df2063e8fbe1c9ad856900d3eafa78f3.ppt
- Количество слайдов: 31

© ABB - 1 2005 Building Automation with System 800 x. A

Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n Arching and reporting n © ABB - 2 n Validation services

Life Sciences Industry: Clean Rooms Protect The Operator © ABB - 3 The Product Environment
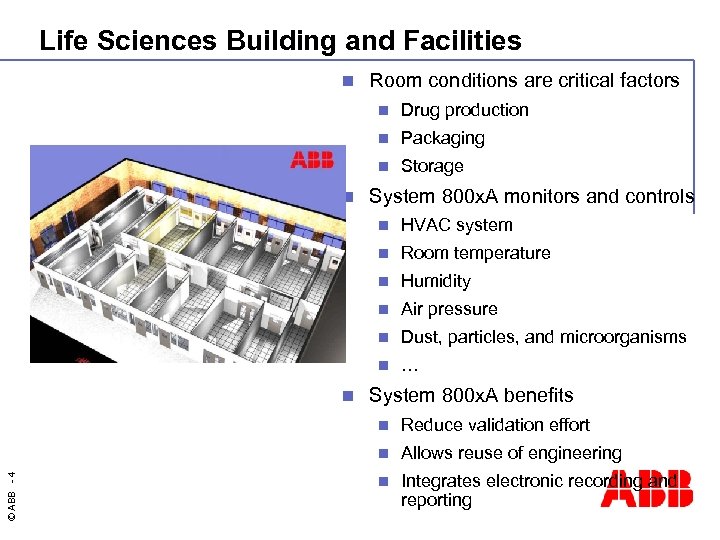
Life Sciences Building and Facilities n Room conditions are critical factors n n Packaging n n Drug production Storage System 800 x. A monitors and controls n n Room temperature n Humidity n Air pressure n Dust, particles, and microorganisms n n HVAC system … System 800 x. A benefits Reduce validation effort n © ABB - 4 n Allows reuse of engineering n Integrates electronic recording and reporting
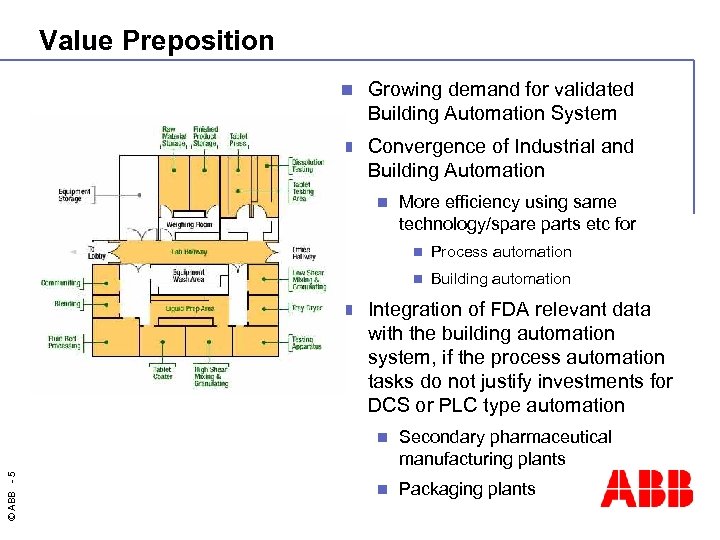
Value Preposition n Growing demand for validated Building Automation System n Convergence of Industrial and Building Automation n More efficiency using same technology/spare parts etc for n n n Process automation Building automation Integration of FDA relevant