a80c6253d56267cd0f49fd90cdf5a034.ppt
- Количество слайдов: 23
 A Prospective multicentre registry, evaluating a real world usage of the Tryton Side Branch Stent: Results of the E-Tryton 150/Benelux- study Prof. P. R. Stella, MD, Ph. D University Medical Center Utrecht On behalf of all investigators
A Prospective multicentre registry, evaluating a real world usage of the Tryton Side Branch Stent: Results of the E-Tryton 150/Benelux- study Prof. P. R. Stella, MD, Ph. D University Medical Center Utrecht On behalf of all investigators
 Potential conflicts of interest Speaker’s name: P. R. Stella, MD, Ph. D I have the following potential conflicts of interest to report: Research contracts: Steering board Tryton FDA study
Potential conflicts of interest Speaker’s name: P. R. Stella, MD, Ph. D I have the following potential conflicts of interest to report: Research contracts: Steering board Tryton FDA study
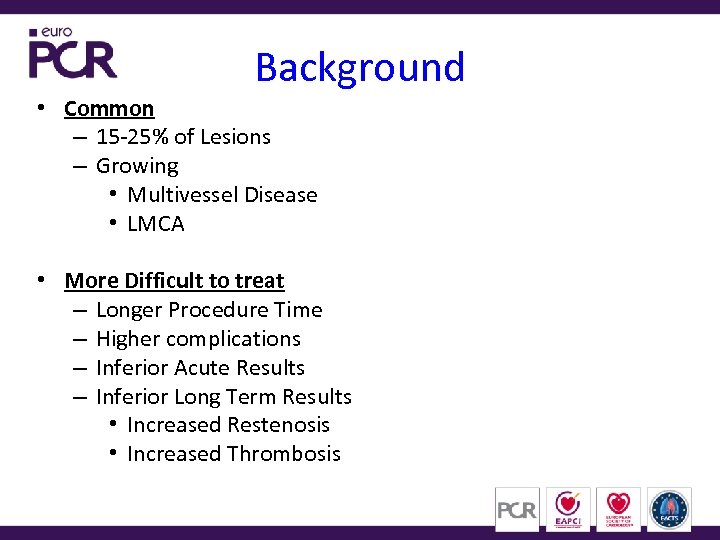 Background • Common – 15 -25% of Lesions – Growing • Multivessel Disease • LMCA • More Difficult to treat – Longer Procedure Time – Higher complications – Inferior Acute Results – Inferior Long Term Results • Increased Restenosis • Increased Thrombosis
Background • Common – 15 -25% of Lesions – Growing • Multivessel Disease • LMCA • More Difficult to treat – Longer Procedure Time – Higher complications – Inferior Acute Results – Inferior Long Term Results • Increased Restenosis • Increased Thrombosis
