вариант наблюдается изменение параметров репликации ДНК • Сверхчувствительность к УФ-свету, • ведущая к появлению красных пятен на коже, переходящих в незаживающие коросты и нередко в рак кожи; неврологические расстройства); поражения век, бровей и глаз. Распространение: 1 случай на 250000 человек в Европе и США; 1 : 40000 человек в Японии
Xeroderma pigmentosum (XP) • XPA, XPB, XPD, XPC, XPE, XPF, XPG , • XP-V не выявлен дефект NER • Дефекты эксцизионной репарации нуклеотидов (нарушение вырезания, застройки брешей и др. ). Разнообразные дефекты репарационных процессов. • При XP-вариант наблюдается изменение параметров репликации ДНК • Сверхчувствительность к УФ-свету, • ведущая к появлению красных пятен на коже, переходящих в незаживающие коросты и нередко в рак кожи; неврологические расстройства); поражения век, бровей и глаз. Распространение: 1 случай на 250000 человек в Европе и США; 1 : 40000 человек в Японии
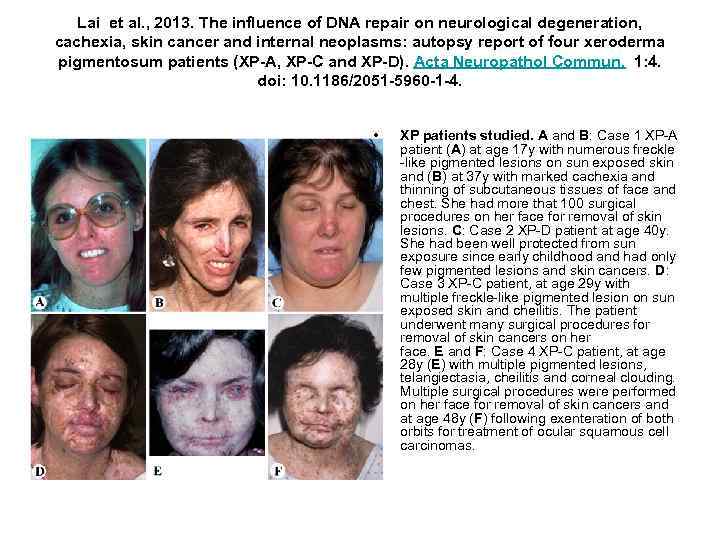 Lai et al. , 2013. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D). Acta Neuropathol Commun. 1: 4. doi: 10. 1186/2051 -5960 -1 -4. • XP patients studied. A and B: Case 1 XP-A patient (A) at age 17 y with numerous freckle -like pigmented lesions on sun exposed skin and (B) at 37 y with marked cachexia and thinning of subcutaneous tissues of face and chest. She had more that 100 surgical procedures on her face for removal of skin lesions. C: Case 2 XP-D patient at age 40 y. She had been well protected from sun exposure since early childhood and had only few pigmented lesions and skin cancers. D: Case 3 XP-C patient, at age 29 y with multiple freckle-like pigmented lesion on sun exposed skin and cheilitis. The patient underwent many surgical procedures for removal of skin cancers on her face. E and F: Case 4 XP-C patient, at age 28 y (E) with multiple pigmented lesions, telangiectasia, cheilitis and corneal clouding. Multiple surgical procedures were performed on her face for removal of skin cancers and at age 48 y (F) following exenteration of both orbits for treatment of ocular squamous cell carcinomas.
Lai et al. , 2013. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D). Acta Neuropathol Commun. 1: 4. doi: 10. 1186/2051 -5960 -1 -4. • XP patients studied. A and B: Case 1 XP-A patient (A) at age 17 y with numerous freckle -like pigmented lesions on sun exposed skin and (B) at 37 y with marked cachexia and thinning of subcutaneous tissues of face and chest. She had more that 100 surgical procedures on her face for removal of skin lesions. C: Case 2 XP-D patient at age 40 y. She had been well protected from sun exposure since early childhood and had only few pigmented lesions and skin cancers. D: Case 3 XP-C patient, at age 29 y with multiple freckle-like pigmented lesion on sun exposed skin and cheilitis. The patient underwent many surgical procedures for removal of skin cancers on her face. E and F: Case 4 XP-C patient, at age 28 y (E) with multiple pigmented lesions, telangiectasia, cheilitis and corneal clouding. Multiple surgical procedures were performed on her face for removal of skin cancers and at age 48 y (F) following exenteration of both orbits for treatment of ocular squamous cell carcinomas.
 Cockayne syndrome (CS) • Autosomal recessive • CSA (WD-repeat protein, β-subunit GTP protein homologue) CSB (ATPase of Swi 2 family), XPB and XPD (helicases in TFII-H), XPG (yeast Rad 2 homologue, endonuclease) 20 (40? ) Bird’s face Loss of subcutaneous fat Skin photosensitivity Neurodegeneration Hypogonadism • • • • UV-sensitivity Impaired transcription-coupled nucleotide excision repair Decreased recovery of transcription after irradiation General RNA-polymerase II transcription defects
Cockayne syndrome (CS) • Autosomal recessive • CSA (WD-repeat protein, β-subunit GTP protein homologue) CSB (ATPase of Swi 2 family), XPB and XPD (helicases in TFII-H), XPG (yeast Rad 2 homologue, endonuclease) 20 (40? ) Bird’s face Loss of subcutaneous fat Skin photosensitivity Neurodegeneration Hypogonadism • • • • UV-sensitivity Impaired transcription-coupled nucleotide excision repair Decreased recovery of transcription after irradiation General RNA-polymerase II transcription defects
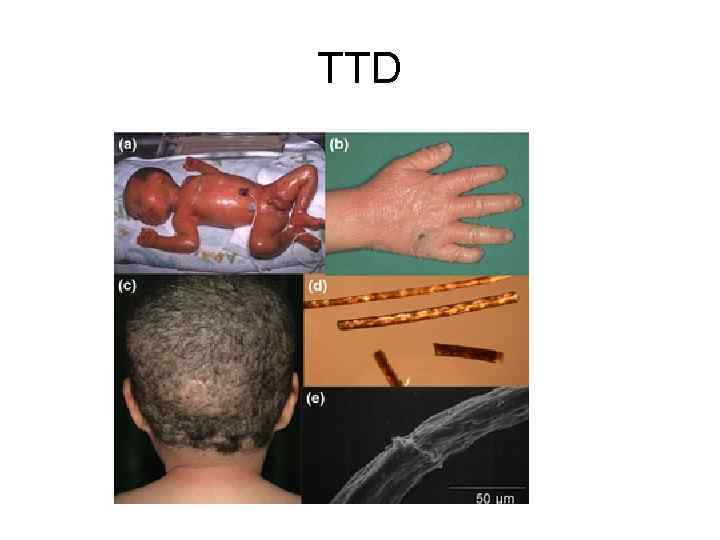 TTD
TTD
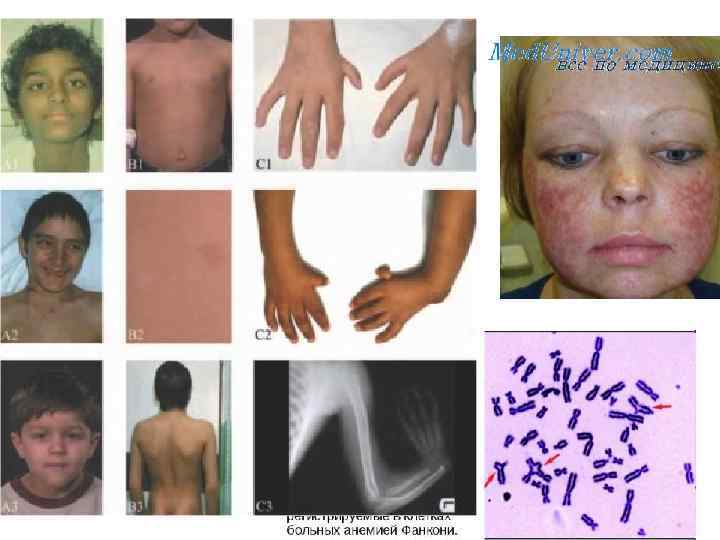
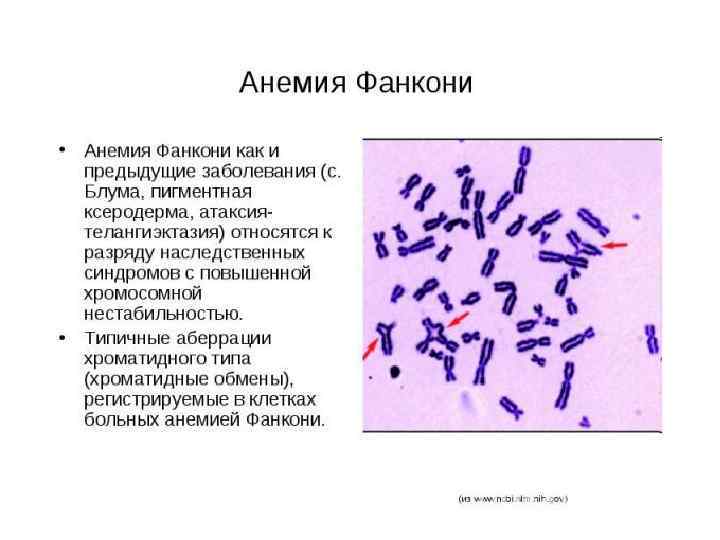
 Анемия Фанкони • FAA, FAB, FAC, FAD, FAE, FAF, FAG -19 групп комплементации • Дефекты репарации повреждений от химических мутагенов и канцерогенов (по не УФ-свста), обусловленные дефектами эндонуклеаз, дефектами распознавания кросс-сшивок ДНК; • пониженная способность к апоптозу после ионизирующего облучения; двукратное удлинение G 2 фазы клеточного цикла Сверхчувствительность к химическим мутагенам и канцерогенам, уменьшение количества всех клеточных элементов крови, различные аномалии врожденных способностей, деформация пальцев и другие виды скелетных нарушений, урогенитальные нарушения, микроцефалия, микрофтальмия, дефекты уха и потеря слуха, сердечные и гастроинтестинальные нарушения
