db66f58e7a596871298befef6c8b041c.ppt
- Количество слайдов: 33

ab initio and Evidence-Based Gene Finding A basic introduction to annotation Wilson Leung 08/2006
Outline n n n What is annotation? ab initio gene finding Genome databases on the web Basics of the UCSC browser Evidence-based gene finding using the UCSC Browser

AAACAACAATCATAAATAGAGGAAGTTTTCGGAATATACGATAAGTGAAATA TCGTTCTTAAAAAAGAGCAAGAACAGTTTAACCATTGAAAACAAGATTATTCC AATAGCCGTAAGAGTTCATTTAATGACGATGGCGGCAAAGTCGAT GAAGGACTAGTCGGAACTGGAAATAGGAATGCGCCAAAAGCTAGTGCAGCT AAACATCAATTGAAACAAGTTTGTACATCGATGCGCGGAGGCGCTTTTCTCT CAGGATGGCTGGGGATGCCAGCACGTTAATCAGGATACCAATTGAGGAGG TGCCCRAGCTCACCEAGAGCCGGCCAATAAGGACCCATCGGGGGGGCCG CTTATGTGGAAGCCAAACATTAAACCATAGGCAACCGATTTGTGGGAATCG AATTTAAGAAACGGCGGTCAGCCACCCGCTCAACAAGTGCCAAAGCCATCT TGGGGGCATACGCCTTCATCAAATTTGGGCGGAACTTGGGGCGAGGACGA TGATGGCGCCGATAGCACCAGCGTTTGGACGGGTCATTCCAHATAEG CACAACGTCTGGTGTTGCAGTCGGTGCCATAGCGCCTGGCEGTTGGCGCC GCTGCTGGTCCCCAATGGGGACAGGCTGTTGGTGTTGGAGTCGG ATCGGCCGGAATGNTAANGAAOTAATCAAATTTTGGCIGACAOAATGNGCA AGTTGCCTTAAACTCGACTGGAAATAACAATGCGCCGGCAACAGGAGCCCT GATTCAGA GCCTGCCGTGGCTCGTCCGAAATGTGGGGACATCATCCTCAGATTGCTCAC AATCATCGGCCGGAATGNTAANGAAOTAATCAAATTTTGGCIGACAOAATGN GCAGATTCAGA ACGTATTAACAAAATGGTCGGCCCCGTTGTTAGTGCAACAGGGTCAAATAT CGCAAGCTCAAATATTGGCCCAAGCGGTGTTGGTTCCGTATCCGGTAATGT CGGGGCACAATGGGGAGCCACACAGGCCGCGTTGGGGCCCCAAGGTATTT CCAAGCAAATCACTGGATGGGAGGAACCACAATCAGATTCAGAATATTAAC AAAATGGTCGGCCCCGTTGTTATGGATAAAAAATTTGTGTCTTCGTACGGAG ATTATGTTGTTAATCAATTTTATTAAGATATTTAAATATGTGTACCTTTC ACGAGAAATTTGCTTACCTTTTCGACACACTTATACAGGTAATA ATTACCTTTTGAGCAATTCGATTTTCATAAAATATACCTAAATCGCATCGTCT ATGAATCTTTGTAATACTTTCGAATTTAATTATTAGTCTACATTAATATTGATA
What to Annotate? n Genes n n Non-coding RNAs n n t. RNAs, mi. RNAs, si. RNAs Regulatory Elements n n Novel genes, known genes, pseudogenes Promoters, enhancers, silencers Repeats n Transposable elements, simple repeats
ab initio Gene Prediction n ab initio = From the Beginning n Gene prediction using only the genomic DNA sequence n n Search for “signals” of protein coding regions Typically uses a probabilistic model • Hidden Markov Model (HMM) n Requires external evidence to verify predictions (m. RNA, ESTs)
Performance of Gene Finders n Most gene-finders can predict prokaryotic genes accurately n However, gene-finders do a poor job of predicting genes in eukaryotes n n Not as much is known about the general properties of eukaryotic genes Splice site recognition, different isoforms