data with the building automation system, if the process automation tasks do not justify investments for DCS or PLC type automation © ABB - 5 n Secondary pharmaceutical manufacturing plants n Packaging plants

© ABB - 6 Floor Plan and Phases
Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n Arching and reporting (environmental and compliance reports) n © ABB - 7 n Validation services
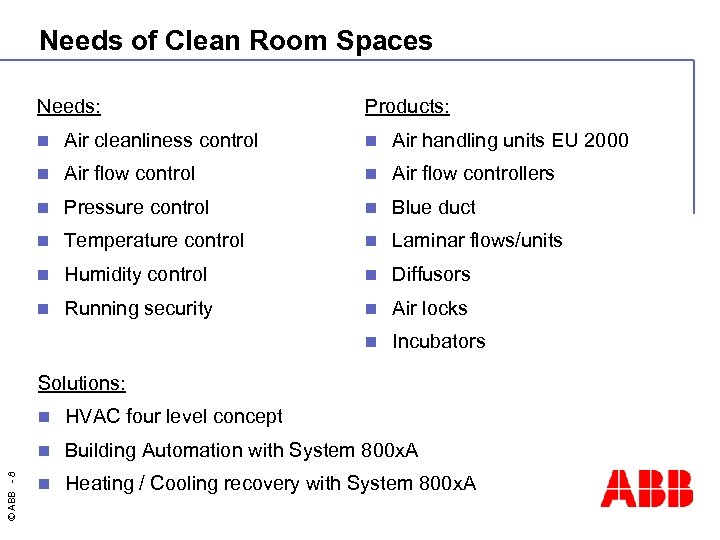
Needs of Clean Room Spaces Needs: Products: n Air cleanliness control n Air handling units EU 2000 n Air flow controllers n Pressure control n Blue duct n Temperature control n Laminar flows/units n Humidity control n Diffusors n Running security n Air locks n Incubators Solutions: HVAC four level concept n © ABB - 8 n Building Automation with System 800 x. A n Heating / Cooling recovery with System 800 x. A
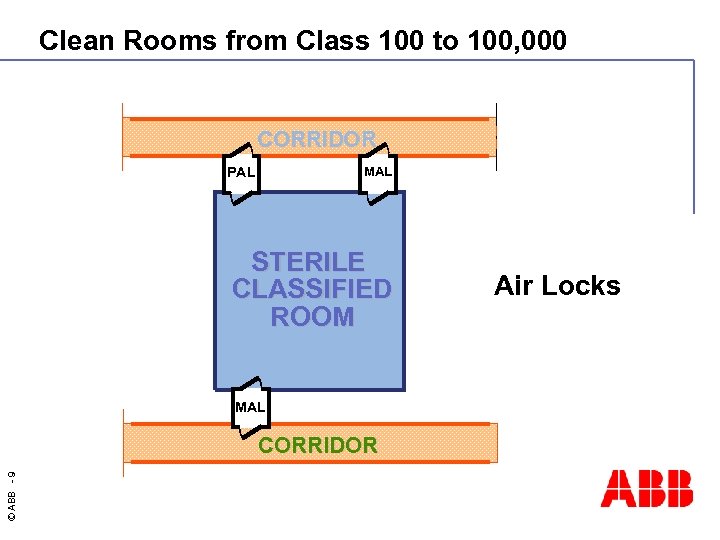
Clean Rooms from Class 100 to 100, 000 CORRIDOR PAL MAL STERILE CLASSIFIED ROOM MAL © ABB - 9 CORRIDOR Air Locks
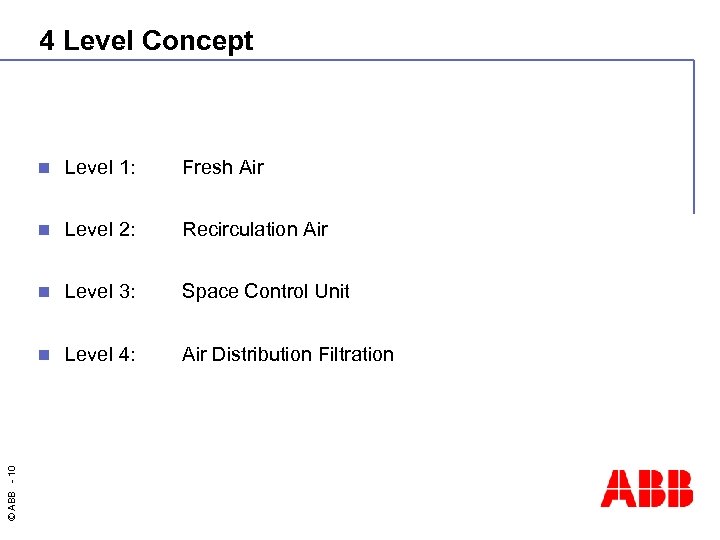
