000eb06e64f5f526856817f112afd8f6.ppt
- Количество слайдов: 70
 A Roadmap for PAT Implementation in Pharmaceutical Manufacturing Robert M. Leasure Principal Scientist Site PAT Champion Pfizer Global Manufacturing 7000 Portage Road, PORT-91 -201 Kalamazoo, MI 49001 (269) 833 -6198 -1 -
A Roadmap for PAT Implementation in Pharmaceutical Manufacturing Robert M. Leasure Principal Scientist Site PAT Champion Pfizer Global Manufacturing 7000 Portage Road, PORT-91 -201 Kalamazoo, MI 49001 (269) 833 -6198 -1 -
 Presentation Outline u Provide some Definitions about PAT • But in the process more Questions will be asked than definitions provided. • Asking the right Questions provides the framework for successful implementations. u Site perspective of a PAT program • Project Selection • Resource Allocation – from site and center support • Steps for Implementation u Examples of PAT Implementations in Kalamazoo Manufacturing Ops • Drug Product Parental Sterile Suspension - improved content uniformity • Drug Product Dissolution Monitoring of Active during p. H adjustment • API Operations Solvent Recovery – improved yield from timely fraction determination. -2 -
Presentation Outline u Provide some Definitions about PAT • But in the process more Questions will be asked than definitions provided. • Asking the right Questions provides the framework for successful implementations. u Site perspective of a PAT program • Project Selection • Resource Allocation – from site and center support • Steps for Implementation u Examples of PAT Implementations in Kalamazoo Manufacturing Ops • Drug Product Parental Sterile Suspension - improved content uniformity • Drug Product Dissolution Monitoring of Active during p. H adjustment • API Operations Solvent Recovery – improved yield from timely fraction determination. -2 -
 Definitions and Questions What is PAT? Process Analytical Technologies Things that come to mind…. . u Probes in Tanks Analyzers in Plant u Automation u Process Data (lots of it) Questions that come to mind…. . u Where are you going to stick that probe? u How are you going to validate that system? u What are you going to do with that data? -3 -
Definitions and Questions What is PAT? Process Analytical Technologies Things that come to mind…. . u Probes in Tanks Analyzers in Plant u Automation u Process Data (lots of it) Questions that come to mind…. . u Where are you going to stick that probe? u How are you going to validate that system? u What are you going to do with that data? -3 -
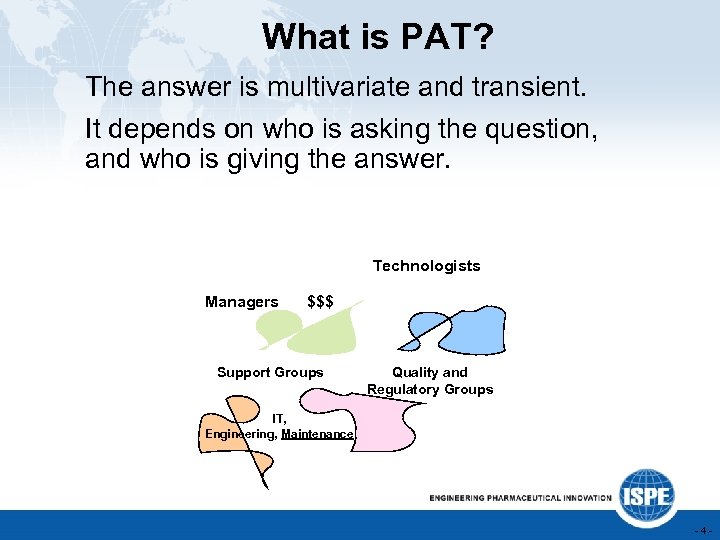 What is PAT? The answer is multivariate and transient. It depends on who is asking the question, and who is giving the answer. Technologists Managers $$$ Support Groups Quality and Regulatory Groups IT, Engineering, Maintenance -4 -
What is PAT? The answer is multivariate and transient. It depends on who is asking the question, and who is giving the answer. Technologists Managers $$$ Support Groups Quality and Regulatory Groups IT, Engineering, Maintenance -4 -
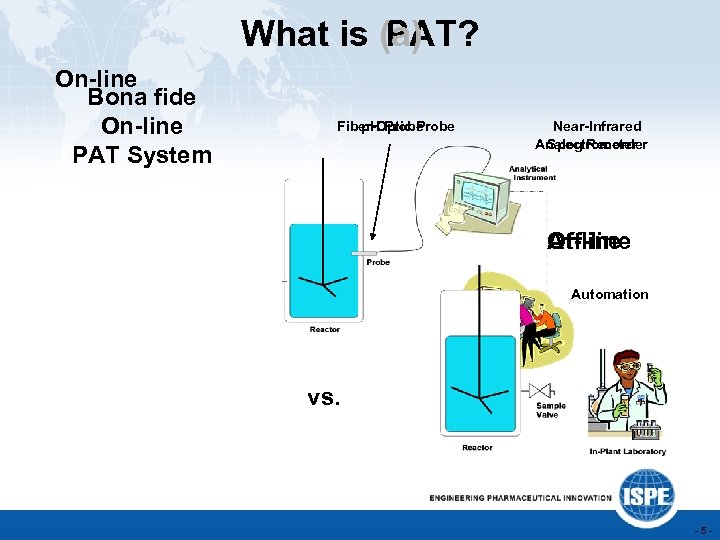 What is (a) PAT? On-line Bona fide On-line PAT System Fiber-Optic Probe p. H Probe Feedback Control Near-Infrared Analog Recorder Spectrometer Off-line At-line Automation vs. -5 -
What is (a) PAT? On-line Bona fide On-line PAT System Fiber-Optic Probe p. H Probe Feedback Control Near-Infrared Analog Recorder Spectrometer Off-line At-line Automation vs. -5 -
 FDA Guidance on PAT FDA Guidance Document on PAT Released in September 2004. http: //www. fda. gov/cder/guidance/6419 fnl. htm Ajaz S. Hussain, Ph. D. Previously Deputy Directory Office of Pharmaceutical Science, CDER, FDA Key proponent for the use of PAT in the pharmaceutical industry. -6 -
FDA Guidance on PAT FDA Guidance Document on PAT Released in September 2004. http: //www. fda. gov/cder/guidance/6419 fnl. htm Ajaz S. Hussain, Ph. D. Previously Deputy Directory Office of Pharmaceutical Science, CDER, FDA Key proponent for the use of PAT in the pharmaceutical industry. -6 -
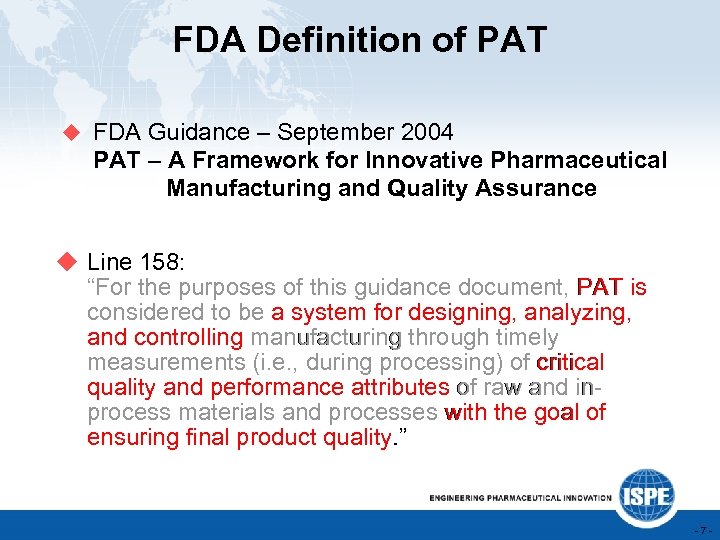 FDA Definition of PAT u FDA Guidance – September 2004 PAT – A Framework for Innovative Pharmaceutical Manufacturing and Quality Assurance u Line 158: “For the purposes of this guidance document, PAT is considered to be a system for designing, analyzing, and controlling manufacturing through timely measurements (i. e. , during processing) of critical quality and performance attributes of raw and inprocess materials and processes with the goal of ensuring final product quality. ” -7 -
FDA Definition of PAT u FDA Guidance – September 2004 PAT – A Framework for Innovative Pharmaceutical Manufacturing and Quality Assurance u Line 158: “For the purposes of this guidance document, PAT is considered to be a system for designing, analyzing, and controlling manufacturing through timely measurements (i. e. , during processing) of critical quality and performance attributes of raw and inprocess materials and processes with the goal of ensuring final product quality. ” -7 -