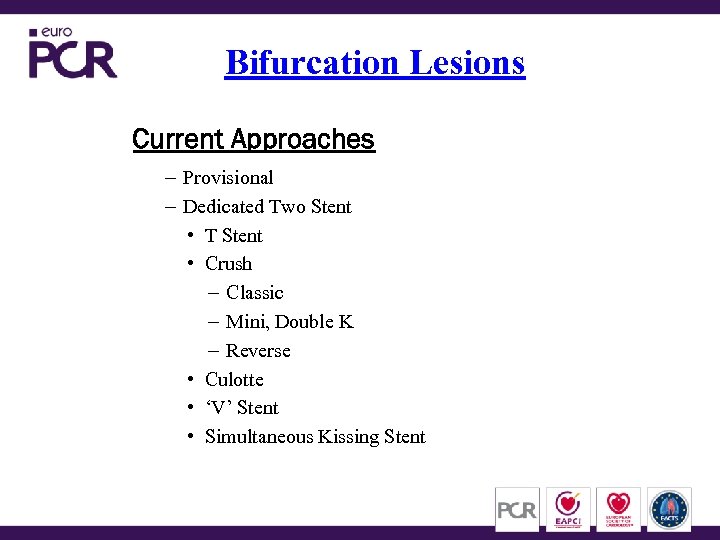 Bifurcation Lesions Current Approaches – Provisional – Dedicated Two Stent • T Stent • Crush – Classic – Mini, Double K – Reverse • Culotte • ‘V’ Stent • Simultaneous Kissing Stent
Bifurcation Lesions Current Approaches – Provisional – Dedicated Two Stent • T Stent • Crush – Classic – Mini, Double K – Reverse • Culotte • ‘V’ Stent • Simultaneous Kissing Stent
 Bifurcation Lesions: Complications • Thrombosis Rate – Incidence: 3. 6 -3. 9% – Hazard Ratio: 4. 6 -6. 5 • Restenosis Rates – Angiographic 20 -40%
Bifurcation Lesions: Complications • Thrombosis Rate – Incidence: 3. 6 -3. 9% – Hazard Ratio: 4. 6 -6. 5 • Restenosis Rates – Angiographic 20 -40%
 Study Objective Assess in a prospective international multicentre singlearm registry, safety and effectiveness at 6 months clinical follow-up of the dedicated Tryton Side Branch Stent
Study Objective Assess in a prospective international multicentre singlearm registry, safety and effectiveness at 6 months clinical follow-up of the dedicated Tryton Side Branch Stent
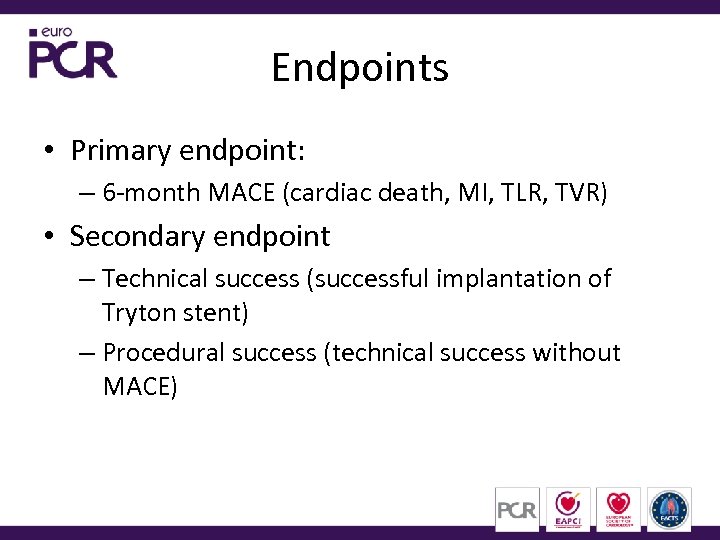 Endpoints • Primary endpoint: – 6 -month MACE (cardiac death, MI, TLR, TVR) • Secondary endpoint – Technical success (successful implantation of Tryton stent) – Procedural success (technical success without MACE)