Анемия Фанкони • FAA, FAB, FAC, FAD, FAE, FAF, FAG -19 групп комплементации • Дефекты репарации повреждений от химических мутагенов и канцерогенов (по не УФ-свста), обусловленные дефектами эндонуклеаз, дефектами распознавания кросс-сшивок ДНК; • пониженная способность к апоптозу после ионизирующего облучения; двукратное удлинение G 2 фазы клеточного цикла Сверхчувствительность к химическим мутагенам и канцерогенам, уменьшение количества всех клеточных элементов крови, различные аномалии врожденных способностей, деформация пальцев и другие виды скелетных нарушений, урогенитальные нарушения, микроцефалия, микрофтальмия, дефекты уха и потеря слуха, сердечные и гастроинтестинальные нарушения
 • Hum Genomics. 2015 Nov 24; 9(1): 32. doi: 10. 1186/s 40246 -015 -0054 -y. • Update of the human and mouse Fanconi anemia genes. • Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. • Fanconi anemia (FA) is a recessively inherited disease manifesting developmental abnormalities, bone marrow failure, and increased risk of malignancies. Whereas FA has been studied for nearly 90 years, only in the last 20 years have increasing numbers of genes been implicated in the pathogenesis associated with this genetic disease. To date, 19 genes have been identified that encode Fanconi anemia complementation group proteins, all of which are named or aliased, using the root symbol "FANC. " Fanconi anemia subtype (FANC) proteins function in a common DNA repair pathway called "the FA pathway, " which is essential for maintaining genomic integrity. The various FANC mutant proteins contribute to distinct steps associated with FA pathogenesis. Herein, we provide a review update of the 19 human FANC and their mouse orthologs, an evolutionary perspective on the FANC genes, and the functional significance of the FA DNA repair pathway in association with clinical disorders. This is an example of a set of genes--known to exist in vertebrates, invertebrates, plants, and yeast--that are grouped together on the basis of shared biochemical and physiological functions, rather than evolutionary phylogeny, and have been named on this basis by the HUGO Gene Nomenclature Committee (HGNC).
• Hum Genomics. 2015 Nov 24; 9(1): 32. doi: 10. 1186/s 40246 -015 -0054 -y. • Update of the human and mouse Fanconi anemia genes. • Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. • Fanconi anemia (FA) is a recessively inherited disease manifesting developmental abnormalities, bone marrow failure, and increased risk of malignancies. Whereas FA has been studied for nearly 90 years, only in the last 20 years have increasing numbers of genes been implicated in the pathogenesis associated with this genetic disease. To date, 19 genes have been identified that encode Fanconi anemia complementation group proteins, all of which are named or aliased, using the root symbol "FANC. " Fanconi anemia subtype (FANC) proteins function in a common DNA repair pathway called "the FA pathway, " which is essential for maintaining genomic integrity. The various FANC mutant proteins contribute to distinct steps associated with FA pathogenesis. Herein, we provide a review update of the 19 human FANC and their mouse orthologs, an evolutionary perspective on the FANC genes, and the functional significance of the FA DNA repair pathway in association with clinical disorders. This is an example of a set of genes--known to exist in vertebrates, invertebrates, plants, and yeast--that are grouped together on the basis of shared biochemical and physiological functions, rather than evolutionary phylogeny, and have been named on this basis by the HUGO Gene Nomenclature Committee (HGNC).
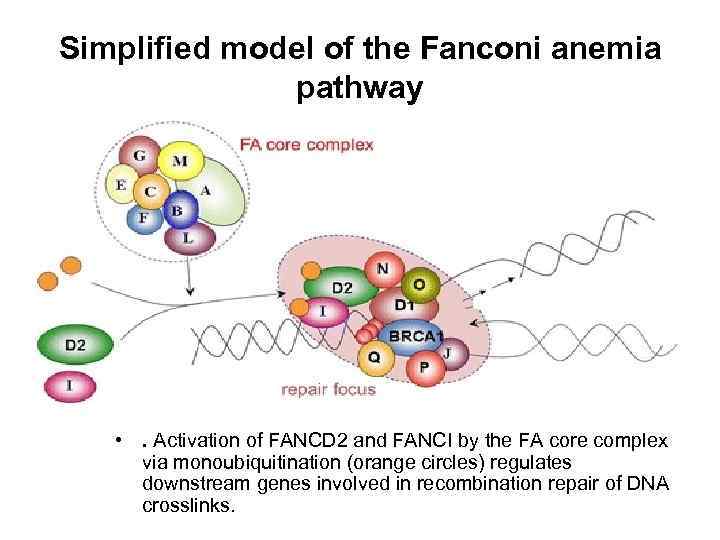
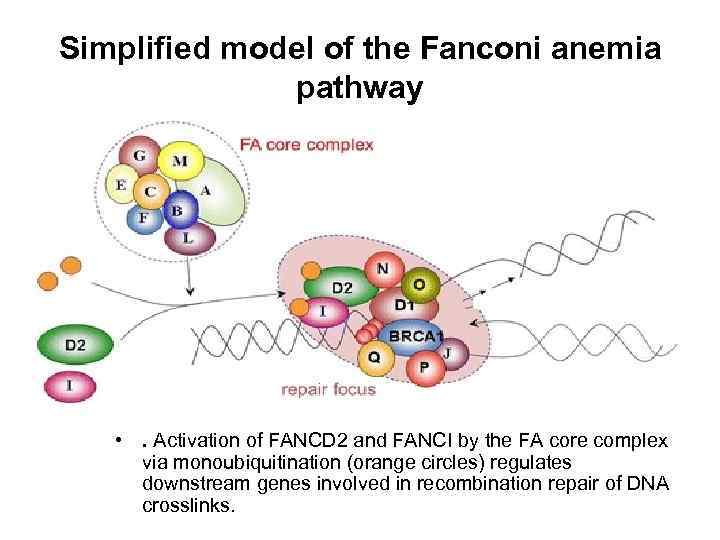 Simplified model of the Fanconi anemia pathway • . Activation of FANCD 2 and FANCI by the FA core complex via monoubiquitination (orange circles) regulates downstream genes involved in recombination repair of DNA crosslinks.
Simplified model of the Fanconi anemia pathway • . Activation of FANCD 2 and FANCI by the FA core complex via monoubiquitination (orange circles) regulates downstream genes involved in recombination repair of DNA crosslinks.
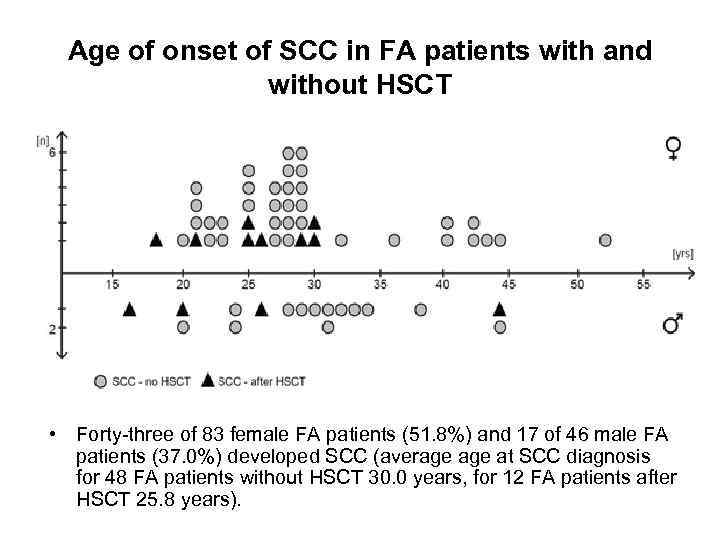 Age of onset of SCC in FA patients with and without HSCT • Forty-three of 83 female FA patients (51. 8%) and 17 of 46 male FA patients (37. 0%) developed SCC (average at SCC diagnosis for 48 FA patients without HSCT 30. 0 years, for 12 FA patients after HSCT 25. 8 years).
Age of onset of SCC in FA patients with and without HSCT • Forty-three of 83 female FA patients (51. 8%) and 17 of 46 male FA patients (37. 0%) developed SCC (average at SCC diagnosis for 48 FA patients without HSCT 30. 0 years, for 12 FA patients after HSCT 25. 8 years).