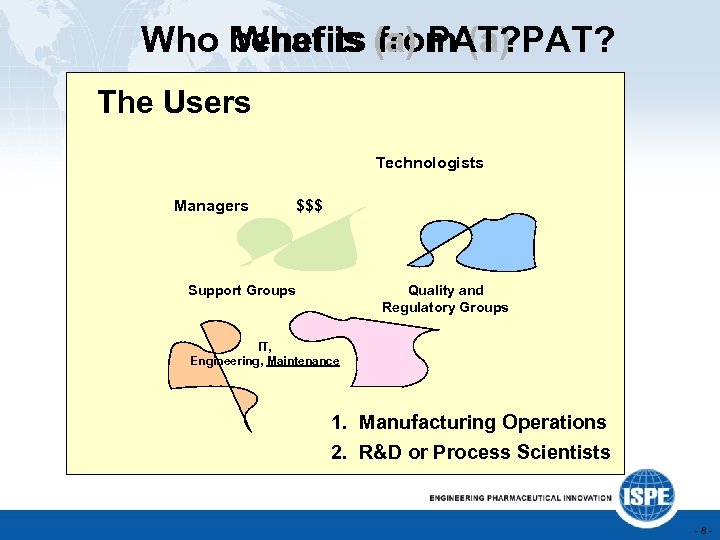 Who benefits (a) PAT? What is from (a) The Users Technologists Managers $$$ Support Groups Quality and Regulatory Groups IT, Engineering, Maintenance 1. Manufacturing Operations 2. R&D or Process Scientists -8 -
Who benefits (a) PAT? What is from (a) The Users Technologists Managers $$$ Support Groups Quality and Regulatory Groups IT, Engineering, Maintenance 1. Manufacturing Operations 2. R&D or Process Scientists -8 -
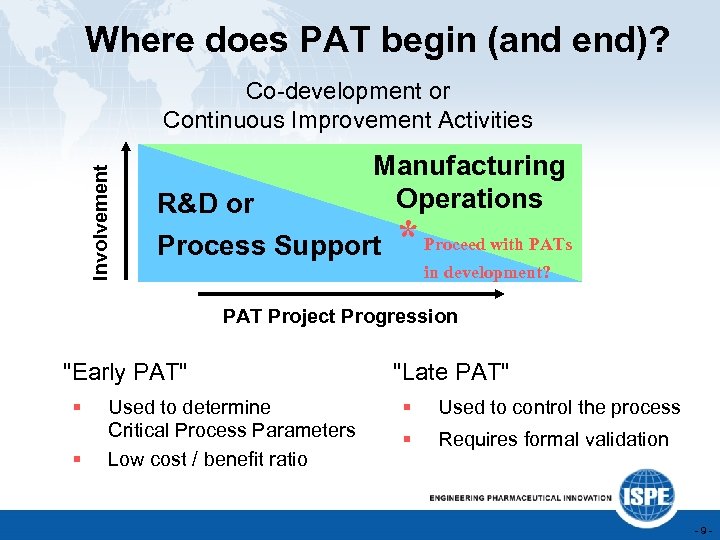 Where does PAT begin (and end)? Involvement Co-development or Continuous Improvement Activities R&D or Manufacturing Operations Process Support * Proceed with PATs in development? PAT Project Progression "Early PAT" § § Used to determine Critical Process Parameters Low cost / benefit ratio "Late PAT" § Used to control the process § Requires formal validation -9 -
Where does PAT begin (and end)? Involvement Co-development or Continuous Improvement Activities R&D or Manufacturing Operations Process Support * Proceed with PATs in development? PAT Project Progression "Early PAT" § § Used to determine Critical Process Parameters Low cost / benefit ratio "Late PAT" § Used to control the process § Requires formal validation -9 -
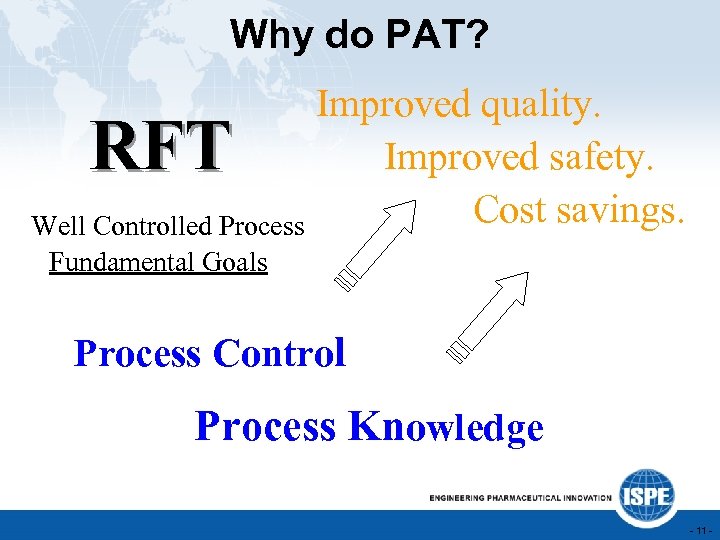 Why do PAT? Improved quality. Improved safety. Cost savings. Well Controlled Process RFT Fundamental Goals Process Control Process Knowledge - 11 -
Why do PAT? Improved quality. Improved safety. Cost savings. Well Controlled Process RFT Fundamental Goals Process Control Process Knowledge - 11 -
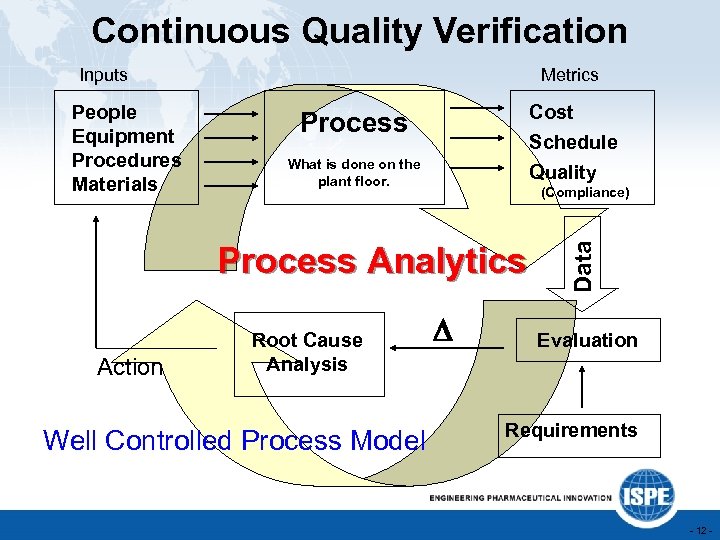 Continuous Quality Verification Inputs Cost Process Schedule Quality What is done on the plant floor. (Compliance) Process Analytics Action Root Cause Analysis Well Controlled Process Model D Data People Equipment Procedures Materials Metrics Evaluation Requirements - 12 -
Continuous Quality Verification Inputs Cost Process Schedule Quality What is done on the plant floor. (Compliance) Process Analytics Action Root Cause Analysis Well Controlled Process Model D Data People Equipment Procedures Materials Metrics Evaluation Requirements - 12 -
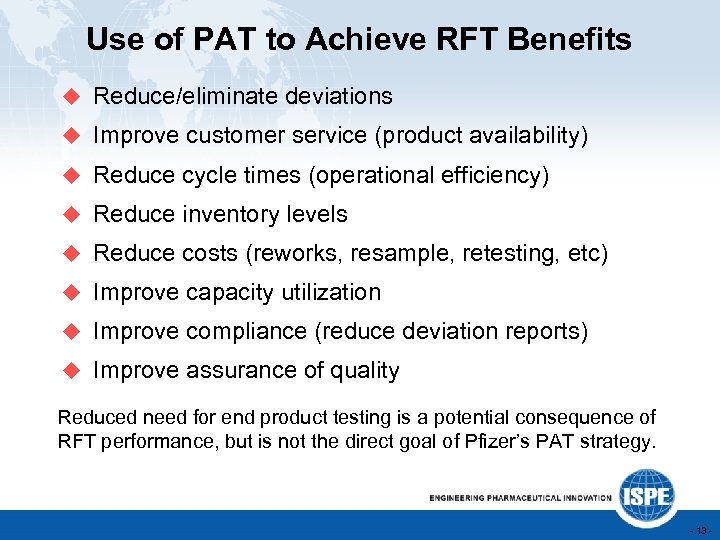 Use of PAT to Achieve RFT Benefits u Reduce/eliminate deviations u Improve customer service (product availability) u Reduce cycle times (operational efficiency) u Reduce inventory levels u Reduce costs (reworks, resample, retesting, etc) u Improve capacity utilization u Improve compliance (reduce deviation reports) u Improve assurance of quality Reduced need for end product testing is a potential consequence of RFT performance, but is not the direct goal of Pfizer’s PAT strategy. - 13 -
Use of PAT to Achieve RFT Benefits u Reduce/eliminate deviations u Improve customer service (product availability) u Reduce cycle times (operational efficiency) u Reduce inventory levels u Reduce costs (reworks, resample, retesting, etc) u Improve capacity utilization u Improve compliance (reduce deviation reports) u Improve assurance of quality Reduced need for end product testing is a potential consequence of RFT performance, but is not the direct goal of Pfizer’s PAT strategy. - 13 -
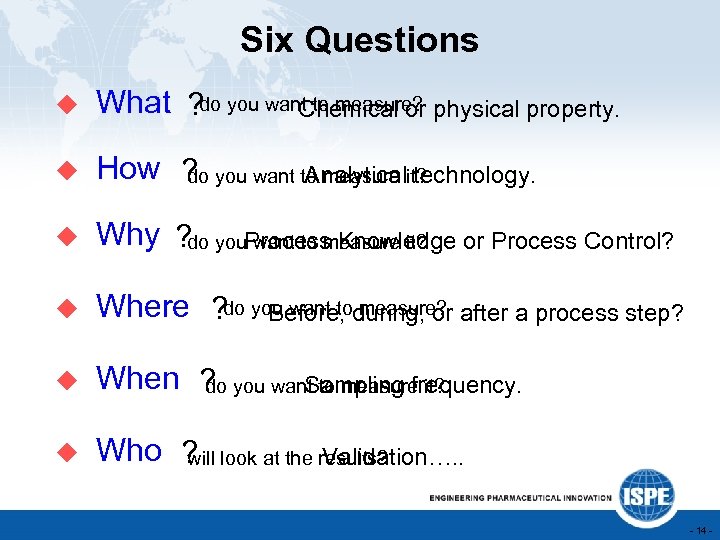 Six Questions u What ? do you want to measure? physical property. Chemical or u How ? you want to measure it? do Analytical technology. u Why ? do you. Process Knowledge or Process Control? want to measure it? u Where ? do you want toduring, or after a process step? Before, measure? u When ? you want to measure it? do Sampling frequency. u Who ? look at the results? will Validation…. . - 14 -
Six Questions u What ? do you want to measure? physical property. Chemical or u How ? you want to measure it? do Analytical technology. u Why ? do you. Process Knowledge or Process Control? want to measure it? u Where ? do you want toduring, or after a process step? Before, measure? u When ? you want to measure it? do Sampling frequency. u Who ? look at the results? will Validation…. . - 14 -
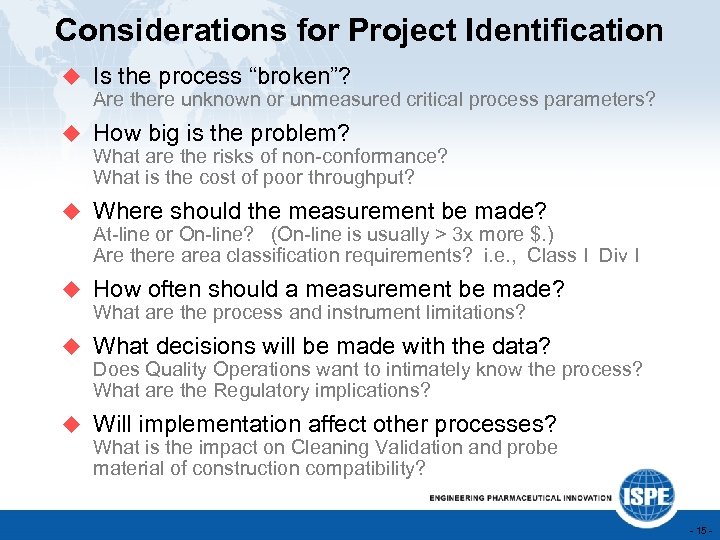 Considerations for Project Identification u Is the process “broken”? Are there unknown or unmeasured critical process parameters? u How big is the problem? What are the risks of non-conformance? What is the cost of poor throughput? u Where should the measurement be made? At-line or On-line? (On-line is usually > 3 x more $. ) Are there area classification requirements? i. e. , Class I Div I u How often should a measurement be made? What are the process and instrument limitations? u What decisions will be made with the data? Does Quality Operations want to intimately know the process? What are the Regulatory implications? u Will implementation affect other processes? What is the impact on Cleaning Validation and probe material of construction compatibility? - 15 -