Endpoints • Primary endpoint: – 6 -month MACE (cardiac death, MI, TLR, TVR) • Secondary endpoint – Technical success (successful implantation of Tryton stent) – Procedural success (technical success without MACE)
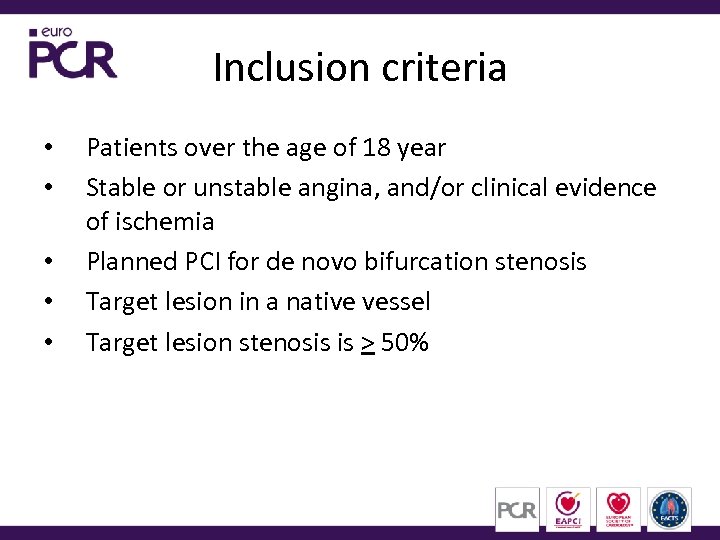 Inclusion criteria • • • Patients over the age of 18 year Stable or unstable angina, and/or clinical evidence of ischemia Planned PCI for de novo bifurcation stenosis Target lesion in a native vessel Target lesion stenosis is > 50%
Inclusion criteria • • • Patients over the age of 18 year Stable or unstable angina, and/or clinical evidence of ischemia Planned PCI for de novo bifurcation stenosis Target lesion in a native vessel Target lesion stenosis is > 50%
 Exclusion criteria • Real world population with limited exclusion criteria: • Contra-indication to dual-antiplatelet therapy • Vessel size MB: <2. 5 mm and >4. 0 mm • Vessel size SB: <2. 25 mm and >3. 5 mm
Exclusion criteria • Real world population with limited exclusion criteria: • Contra-indication to dual-antiplatelet therapy • Vessel size MB: <2. 5 mm and >4. 0 mm • Vessel size SB: <2. 25 mm and >3. 5 mm
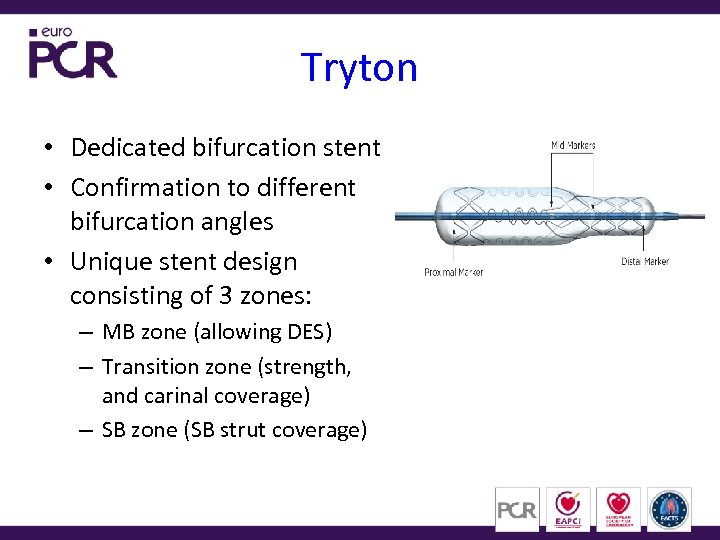 Tryton • Dedicated bifurcation stent • Confirmation to different bifurcation angles • Unique stent design consisting of 3 zones: – MB zone (allowing DES) – Transition zone (strength, and carinal coverage) – SB zone (SB strut coverage)
