a314b44014e04198f1485999311ff230.ppt
- Количество слайдов: 21

A Phase 3 International, Randomized, Double-Blind, Placebo-Controlled Trial Evaluating the Efficacy and Safety Of Orbofiban in Patients with Unstable Coronary Syndromes Cannon CP et al. Circulation 2000; 102: 149 -156 (and www. circ. ahajournals. org r 23 -r 35).

Need for Long-Term Antiplatelet Therapy l Markers of platelet activation persist 1 month post ACS - Ault K, et al. P selectin in TIMI 12 trial. JACC 1999; 33: 634 -639. l Angioscopy: Thrombus persists 1 month post ACS - Van Belle, et al. Circulation 1998; 97: 26 -33 l Events persist beyond acute period: In the TIMI 3 Registry, Death/MI/Rec Ischemia - In-hospital = 10. 5% - One year = 28. 3% l Benefit of IIb/IIIa inhibition achieved only during IV infusion period (PURSUIT, PRISM-PLUS)
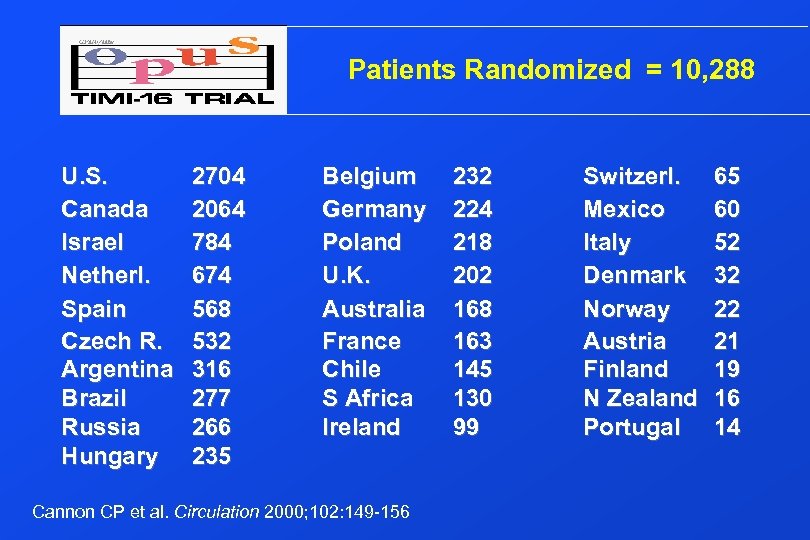