4 Level Concept Level 1: Fresh Air n Level 2: Recirculation Air n Level 3: Space Control Unit n © ABB - 10 n Level 4: Air Distribution Filtration
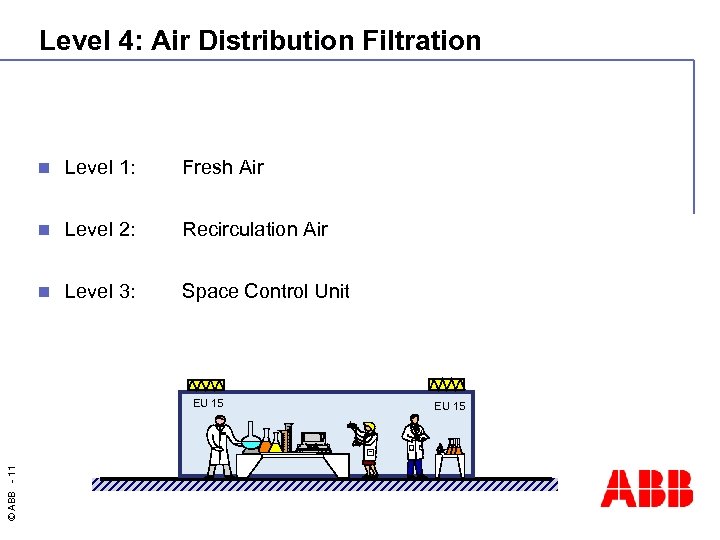
Level 4: Air Distribution Filtration n Level 1: Fresh Air n Level 2: Recirculation Air n Level 3: Space Control Unit © ABB - 11 EU 15
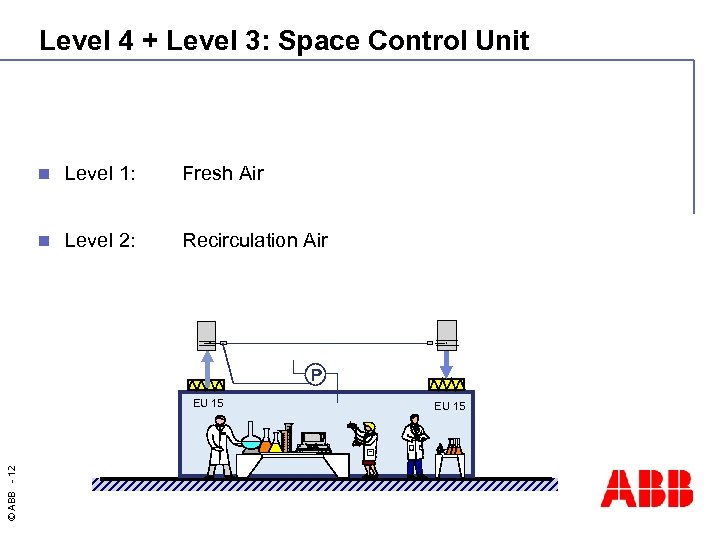
Level 4 + Level 3: Space Control Unit n Level 1: Fresh Air n Level 2: Recirculation Air P © ABB - 12 EU 15
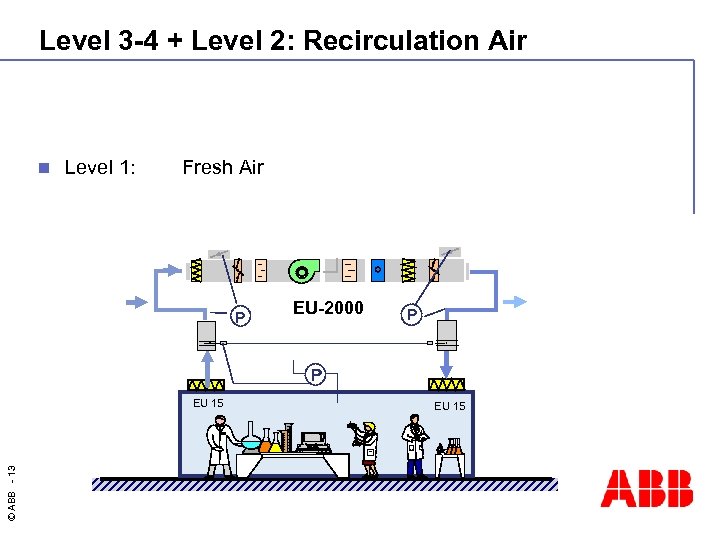
Level 3 -4 + Level 2: Recirculation Air n Level 1: Fresh Air - P EU-2000 P P © ABB - 13 EU 15
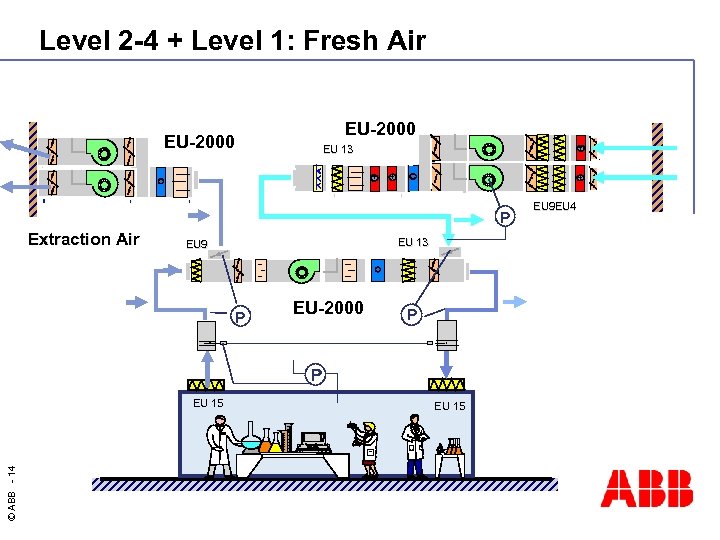
Level 2 -4 + Level 1: Fresh Air EU-2000 + EU-2000 EU 13 + - + + - P Extraction Air EU 13 EU 9 - P EU-2000 P P © ABB - 14 EU 15 EU 9 EU 4

Building Automation with System 800 x. A Conditions are continuously monitored: n Alarms and Events indicate n n n Predefined limits are exceeded Conditions are violated Alarms n n Visible in all displays n n Automatically created Single alarm and alarm group indication Graphic Display n n Example: Air Unit Ground Floor Schedule n Weekly © ABB - 15 n User Access Control n Change Control n Reporting
Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n Arching and reporting (environmental and compliance reports) n © ABB - 16 n Validation services
GMP - Buildings and Facilities n Suitable location n Suitable construction n Suitable size/layout (to prevent cross-contamination) n Adequate HVAC n Adequate lighting n Maintainable © ABB - 17 n Cleanable Glossary: GMP: Good Manufacturing Practice

GMP - Buildings and Facilities Issues Contamination Micro-organisms Material Flow Cross-contamination Dirt Particles Process/Product © ABB - 18 Moisture Temperature Personnel Flow Cross-contamination
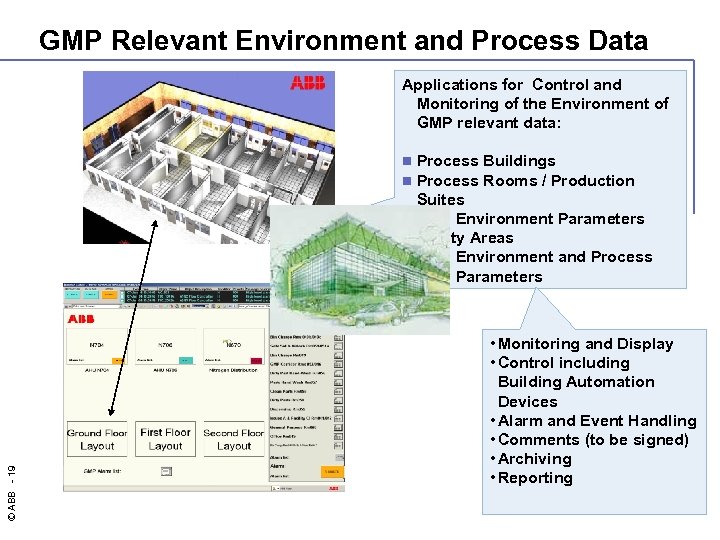
GMP Relevant Environment and Process Data Applications for Control and Monitoring of the Environment of GMP relevant data: Process Buildings Process Rooms / Production Suites n Environment Parameters n Utility Areas n Environment and Process Parameters © ABB - 19 n n • Monitoring and Display • Control including Building Automation Devices • Alarm and Event Handling • Comments (to be signed) • Archiving • Reporting
Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n FDA 21 CFR Part 11 Support n Arching and reporting (environmental and compliance reports) n © ABB - 20 n Validation services
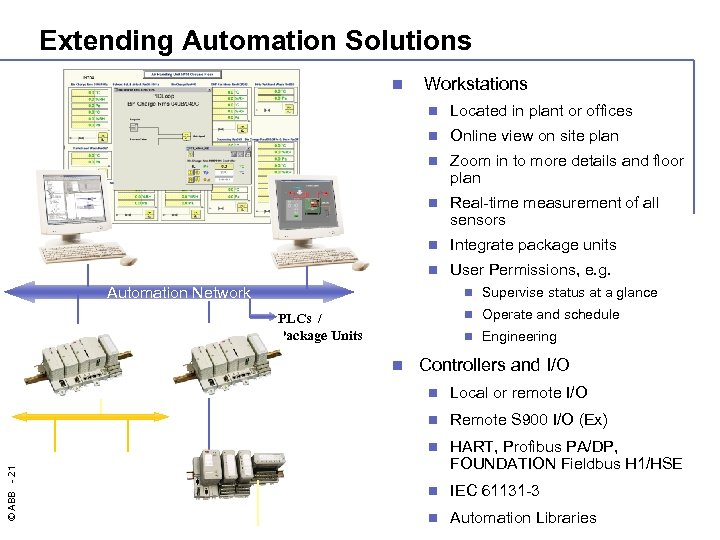
Extending Automation Solutions n Workstations n Located in plant or offices n Online view on site plan n Zoom in to more details and floor plan n Real-time measurement of all sensors n Integrate package units n User Permissions, e. g. Automation Network n Supervise status at a glance n Operate and schedule PLCs / Package Units n Engineering n Controllers and I/O Local or remote I/O n Remote S 900 I/O (Ex) n © ABB - 21 n HART, Profibus PA/DP, FOUNDATION Fieldbus H 1/HSE n IEC 61131 -3 n Automation Libraries
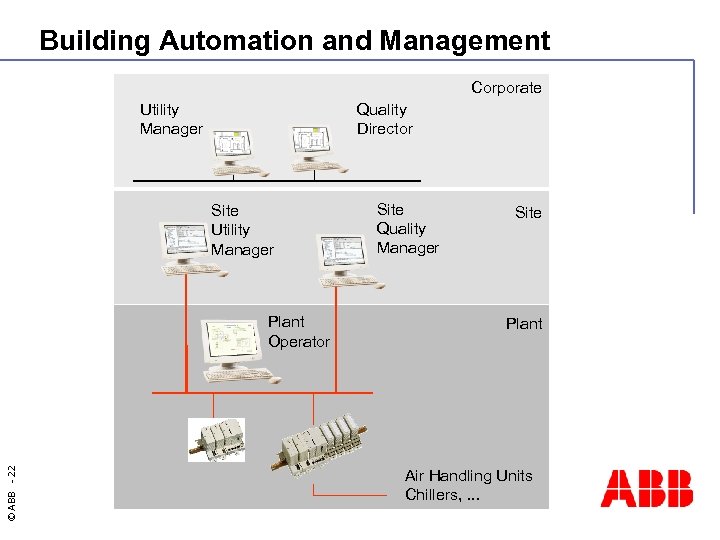
Building Automation and Management Corporate Utility Manager Quality Director Site Utility Manager © ABB - 22 Plant Operator Site Quality Manager Site Plant Air Handling Units Chillers, . . .