ab initio Gene Finders n Examples: n Glimmer for prokaryotic gene predictions • (S. Salzberg, A. Delcher, S. Kasif, and O. White 1998) n Genscan for eukaryotic gene predictions • (Burge and Karlin 1997) n We will use Genscan for our chimpanzee and Drosophila annotations
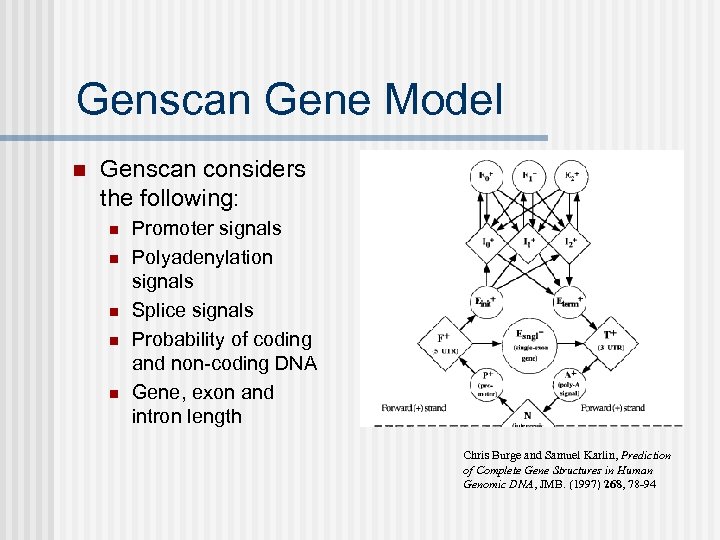
Genscan Gene Model n Genscan considers the following: n n n Promoter signals Polyadenylation signals Splice signals Probability of coding and non-coding DNA Gene, exon and intron length Chris Burge and Samuel Karlin, Prediction of Complete Gene Structures in Human Genomic DNA, JMB. (1997) 268, 78 -94
Common Problems n Common problems with gene finders n n n Fusing neighboring genes Spliting a single gene Miss exons or entire genes Overpredict exons or genes Other challenges n n Nested genes Noncanonical splice spites Pseudogenes Different isoforms of same gene
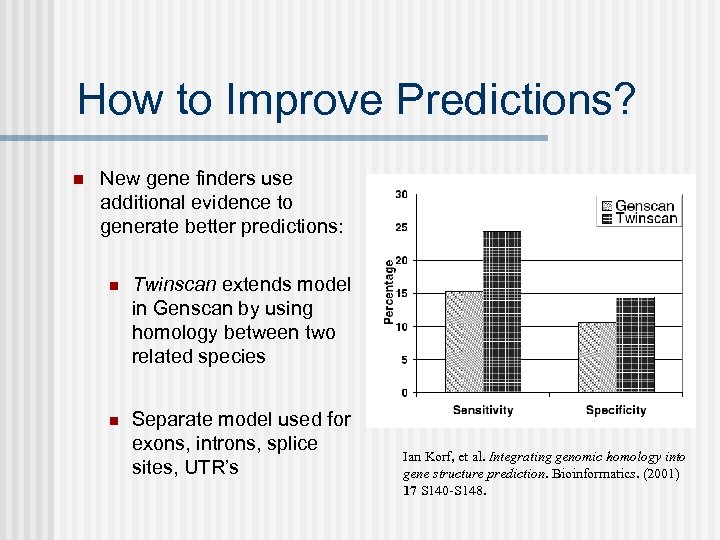
How to Improve Predictions? n New gene finders use additional evidence to generate better predictions: n Twinscan extends model in Genscan by using homology between two related species n Separate model used for exons, introns, splice sites, UTR’s Ian Korf, et al. Integrating genomic homology into gene structure prediction. Bioinformatics. (2001) 17 S 140 -S 148.
How to Improve Predictions? n Improve algorithms n n Use multiple closely-related species (N-scan) Computational predictions with biological evidence (ESTs, c. DNAs) Filter psuedogenes (PPFinder) Manual annotation n n Collect evidence from multiple biological and computational sources to create gene models This method still generates the best annotations
Web Databases n Comprehensive databases n n NCBI, EBI, DDBJ Ensembl UCSC Genome Browser Species-specific databases n n Flybase (Drosophila) Wormbase (C. elegans)
Introduction to Ensembl Available at http: //www. ensembl. org
What is Ensembl? n Ensembl is a joint project between the European Bioinformatics Institute (EBI) and the Wellcome Trust Sanger Institute n Ensembl seeks to develop an automated system for the production and maintenance of annotations on eukaryotic genomes n These annotations should also be easily accessible to researchers
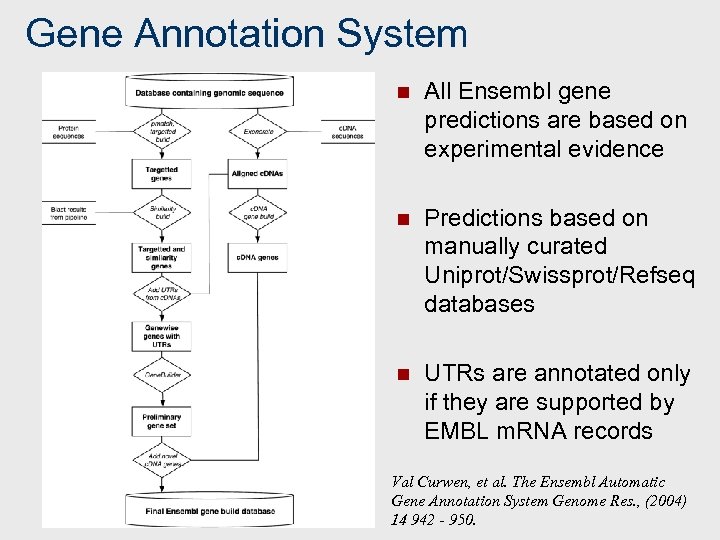
Gene Annotation System n All Ensembl gene predictions are based on experimental evidence n Predictions based on manually curated Uniprot/Swissprot/Refseq databases n UTRs are annotated only if they are supported by EMBL m. RNA records Val Curwen, et al. The Ensembl Automatic Gene Annotation System Genome Res. , (2004) 14 942 - 950.
Introduction to the UCSC Browser http: //genome. ucsc. edu