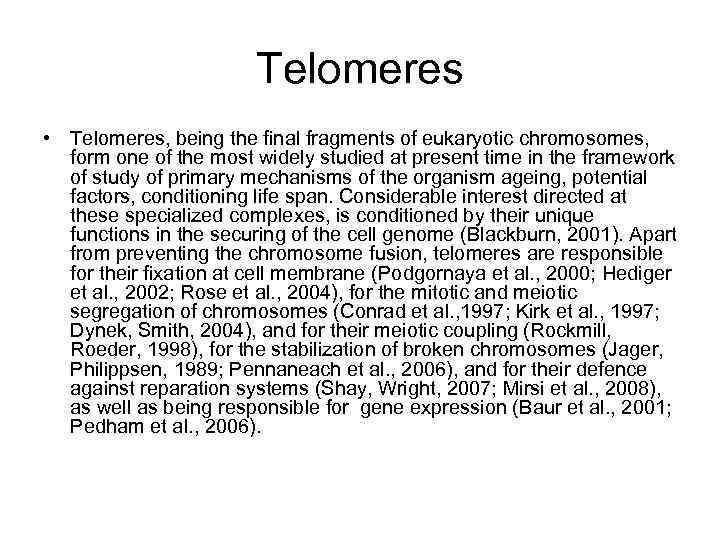 Telomeres • Telomeres, being the final fragments of eukaryotic chromosomes, form one of the most widely studied at present time in the framework of study of primary mechanisms of the organism ageing, potential factors, conditioning life span. Considerable interest directed at these specialized complexes, is conditioned by their unique functions in the securing of the cell genome (Blackburn, 2001). Apart from preventing the chromosome fusion, telomeres are responsible for their fixation at cell membrane (Podgornaya et al. , 2000; Hediger et al. , 2002; Rose et al. , 2004), for the mitotic and meiotic segregation of chromosomes (Conrad et al. , 1997; Kirk et al. , 1997; Dynek, Smith, 2004), and for their meiotic coupling (Rockmill, Roeder, 1998), for the stabilization of broken chromosomes (Jager, Philippsen, 1989; Pennaneach et al. , 2006), and for their defence against reparation systems (Shay, Wright, 2007; Mirsi et al. , 2008), as well as being responsible for gene expression (Baur et al. , 2001; Pedham et al. , 2006).
Telomeres • Telomeres, being the final fragments of eukaryotic chromosomes, form one of the most widely studied at present time in the framework of study of primary mechanisms of the organism ageing, potential factors, conditioning life span. Considerable interest directed at these specialized complexes, is conditioned by their unique functions in the securing of the cell genome (Blackburn, 2001). Apart from preventing the chromosome fusion, telomeres are responsible for their fixation at cell membrane (Podgornaya et al. , 2000; Hediger et al. , 2002; Rose et al. , 2004), for the mitotic and meiotic segregation of chromosomes (Conrad et al. , 1997; Kirk et al. , 1997; Dynek, Smith, 2004), and for their meiotic coupling (Rockmill, Roeder, 1998), for the stabilization of broken chromosomes (Jager, Philippsen, 1989; Pennaneach et al. , 2006), and for their defence against reparation systems (Shay, Wright, 2007; Mirsi et al. , 2008), as well as being responsible for gene expression (Baur et al. , 2001; Pedham et al. , 2006).
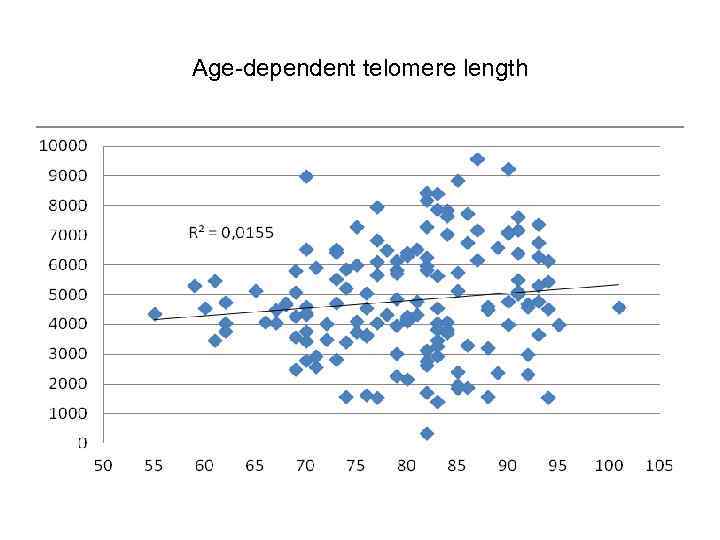 Age-dependent telomere length
Age-dependent telomere length
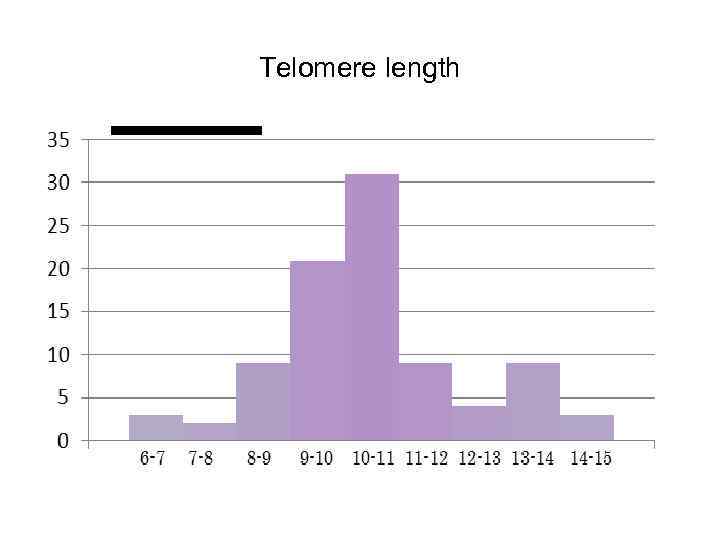 Telomere length
Telomere length
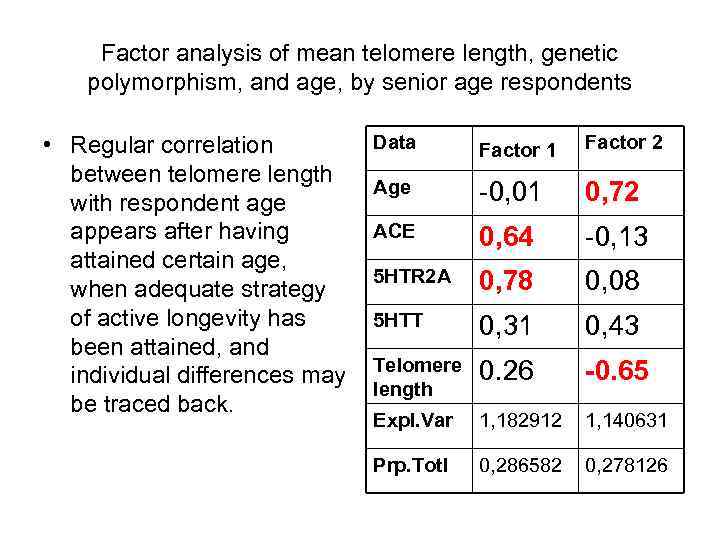 Factor analysis of mean telomere length, genetic polymorphism, and age, by senior age respondents • Regular correlation between telomere length with respondent age appears after having attained certain age, when adequate strategy of active longevity has been attained, and individual differences may be traced back. Data Factor 1 Factor 2 Age -0, 01 0, 72 АСЕ 0, 64 -0, 13 5 НТR 2 А 0, 78 0, 08 5 НТТ 0, 31 0, 43 Telomere length 0. 26 -0. 65 Expl. Var 1, 182912 1, 140631 Prp. Totl 0, 286582 0, 278126
Factor analysis of mean telomere length, genetic polymorphism, and age, by senior age respondents • Regular correlation between telomere length with respondent age appears after having attained certain age, when adequate strategy of active longevity has been attained, and individual differences may be traced back. Data Factor 1 Factor 2 Age -0, 01 0, 72 АСЕ 0, 64 -0, 13 5 НТR 2 А 0, 78 0, 08 5 НТТ 0, 31 0, 43 Telomere length 0. 26 -0. 65 Expl. Var 1, 182912 1, 140631 Prp. Totl 0, 286582 0, 278126
 Запретить
Запретить

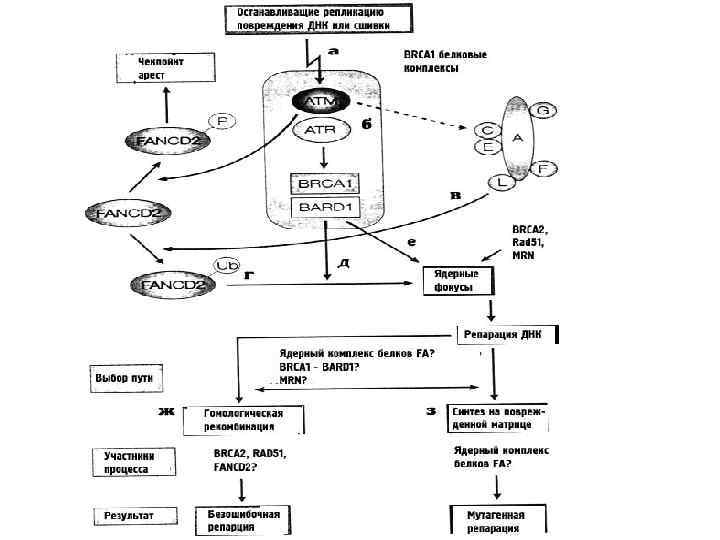
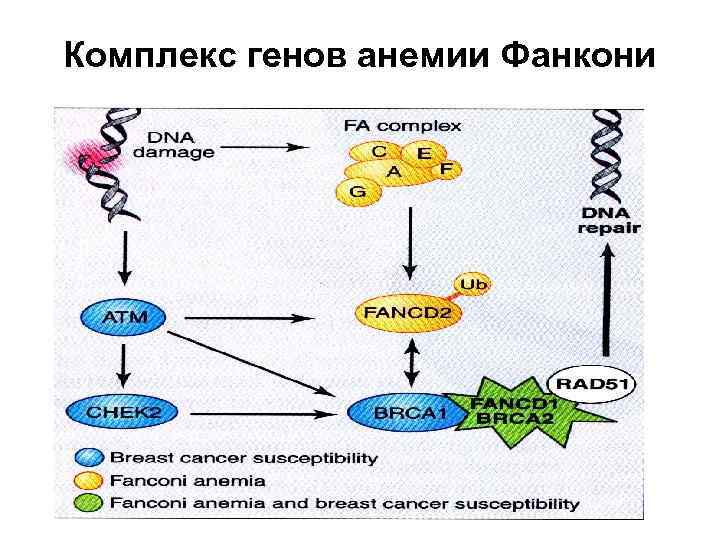 Комплекс генов анемии Фанкони
Комплекс генов анемии Фанкони
 Simplified model of the Fanconi anemia pathway • . Activation of FANCD 2 and FANCI by the FA core complex via monoubiquitination (orange circles) regulates downstream genes involved in recombination repair of DNA crosslinks.