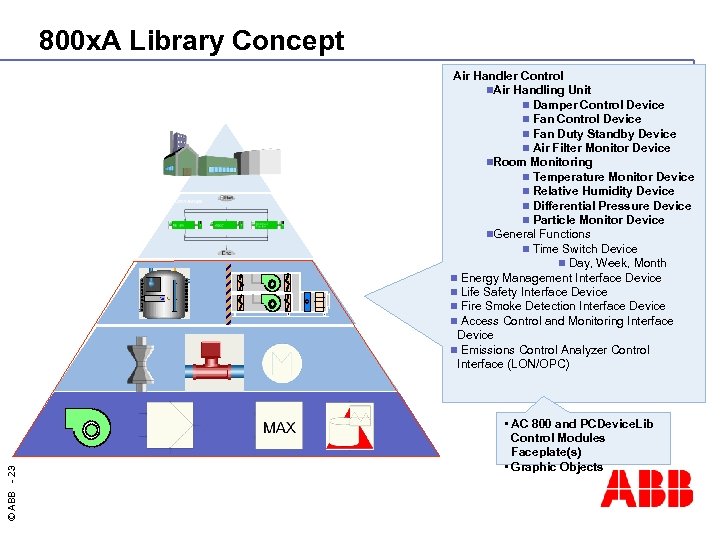
800 x. A Library Concept © ABB - 23 Air Handler Control n. Air Handling Unit n Damper Control Device n Fan Duty Standby Device n Air Filter Monitor Device n. Room Monitoring n Temperature Monitor Device n Relative Humidity Device n Differential Pressure Device n Particle Monitor Device n. General Functions n Time Switch Device n Day, Week, Month n Energy Management Interface Device n Life Safety Interface Device n Fire Smoke Detection Interface Device n Access Control and Monitoring Interface Device n Emissions Control Analyzer Control Interface (LON/OPC) • AC 800 and PCDevice. Lib Control Modules Faceplate(s) • Graphic Objects
Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n FDA 21 CFR Part 11 Support n Arching and reporting (environmental and compliance reports) n © ABB - 24 n Validation services
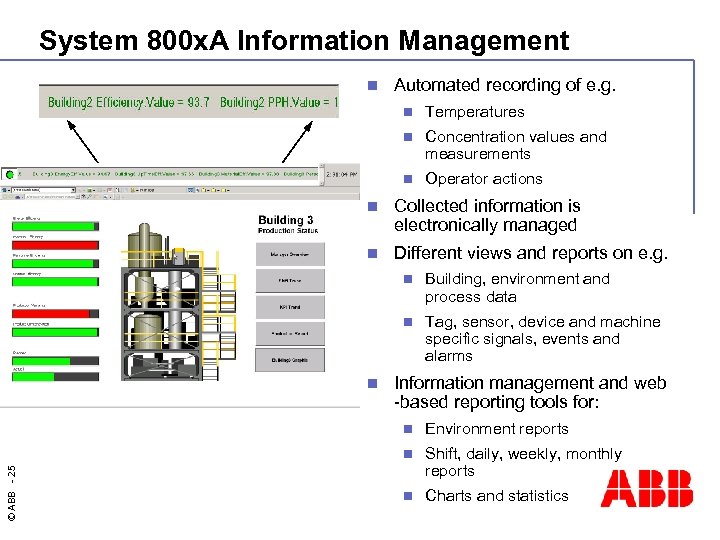
System 800 x. A Information Management n Automated recording of e. g. n Temperatures n Concentration values and measurements n Operator actions n Collected information is electronically managed n Different views and reports on e. g. n n n Building, environment and process data Tag, sensor, device and machine specific signals, events and alarms Information management and web -based reporting tools for: Environment reports n © ABB - 25 n Shift, daily, weekly, monthly reports n Charts and statistics
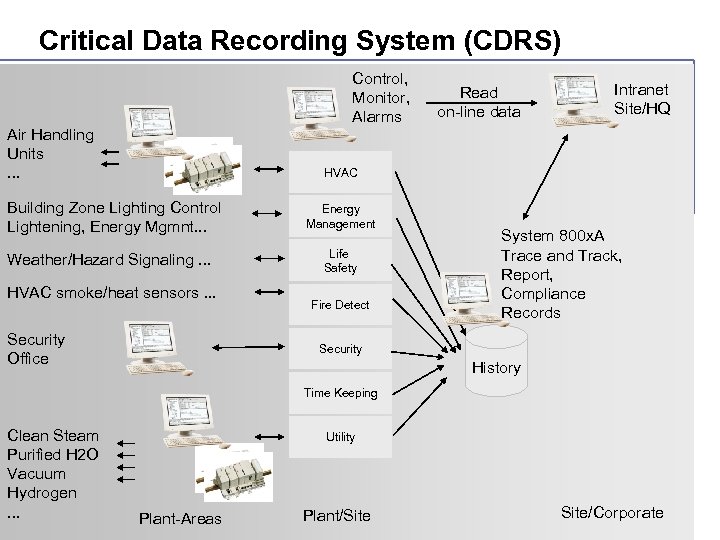
Critical Data Recording System (CDRS) Control, Monitor, Alarms Air Handling Units. . . Read on-line data Intranet Site/HQ HVAC Building Zone Lighting Control Lightening, Energy Mgmnt. . . Weather/Hazard Signaling. . . HVAC smoke/heat sensors. . . Security Office Energy Management Life Safety Fire Detect System 800 x. A Trace and Track, Report, Compliance Records Security History Time Keeping © ABB - 26 Clean Steam Purified H 2 O Vacuum Hydrogen. . . Utility Plant-Areas Plant/Site/Corporate
