6241d2dadc8ffe9ac580fdfb32be5e28.ppt
- Количество слайдов: 1
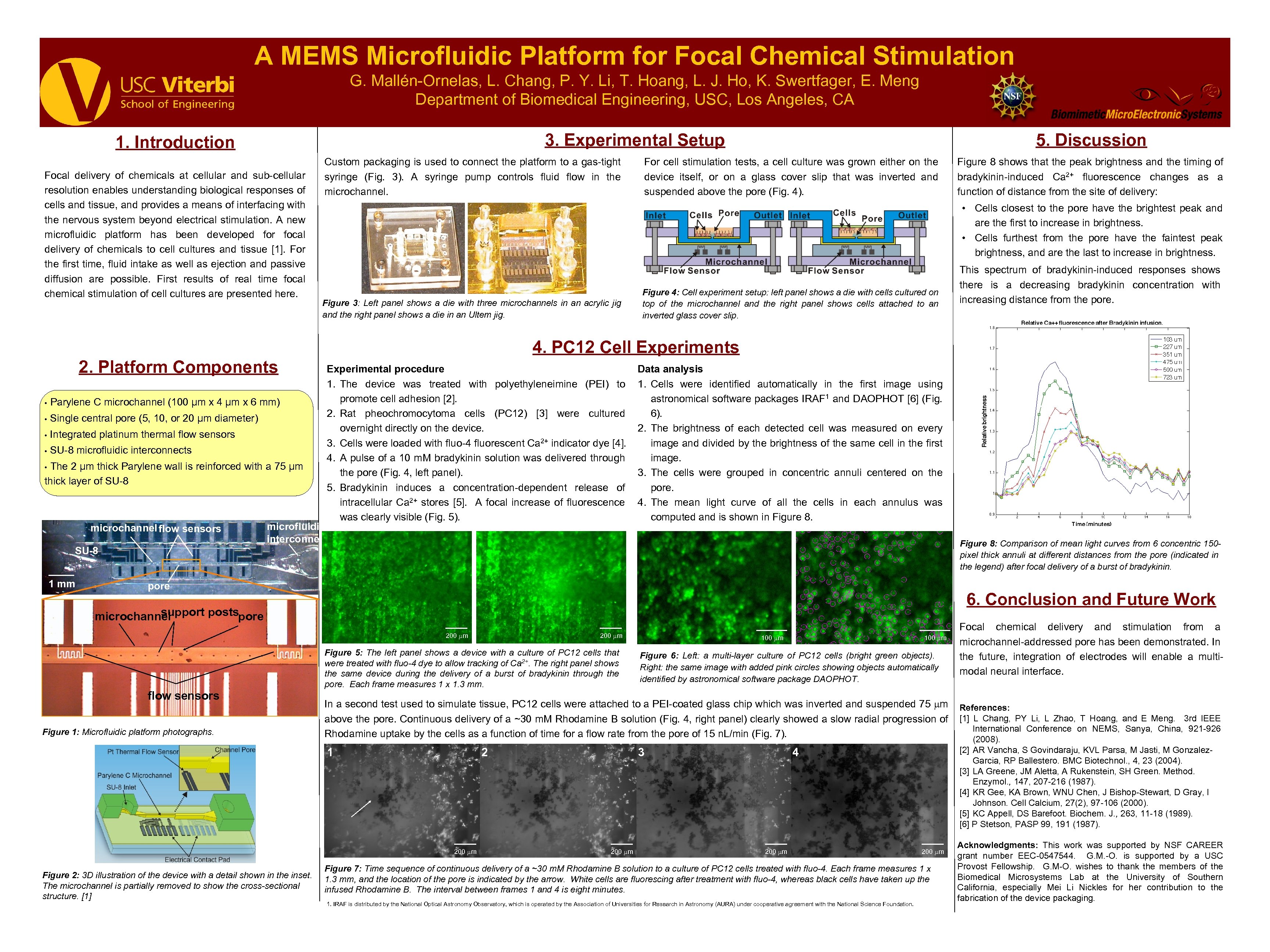 A MEMS Microfluidic Platform for Focal Chemical Stimulation G. Mallén-Ornelas, L. Chang, P. Y. Li, T. Hoang, L. J. Ho, K. Swertfager, E. Meng Department of Biomedical Engineering, USC, Los Angeles, CA 3. Experimental Setup 1. Introduction Focal delivery of chemicals at cellular and sub-cellular resolution enables understanding biological responses of cells and tissue, and provides a means of interfacing with the nervous system beyond electrical stimulation. A new microfluidic platform has been developed for focal delivery of chemicals to cell cultures and tissue [1]. For the first time, fluid intake as well as ejection and passive diffusion are possible. First results of real time focal chemical stimulation of cell cultures are presented here. Custom packaging is used to connect the platform to a gas-tight syringe (Fig. 3). A syringe pump controls fluid flow in the microchannel. 5. Discussion For cell stimulation tests, a cell culture was grown either on the device itself, or on a glass cover slip that was inverted and suspended above the pore (Fig. 4). Figure 8 shows that the peak brightness and the timing of bradykinin-induced Ca 2+ fluorescence changes as a function of distance from the site of delivery: • Cells closest to the pore have the brightest peak and are the first to increase in brightness. • Cells furthest from the pore have the faintest peak brightness, and are the last to increase in brightness. Figure 3: Left panel shows a die with three microchannels in an acrylic jig and the right panel shows a die in an Ultem jig. Figure 4: Cell experiment setup: left panel shows a die with cells cultured on top of the microchannel and the right panel shows cells attached to an inverted glass cover slip. This spectrum of bradykinin-induced responses shows there is a decreasing bradykinin concentration with increasing distance from the pore. 4. PC 12 Cell Experiments 2. Platform Components • Parylene C microchannel (100 μm x 4 μm x 6 mm) • Single central pore (5, 10, or 20 μm diameter) • Integrated platinum thermal flow sensors • SU-8 microfluidic interconnects The 2 μm thick Parylene wall is reinforced with a 75 μm thick layer of SU-8 • microchannel flow sensors Experimental procedure 1. The device was treated with polyethyleneimine (PEI) to promote cell adhesion [2]. 2. Rat pheochromocytoma cells (PC 12) [3] were cultured overnight directly on the device. 3. Cells were loaded with fluo-4 fluorescent Ca 2+ indicator dye [4]. 4. A pulse of a 10 m. M bradykinin solution was delivered through the pore (Fig. 4, left panel). 5. Bradykinin induces a concentration-dependent release of intracellular Ca 2+ stores [5]. A focal increase of fluorescence was clearly visible (Fig. 5). microfluidic interconnect Data analysis 1. Cells were identified automatically in the first image using astronomical software packages IRAF 1 and DAOPHOT [6] (Fig. 6). 2. The brightness of each detected cell was measured on every image and divided by the brightness of the same cell in the first image. 