Considerations for Project Identification u Is the process “broken”? Are there unknown or unmeasured critical process parameters? u How big is the problem? What are the risks of non-conformance? What is the cost of poor throughput? u Where should the measurement be made? At-line or On-line? (On-line is usually > 3 x more $. ) Are there area classification requirements? i. e. , Class I Div I u How often should a measurement be made? What are the process and instrument limitations? u What decisions will be made with the data? Does Quality Operations want to intimately know the process? What are the Regulatory implications? u Will implementation affect other processes? What is the impact on Cleaning Validation and probe material of construction compatibility? - 15 -
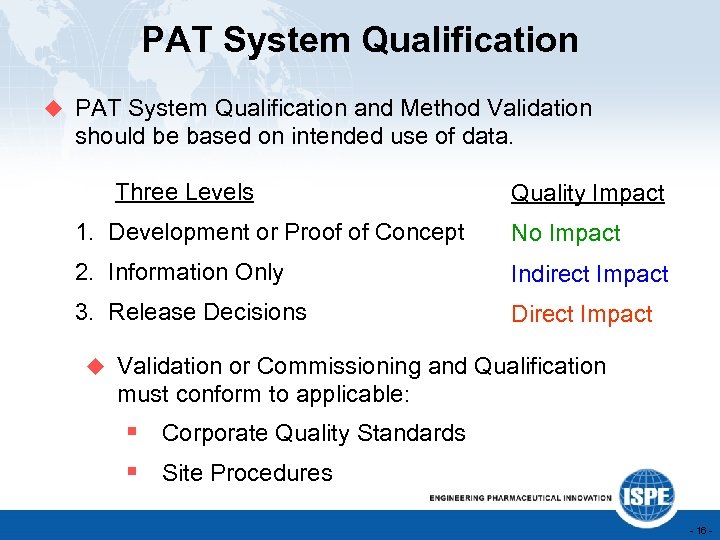 PAT System Qualification u PAT System Qualification and Method Validation should be based on intended use of data. Three Levels Quality Impact 1. Development or Proof of Concept No Impact 2. Information Only Indirect Impact 3. Release Decisions Direct Impact u Validation or Commissioning and Qualification must conform to applicable: § Corporate Quality Standards § Site Procedures - 16 -
PAT System Qualification u PAT System Qualification and Method Validation should be based on intended use of data. Three Levels Quality Impact 1. Development or Proof of Concept No Impact 2. Information Only Indirect Impact 3. Release Decisions Direct Impact u Validation or Commissioning and Qualification must conform to applicable: § Corporate Quality Standards § Site Procedures - 16 -
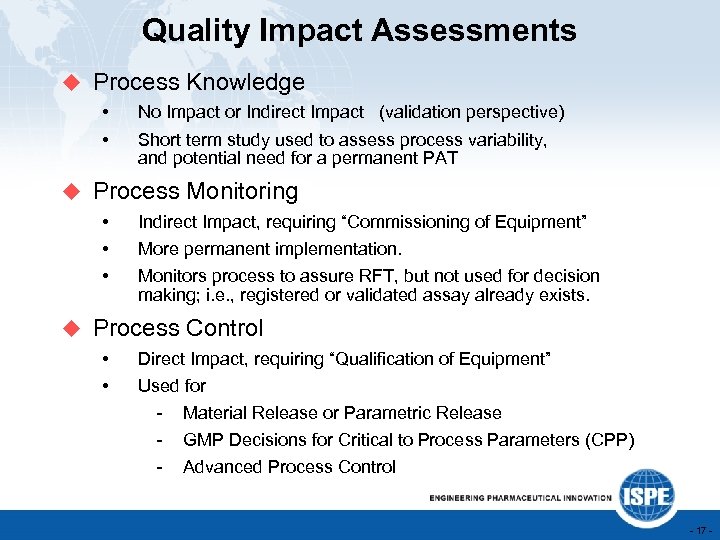 Quality Impact Assessments u Process Knowledge • • No Impact or Indirect Impact (validation perspective) Short term study used to assess process variability, and potential need for a permanent PAT u Process Monitoring • • • Indirect Impact, requiring “Commissioning of Equipment” More permanent implementation. Monitors process to assure RFT, but not used for decision making; i. e. , registered or validated assay already exists. u Process Control • • Direct Impact, requiring “Qualification of Equipment” Used for - Material Release or Parametric Release GMP Decisions for Critical to Process Parameters (CPP) Advanced Process Control - 17 -
Quality Impact Assessments u Process Knowledge • • No Impact or Indirect Impact (validation perspective) Short term study used to assess process variability, and potential need for a permanent PAT u Process Monitoring • • • Indirect Impact, requiring “Commissioning of Equipment” More permanent implementation. Monitors process to assure RFT, but not used for decision making; i. e. , registered or validated assay already exists. u Process Control • • Direct Impact, requiring “Qualification of Equipment” Used for - Material Release or Parametric Release GMP Decisions for Critical to Process Parameters (CPP) Advanced Process Control - 17 -
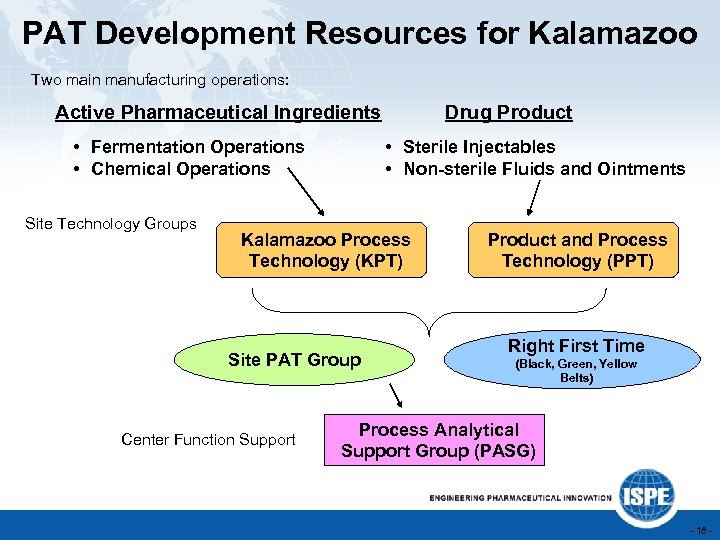 PAT Development Resources for Kalamazoo Two main manufacturing operations: Active Pharmaceutical Ingredients • Fermentation Operations • Chemical Operations Site Technology Groups Drug Product • Sterile Injectables • Non-sterile Fluids and Ointments Kalamazoo Process Technology (KPT) Site PAT Group Center Function Support Product and Process Technology (PPT) Right First Time (Black, Green, Yellow Belts) Process Analytical Support Group (PASG) - 18 -
PAT Development Resources for Kalamazoo Two main manufacturing operations: Active Pharmaceutical Ingredients • Fermentation Operations • Chemical Operations Site Technology Groups Drug Product • Sterile Injectables • Non-sterile Fluids and Ointments Kalamazoo Process Technology (KPT) Site PAT Group Center Function Support Product and Process Technology (PPT) Right First Time (Black, Green, Yellow Belts) Process Analytical Support Group (PASG) - 18 -
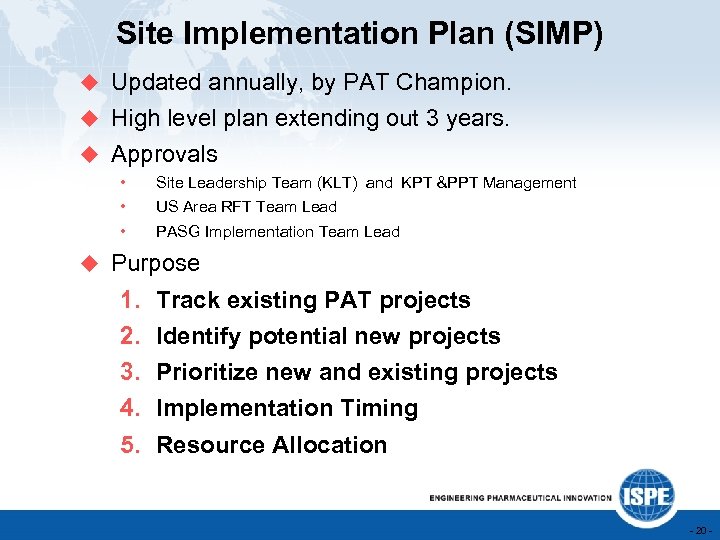 Site Implementation Plan (SIMP) u Updated annually, by PAT Champion. u High level plan extending out 3 years. u Approvals • • • Site Leadership Team (KLT) and KPT &PPT Management US Area RFT Team Lead PASG Implementation Team Lead u Purpose 1. 2. 3. 4. 5. Track existing PAT projects Identify potential new projects Prioritize new and existing projects Implementation Timing Resource Allocation - 20 -
Site Implementation Plan (SIMP) u Updated annually, by PAT Champion. u High level plan extending out 3 years. u Approvals • • • Site Leadership Team (KLT) and KPT &PPT Management US Area RFT Team Lead PASG Implementation Team Lead u Purpose 1. 2. 3. 4. 5. Track existing PAT projects Identify potential new projects Prioritize new and existing projects Implementation Timing Resource Allocation - 20 -
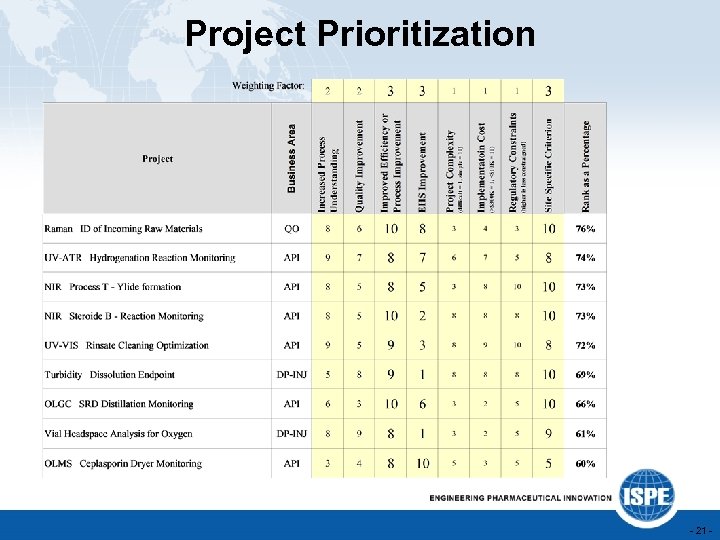 Project Prioritization - 21 -
Project Prioritization - 21 -
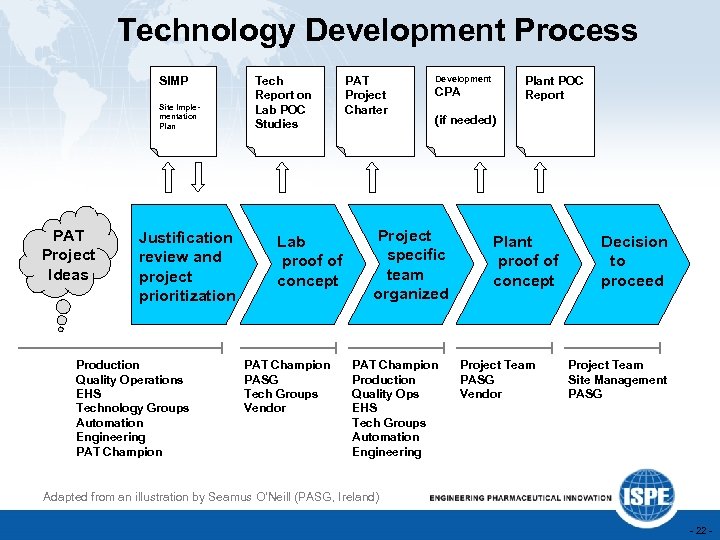 Technology Development Process SIMP Site Implementation Plan PAT Project Ideas Justification review and project prioritization Production Quality Operations EHS Technology Groups Automation Engineering PAT Champion Tech Report on Lab POC Studies Lab proof of concept PAT Champion PASG Tech Groups Vendor PAT Project Charter Development Plant POC Report CPA (if needed) Project specific team organized PAT Champion Production Quality Ops EHS Tech Groups Automation Engineering Plant proof of concept Project Team PASG Vendor Decision to proceed Project Team Site Management PASG Adapted from an illustration by Seamus O’Neill (PASG, Ireland) - 22 -