Tryton • Dedicated bifurcation stent • Confirmation to different bifurcation angles • Unique stent design consisting of 3 zones: – MB zone (allowing DES) – Transition zone (strength, and carinal coverage) – SB zone (SB strut coverage)
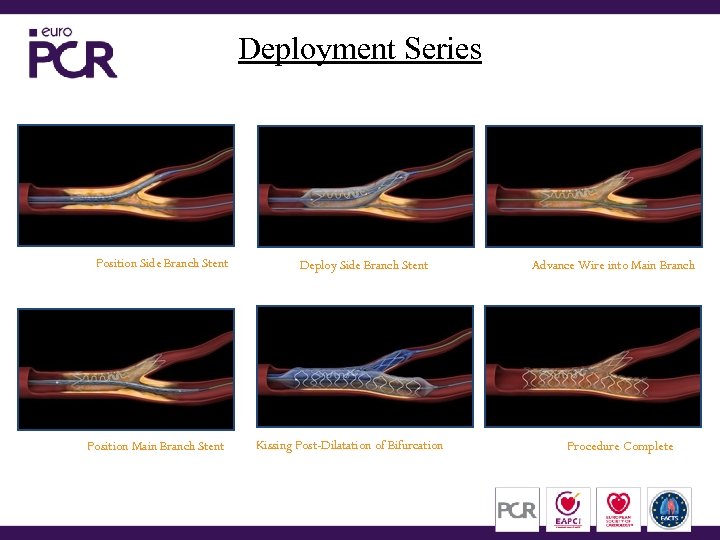 Deployment Series Position Side Branch Stent Position Main Branch Stent Deploy Side Branch Stent Kissing Post-Dilatation of Bifurcation Advance Wire into Main Branch Procedure Complete
Deployment Series Position Side Branch Stent Position Main Branch Stent Deploy Side Branch Stent Kissing Post-Dilatation of Bifurcation Advance Wire into Main Branch Procedure Complete
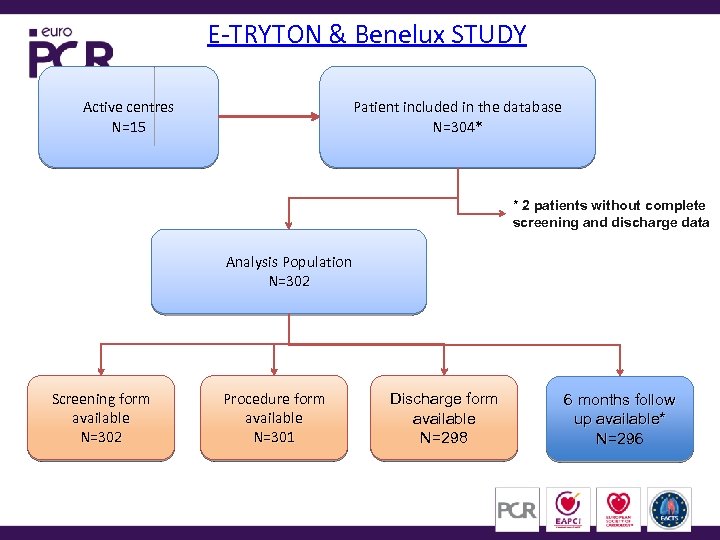 E-TRYTON & Benelux STUDY Active centres N=15 Patient included in the database N=304* * 2 patients without complete screening and discharge data Analysis Population N=302 Screening form available N=302 Procedure form available N=301 Discharge form available N=298 6 months follow up available* N=296
E-TRYTON & Benelux STUDY Active centres N=15 Patient included in the database N=304* * 2 patients without complete screening and discharge data Analysis Population N=302 Screening form available N=302 Procedure form available N=301 Discharge form available N=298 6 months follow up available* N=296