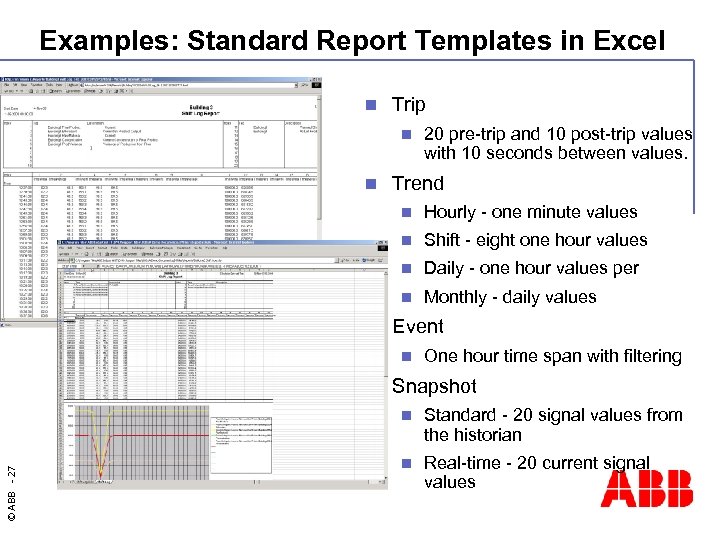
Examples: Standard Report Templates in Excel n Trip n n 20 pre-trip and 10 post-trip values with 10 seconds between values. Trend n n Shift - eight one hour values n Daily - one hour values per n n Hourly - one minute values Monthly - daily values Event n n One hour time span with filtering Snapshot © ABB - 27 n Standard - 20 signal values from the historian n Real-time - 20 current signal values
Building Automation with 800 x. A Introduction and overview n Clean rooms n GMP relevant environment and process data n Engineering, operation and control n FDA 21 CFR Part 11 Support n Arching and reporting (environmental and compliance reports) n © ABB - 28 n Validation services

Facility and Equipment Validation Options Full Validation Service – Management and Execution n Watchdog Service – Independence and Assurance n Document Review Service – Low Cost Option n Consultancy – Risk Based Approach n Qualification including Performance Qualification n Training n © ABB - 29 n Validation Procedures

Customer Benefits n Achievement of product quality and safety in the manufacturing process n Meeting of GMP, validation and FDA 21 CFR Part 11 requirements n Coupling of manufacturing and building automation allows integrated reporting and reporting n Reduces validation effort n Reduces costs of compliance n Our customers find the partner who n is committed to open systems, and can integrate it n with the Manufacturing Executions System for secondary pharmaceutical production n © ABB - 30 n with control systems in the primary / bulk production area has worldwide support capabilities

Thank You! © ABB - 31 Please visit us at www. abb. com/Life. Sciences
df2063e8fbe1c9ad856900d3eafa78f3.ppt