Technology Development Process SIMP Site Implementation Plan PAT Project Ideas Justification review and project prioritization Production Quality Operations EHS Technology Groups Automation Engineering PAT Champion Tech Report on Lab POC Studies Lab proof of concept PAT Champion PASG Tech Groups Vendor PAT Project Charter Development Plant POC Report CPA (if needed) Project specific team organized PAT Champion Production Quality Ops EHS Tech Groups Automation Engineering Plant proof of concept Project Team PASG Vendor Decision to proceed Project Team Site Management PASG Adapted from an illustration by Seamus O’Neill (PASG, Ireland) - 22 -
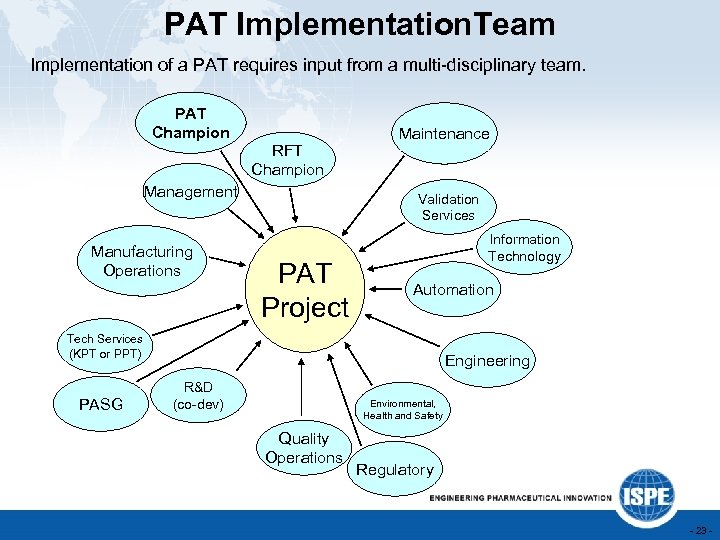 PAT Implementation. Team Implementation of a PAT requires input from a multi-disciplinary team. PAT Champion RFT Champion Management Manufacturing Operations Maintenance Validation Services PAT Project Information Technology Automation Tech Services (KPT or PPT) PASG Engineering R&D (co-dev) Environmental, Health and Safety Quality Operations Regulatory - 23 -
PAT Implementation. Team Implementation of a PAT requires input from a multi-disciplinary team. PAT Champion RFT Champion Management Manufacturing Operations Maintenance Validation Services PAT Project Information Technology Automation Tech Services (KPT or PPT) PASG Engineering R&D (co-dev) Environmental, Health and Safety Quality Operations Regulatory - 23 -
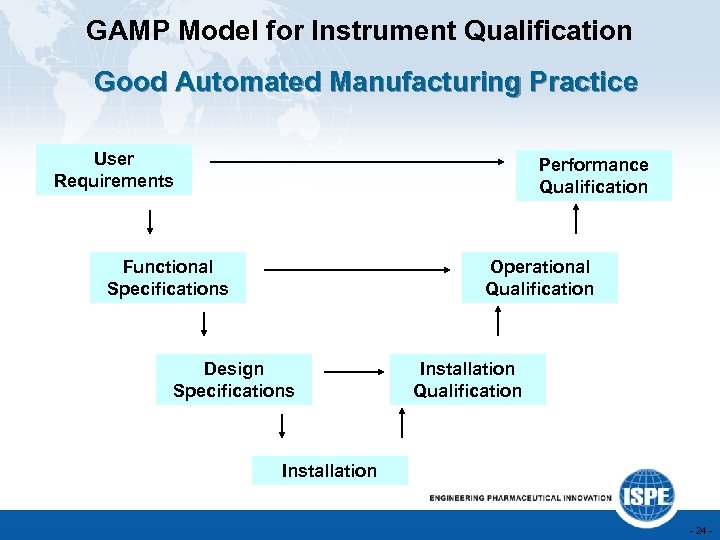 GAMP Model for Instrument Qualification Good Automated Manufacturing Practice User Requirements Performance Qualification Functional Specifications Operational Qualification Design Specifications Installation Qualification Installation - 24 -
GAMP Model for Instrument Qualification Good Automated Manufacturing Practice User Requirements Performance Qualification Functional Specifications Operational Qualification Design Specifications Installation Qualification Installation - 24 -
 Q More uestions What are you going to do with the data? u Is the information used for material release? u Do components come into direct contact with product? u Is there a GMP Impact? u Is there a Regulatory Impact? u Does the system affect product quality? u What if the system fails? u How should the data be archived? u Etcetera (ca. 14 questions for a system level impact assessment) Really asking: Is the PAT for Process Answer: Knowledge or Process Control ? Quality Impact Assessment document - 25 -
Q More uestions What are you going to do with the data? u Is the information used for material release? u Do components come into direct contact with product? u Is there a GMP Impact? u Is there a Regulatory Impact? u Does the system affect product quality? u What if the system fails? u How should the data be archived? u Etcetera (ca. 14 questions for a system level impact assessment) Really asking: Is the PAT for Process Answer: Knowledge or Process Control ? Quality Impact Assessment document - 25 -
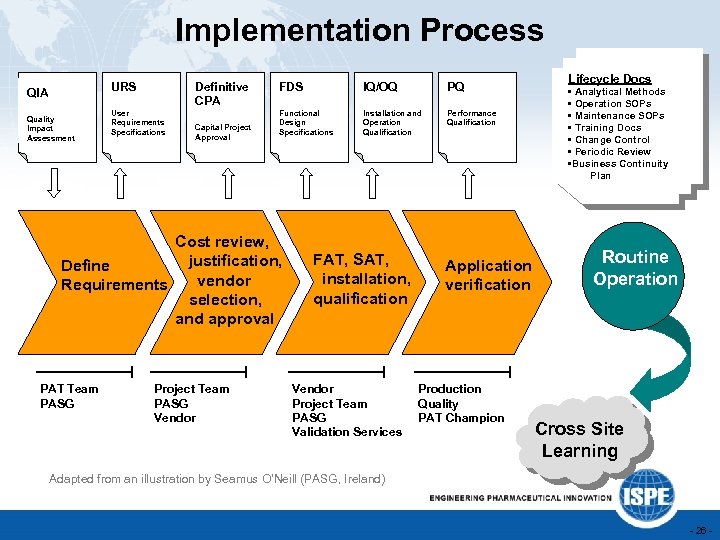 Implementation Process URS QIA Quality Impact Assessment Definitive CPA User Requirements Specifications Capital Project Approval FDS IQ/OQ PQ Functional Design Specifications Installation and Operation Qualification Performance Qualification Cost review, justification, Define vendor Requirements selection, and approval PAT Team PASG Project Team PASG Vendor FAT, SAT, installation, qualification Vendor Project Team PASG Validation Services Application verification Production Quality PAT Champion Lifecycle Docs • Analytical Methods • Operation SOPs • Maintenance SOPs • Training Docs • Change Control • Periodic Review • Business Continuity Plan Routine Ready for Routine Operation? Cross Site Learning Adapted from an illustration by Seamus O’Neill (PASG, Ireland) - 26 -
Implementation Process URS QIA Quality Impact Assessment Definitive CPA User Requirements Specifications Capital Project Approval FDS IQ/OQ PQ Functional Design Specifications Installation and Operation Qualification Performance Qualification Cost review, justification, Define vendor Requirements selection, and approval PAT Team PASG Project Team PASG Vendor FAT, SAT, installation, qualification Vendor Project Team PASG Validation Services Application verification Production Quality PAT Champion Lifecycle Docs • Analytical Methods • Operation SOPs • Maintenance SOPs • Training Docs • Change Control • Periodic Review • Business Continuity Plan Routine Ready for Routine Operation? Cross Site Learning Adapted from an illustration by Seamus O’Neill (PASG, Ireland) - 26 -