Patients Randomized = 10, 288 U. S. Canada Israel Netherl. Spain Czech R. Argentina Brazil Russia Hungary 2704 2064 784 674 568 532 316 277 266 235 Belgium Germany Poland U. K. Australia France Chile S Africa Ireland Cannon CP et al. Circulation 2000; 102: 149 -156 232 224 218 202 168 163 145 130 99 Switzerl. Mexico Italy Denmark Norway Austria Finland N Zealand Portugal 65 60 52 32 22 21 19 16 14

Central Units TIMI Study Chairman’s Office Eugene Braunwald, MD Christopher Cannon, MD Carolyn Mc. Cabe, BS Nottingham Clinical Trial Data Centre Allan Skene, Ph. D Robert Wilcox, MD Andrew Foxley Andrew Charlesworth Sponsor - G. D. Searle: John Alexander, MD Robert Anders, Pharm. D Daniel Burns Cannon CP et al. Circulation 2000; 102: 149 -156
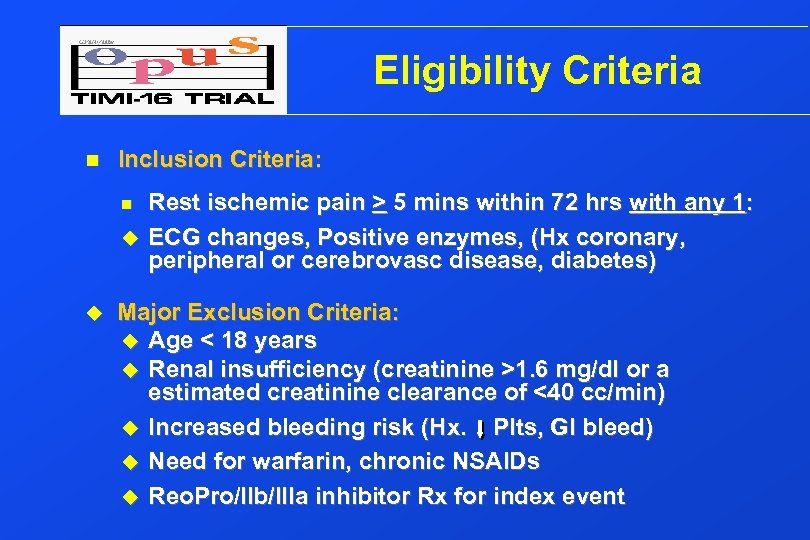