Simplified model of the Fanconi anemia pathway • . Activation of FANCD 2 and FANCI by the FA core complex via monoubiquitination (orange circles) regulates downstream genes involved in recombination repair of DNA crosslinks.
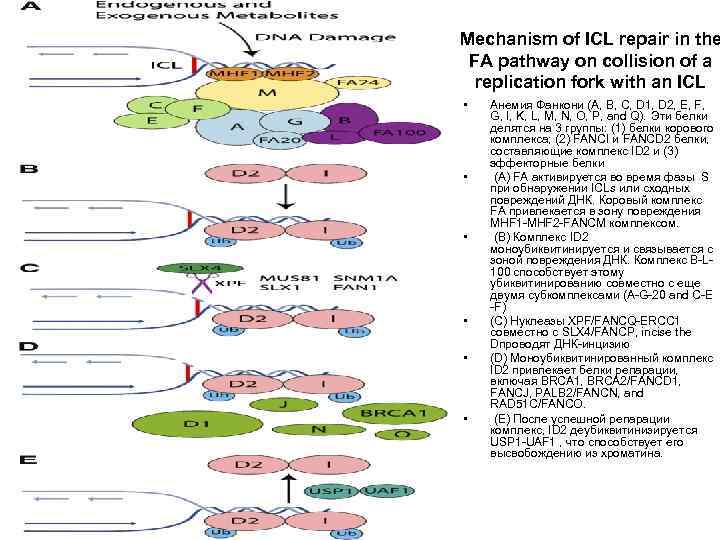 Mechanism of ICL repair in the FA pathway on collision of a replication fork with an ICL • • • Анемия Фанкони (A, B, C, D 1, D 2, E, F, G, I, K, L, M, N, O, P, and Q). Эти белки делятся на 3 группы: (1) белки корового комплекса; (2) FANCI и FANCD 2 белки, составляющие комплекс ID 2 и (3) эффекторные белки (A) FA активируется во время фазы S при обнаружении ICLs или сходных повреждений ДНК. Коровый комплекс FA привлекается в зону повреждения MHF 1 -MHF 2 -FANCM комплексом. (B) Комплекс ID 2 моноубиквитинируется и связывается с зоной повреждения ДНК. Комплекс B-L 100 способствует этому убиквитинированию совместно с еще двумя субкомплексами (A-G-20 and C-E -F) (C) Нуклеазы XPF/FANCQ-ERCC 1 совместно с SLX 4/FANCP, incise the Dпроводят ДНК-инцизию (D) Моноубиквитинированный комплекс ID 2 привлекает белки репарации, включая BRCA 1, BRCA 2/FANCD 1, FANCJ, PALB 2/FANCN, and RAD 51 C/FANCO. (E) После успешной репарации комплекс, ID 2 деубиквитинизируется USP 1 -UAF 1 , что способствует его высвобождению из хроматина.
Mechanism of ICL repair in the FA pathway on collision of a replication fork with an ICL • • • Анемия Фанкони (A, B, C, D 1, D 2, E, F, G, I, K, L, M, N, O, P, and Q). Эти белки делятся на 3 группы: (1) белки корового комплекса; (2) FANCI и FANCD 2 белки, составляющие комплекс ID 2 и (3) эффекторные белки (A) FA активируется во время фазы S при обнаружении ICLs или сходных повреждений ДНК. Коровый комплекс FA привлекается в зону повреждения MHF 1 -MHF 2 -FANCM комплексом. (B) Комплекс ID 2 моноубиквитинируется и связывается с зоной повреждения ДНК. Комплекс B-L 100 способствует этому убиквитинированию совместно с еще двумя субкомплексами (A-G-20 and C-E -F) (C) Нуклеазы XPF/FANCQ-ERCC 1 совместно с SLX 4/FANCP, incise the Dпроводят ДНК-инцизию (D) Моноубиквитинированный комплекс ID 2 привлекает белки репарации, включая BRCA 1, BRCA 2/FANCD 1, FANCJ, PALB 2/FANCN, and RAD 51 C/FANCO. (E) После успешной репарации комплекс, ID 2 деубиквитинизируется USP 1 -UAF 1 , что способствует его высвобождению из хроматина.
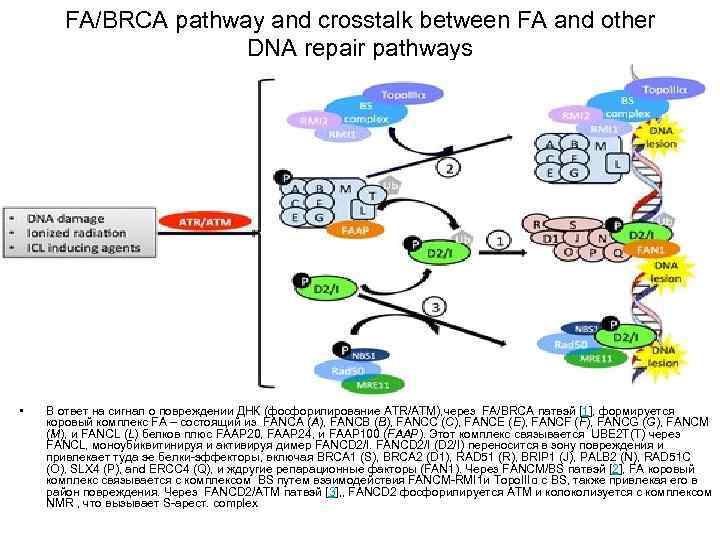 FA/BRCA pathway and crosstalk between FA and other DNA repair pathways • В ответ на сигнал о повреждении ДНК (фосфорилирование ATR/ATM), через FA/BRCA патвэй [1], формируется коровый комплекс FA – состоящий из FANCA (A), FANCB (B), FANCC (C), FANCE (E), FANCF (F), FANCG (G), FANCM (M), и FANCL (L) белков плюс FAAP 20, FAAP 24, и FAAP 100 (FAAP). Этот комплекс связывается UBE 2 T(T) через FANCL, моноубиквитинируя и активируя димер FANCD 2/I (D 2/I) переносится в зону повреждения и привлекает туда эе белки-эффекторы, включая BRCA 1 (S), BRCA 2 (D 1), RAD 51 (R), BRIP 1 (J), PALB 2 (N), RAD 51 C (O), SLX 4 (P), and ERCC 4 (Q), и ждругие репарационные факторы (FAN 1). Через FANCM/BS патвэй [2], FA коровый комплекс связывается с комплексом BS путем взаимодействия FANCM-RMI 1 и Topo. IIIα с BS, также привлекая его в район повреждения. Через FANCD 2/ATM патвэй [3], , FANCD 2 фосфорилируется ATM и колоколизуется с комплексом NMR , что вызывает S-арест. complex
FA/BRCA pathway and crosstalk between FA and other DNA repair pathways • В ответ на сигнал о повреждении ДНК (фосфорилирование ATR/ATM), через FA/BRCA патвэй [1], формируется коровый комплекс FA – состоящий из FANCA (A), FANCB (B), FANCC (C), FANCE (E), FANCF (F), FANCG (G), FANCM (M), и FANCL (L) белков плюс FAAP 20, FAAP 24, и FAAP 100 (FAAP). Этот комплекс связывается UBE 2 T(T) через FANCL, моноубиквитинируя и активируя димер FANCD 2/I (D 2/I) переносится в зону повреждения и привлекает туда эе белки-эффекторы, включая BRCA 1 (S), BRCA 2 (D 1), RAD 51 (R), BRIP 1 (J), PALB 2 (N), RAD 51 C (O), SLX 4 (P), and ERCC 4 (Q), и ждругие репарационные факторы (FAN 1). Через FANCM/BS патвэй [2], FA коровый комплекс связывается с комплексом BS путем взаимодействия FANCM-RMI 1 и Topo. IIIα с BS, также привлекая его в район повреждения. Через FANCD 2/ATM патвэй [3], , FANCD 2 фосфорилируется ATM и колоколизуется с комплексом NMR , что вызывает S-арест. complex