3. The cells were grouped in concentric annuli centered on the pore. 4. The mean light curve of all the cells in each annulus was computed and is shown in Figure 8: Comparison of mean light curves from 6 concentric 150 pixel thick annuli at different distances from the pore (indicated in the legend) after focal delivery of a burst of bradykinin. SU-8 1 mm pore 6. Conclusion and Future Work support postspore microchannel 200 m flow sensors Figure 1: Microfluidic platform photographs. 200 m Figure 5: The left panel shows a device with a culture of PC 12 cells that were treated with fluo-4 dye to allow tracking of Ca 2+. The right panel shows the same device during the delivery of a burst of bradykinin through the pore. Each frame measures 1 x 1. 3 mm. 100 m Figure 6: Left: a multi-layer culture of PC 12 cells (bright green objects). Right: the same image with added pink circles showing objects automatically identified by astronomical software package DAOPHOT. In a second test used to simulate tissue, PC 12 cells were attached to a PEI-coated glass chip which was inverted and suspended 75 m above the pore. Continuous delivery of a ~30 m. M Rhodamine B solution (Fig. 4, right panel) clearly showed a slow radial progression of Rhodamine uptake by the cells as a function of time for a flow rate from the pore of 15 n. L/min (Fig. 7). microchannel 1 2 SU-8 3 4 1 mm 200 m Figure 2: 3 D illustration of the device with a detail shown in the inset. The microchannel is partially removed to show the cross-sectional structure. [1] 200 m Figure 7: Time sequence of continuous delivery of a ~30 m. M Rhodamine B solution to a culture of PC 12 cells treated with fluo-4. Each frame measures 1 x 1. 3 mm, and the location of the pore is indicated by the arrow. White cells are fluorescing after treatment with fluo-4, whereas black cells have taken up the infused Rhodamine B. The interval between frames 1 and 4 is eight minutes. 1. IRAF is distributed by the National Optical Astronomy Observatory, which is operated by the Association of Universities for Research in Astronomy (AURA) under cooperative agreement with the National Science Foundation. Focal chemical delivery and stimulation from a microchannel-addressed pore has been demonstrated. In the future, integration of electrodes will enable a multimodal neural interface. References: [1] L Chang, PY Li, L Zhao, T Hoang, and E Meng. 3 rd IEEE International Conference on NEMS, Sanya, China, 921 -926 (2008). [2] AR Vancha, S Govindaraju, KVL Parsa, M Jasti, M Gonzalez. Garcia, RP Ballestero. BMC Biotechnol. , 4, 23 (2004). [3] LA Greene, JM Aletta, A Rukenstein, SH Green. Method. Enzymol. , 147, 207 -216 (1987). [4] KR Gee, KA Brown, WNU Chen, J Bishop-Stewart, D Gray, I Johnson. Cell Calcium, 27(2), 97 -106 (2000). [5] KC Appell, DS Barefoot. Biochem. J. , 263, 11 -18 (1989). [6] P Stetson, PASP 99, 191 (1987). Acknowledgments: This work was supported by NSF CAREER grant number EEC-0547544. G. M. -O. is supported by a USC Provost Fellowship. G. M-O. wishes to thank the members of the Biomedical Microsystems Lab at the University of Southern California, especially Mei Li Nickles for her contribution to the fabrication of the device packaging.