Eligibility Criteria n Inclusion Criteria: n u u Rest ischemic pain > 5 mins within 72 hrs with any 1: ECG changes, Positive enzymes, (Hx coronary, peripheral or cerebrovasc disease, diabetes) Major Exclusion Criteria: u Age < 18 years u Renal insufficiency (creatinine >1. 6 mg/dl or a estimated creatinine clearance of <40 cc/min) u Increased bleeding risk (Hx. Plts, GI bleed) u Need for warfarin, chronic NSAIDs u Reo. Pro/IIb/IIIa inhibitor Rx for index event
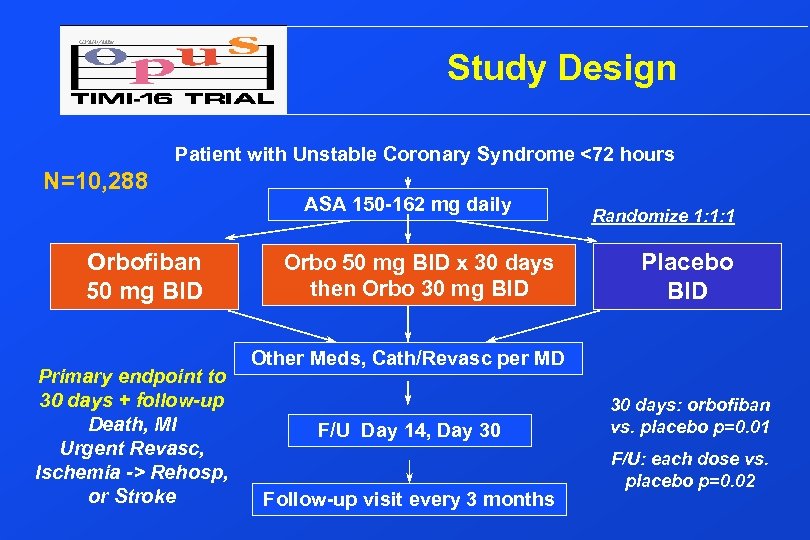
Study Design Patient with Unstable Coronary Syndrome <72 hours N=10, 288 Orbofiban 50 mg BID Primary endpoint to 30 days + follow-up Death, MI Urgent Revasc, Ischemia -> Rehosp, or Stroke ASA 150 -162 mg daily Orbo 50 mg BID x 30 days then Orbo 30 mg BID Randomize 1: 1: 1 Placebo BID Other Meds, Cath/Revasc per MD F/U Day 14, Day 30 Follow-up visit every 3 months 30 days: orbofiban vs. placebo p=0. 01 F/U: each dose vs. placebo p=0. 02