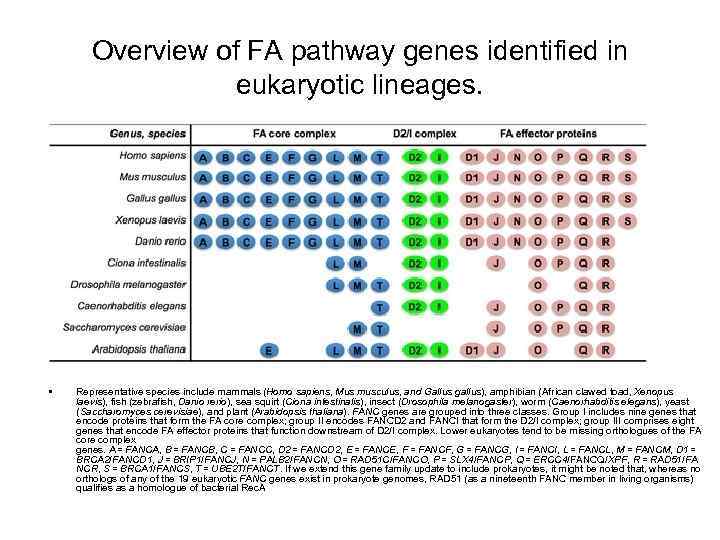 Overview of FA pathway genes identified in eukaryotic lineages. • Representative species include mammals (Homo sapiens, Mus musculus, and Gallus gallus), amphibian (African clawed toad, Xenopus laevis), fish (zebrafish, Danio rerio), sea squirt (Ciona intestinalis), insect (Drosophila melanogaster), worm (Caenorhabditis elegans), yeast (Saccharomyces cerevisiae), and plant (Arabidopsis thaliana). FANC genes are grouped into three classes. Group I includes nine genes that encode proteins that form the FA core complex; group II encodes FANCD 2 and FANCI that form the D 2/I complex; group III comprises eight genes that encode FA effector proteins that function downstream of D 2/I complex. Lower eukaryotes tend to be missing orthologues of the FA core complex genes. A = FANCA, B = FANCB, C = FANCC, D 2 = FANCD 2, E = FANCE, F = FANCF, G = FANCG, I = FANCI, L = FANCL, M = FANCM, D 1 = BRCA 2/FANCD 1, J = BRIP 1/FANCJ, N = PALB 2/FANCN, O = RAD 51 C/FANCO, P = SLX 4/FANCP, Q = ERCC 4/FANCQ/XPF, R = RAD 51/FA NCR, S = BRCA 1/FANCS, T = UBE 2 T/FANCT. If we extend this gene family update to include prokaryotes, it might be noted that, whereas no orthologs of any of the 19 eukaryotic FANC genes exist in prokaryote genomes, RAD 51 (as a nineteenth FANC member in living organisms) qualifies as a homologue of bacterial Rec. A
Overview of FA pathway genes identified in eukaryotic lineages. • Representative species include mammals (Homo sapiens, Mus musculus, and Gallus gallus), amphibian (African clawed toad, Xenopus laevis), fish (zebrafish, Danio rerio), sea squirt (Ciona intestinalis), insect (Drosophila melanogaster), worm (Caenorhabditis elegans), yeast (Saccharomyces cerevisiae), and plant (Arabidopsis thaliana). FANC genes are grouped into three classes. Group I includes nine genes that encode proteins that form the FA core complex; group II encodes FANCD 2 and FANCI that form the D 2/I complex; group III comprises eight genes that encode FA effector proteins that function downstream of D 2/I complex. Lower eukaryotes tend to be missing orthologues of the FA core complex genes. A = FANCA, B = FANCB, C = FANCC, D 2 = FANCD 2, E = FANCE, F = FANCF, G = FANCG, I = FANCI, L = FANCL, M = FANCM, D 1 = BRCA 2/FANCD 1, J = BRIP 1/FANCJ, N = PALB 2/FANCN, O = RAD 51 C/FANCO, P = SLX 4/FANCP, Q = ERCC 4/FANCQ/XPF, R = RAD 51/FA NCR, S = BRCA 1/FANCS, T = UBE 2 T/FANCT. If we extend this gene family update to include prokaryotes, it might be noted that, whereas no orthologs of any of the 19 eukaryotic FANC genes exist in prokaryote genomes, RAD 51 (as a nineteenth FANC member in living organisms) qualifies as a homologue of bacterial Rec. A
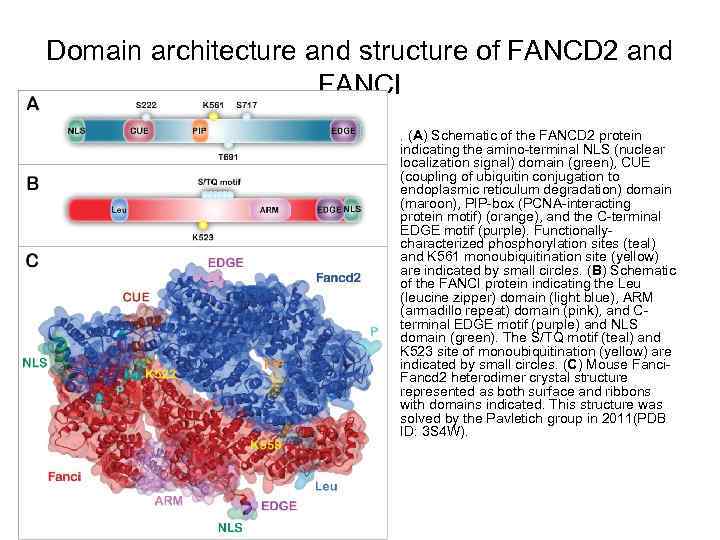 Domain architecture and structure of FANCD 2 and FANCI • . (A) Schematic of the FANCD 2 protein indicating the amino-terminal NLS (nuclear localization signal) domain (green), CUE (coupling of ubiquitin conjugation to endoplasmic reticulum degradation) domain (maroon), PIP-box (PCNA-interacting protein motif) (orange), and the C-terminal EDGE motif (purple). Functionallycharacterized phosphorylation sites (teal) and K 561 monoubiquitination site (yellow) are indicated by small circles. (B) Schematic of the FANCI protein indicating the Leu (leucine zipper) domain (light blue), ARM (armadillo repeat) domain (pink), and Cterminal EDGE motif (purple) and NLS domain (green). The S/TQ motif (teal) and K 523 site of monoubiquitination (yellow) are indicated by small circles. (C) Mouse Fanci. Fancd 2 heterodimer crystal structure represented as both surface and ribbons with domains indicated. This structure was solved by the Pavletich group in 2011(PDB ID: 3 S 4 W).
Domain architecture and structure of FANCD 2 and FANCI • . (A) Schematic of the FANCD 2 protein indicating the amino-terminal NLS (nuclear localization signal) domain (green), CUE (coupling of ubiquitin conjugation to endoplasmic reticulum degradation) domain (maroon), PIP-box (PCNA-interacting protein motif) (orange), and the C-terminal EDGE motif (purple). Functionallycharacterized phosphorylation sites (teal) and K 561 monoubiquitination site (yellow) are indicated by small circles. (B) Schematic of the FANCI protein indicating the Leu (leucine zipper) domain (light blue), ARM (armadillo repeat) domain (pink), and Cterminal EDGE motif (purple) and NLS domain (green). The S/TQ motif (teal) and K 523 site of monoubiquitination (yellow) are indicated by small circles. (C) Mouse Fanci. Fancd 2 heterodimer crystal structure represented as both surface and ribbons with domains indicated. This structure was solved by the Pavletich group in 2011(PDB ID: 3 S 4 W).
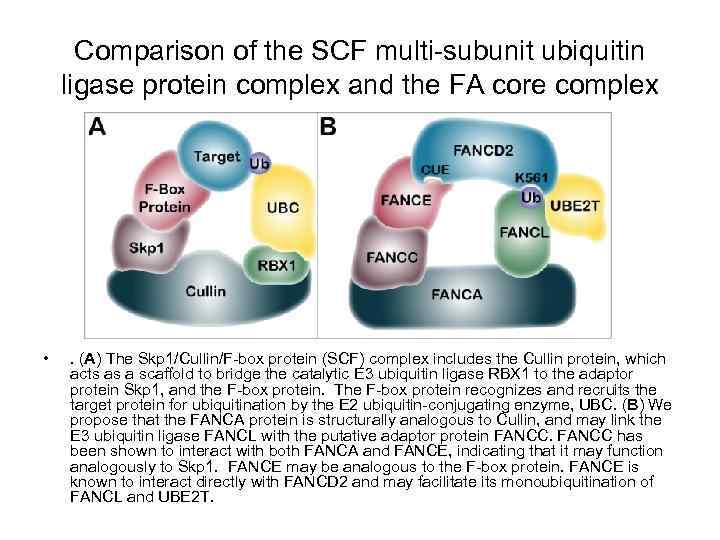 Comparison of the SCF multi-subunit ubiquitin ligase protein complex and the FA core complex • . (A) The Skp 1/Cullin/F-box protein (SCF) complex includes the Cullin protein, which acts as a scaffold to bridge the catalytic E 3 ubiquitin ligase RBX 1 to the adaptor protein Skp 1, and the F-box protein. The F-box protein recognizes and recruits the target protein for ubiquitination by the E 2 ubiquitin-conjugating enzyme, UBC. (B) We propose that the FANCA protein is structurally analogous to Cullin, and may link the E 3 ubiquitin ligase FANCL with the putative adaptor protein FANCC has been shown to interact with both FANCA and FANCE, indicating that it may function analogously to Skp 1. FANCE may be analogous to the F-box protein. FANCE is known to interact directly with FANCD 2 and may facilitate its monoubiquitination of FANCL and UBE 2 T.