 Example #1 – CU in a Sterile Suspension u Application: Drug Product Sterile Aqueous Suspension u Quality Impact: No Impact, Process Knowledge (product was not for sale) u Objective: Improved Content Uniformity during later stages of filling operation. u Project: RFT and Continuous Improvement Black Belt project to provide suggested process changes for improved content uniformity. - 27 -
Example #1 – CU in a Sterile Suspension u Application: Drug Product Sterile Aqueous Suspension u Quality Impact: No Impact, Process Knowledge (product was not for sale) u Objective: Improved Content Uniformity during later stages of filling operation. u Project: RFT and Continuous Improvement Black Belt project to provide suggested process changes for improved content uniformity. - 27 -
 Drug Product – Sterile Injectable u Parenteral Suspension u Solid • Drug (20 - 150 mg/m. L) u Vehicle • Water (> 95%) • Surfactants • Preservative u 2 m. L vial with 1. 2 m. L fill - 28 -
Drug Product – Sterile Injectable u Parenteral Suspension u Solid • Drug (20 - 150 mg/m. L) u Vehicle • Water (> 95%) • Surfactants • Preservative u 2 m. L vial with 1. 2 m. L fill - 28 -
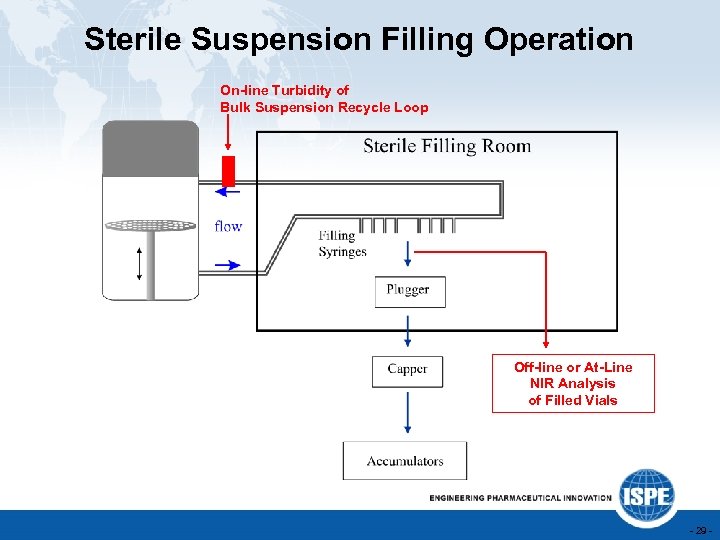 Sterile Suspension Filling Operation On-line Turbidity of Bulk Suspension Recycle Loop Off-line or At-Line NIR Analysis of Filled Vials - 29 -
Sterile Suspension Filling Operation On-line Turbidity of Bulk Suspension Recycle Loop Off-line or At-Line NIR Analysis of Filled Vials - 29 -
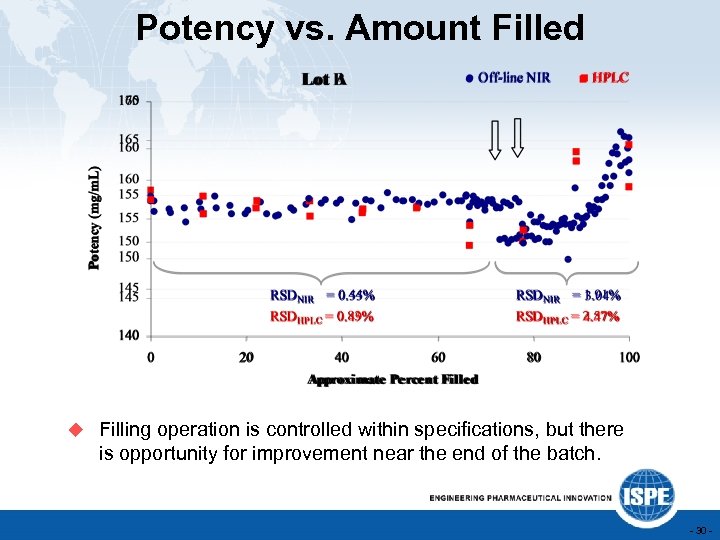 Potency vs. Amount Filled u Filling operation is controlled within specifications, but there is opportunity for improvement near the end of the batch. - 30 -
Potency vs. Amount Filled u Filling operation is controlled within specifications, but there is opportunity for improvement near the end of the batch. - 30 -
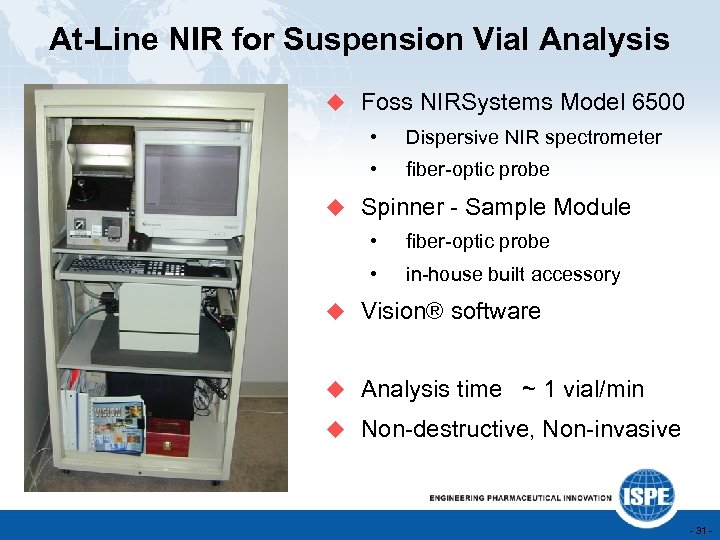 At-Line NIR for Suspension Vial Analysis u Foss NIRSystems Model 6500 • Dispersive NIR spectrometer • fiber-optic probe u Spinner - Sample Module • fiber-optic probe • in-house built accessory u Vision® software u Analysis time ~ 1 vial/min u Non-destructive, Non-invasive - 31 -
At-Line NIR for Suspension Vial Analysis u Foss NIRSystems Model 6500 • Dispersive NIR spectrometer • fiber-optic probe u Spinner - Sample Module • fiber-optic probe • in-house built accessory u Vision® software u Analysis time ~ 1 vial/min u Non-destructive, Non-invasive - 31 -
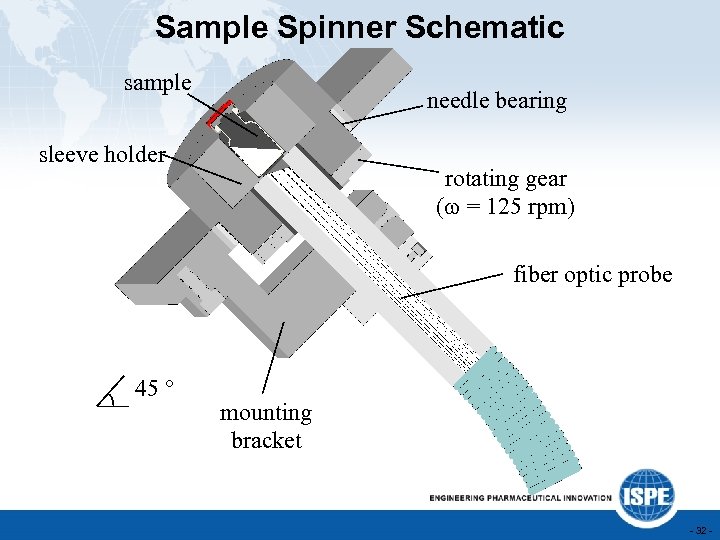 Sample Spinner Schematic sample needle bearing sleeve holder rotating gear (w = 125 rpm) fiber optic probe 45 ° mounting bracket - 32 -
Sample Spinner Schematic sample needle bearing sleeve holder rotating gear (w = 125 rpm) fiber optic probe 45 ° mounting bracket - 32 -
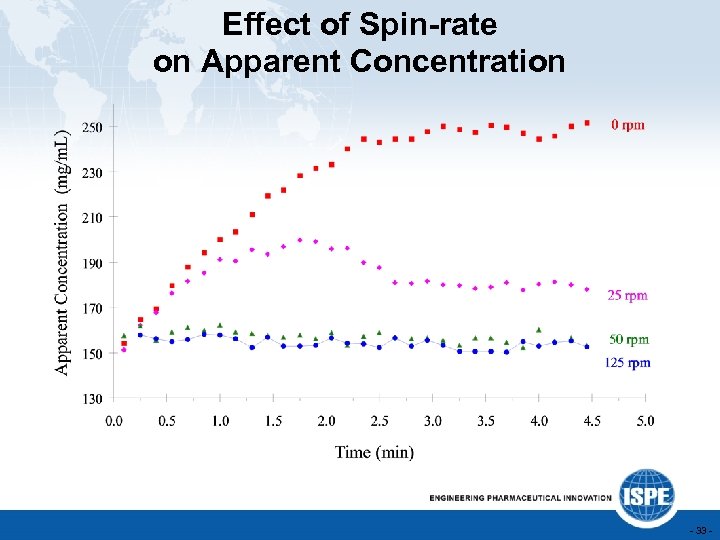 Effect of Spin-rate on Apparent Concentration - 33 -
Effect of Spin-rate on Apparent Concentration - 33 -
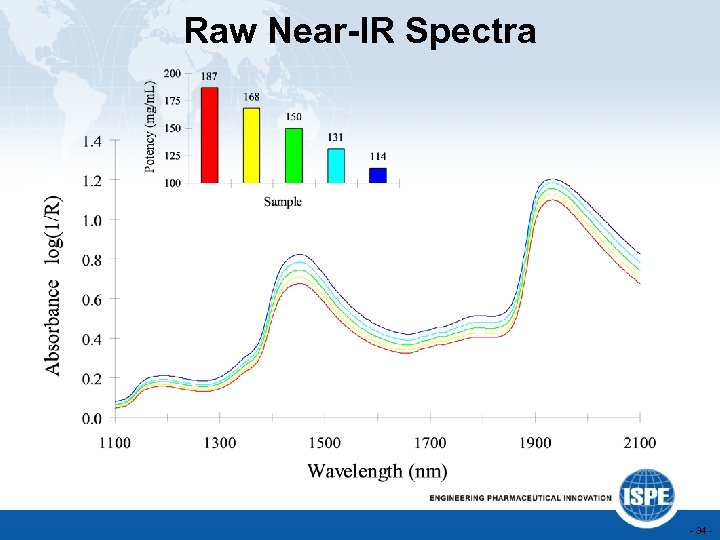 Raw Near-IR Spectra - 34 -
Raw Near-IR Spectra - 34 -
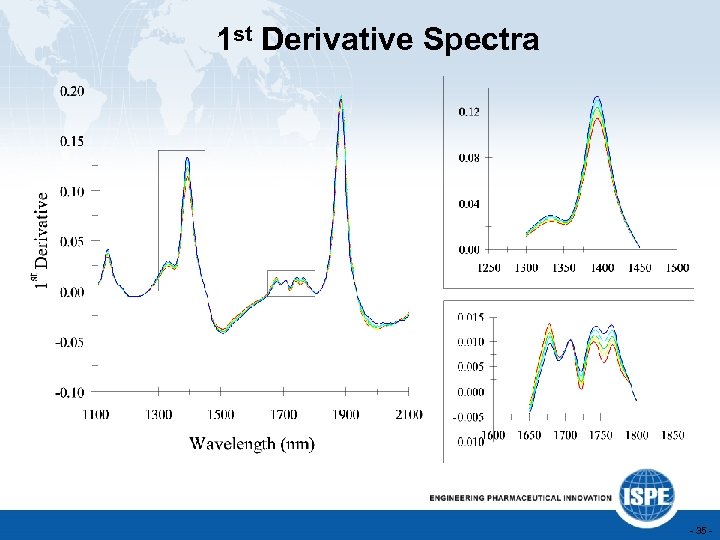 1 st Derivative Spectra - 35 -
1 st Derivative Spectra - 35 -
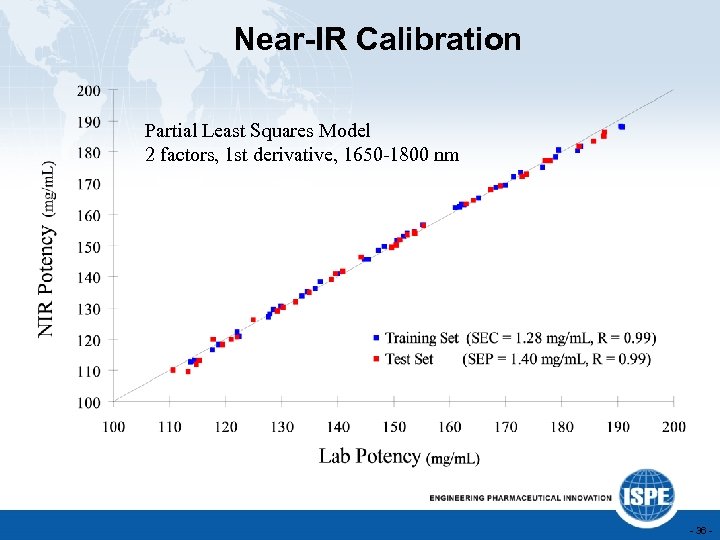 Near-IR Calibration Partial Least Squares Model 2 factors, 1 st derivative, 1650 -1800 nm - 36 -
Near-IR Calibration Partial Least Squares Model 2 factors, 1 st derivative, 1650 -1800 nm - 36 -
 Optek Turbidity Sensor 1. Sensor Body 2. Windows 3. NIR Filter 4. Photo Diode 5. Optics Module 6. Tungsten Lamp - 37 -
Optek Turbidity Sensor 1. Sensor Body 2. Windows 3. NIR Filter 4. Photo Diode 5. Optics Module 6. Tungsten Lamp - 37 -
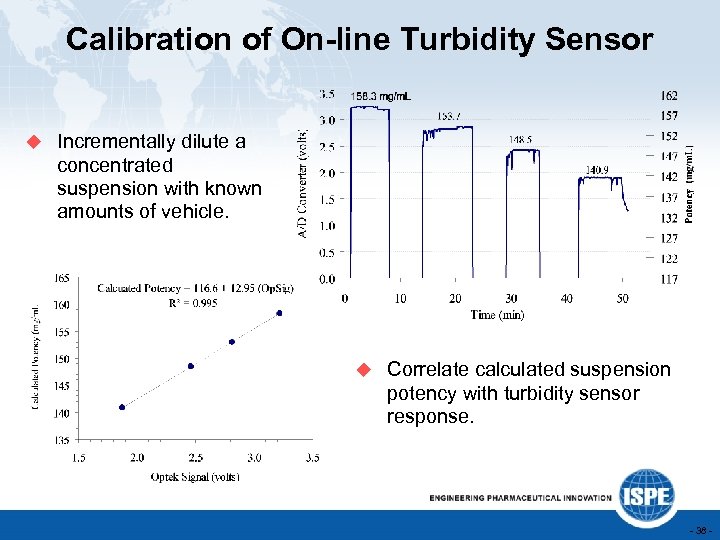 Calibration of On-line Turbidity Sensor u Incrementally dilute a concentrated suspension with known amounts of vehicle. u Correlate calculated suspension potency with turbidity sensor response. - 38 -
Calibration of On-line Turbidity Sensor u Incrementally dilute a concentrated suspension with known amounts of vehicle. u Correlate calculated suspension potency with turbidity sensor response. - 38 -
 DOE Study using On-line Turbidity u RFT Black Belt Project to improve Content Uniformity by optimizing filling parameters. u 6 factor DOE study was conducted varying mixing time, mixing power, recirculation flow-rate, etc. Tommy Garner - 39 -
DOE Study using On-line Turbidity u RFT Black Belt Project to improve Content Uniformity by optimizing filling parameters. u 6 factor DOE study was conducted varying mixing time, mixing power, recirculation flow-rate, etc. Tommy Garner - 39 -
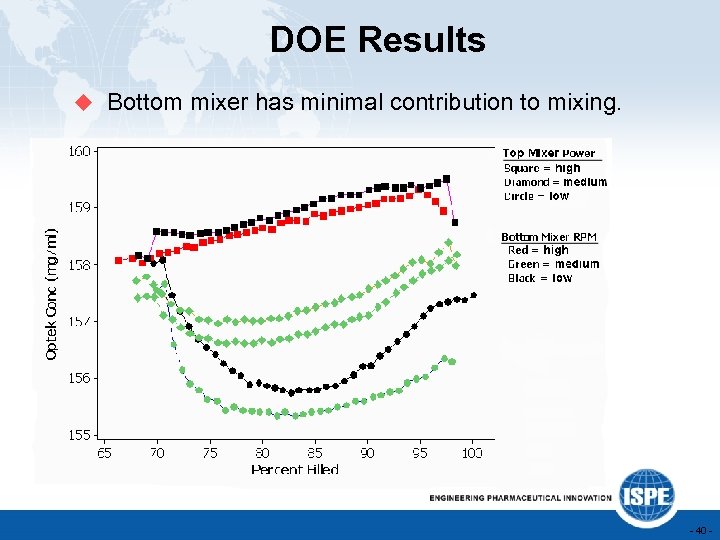 DOE Results u Bottom mixer has minimal contribution to mixing. - 40 -
DOE Results u Bottom mixer has minimal contribution to mixing. - 40 -
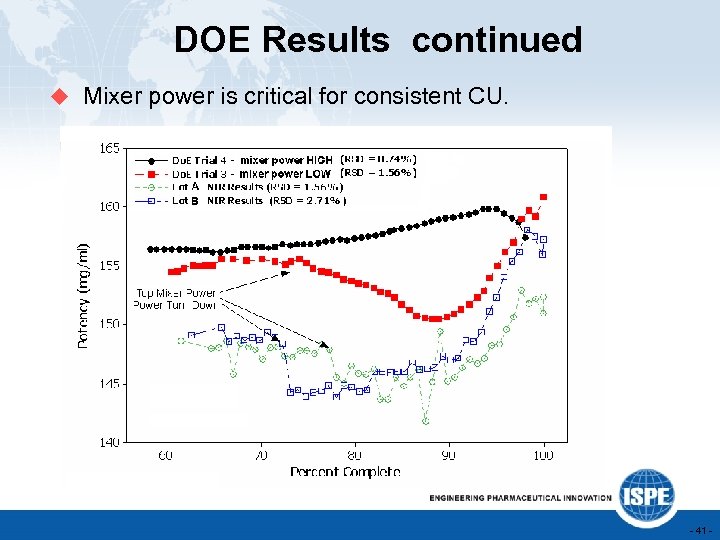 DOE Results continued u Mixer power is critical for consistent CU. - 41 -
DOE Results continued u Mixer power is critical for consistent CU. - 41 -
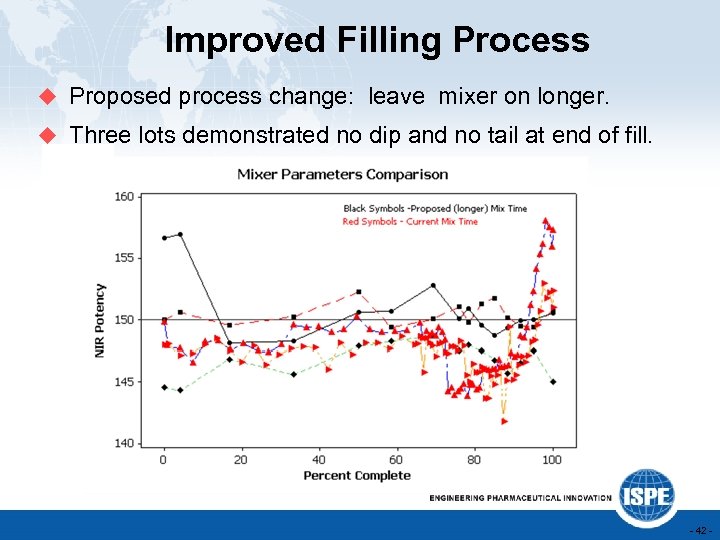 Improved Filling Process u Proposed process change: leave mixer on longer. u Three lots demonstrated no dip and no tail at end of fill. - 42 -
Improved Filling Process u Proposed process change: leave mixer on longer. u Three lots demonstrated no dip and no tail at end of fill. - 42 -