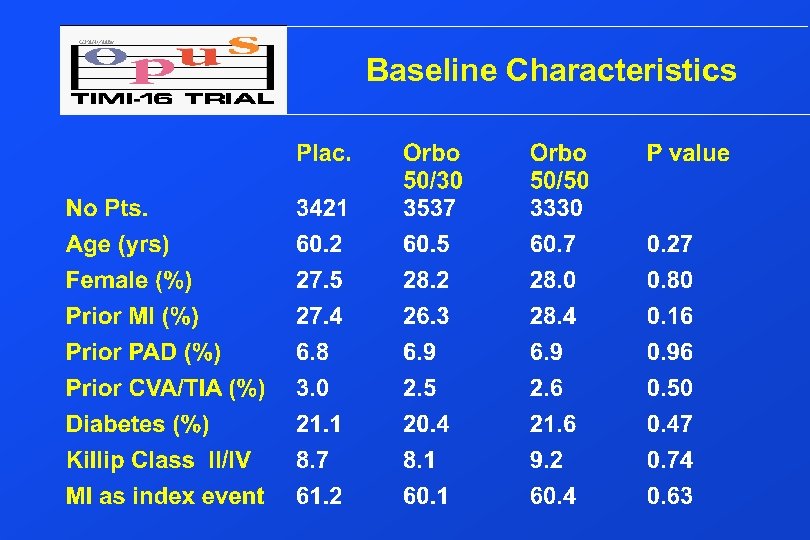
Baseline Characteristics
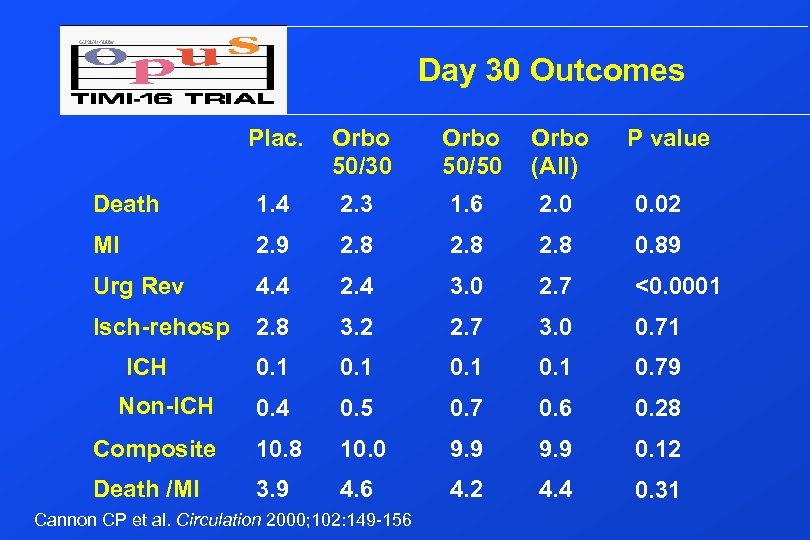
Day 30 Outcomes Plac. Orbo 50/30 Orbo 50/50 Orbo (All) Death 1. 4 2. 3 1. 6 2. 0 0. 02 MI 2. 9 2. 8 0. 89 Urg Rev 4. 4 2. 4 3. 0 2. 7 <0. 0001 Isch-rehosp 2. 8 3. 2 2. 7 3. 0 0. 71 0. 1 0. 79 0. 4 0. 5 0. 7 0. 6 0. 28 Composite 10. 8 10. 0 9. 9 0. 12 Death /MI 3. 9 4. 6 4. 2 4. 4 0. 31 ICH Non-ICH Cannon CP et al. Circulation 2000; 102: 149 -156 P value
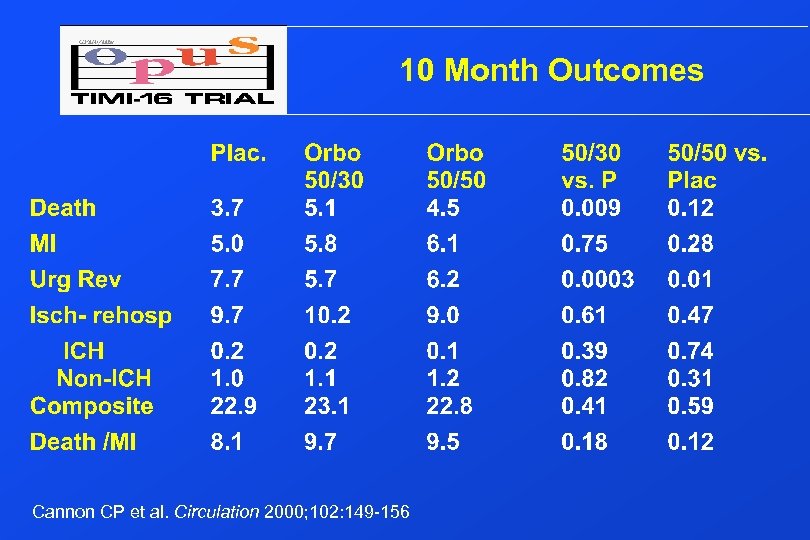
10 Month Outcomes Cannon CP et al. Circulation 2000; 102: 149 -156
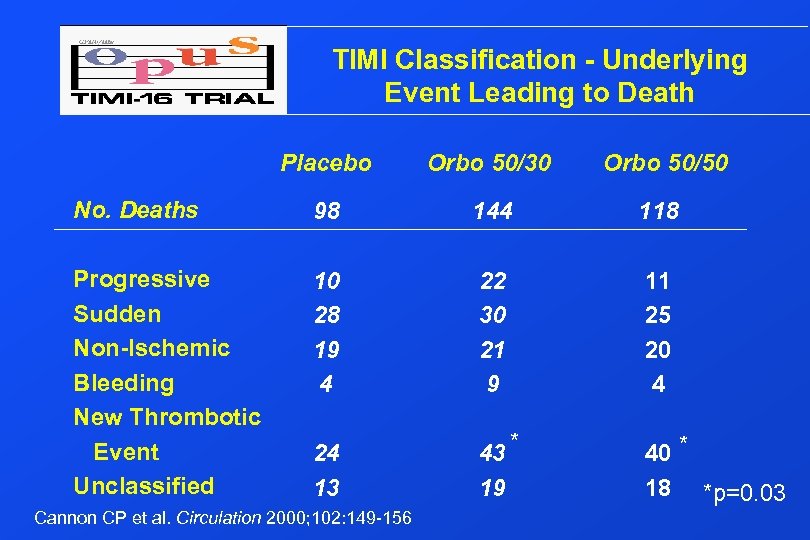
TIMI Classification - Underlying Event Leading to Death Placebo Orbo 50/30 Orbo 50/50 No. Deaths 98 144 118 Progressive Sudden Non-Ischemic Bleeding New Thrombotic Event Unclassified 10 28 19 4 22 30 21 9 11 25 20 4 24 13 43 * 19 40 * 18 *p=0. 