Comparison of the SCF multi-subunit ubiquitin ligase protein complex and the FA core complex • . (A) The Skp 1/Cullin/F-box protein (SCF) complex includes the Cullin protein, which acts as a scaffold to bridge the catalytic E 3 ubiquitin ligase RBX 1 to the adaptor protein Skp 1, and the F-box protein. The F-box protein recognizes and recruits the target protein for ubiquitination by the E 2 ubiquitin-conjugating enzyme, UBC. (B) We propose that the FANCA protein is structurally analogous to Cullin, and may link the E 3 ubiquitin ligase FANCL with the putative adaptor protein FANCC has been shown to interact with both FANCA and FANCE, indicating that it may function analogously to Skp 1. FANCE may be analogous to the F-box protein. FANCE is known to interact directly with FANCD 2 and may facilitate its monoubiquitination of FANCL and UBE 2 T.
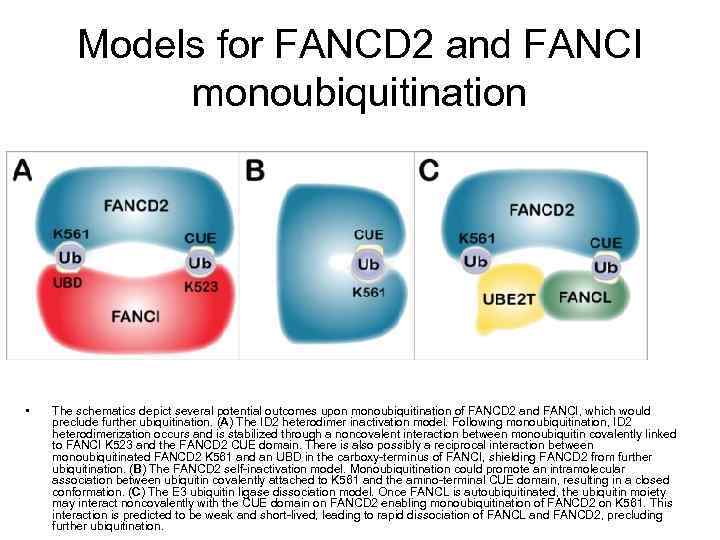 Models for FANCD 2 and FANCI monoubiquitination • The schematics depict several potential outcomes upon monoubiquitination of FANCD 2 and FANCI, which would preclude further ubiquitination. (A) The ID 2 heterodimer inactivation model. Following monoubiquitination, ID 2 heterodimerization occurs and is stabilized through a noncovalent interaction between monoubiquitin covalently linked to FANCI K 523 and the FANCD 2 CUE domain. There is also possibly a reciprocal interaction between monoubiquitinated FANCD 2 K 561 and an UBD in the carboxy-terminus of FANCI, shielding FANCD 2 from further ubiquitination. (B) The FANCD 2 self-inactivation model. Monoubiquitination could promote an intramolecular association between ubiquitin covalently attached to K 561 and the amino-terminal CUE domain, resulting in a closed conformation. (C) The E 3 ubiquitin ligase dissociation model. Once FANCL is autoubiquitinated, the ubiquitin moiety may interact noncovalently with the CUE domain on FANCD 2 enabling monoubiquitination of FANCD 2 on K 561. This interaction is predicted to be weak and short-lived, leading to rapid dissociation of FANCL and FANCD 2, precluding further ubiquitination.
Models for FANCD 2 and FANCI monoubiquitination • The schematics depict several potential outcomes upon monoubiquitination of FANCD 2 and FANCI, which would preclude further ubiquitination. (A) The ID 2 heterodimer inactivation model. Following monoubiquitination, ID 2 heterodimerization occurs and is stabilized through a noncovalent interaction between monoubiquitin covalently linked to FANCI K 523 and the FANCD 2 CUE domain. There is also possibly a reciprocal interaction between monoubiquitinated FANCD 2 K 561 and an UBD in the carboxy-terminus of FANCI, shielding FANCD 2 from further ubiquitination. (B) The FANCD 2 self-inactivation model. Monoubiquitination could promote an intramolecular association between ubiquitin covalently attached to K 561 and the amino-terminal CUE domain, resulting in a closed conformation. (C) The E 3 ubiquitin ligase dissociation model. Once FANCL is autoubiquitinated, the ubiquitin moiety may interact noncovalently with the CUE domain on FANCD 2 enabling monoubiquitination of FANCD 2 on K 561. This interaction is predicted to be weak and short-lived, leading to rapid dissociation of FANCL and FANCD 2, precluding further ubiquitination.
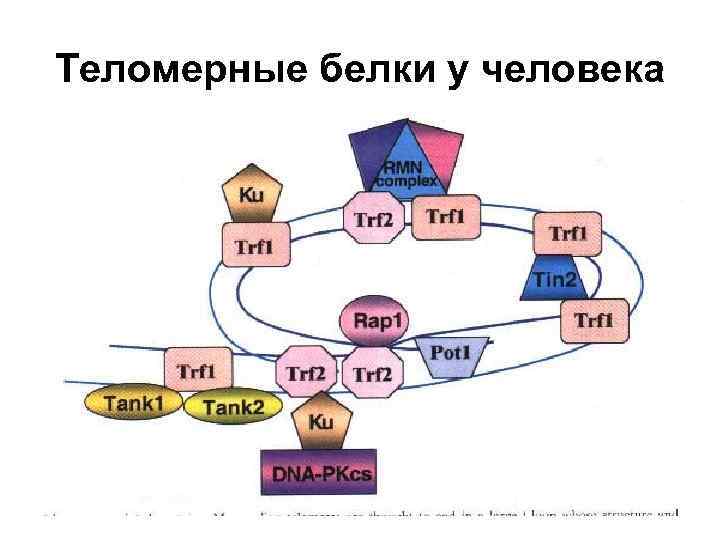 Теломерные белки у человека
Теломерные белки у человека
 • Data acquired in the course of study of primary fibroblasts from humans, are less contradictory, and provide a picture which is much more stable. It is necessary to assess more accurately the character of dispersion of all markers studied by decades, and to form a reliable scale. • It is just this observation which demonstrates that progeria is a deeply pathological aging and its analogy with the natural aging is limited.
• Data acquired in the course of study of primary fibroblasts from humans, are less contradictory, and provide a picture which is much more stable. It is necessary to assess more accurately the character of dispersion of all markers studied by decades, and to form a reliable scale. • It is just this observation which demonstrates that progeria is a deeply pathological aging and its analogy with the natural aging is limited.