 Advantages offered by On-Line Turbidity u Improved temporal sampling resolution. u Cost savings, by reducing or eliminating the need to perform off-line analysis by NIR or HPLC. Note: HPLC analysis by routine labs is ca. $100/analysis. u Eliminated error of taking “grab” samples for off-line analysis. This was found to be significant, if the sampling line is not properly configured, due to settling. u Time savings - ability to perform several parts of the DOE during the same run, i. e. , ability to see when system has become perturbed or equilibrated. - 43 -
Advantages offered by On-Line Turbidity u Improved temporal sampling resolution. u Cost savings, by reducing or eliminating the need to perform off-line analysis by NIR or HPLC. Note: HPLC analysis by routine labs is ca. $100/analysis. u Eliminated error of taking “grab” samples for off-line analysis. This was found to be significant, if the sampling line is not properly configured, due to settling. u Time savings - ability to perform several parts of the DOE during the same run, i. e. , ability to see when system has become perturbed or equilibrated. - 43 -
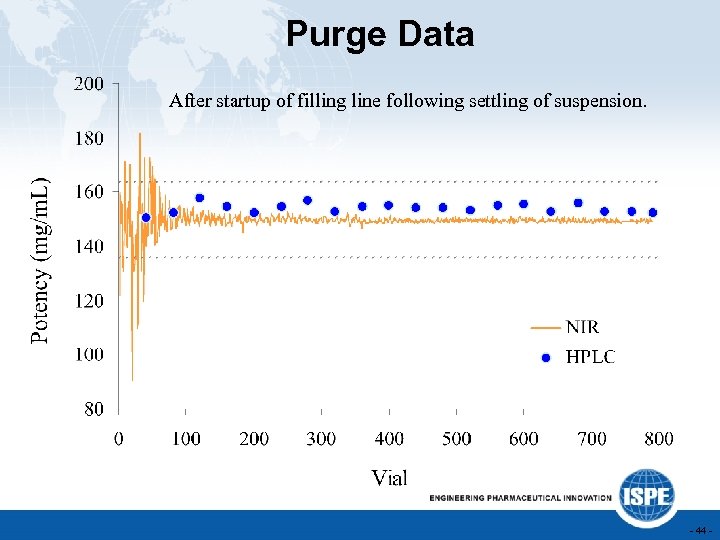 Purge Data After startup of filling line following settling of suspension. - 44 -
Purge Data After startup of filling line following settling of suspension. - 44 -
 Nyquist-Shannon Sampling Theorem The sampling rate must be twice the maximum frequency component of the "signal" being measured, otherwise aliasing will occur. fsampling = 2 fsignal Graphical representations see Aliasing. Bruno A. Olshausen, PCS 129 – Sensory Processes, Oct 10, 2000. http: //redwood. ucdavis. edu/bruno/npb 261/aliasing. pdf - 45 -
Nyquist-Shannon Sampling Theorem The sampling rate must be twice the maximum frequency component of the "signal" being measured, otherwise aliasing will occur. fsampling = 2 fsignal Graphical representations see Aliasing. Bruno A. Olshausen, PCS 129 – Sensory Processes, Oct 10, 2000. http: //redwood. ucdavis. edu/bruno/npb 261/aliasing. pdf - 45 -
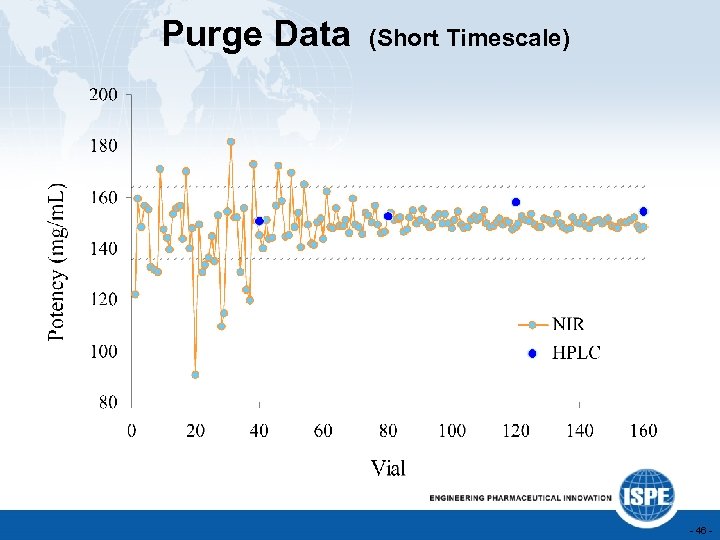 Purge Data (Short Timescale) - 46 -
Purge Data (Short Timescale) - 46 -
 USP Compendial CU Testing <905> “Uniformity of Dosage Units” in USP-NF u Stage 1 Acceptance Criteria Assay 10 samples, i. e. , n = 10 Pass if RSD ≤ 6. 0% and no value is outside 85% to 115% claim. Fail if one or more value is outside 75% to 125% claim. u Stage 2 Acceptance Criteria Assay 20 more samples, i. e. , n = 30 Pass if RSD ≤ 7. 8%, no more than one value is outside 85% to 115% claim, and no value is outside 75% to 125% claim. u Statistics are based on a small sample population; i. e. , analytical testing with low statistical power. - 47 -
USP Compendial CU Testing <905> “Uniformity of Dosage Units” in USP-NF u Stage 1 Acceptance Criteria Assay 10 samples, i. e. , n = 10 Pass if RSD ≤ 6. 0% and no value is outside 85% to 115% claim. Fail if one or more value is outside 75% to 125% claim. u Stage 2 Acceptance Criteria Assay 20 more samples, i. e. , n = 30 Pass if RSD ≤ 7. 8%, no more than one value is outside 85% to 115% claim, and no value is outside 75% to 125% claim. u Statistics are based on a small sample population; i. e. , analytical testing with low statistical power. - 47 -
 CU Testing Criteria for Large N u USP <905> is unsuitable for data sets comprised of large sample populations. u Proposed Acceptance Criteria outlined in article: Sandell D. , Vukovinsky K. , Diener M. , Hofer J. , Pazdan J. , and Timmermans J. Development of a Content Uniformity Test Suitable for Large Sample Size. Drug Information Journal, Vol 40, pp. 337 -344, 2006. - 48 -
CU Testing Criteria for Large N u USP <905> is unsuitable for data sets comprised of large sample populations. u Proposed Acceptance Criteria outlined in article: Sandell D. , Vukovinsky K. , Diener M. , Hofer J. , Pazdan J. , and Timmermans J. Development of a Content Uniformity Test Suitable for Large Sample Size. Drug Information Journal, Vol 40, pp. 337 -344, 2006. - 48 -
 Example #2 – DP Dissolution Monitoring u Objective Provide a non-qualitative means of assessing completion of API dissolution during compounding prior to aseptic filtration. u Quality Impact Assessment Indirect Impact. Current IPC is by monitoring p. H. u Key Players Justine Mc. Kenzie Project Management Bob Witteman Greenbelt, Manufacturing Engineer Tim Wang Kalamazoo Injectable Manufacturing Bob Leasure Site PAT Support - 49 -
Example #2 – DP Dissolution Monitoring u Objective Provide a non-qualitative means of assessing completion of API dissolution during compounding prior to aseptic filtration. u Quality Impact Assessment Indirect Impact. Current IPC is by monitoring p. H. u Key Players Justine Mc. Kenzie Project Management Bob Witteman Greenbelt, Manufacturing Engineer Tim Wang Kalamazoo Injectable Manufacturing Bob Leasure Site PAT Support - 49 -