03 Cannon CP et al. Circulation 2000; 102: 149 -156
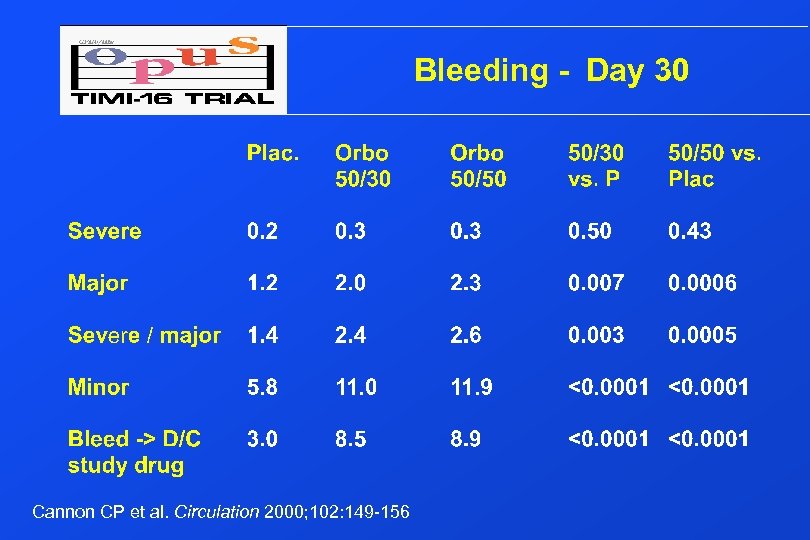
Bleeding - Day 30 Cannon CP et al. Circulation 2000; 102: 149 -156
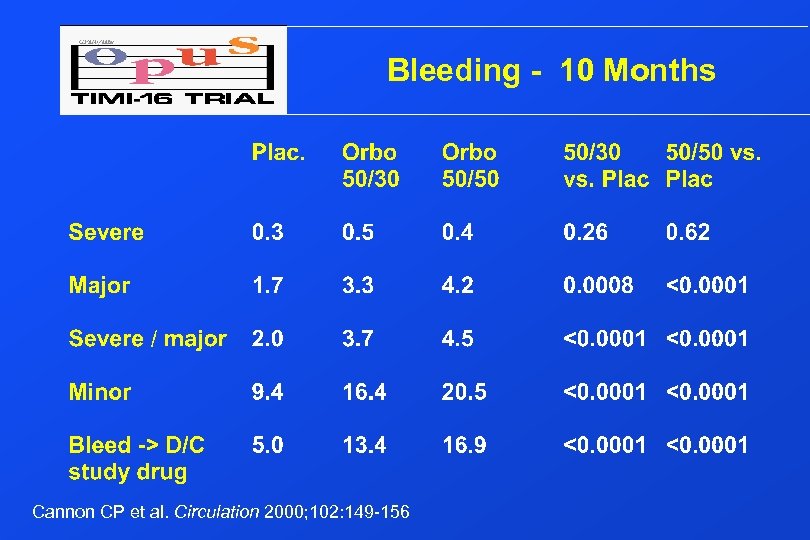
Bleeding - 10 Months Cannon CP et al. Circulation 2000; 102: 149 -156
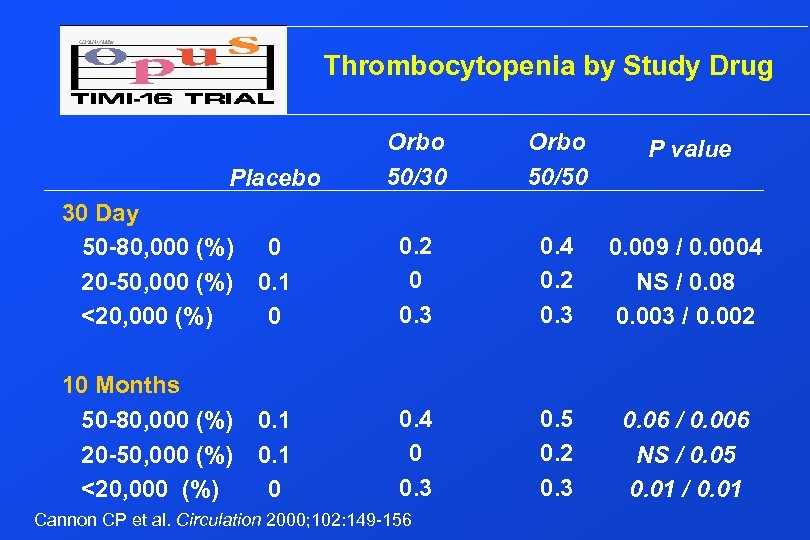
Thrombocytopenia by Study Drug Placebo Orbo 50/30 Orbo 50/50 P value 30 Day 50 -80, 000 (%) 20 -50, 000 (%) <20, 000 (%) 0 0. 1 0 0. 2 0 0. 3 0. 4 0. 2 0. 3 0. 009 / 0. 0004 NS / 0. 08 0. 003 / 0. 002 10 Months 50 -80, 000 (%) 20 -50, 000 (%) <20, 000 (%) 0. 1 0 0. 4 0 0. 3 0. 5 0. 2 0. 3 0. 06 / 0. 006 NS / 0. 05 0. 01 / 0. 01 Cannon CP et al. Circulation 2000; 102: 149 -156
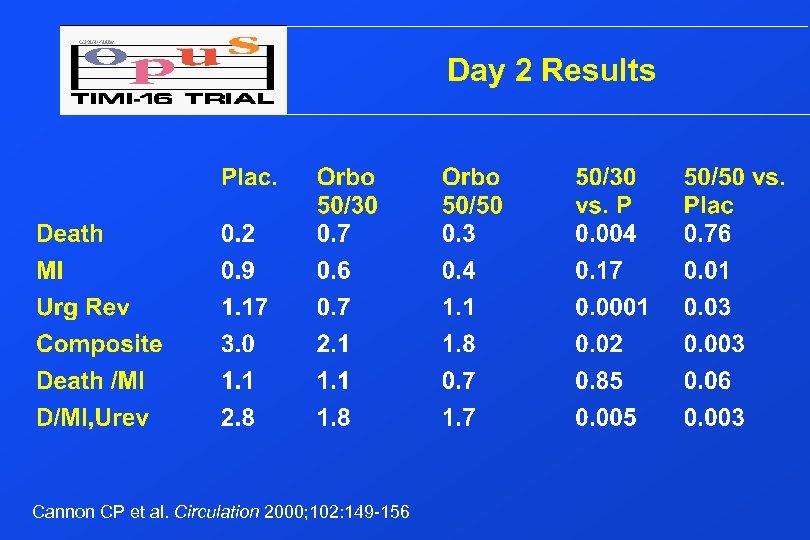
Day 2 Results Cannon CP et al. Circulation 2000; 102: 149 -156