UCSC Browser Developers n UCSC Browser is created by the Genome Bioinformatics Group of UC Santa Cruz n Development team: http: //genome. ucsc. edu/staff. html n n UCSC Browser was initially created for the human genome project n n Led by Jim Kent and David Haussler It has since been adapted for many other organisms We have set up local version of the UCSC Browser for Bio 4342
Functions of UCSC Browser n Functionalities of UCSC Browser n n Genome Browser - views of genomic regions BLAT - BLAST-Like Alignment Tool Table Browser - SQL access to genomic data Training section on the UCSC web site n http: //openhelix. com/ucscmaterials. shtml • Pre-recorded tutorial (presentation slide set) • Reference cards

Chimp BAC Analysis n Goal: Annotation of one of the features in a 170 kb chimpanzee BAC n A more detailed walkthrough is available in your binder n Genscan was run on the repeat-masked BAC using the vertebrate parameter set (GENSCAN_Chimp. BAC. html) n n n Predicts 8 genes within this BAC By default, Genscan also predicts promoter and poly-A sites; however, these are generally unreliable Output consists of map, summary table, peptide and coding sequences of the predicted genes
Chimp BAC Analysis n Analysis of Gene 1 (423 coding bases): n n Use the predicted peptide sequence to evaluate the validity of Genscan prediction blastp of predicted peptide against the nr database n Typically uses the NCBI BLAST page: • http: //www. ncbi. nlm. nih. gov/blast/ n n Choose blastp and search against nr For the purpose of this tutorial, open blastp. Gene 1. html