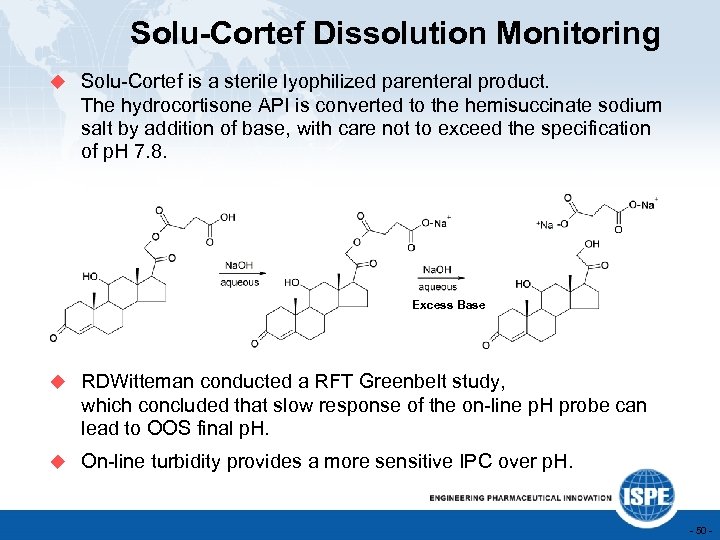 Solu-Cortef Dissolution Monitoring u Solu-Cortef is a sterile lyophilized parenteral product. The hydrocortisone API is converted to the hemisuccinate sodium salt by addition of base, with care not to exceed the specification of p. H 7. 8. Excess Base u RDWitteman conducted a RFT Greenbelt study, which concluded that slow response of the on-line p. H probe can lead to OOS final p. H. u On-line turbidity provides a more sensitive IPC over p. H. - 50 -
Solu-Cortef Dissolution Monitoring u Solu-Cortef is a sterile lyophilized parenteral product. The hydrocortisone API is converted to the hemisuccinate sodium salt by addition of base, with care not to exceed the specification of p. H 7. 8. Excess Base u RDWitteman conducted a RFT Greenbelt study, which concluded that slow response of the on-line p. H probe can lead to OOS final p. H. u On-line turbidity provides a more sensitive IPC over p. H. - 50 -
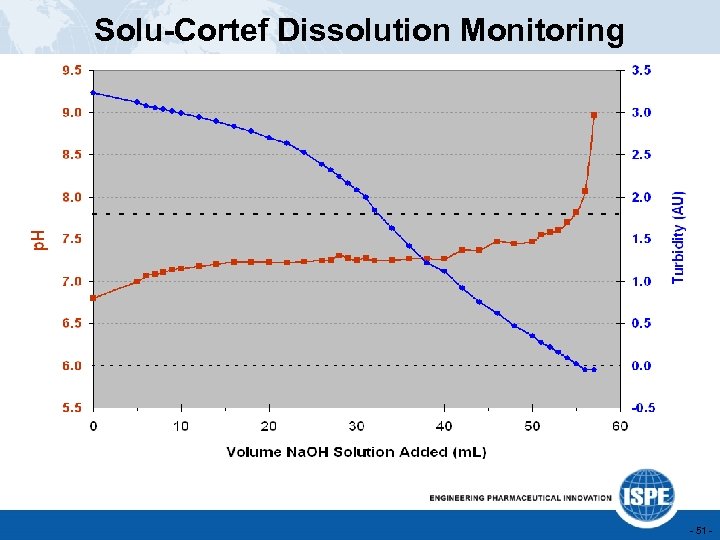 Solu-Cortef Dissolution Monitoring - 51 -
Solu-Cortef Dissolution Monitoring - 51 -
 Optek Forward Scatter Turbidity Probe Optek Model AS 16 -N Single Channel Photometer • Forward scatter Turbidity Probe • Operates in NIR from 730 to 970 nm • OPL from 1 to 40 mm • Aseptic Ingold or Triclover fittings • Analog controller, 4 -20 m. A I/O (no computer) • ca. $10 K - 52 -
Optek Forward Scatter Turbidity Probe Optek Model AS 16 -N Single Channel Photometer • Forward scatter Turbidity Probe • Operates in NIR from 730 to 970 nm • OPL from 1 to 40 mm • Aseptic Ingold or Triclover fittings • Analog controller, 4 -20 m. A I/O (no computer) • ca. $10 K - 52 -
 Implementation Plans u Optek Turbidity Probes have been installed in two CIP compounding tanks in Kalamazoo’s new aseptic production facility. u C&Q of the analyzers is underway as part of the validation of the new production facilities. u Current plans are for the equipment to be used for indirect impact process monitoring. u Use of the equipment for direct impact process control will be evaluated after additional process knowledge is gained and with consideration of benefits from RFT and Lean manufacturing. - 53 -
Implementation Plans u Optek Turbidity Probes have been installed in two CIP compounding tanks in Kalamazoo’s new aseptic production facility. u C&Q of the analyzers is underway as part of the validation of the new production facilities. u Current plans are for the equipment to be used for indirect impact process monitoring. u Use of the equipment for direct impact process control will be evaluated after additional process knowledge is gained and with consideration of benefits from RFT and Lean manufacturing. - 53 -
 Example #3 – API Solvent Recovery u Application: Cost Savings by Improving Yield for Solvent Recovery in API Operations u Quality Impact: Direct Impact u Issues: Relatively slow determination of cut for collecting product fraction. Based on In-Plant Lab GC analysis. u Project: Install On-line Gas Chromatographic analysis with associated automation. (as deemed by QO) - 54 -
Example #3 – API Solvent Recovery u Application: Cost Savings by Improving Yield for Solvent Recovery in API Operations u Quality Impact: Direct Impact u Issues: Relatively slow determination of cut for collecting product fraction. Based on In-Plant Lab GC analysis. u Project: Install On-line Gas Chromatographic analysis with associated automation. (as deemed by QO) - 54 -
 OLGC Installation u One of seven solvent recovery columns at the site. u Column #5 is used to recover seven different solvents. • • Methylene Chloride • Ethyl Acetate • THF • DMAP (THF containing alcohols) • Toluene • u DMF Acetone Photo shows • Column • Still Pot • In-Plant Lab - 55 -
OLGC Installation u One of seven solvent recovery columns at the site. u Column #5 is used to recover seven different solvents. • • Methylene Chloride • Ethyl Acetate • THF • DMAP (THF containing alcohols) • Toluene • u DMF Acetone Photo shows • Column • Still Pot • In-Plant Lab - 55 -
 Existing At-line GC Assay u Performed by manufacturing operators in the “In-Plant Lab” (IPL) u Analysis is time consuming due to manual steps: • Collect sample • Transport to IPL • Sample preparation and injection • Assay runtime, as long as 45 minutes depending on solvent u Prompt for manual analysis is based on column temperatures and “wait” times indicated in Master Record - 56 -
Existing At-line GC Assay u Performed by manufacturing operators in the “In-Plant Lab” (IPL) u Analysis is time consuming due to manual steps: • Collect sample • Transport to IPL • Sample preparation and injection • Assay runtime, as long as 45 minutes depending on solvent u Prompt for manual analysis is based on column temperatures and “wait” times indicated in Master Record - 56 -
 Siemens Maxum II On-line GC Dual Oven, Isothermal GC Calibration Standard Sampling Valves - 57 -
Siemens Maxum II On-line GC Dual Oven, Isothermal GC Calibration Standard Sampling Valves - 57 -
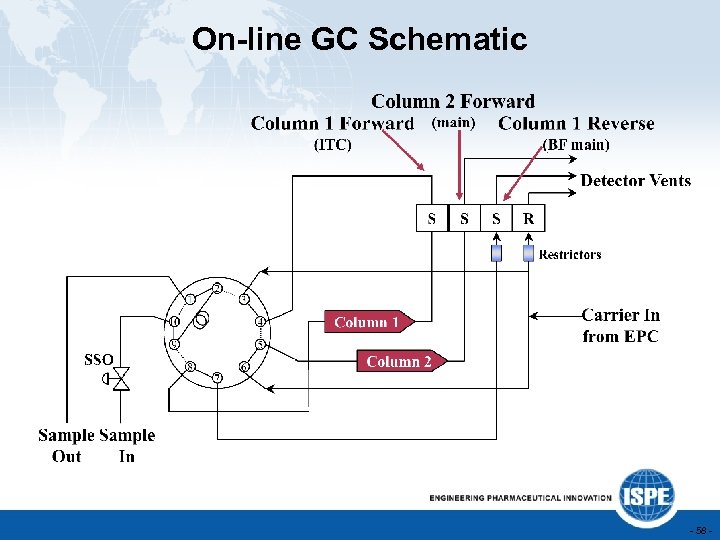 On-line GC Schematic - 58 -