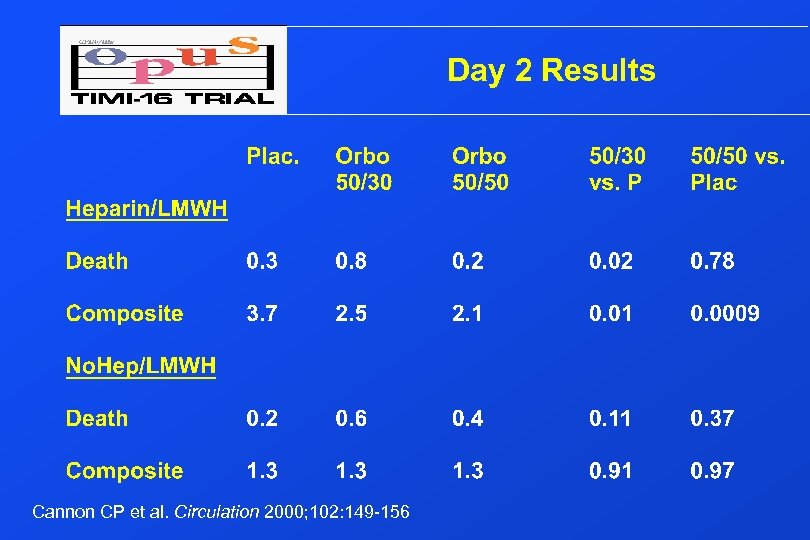
Day 2 Results Cannon CP et al. Circulation 2000; 102: 149 -156
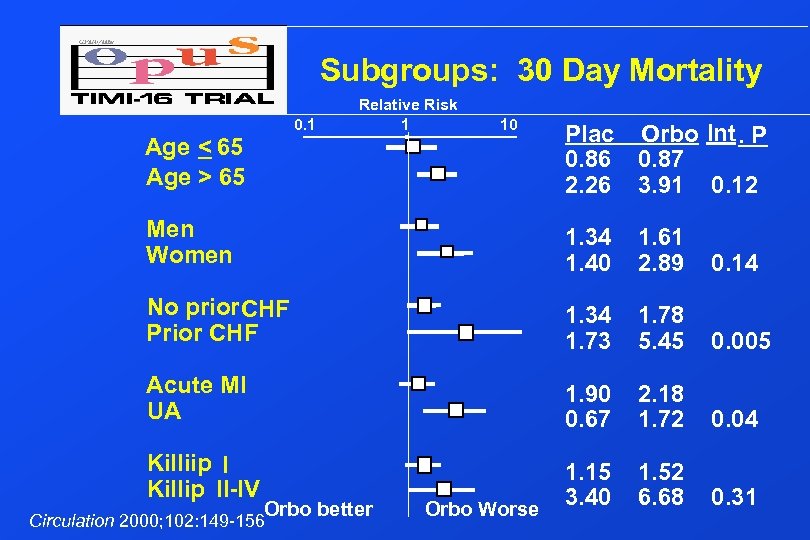
Subgroups: 30 Day Mortality 0. 1 Relative Risk 1 10 Age < 65 Age > 65 Plac Orbo Int. P 0. 86 0. 87 2. 26 3. 91 0. 12 Men Women 1. 34 1. 40 1. 61 2. 89 0. 14 No prior CHF Prior CHF 1. 34 1. 73 1. 78 5. 45 0. 005 Acute MI UA 1. 90 0. 67 2. 18 1. 72 0. 04 Killiip I Killip II-IV 1. 15 3. 40 1. 52 6. 68 0. 31 Orbo better Circulation 2000; 102: 149 -156 Orbo Worse
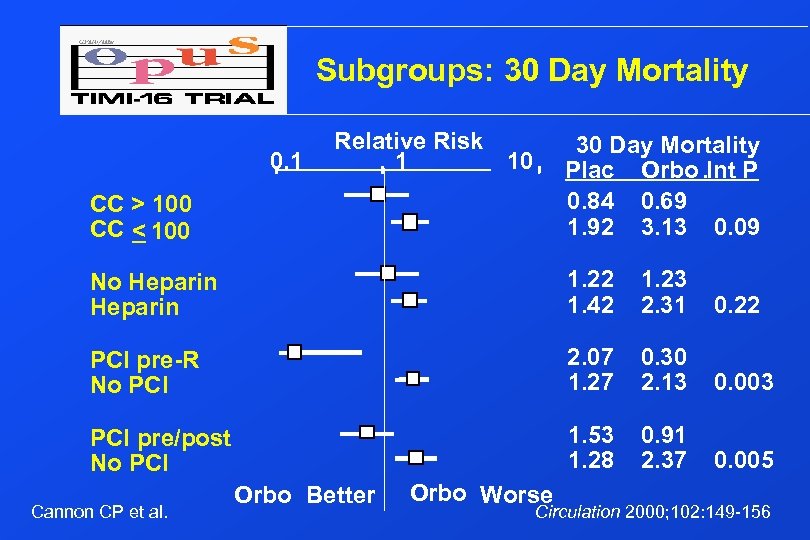
Subgroups: 30 Day Mortality Relative Risk 10 1 CC > 100 CC < 100 30 Day Mortality Plac Orbo. Int P 0. 84 0. 69 1. 92 3. 13 0. 09 No Heparin 1. 22 1. 42 1. 23 2. 31 0. 22 PCI pre-R No PCI 2. 07 1. 27 0. 30 2. 13 0. 003 PCI pre/post No PCI 1. 53 1. 28 0. 91 2. 37 0. 005 0. 1 Cannon CP et al. Orbo Better Orbo Worse Circulation 2000; 102: 149 -156