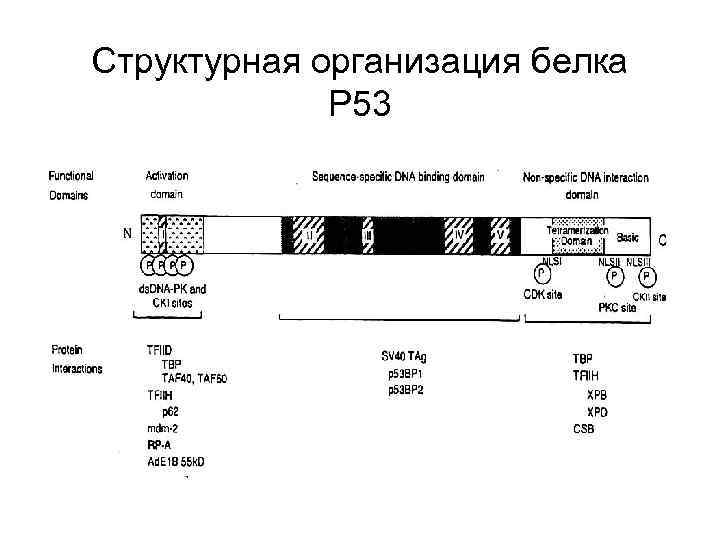 Структурная организация белка Р 53
Структурная организация белка Р 53
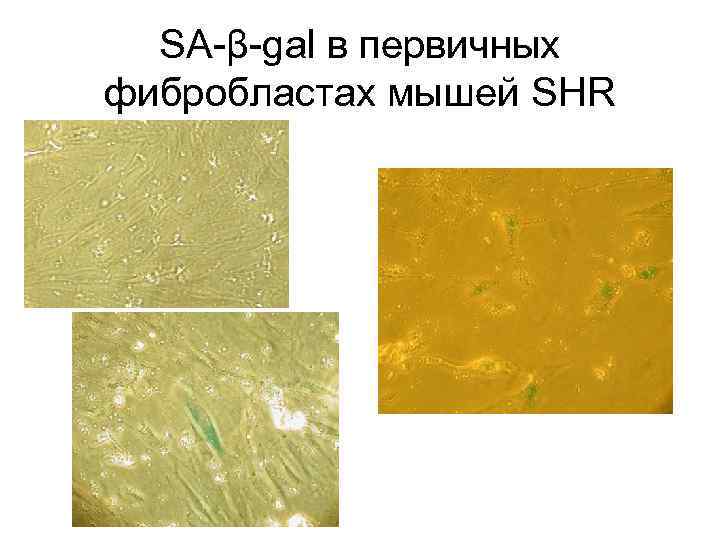 SA-β-gal в первичных фибробластах мышей SHR
SA-β-gal в первичных фибробластах мышей SHR
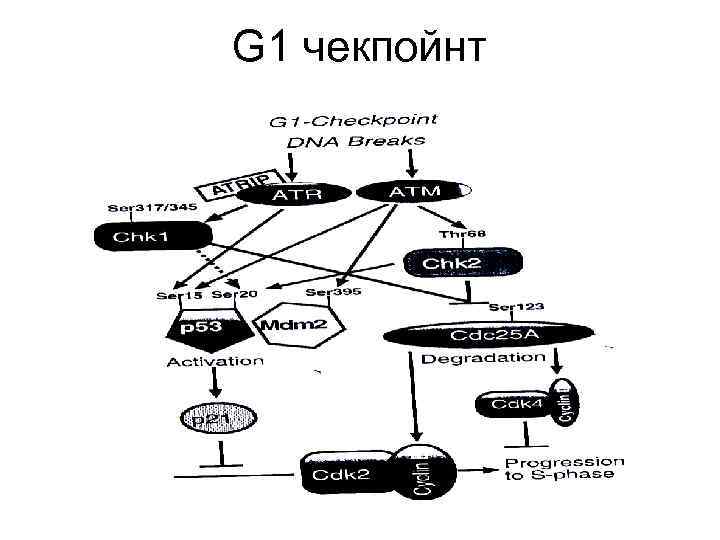 G 1 чекпойнт
G 1 чекпойнт
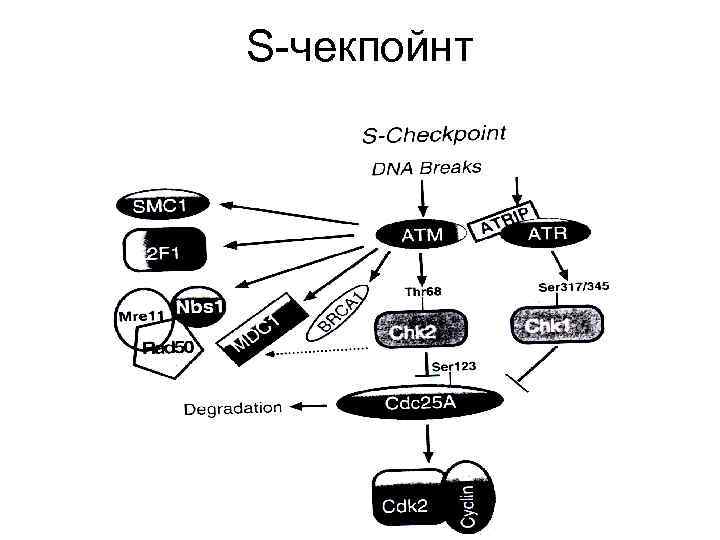 S-чекпойнт
S-чекпойнт
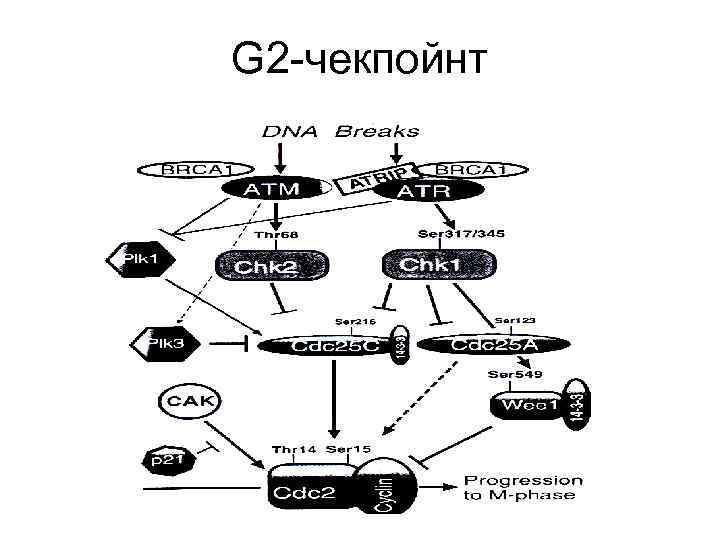 G 2 -чекпойнт
G 2 -чекпойнт
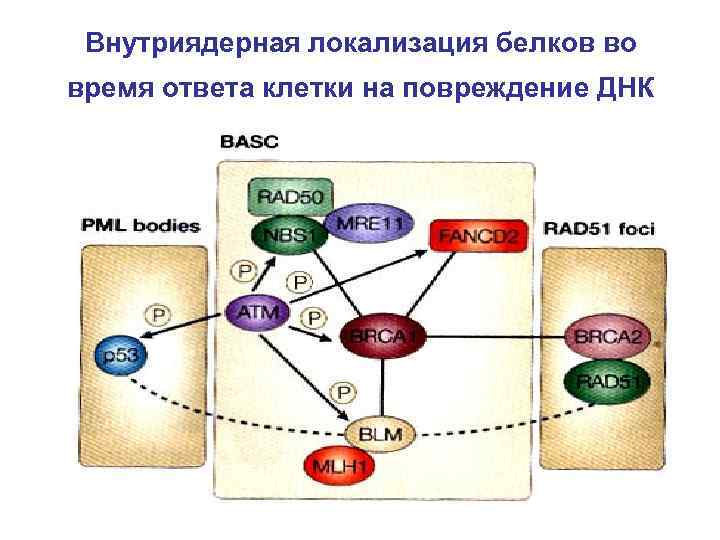 Внутриядерная локализация белков во время ответа клетки на повреждение ДНК
Внутриядерная локализация белков во время ответа клетки на повреждение ДНК
 Механизмы G 2 -ареста • АТМ активирует СНК 2 через фосфорелирование триптофана в 68 положении, которая в свою очередь фосфорелирует серин в 215 положении у CDC 25 C, что приводит к блокированию ее функций. Фосфорелированная форма CDC 25 C связывается с белком 14 -3 -3σ, что поддерживает еe каталитическую неактивность и способствует переходу в цитоплазму и секвестрированию. • Вторая ветвь G 2 -чекпойнта опосредуется через ATR/CHK 1 активацию. При этом пути одновременно фосфорелируется-выключается белок CDC 25 А, а также фосфорелируется серин-549 белка Wee 1 (пртеинкиназа), что облегчает его связывание с тем же белком 14 -3 -3σ и приводит к усилению ингибиторной активности киназ по отношению к CDC 2(CDK 1). Это придает второй ветви большую гибкость в контроле и консолидации G 2 -ареста.
Механизмы G 2 -ареста • АТМ активирует СНК 2 через фосфорелирование триптофана в 68 положении, которая в свою очередь фосфорелирует серин в 215 положении у CDC 25 C, что приводит к блокированию ее функций. Фосфорелированная форма CDC 25 C связывается с белком 14 -3 -3σ, что поддерживает еe каталитическую неактивность и способствует переходу в цитоплазму и секвестрированию. • Вторая ветвь G 2 -чекпойнта опосредуется через ATR/CHK 1 активацию. При этом пути одновременно фосфорелируется-выключается белок CDC 25 А, а также фосфорелируется серин-549 белка Wee 1 (пртеинкиназа), что облегчает его связывание с тем же белком 14 -3 -3σ и приводит к усилению ингибиторной активности киназ по отношению к CDC 2(CDK 1). Это придает второй ветви большую гибкость в контроле и консолидации G 2 -ареста.
 Механизмы G 2 -ареста(2) • После облучения резко падает уровень м. РНК циклина В, возможно из-за ее повышенной нестабильности, причем этот эффект определяет протяженность G 2 ареста. • Циклин В во время G 1 и S фаз имеет цитоплазматическую локализацию и перемещается в ядро только к началу митоза. Белок 14 -3 -3σ приводит к секвестрированию циклина В в цитоплазме в ответ на повреждение ДНК.
Механизмы G 2 -ареста(2) • После облучения резко падает уровень м. РНК циклина В, возможно из-за ее повышенной нестабильности, причем этот эффект определяет протяженность G 2 ареста. • Циклин В во время G 1 и S фаз имеет цитоплазматическую локализацию и перемещается в ядро только к началу митоза. Белок 14 -3 -3σ приводит к секвестрированию циклина В в цитоплазме в ответ на повреждение ДНК.
 Механизмы G 2 -ареста(3) • PLK 1 и PLK 3 (Polo-like kinase). Белки этого семейства принимают активное участие в митозе, включая вход и выход из него. PLK 1 является позитивным регулятором CDC 25 C-активности в необлученных клетках и, специфически фосфорелируя ее, способствует вхождению в митоз. PLK 3, напротив, активируется АТМ в ответ на повреждение ДНК, взаимодействует с CDC 25 C, фосфорелируя ее по серину-216, что приводит к ингибированию ее активности.