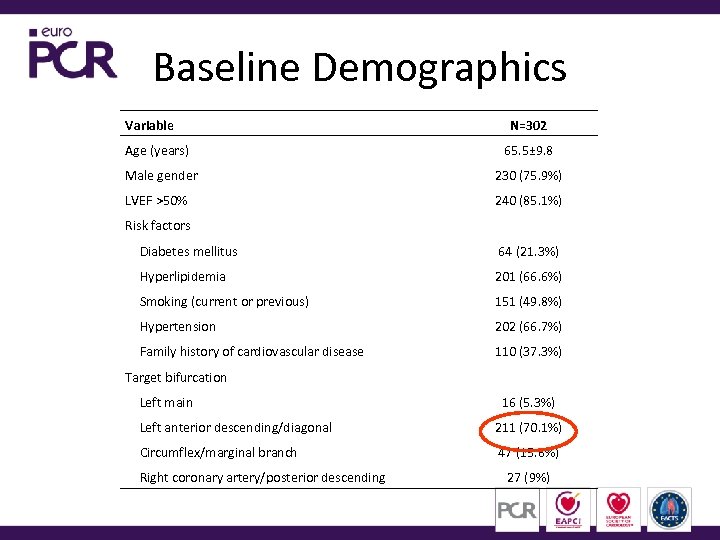 Baseline Demographics Variable Age (years) N=302 65. 5± 9. 8 Male gender 230 (75. 9%) LVEF >50% 240 (85. 1%) Risk factors Diabetes mellitus 64 (21. 3%) Hyperlipidemia 201 (66. 6%) Smoking (current or previous) 151 (49. 8%) Hypertension 202 (66. 7%) Family history of cardiovascular disease 110 (37. 3%) Target bifurcation Left main 16 (5. 3%) Left anterior descending/diagonal 211 (70. 1%) Circumflex/marginal branch 47 (15. 6%) Right coronary artery/posterior descending 27 (9%)
Baseline Demographics Variable Age (years) N=302 65. 5± 9. 8 Male gender 230 (75. 9%) LVEF >50% 240 (85. 1%) Risk factors Diabetes mellitus 64 (21. 3%) Hyperlipidemia 201 (66. 6%) Smoking (current or previous) 151 (49. 8%) Hypertension 202 (66. 7%) Family history of cardiovascular disease 110 (37. 3%) Target bifurcation Left main 16 (5. 3%) Left anterior descending/diagonal 211 (70. 1%) Circumflex/marginal branch 47 (15. 6%) Right coronary artery/posterior descending 27 (9%)
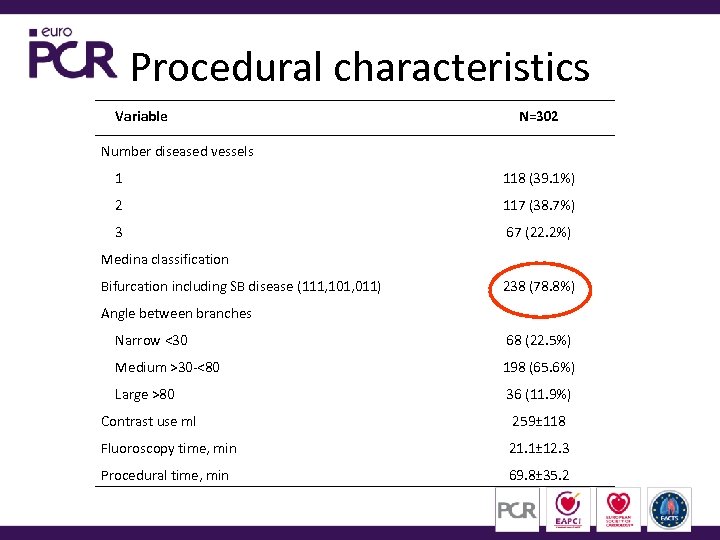 Procedural characteristics Variable N=302 Number diseased vessels 1 118 (39. 1%) 2 117 (38. 7%) 3 67 (22. 2%) Medina classification Bifurcation including SB disease (111, 101, 011) 238 (78. 8%) Angle between branches Narrow <30 68 (22. 5%) Medium >30 -<80 198 (65. 6%) Large >80 36 (11. 9%) Contrast use ml 259± 118 Fluoroscopy time, min 21. 1± 12. 3 Procedural time, min 69. 8± 35. 2