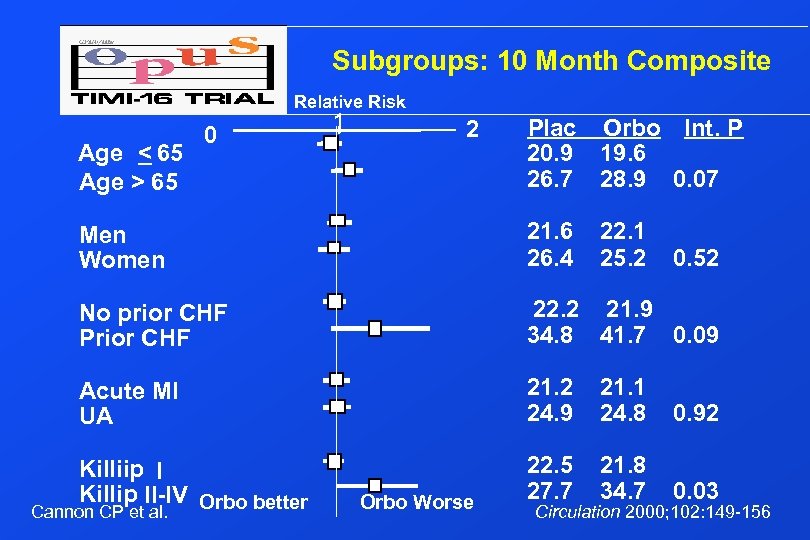
Subgroups: 10 Month Composite Age < 65 Age > 65 0 Relative Risk 1 2 Plac Orbo Int. P 20. 9 19. 6 26. 7 28. 9 0. 07 Men Women 21. 6 26. 4 No prior CHF Prior CHF 22. 2 21. 9 34. 8 41. 7 0. 09 Acute MI UA 21. 2 24. 9 21. 1 24. 8 0. 92 22. 5 27. 7 21. 8 34. 7 0. 03 Killiip I Killip II-IV Orbo better Cannon CP et al. Orbo Worse 22. 1 25. 2 0. 52 Circulation 2000; 102: 149 -156
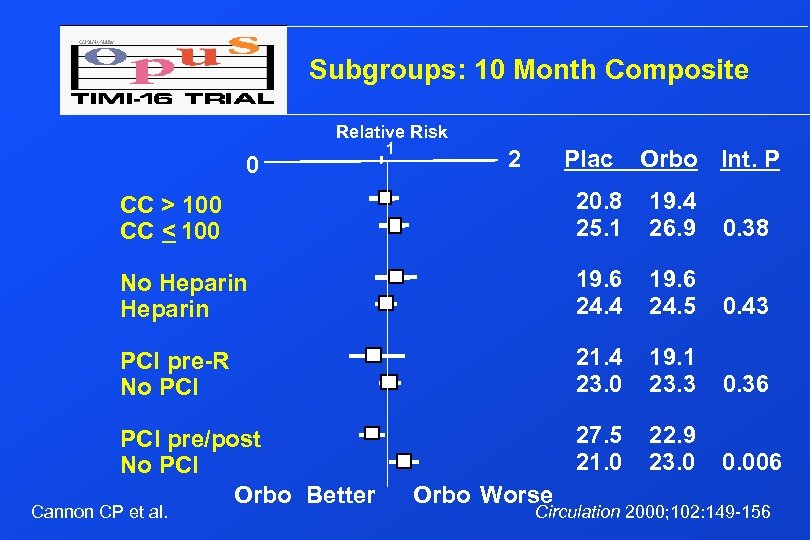
Subgroups: 10 Month Composite Relative Risk 0 1 2 Plac Orbo Int. P CC > 100 CC < 100 20. 8 25. 1 19. 4 26. 9 0. 38 No Heparin 19. 6 24. 4 19. 6 24. 5 0. 43 PCI pre-R No PCI 21. 4 23. 0 19. 1 23. 3 0. 36 PCI pre/post No PCI Orbo Better 27. 5 21. 0 22. 9 23. 0 0. 006 Cannon CP et al. Orbo Worse Circulation 2000; 102: 149 -156
Summary 1. Orbofiban: 1. 2. Greater benefit in PCI 3. 2. Minimal efficacy benefit overall in ACS Substudies: P-selectin, FGN binding mortality, 1. 2. thrombotic events 3. 3. Small absolute % -> ? Prothrombotic, ? Unstable patients Major bleeding, thrombocytopenia higher, but in acceptable range Cannon CP et al. Circulation 2000; 102: 149 -156

Potential Explanations Future Directions l PK/PD variability, peak trough èLonger T 1/2, ? Adjust with platelet monitoring l Only modest benefit in non-PCI ACS patients l Recurrent events not platelet -mediated? - unlikely l Concomitant Rx beyond ASA? è? è? l Clopidogrel to Platelet Activation, Antithrombin to inhibit clotting cascade ? Prothrombotic effects: èNeed Cannon CP et al. drugs with tight binding Circulation 2000; 102: 149 -156
a314b44014e04198f1485999311ff230.ppt