Механизмы G 2 -ареста(3) • PLK 1 и PLK 3 (Polo-like kinase). Белки этого семейства принимают активное участие в митозе, включая вход и выход из него. PLK 1 является позитивным регулятором CDC 25 C-активности в необлученных клетках и, специфически фосфорелируя ее, способствует вхождению в митоз. PLK 3, напротив, активируется АТМ в ответ на повреждение ДНК, взаимодействует с CDC 25 C, фосфорелируя ее по серину-216, что приводит к ингибированию ее активности.
 Механизмы G 2 -ареста(4) • Остановить вход в митоз при наличии повреждений в ДНК может взаимодействие PCNA c Р 21, CDC 25 C, и CDC 2(Cdk 1)-циклин В, но не одновременное, а последовательное. Связывание Р 21 и CDC 25 C с комплексом PCNA-CDC 2 -циклин В является совершенно особым и не позволяет CDC 25 C дефосфорелировать CDК 1 для активации митоза. Р 21 может блокировать САК, которая активирует CDК 1 путем фосфорелирования триптофана в 161 положении.
Механизмы G 2 -ареста(4) • Остановить вход в митоз при наличии повреждений в ДНК может взаимодействие PCNA c Р 21, CDC 25 C, и CDC 2(Cdk 1)-циклин В, но не одновременное, а последовательное. Связывание Р 21 и CDC 25 C с комплексом PCNA-CDC 2 -циклин В является совершенно особым и не позволяет CDC 25 C дефосфорелировать CDК 1 для активации митоза. Р 21 может блокировать САК, которая активирует CDК 1 путем фосфорелирования триптофана в 161 положении.
 Механизмы G 2 -ареста(5) • Роль Р 53 в поддержании G 2 -ареста состоит в том, что он активирует транскрипцию трех вовлеченных в него белков: GADD 45, P 21 и 14 -3 -3σ и подавляет транскрипцию CDC 2(CDK 1) и циклина В. • Есть данные о вовлеченности в G 2 -арест BRCA 1, опосредованно через ATM/ATR или напрямую, через активацию CHK 1, но механизм этого остается неясным. • G 2 -реакция клетки на облучение зависит от фазы цикла, в которую это произошло.
Механизмы G 2 -ареста(5) • Роль Р 53 в поддержании G 2 -ареста состоит в том, что он активирует транскрипцию трех вовлеченных в него белков: GADD 45, P 21 и 14 -3 -3σ и подавляет транскрипцию CDC 2(CDK 1) и циклина В. • Есть данные о вовлеченности в G 2 -арест BRCA 1, опосредованно через ATM/ATR или напрямую, через активацию CHK 1, но механизм этого остается неясным. • G 2 -реакция клетки на облучение зависит от фазы цикла, в которую это произошло.
 Механизмы G 2 -ареста(6) • G 2 чекпойнт-ответ разделяется на два различных пути. Один начинается сразу же после облучения, захватывает клетки, облученные непосредственно в G 2 -фазе и является АТМ-зависимым, проходящим и независимым от дозы. Он приводит к резкому снижению митотического индекса. • Второй, который развивается позже, в клетках, облученных на более ранних стадиях клеточного цикла, является АТМ-независимым, зато зависимым от дозы и приводит к накоплению клеток в фазе G 2.
Механизмы G 2 -ареста(6) • G 2 чекпойнт-ответ разделяется на два различных пути. Один начинается сразу же после облучения, захватывает клетки, облученные непосредственно в G 2 -фазе и является АТМ-зависимым, проходящим и независимым от дозы. Он приводит к резкому снижению митотического индекса. • Второй, который развивается позже, в клетках, облученных на более ранних стадиях клеточного цикла, является АТМ-независимым, зато зависимым от дозы и приводит к накоплению клеток в фазе G 2.
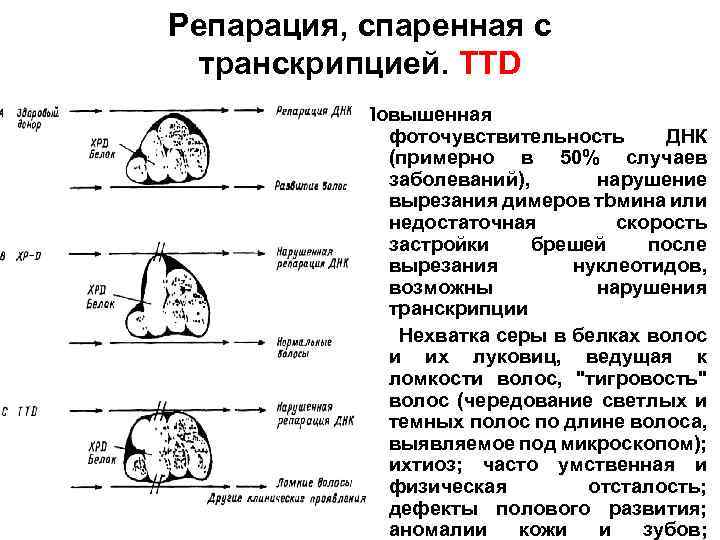 Репарация, спаренная с транскрипцией. TTD Повышенная фоточувствительность ДНК (примерно в 50% случаев заболеваний), нарушение вырезания димеров тbмина или недостаточная скорость застройки брешей после вырезания нуклеотидов, возможны нарушения транскрипции Нехватка серы в белках волос и их луковиц, ведущая к ломкости волос, "тигровость" волос (чередование светлых и темных полос по длине волоса, выявляемое под микроскопом); ихтиоз; часто умственная и физическая отсталость; дефекты полового развития; аномалии кожи и зубов;
Репарация, спаренная с транскрипцией. TTD Повышенная фоточувствительность ДНК (примерно в 50% случаев заболеваний), нарушение вырезания димеров тbмина или недостаточная скорость застройки брешей после вырезания нуклеотидов, возможны нарушения транскрипции Нехватка серы в белках волос и их луковиц, ведущая к ломкости волос, "тигровость" волос (чередование светлых и темных полос по длине волоса, выявляемое под микроскопом); ихтиоз; часто умственная и физическая отсталость; дефекты полового развития; аномалии кожи и зубов;
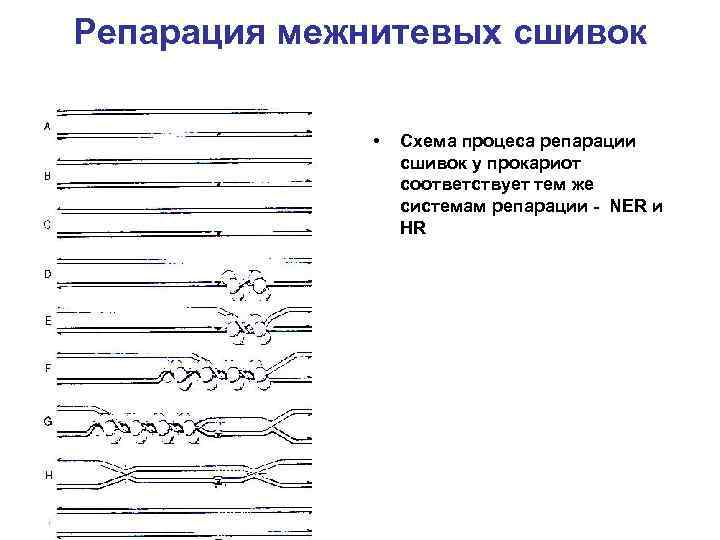 Репарация межнитевых сшивок • Схема процеса репарации сшивок у прокариот соответствует тем же системам репарации - NER и HR
Репарация межнитевых сшивок • Схема процеса репарации сшивок у прокариот соответствует тем же системам репарации - NER и HR
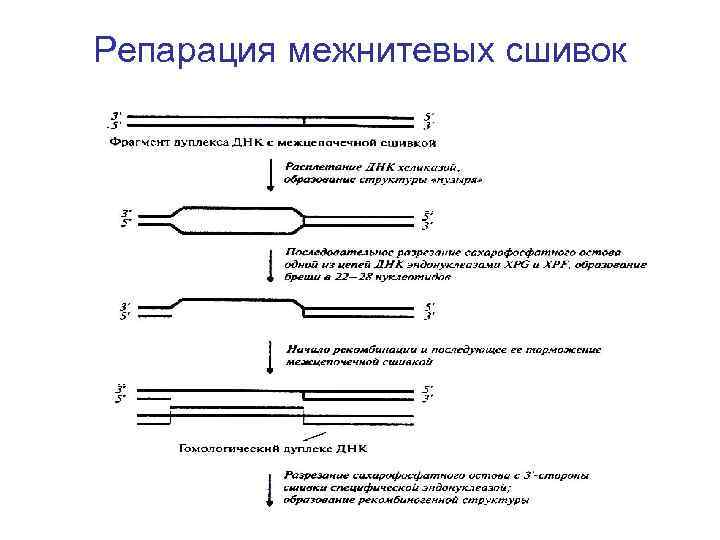 Репарация межнитевых сшивок
Репарация межнитевых сшивок
 Репарация межнитевых сшивок
Репарация межнитевых сшивок