On-line GC Schematic - 58 -
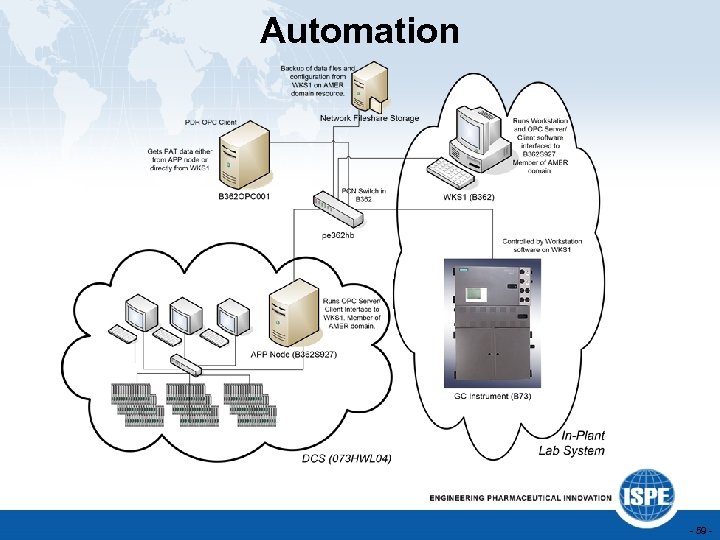 Automation - 59 -
Automation - 59 -
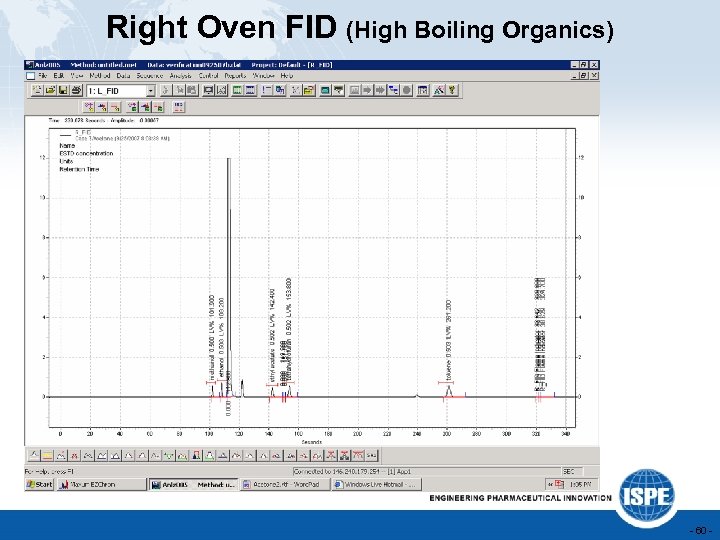 Right Oven FID (High Boiling Organics) - 60 -
Right Oven FID (High Boiling Organics) - 60 -
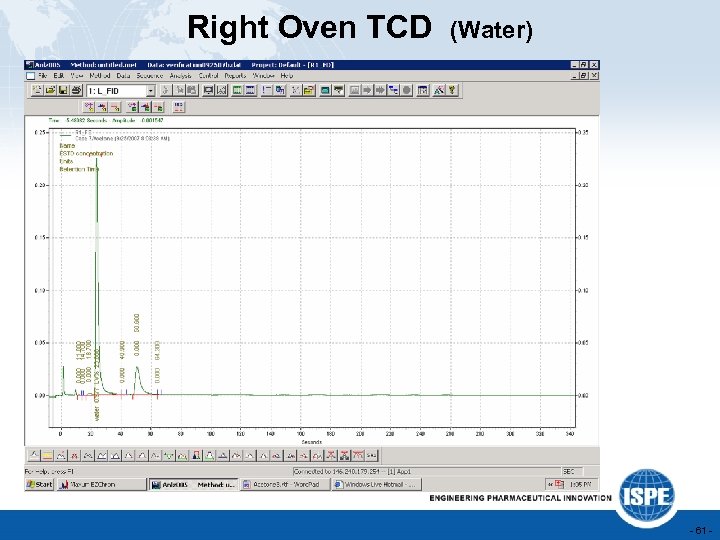 Right Oven TCD (Water) - 61 -
Right Oven TCD (Water) - 61 -
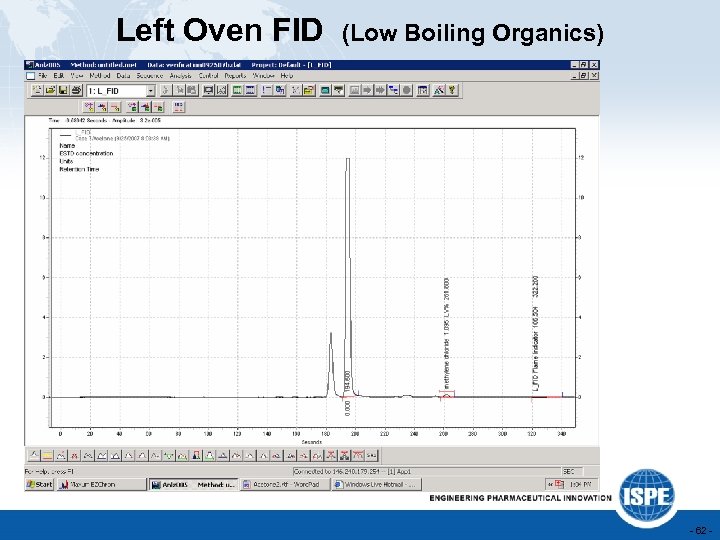 Left Oven FID (Low Boiling Organics) - 62 -
Left Oven FID (Low Boiling Organics) - 62 -
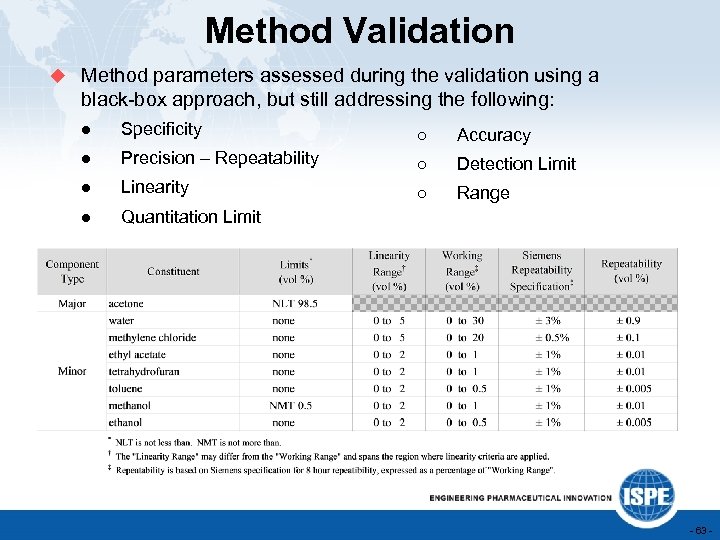 Method Validation u Method parameters assessed during the validation using a black-box approach, but still addressing the following: ● Specificity ○ Accuracy ● Precision – Repeatability ○ Detection Limit ● Linearity ○ Range ● Quantitation Limit - 63 -
Method Validation u Method parameters assessed during the validation using a black-box approach, but still addressing the following: ● Specificity ○ Accuracy ● Precision – Repeatability ○ Detection Limit ● Linearity ○ Range ● Quantitation Limit - 63 -
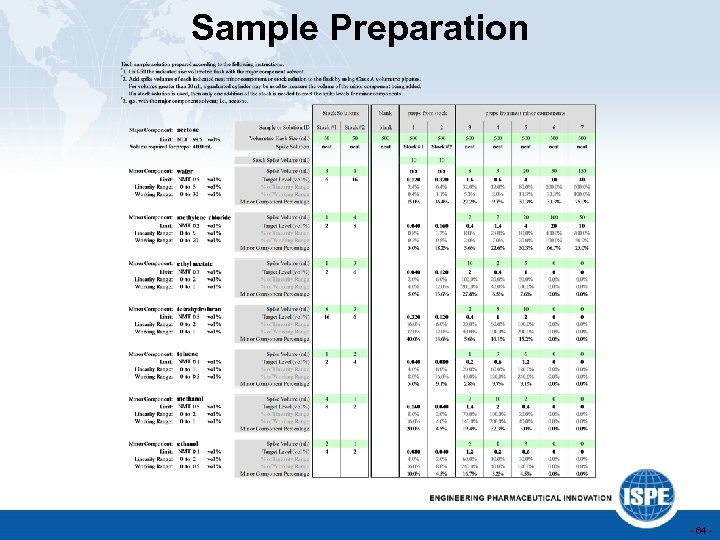 Sample Preparation - 64 -
Sample Preparation - 64 -
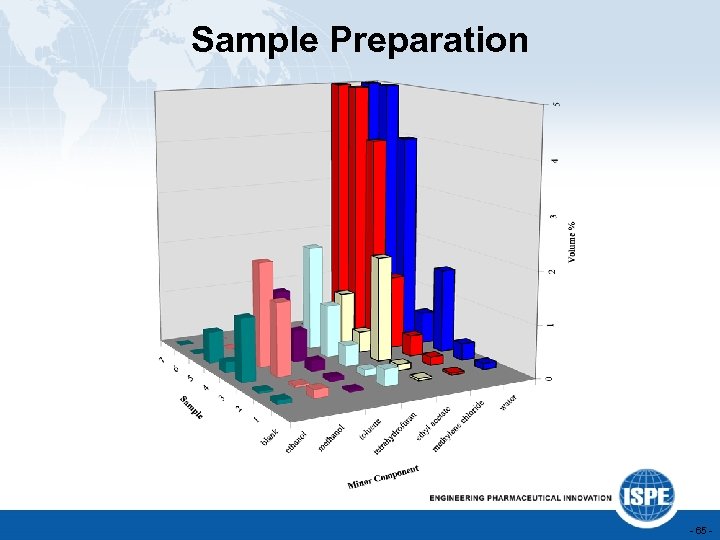 Sample Preparation - 65 -
Sample Preparation - 65 -
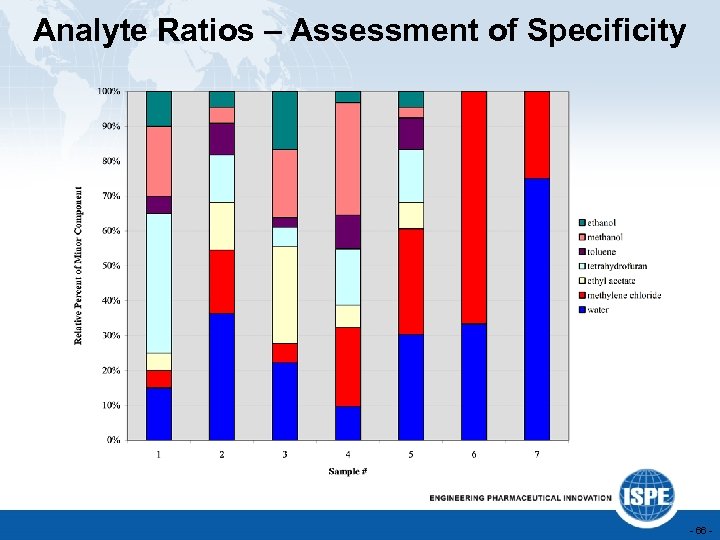 Analyte Ratios – Assessment of Specificity - 66 -
Analyte Ratios – Assessment of Specificity - 66 -
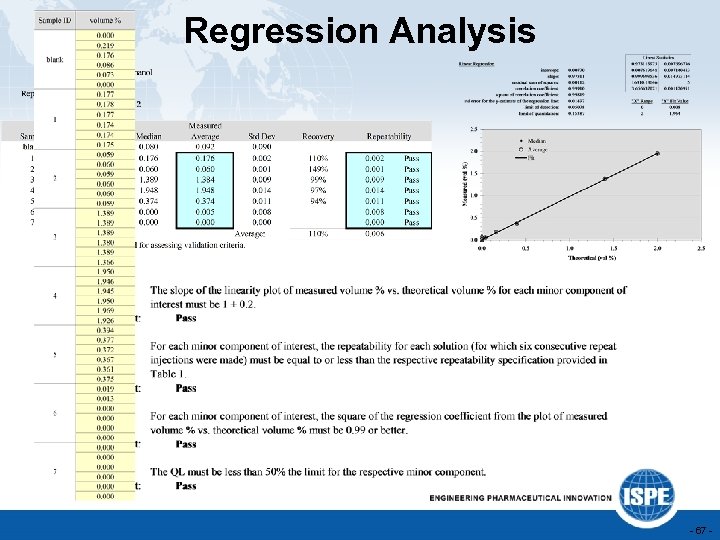 Regression Analysis - 67 -
Regression Analysis - 67 -
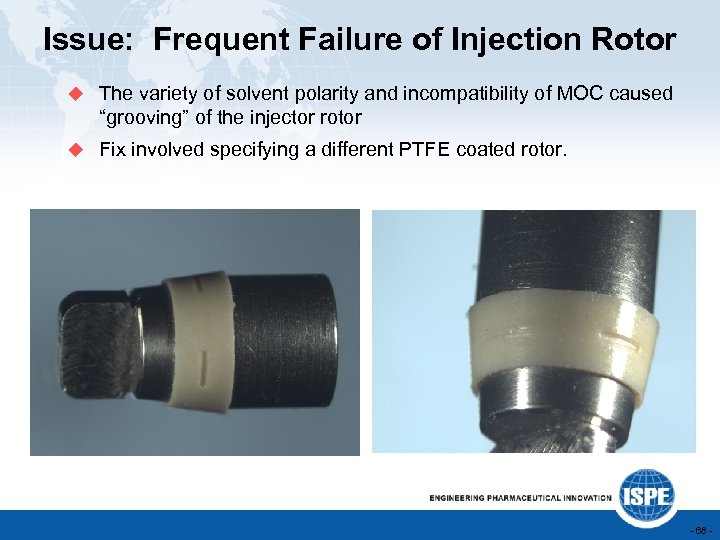 Issue: Frequent Failure of Injection Rotor u The variety of solvent polarity and incompatibility of MOC caused “grooving” of the injector rotor u Fix involved specifying a different PTFE coated rotor. - 68 -
Issue: Frequent Failure of Injection Rotor u The variety of solvent polarity and incompatibility of MOC caused “grooving” of the injector rotor u Fix involved specifying a different PTFE coated rotor. - 68 -
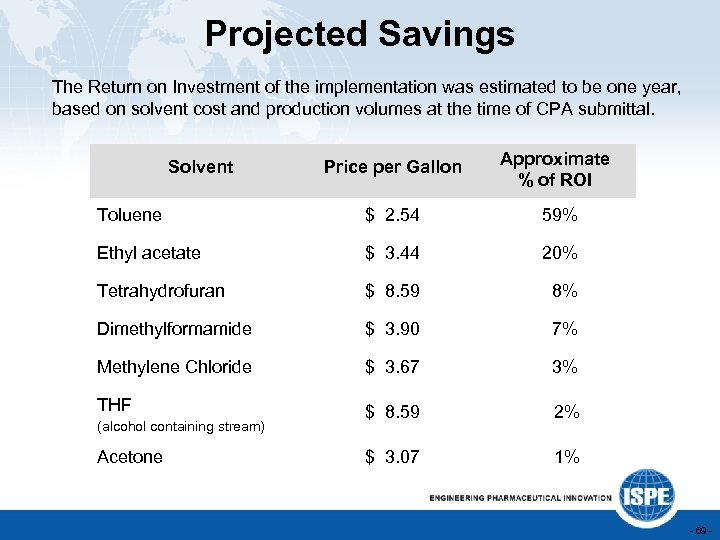 Projected Savings The Return on Investment of the implementation was estimated to be one year, based on solvent cost and production volumes at the time of CPA submittal. Price per Gallon Approximate % of ROI Toluene $ 2. 54 59% Ethyl acetate $ 3. 44 20% Tetrahydrofuran $ 8. 59 8% Dimethylformamide $ 3. 90 7% Methylene Chloride $ 3. 67 3% THF $ 8. 59 2% $ 3. 07 1% Solvent (alcohol containing stream) Acetone - 69 -
Projected Savings The Return on Investment of the implementation was estimated to be one year, based on solvent cost and production volumes at the time of CPA submittal. Price per Gallon Approximate % of ROI Toluene $ 2. 54 59% Ethyl acetate $ 3. 44 20% Tetrahydrofuran $ 8. 59 8% Dimethylformamide $ 3. 90 7% Methylene Chloride $ 3. 67 3% THF $ 8. 59 2% $ 3. 07 1% Solvent (alcohol containing stream) Acetone - 69 -
 Lessons Learned u Stick to the Plan Do not deviate from define validation approach established at the beginning of the project; otherwise the project may be delayed. u Train Appropriate Personnel Appropriately Cross-train key users for daily care and troubleshooting of the instrument. User training should be budgeted as part of the project scope. u Keep it Simple Depending on the technology, analysis of multiple streams/products may present challenges and additional overhead. - 70 -
Lessons Learned u Stick to the Plan Do not deviate from define validation approach established at the beginning of the project; otherwise the project may be delayed. u Train Appropriate Personnel Appropriately Cross-train key users for daily care and troubleshooting of the instrument. User training should be budgeted as part of the project scope. u Keep it Simple Depending on the technology, analysis of multiple streams/products may present challenges and additional overhead. - 70 -
 Acknowledgements u Drug Product Suspension CU • Tom Garner - RFT Black Belt and Project Manager u Drug Product Dissolution Monitoring • Robert Wittemann - RFT Green Belt and Production Engineer • Tim Wang - PPT Production Engineering u On-line GC for Solvent Recovery • Brad Diehl - PASG Implementation Support • Frank Sistare - PGM Groton • Joe Geiger - Production Engineering Solvent Recovery • Jeff Terpstra - Project Management • Pete Miilu, Marc Surprenant - IT Automation • Donald Zeilenga • Scott Wagenaar, Kurt Holton - Production Operations • Andrew Meister - KPT and Site PAT Support - Instrumentation Maintenance - 71 -
Acknowledgements u Drug Product Suspension CU • Tom Garner - RFT Black Belt and Project Manager u Drug Product Dissolution Monitoring • Robert Wittemann - RFT Green Belt and Production Engineer • Tim Wang - PPT Production Engineering u On-line GC for Solvent Recovery • Brad Diehl - PASG Implementation Support • Frank Sistare - PGM Groton • Joe Geiger - Production Engineering Solvent Recovery • Jeff Terpstra - Project Management • Pete Miilu, Marc Surprenant - IT Automation • Donald Zeilenga • Scott Wagenaar, Kurt Holton - Production Operations • Andrew Meister - KPT and Site PAT Support - Instrumentation Maintenance - 71 -
 - 72 -
- 72 -