Procedural characteristics Variable N=302 Number diseased vessels 1 118 (39. 1%) 2 117 (38. 7%) 3 67 (22. 2%) Medina classification Bifurcation including SB disease (111, 101, 011) 238 (78. 8%) Angle between branches Narrow <30 68 (22. 5%) Medium >30 -<80 198 (65. 6%) Large >80 36 (11. 9%) Contrast use ml 259± 118 Fluoroscopy time, min 21. 1± 12. 3 Procedural time, min 69. 8± 35. 2
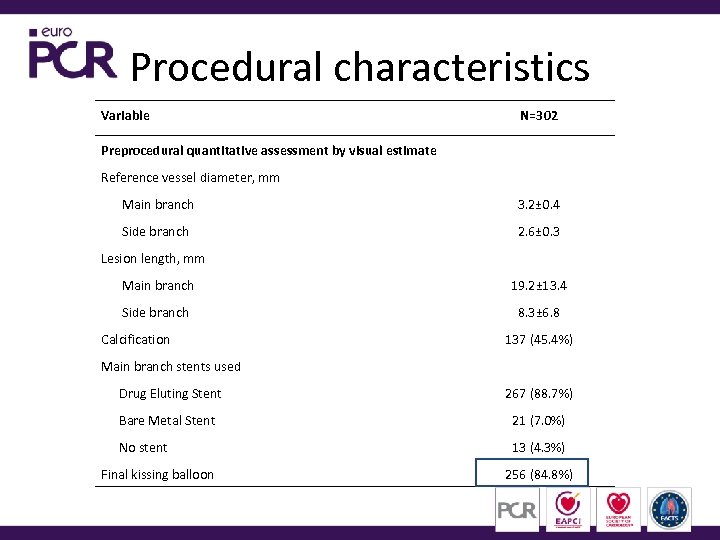 Procedural characteristics Variable N=302 Preprocedural quantitative assessment by visual estimate Reference vessel diameter, mm Main branch 3. 2± 0. 4 Side branch 2. 6± 0. 3 Lesion length, mm Main branch 19. 2± 13. 4 Side branch 8. 3± 6. 8 Calcification 137 (45. 4%) Main branch stents used Drug Eluting Stent 267 (88. 7%) Bare Metal Stent 21 (7. 0%) No stent 13 (4. 3%) Final kissing balloon 256 (84. 8%)
Procedural characteristics Variable N=302 Preprocedural quantitative assessment by visual estimate Reference vessel diameter, mm Main branch 3. 2± 0. 4 Side branch 2. 6± 0. 3 Lesion length, mm Main branch 19. 2± 13. 4 Side branch 8. 3± 6. 8 Calcification 137 (45. 4%) Main branch stents used Drug Eluting Stent 267 (88. 7%) Bare Metal Stent 21 (7. 0%) No stent 13 (4. 3%) Final kissing balloon 256 (84. 8%)
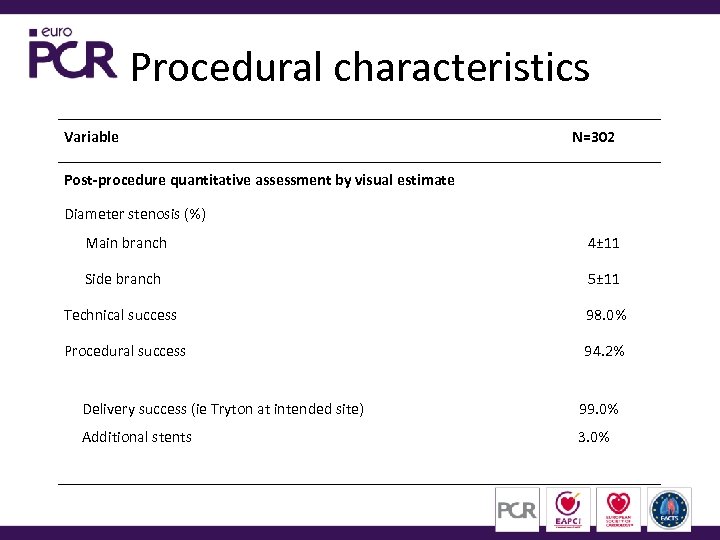 Procedural characteristics Variable N=302 Post-procedure quantitative assessment by visual estimate Diameter stenosis (%) Main branch 4± 11 Side branch 5± 11 Technical success 98. 0% Procedural success 94. 2% Delivery success (ie Tryton at intended site) 99. 0% Additional stents 3. 0%