Interpreting blastp Output n Many significant hits to the nr database that cover the entire length of the predicted protein n Do not rely on hits that have accession numbers starting with XP n n n XP indicates Ref. Seq without experimental confirmation NP indicates Ref. Seq that has been validated by the NCBI staff Click on the Score for the second hit in the blastp output (gb|AAH 70482. 1) n Indicates hit to human HMGB 3 protein
Investigating HMGB 3 Alignment The full HMGB 3 protein has length of 200 aa n However, our predicted peptide only has 140 aa n n Possible explanations: 1. Genscan mispredicted the gene • 2. Missed part of the real chimp protein Genscan predicted the gene correctly • • Pseudogene that has acquired an in-frame stop codon Functional protein in chimp that lacks one or more functional domains when compared to the human version
Analysis using UCSC Browser n Go back to Genscan output page and copy the first predicted coding sequence n Navigate to UCSC browser @ http: //genome. ucsc. edu n Click on “BLAT” n n n Select the human genome (May 2004 assembly) Paste the coding sequence into the text box Click “submit”
Human BLAT Results n Predicted sequence matches to many places in the human genome n n n Click on “browser” for the top hit (on chromosome 7) n n n Top hit shows sequence identity of 99. 1% between our sequence and the human sequence Next best match has identity of 93. 6%, below what we expect for human / chimp orthologs (98. 5% identical) The genome browser for this region in human chromosome 7 should now appear Navigation buttons are on the top menu bar Reinitialize the browser by clicking on “hide all”

Adjusting Display Options n Adjust following tracks to “pack” n Under “Mapping and Sequencing Tracks”: • Blat Sequence n Adjust following tracks to “dense” n Under “Gene and Gene Prediction Tracks” • Known Genes, Ref. Seq, Ensembl Genes, N-scan, SGP Genes, Genscan Genes n Under “m. RNA and EST Tracks” • Human m. RNAs, Spliced ESTs, Human ESTs, Other ESTs n Under “Comparative Genomics” • Mouse Net n Under “Variation and Repeats” • Repeat. Masker
Human Genome Browser n Hit “refresh” and look at new image; zoom out 3 x to get a broader view n There are no known genes in this region n n Only evidence is from hypothetical genes predicted by SGP and Genscan SGP predicted a larger gene with two exons There also no known human m. RNA or human ESTs in the aligned region However, there are ESTs from other organisms

Investigate Partial Match n Go to Gen. Bank record for the human HMGB 3 protein (using the BLAST result) n Click on the “Display” button and select “FASTA” to obtain the sequence n Go back to the BLAT search to search this sequence against the human genome assembly (May 2004)
BLAT search of human HMGB 3 n Notice the match to part of human chromosome 7 we observed previously is only the 7 th best match (identity of 88%) n n Consistent with one of our hypotheses that our predicted protein is a paralog Click on “browser” to see corresponding sequence on human chromosome 7 n n BLAT results overlap Genscan prediction but extend both ends Why would Genscan predict a shorter gene?

Examining Alignment n Now we need to examine the alignment: n n In general, the alignment looks good except for a few changes n n Go back to previous page and click on “details” However, when examining some of the unmatched (black) regions, notice there is a “tag” - a stop codon. Confirm predicted protein is in frame relative to human chromosome 7 by n Looking at the side-by-side alignment
Confirming Pseudogene n Side-by-side alignment color scheme n n Lines = match Green = similar amino acids Red = dissimilar amino acids We noticed a red “X” (stop codon) aligning to a “Y” (tyrosine) in the human sequence
Confirming Pseudogene n Alignment after stop codon showed no deterioration in similarity suggest our prediction is a recently retrotransposed pseudogene n To confirm hypothesis, go back to BLAT results and get the top hit (100% identity on chromosome X) n The real HMGB 3 gene in human is a 4 -exon gene!
Conclusions n Based on evidence accumulated: n As a c. DNA, the four-exon HMGB 3 gene was retrotransposed n It then acquired a stop codon mutation prior to the split of the chimpanzee and human lineages n Retrotransposition event is relatively recent • Pseudogene still retains 88. 8% sequence identity to source protein

Questions?
db66f58e7a596871298befef6c8b041c.ppt