Procedural characteristics Variable N=302 Post-procedure quantitative assessment by visual estimate Diameter stenosis (%) Main branch 4± 11 Side branch 5± 11 Technical success 98. 0% Procedural success 94. 2% Delivery success (ie Tryton at intended site) 99. 0% Additional stents 3. 0%
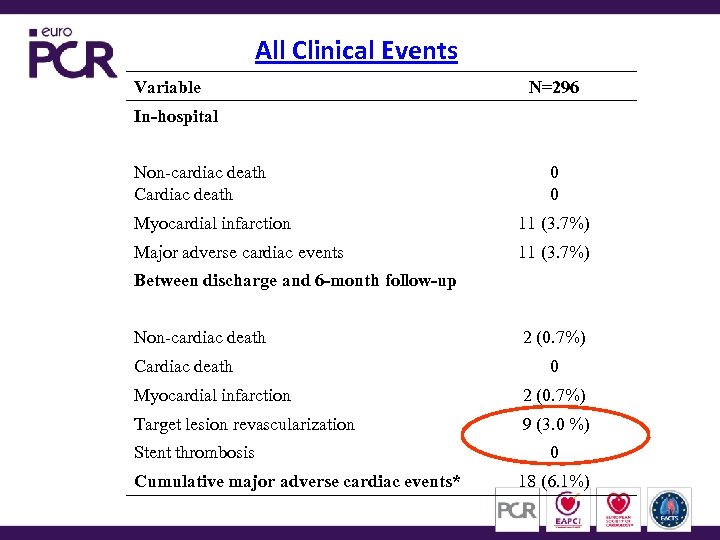 All Clinical Events Variable N=296 In-hospital Non-cardiac death Cardiac death 0 0 Myocardial infarction 11 (3. 7%) Major adverse cardiac events 11 (3. 7%) Between discharge and 6 -month follow-up Non-cardiac death Cardiac death 2 (0. 7%) 0 Myocardial infarction 2 (0. 7%) Target lesion revascularization 9 (3. 0 %) Stent thrombosis Cumulative major adverse cardiac events* 0 18 (6. 1%)
All Clinical Events Variable N=296 In-hospital Non-cardiac death Cardiac death 0 0 Myocardial infarction 11 (3. 7%) Major adverse cardiac events 11 (3. 7%) Between discharge and 6 -month follow-up Non-cardiac death Cardiac death 2 (0. 7%) 0 Myocardial infarction 2 (0. 7%) Target lesion revascularization 9 (3. 0 %) Stent thrombosis Cumulative major adverse cardiac events* 0 18 (6. 1%)
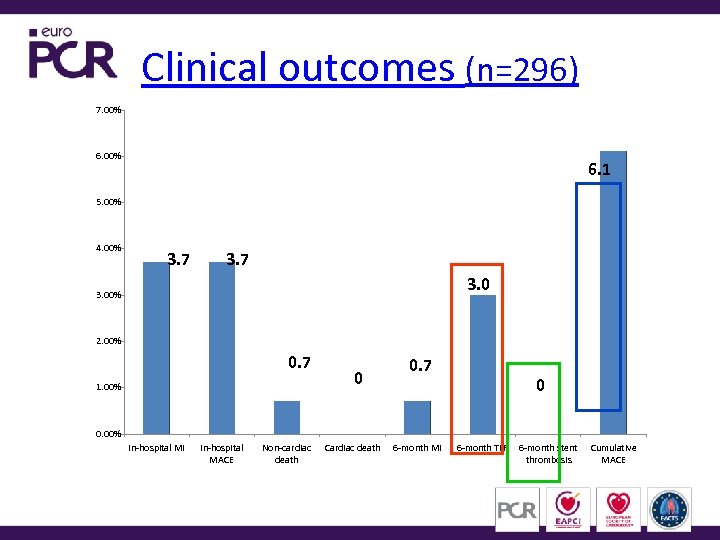 Clinical outcomes (n=296) 7. 00% 6. 1 5. 00% 4. 00% 3. 7 3. 00% 2. 00% 0. 7 1. 00% 0 0. 7 0 0. 00% In-hospital MI In-hospital MACE Non-cardiac death Cardiac death 6 -month MI 6 -month TLR 6 -month stent thrombosis Cumulative MACE
Clinical outcomes (n=296) 7. 00% 6. 1 5. 00% 4. 00% 3. 7 3. 00% 2. 00% 0. 7 1. 00% 0 0. 7 0 0. 00% In-hospital MI In-hospital MACE Non-cardiac death Cardiac death 6 -month MI 6 -month TLR 6 -month stent thrombosis Cumulative MACE
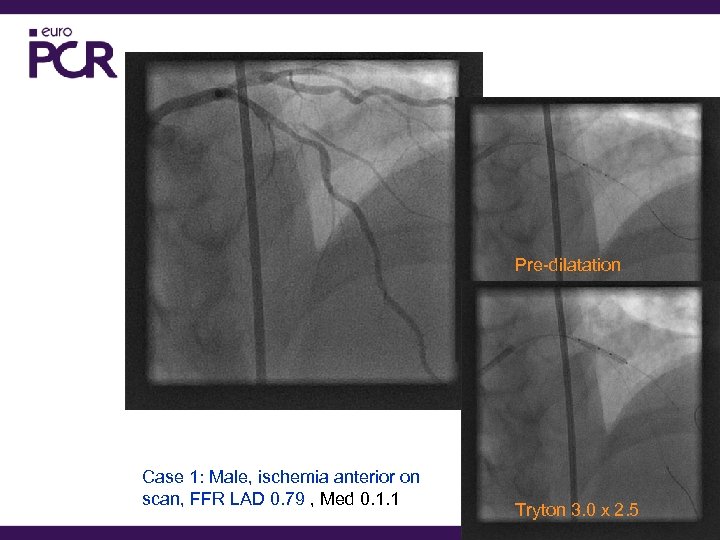 Pre-dilatation Case 1: Male, ischemia anterior on scan, FFR LAD 0. 79 , Med 0. 1. 1 Tryton 3. 0 x 2. 5
Pre-dilatation Case 1: Male, ischemia anterior on scan, FFR LAD 0. 79 , Med 0. 1. 1 Tryton 3. 0 x 2. 5
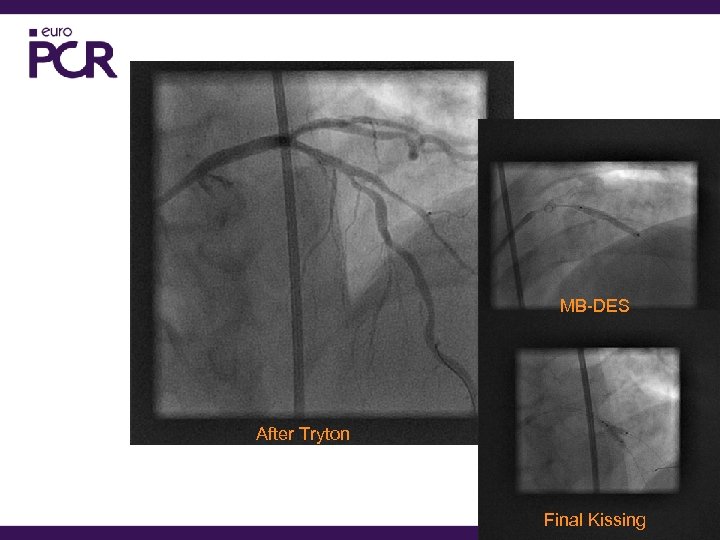 MB-DES After Tryton Final Kissing
MB-DES After Tryton Final Kissing
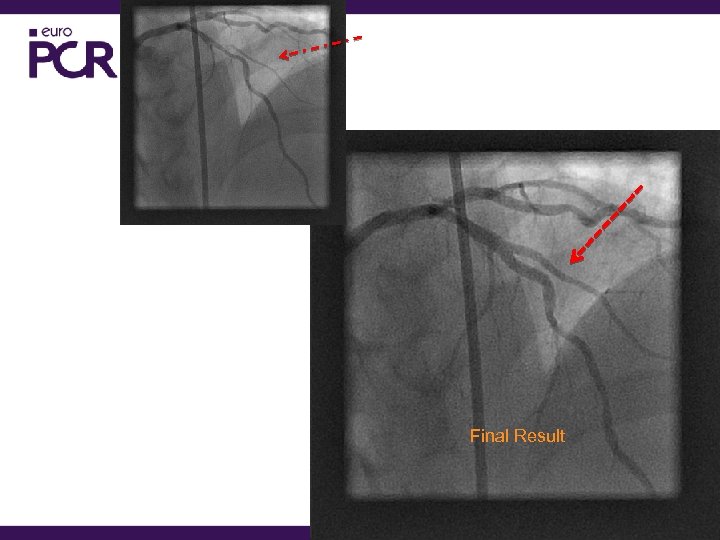 Final Result
Final Result
 Conclusion In this –large- prospective real world registry, bifurcation stenting with the Tryton dedicated SB stent is safe and feasible, with high technical and procedural success rates. Moreover, mid-term outcomes are very promising with very low TLR and MACE rates without the occurrence of stent thrombosis at 6 -month follow-up.
Conclusion In this –large- prospective real world registry, bifurcation stenting with the Tryton dedicated SB stent is safe and feasible, with high technical and procedural success rates. Moreover, mid-term outcomes are very promising with very low TLR and MACE rates without the occurrence of stent thrombosis at 6 -month follow-up.
 Heart Lung Centre Utrecht - UMCU September 21 TCT 2010 23
Heart Lung Centre Utrecht - UMCU September 21 TCT 2010 23