A MEMS Microfluidic Platform for Focal Chemical Stimulation G. Mallén-Ornelas, L. Chang, P. Y. Li, T. Hoang, L. J. Ho, K. Swertfager, E. Meng Department of Biomedical Engineering, USC, Los Angeles, CA 3. Experimental Setup 1. Introduction Focal delivery of chemicals at cellular and sub-cellular resolution enables understanding biological responses of cells and tissue, and provides a means of interfacing with the nervous system beyond electrical stimulation. A new microfluidic platform has been developed for focal delivery of chemicals to cell cultures and tissue [1]. For the first time, fluid intake as well as ejection and passive diffusion are possible. First results of real time focal chemical stimulation of cell cultures are presented here. Custom packaging is used to connect the platform to a gas-tight syringe (Fig. 3). A syringe pump controls fluid flow in the microchannel. 5. Discussion For cell stimulation tests, a cell culture was grown either on the device itself, or on a glass cover slip that was inverted and suspended above the pore (Fig. 4). Figure 8 shows that the peak brightness and the timing of bradykinin-induced Ca 2+ fluorescence changes as a function of distance from the site of delivery: • Cells closest to the pore have the brightest peak and are the first to increase in brightness. • Cells furthest from the pore have the faintest peak brightness, and are the last to increase in brightness. Figure 3: Left panel shows a die with three microchannels in an acrylic jig and the right panel shows a die in an Ultem jig. Figure 4: Cell experiment setup: left panel shows a die with cells cultured on top of the microchannel and the right panel shows cells attached to an inverted glass cover slip. This spectrum of bradykinin-induced responses shows there is a decreasing bradykinin concentration with increasing distance from the pore. 4. PC 12 Cell Experiments 2. Platform Components • Parylene C microchannel (100 μm x 4 μm x 6 mm) • Single central pore (5, 10, or 20 μm diameter) • Integrated platinum thermal flow sensors • SU-8 microfluidic interconnects The 2 μm thick Parylene wall is reinforced with a 75 μm thick layer of SU-8 • microchannel flow sensors Experimental procedure 1. The device was treated with polyethyleneimine (PEI) to promote cell adhesion [2]. 2. Rat pheochromocytoma cells (PC 12) [3] were cultured overnight directly on the device. 3. Cells were loaded with fluo-4 fluorescent Ca 2+ indicator dye [4]. 4. A pulse of a 10 m. M bradykinin solution was delivered through the pore (Fig. 4, left panel). 5. Bradykinin induces a concentration-dependent release of intracellular Ca 2+ stores [5]. A focal increase of fluorescence was clearly visible (Fig. 5). microfluidic interconnect Data analysis 1. Cells were identified automatically in the first image using astronomical software packages IRAF 1 and DAOPHOT [6] (Fig. 6). 2. The brightness of each detected cell was measured on every image and divided by the brightness of the same cell in the first image. 3. The cells were grouped in concentric annuli centered on the pore. 4. The mean light curve of all the cells in each annulus was computed and is shown in Figure 8: Comparison of mean light curves from 6 concentric 150 pixel thick annuli at different distances from the pore (indicated in the legend) after focal delivery of a burst of bradykinin. SU-8 1 mm pore 6. Conclusion and Future Work support postspore microchannel 200 m flow sensors Figure 1: Microfluidic platform photographs. 200 m Figure 5: The left panel shows a device with a culture of PC 12 cells that were treated with fluo-4 dye to allow tracking of Ca 2+. The right panel shows the same device during the delivery of a burst of bradykinin through the pore. Each frame measures 1 x 1. 3 mm. 100 m Figure 6: Left: a multi-layer culture of PC 12 cells (bright green objects). Right: the same image with added pink circles showing objects automatically identified by astronomical software package DAOPHOT. In a second test used to simulate tissue, PC 12 cells were attached to a PEI-coated glass chip which was inverted and suspended 75 m above the pore. Continuous delivery of a ~30 m. M Rhodamine B solution (Fig. 4, right panel) clearly showed a slow radial progression of Rhodamine uptake by the cells as a function of time for a flow rate from the pore of 15 n. L/min (Fig. 7). microchannel 1 2 SU-8 3 4 1 mm 200 m Figure 2: 3 D illustration of the device with a detail shown in the inset. The microchannel is partially removed to show the cross-sectional structure. [1] 200 m Figure 7: Time sequence of continuous delivery of a ~30 m. M Rhodamine B solution to a culture of PC 12 cells treated with fluo-4. Each frame measures 1 x 1. 3 mm, and the location of the pore is indicated by the arrow. White cells are fluorescing after treatment with fluo-4, whereas black cells have taken up the infused Rhodamine B. The interval between frames 1 and 4 is eight minutes. 1. IRAF is distributed by the National Optical Astronomy Observatory, which is operated by the Association of Universities for Research in Astronomy (AURA) under cooperative agreement with the National Science Foundation. Focal chemical delivery and stimulation from a microchannel-addressed pore has been demonstrated. In the future, integration of electrodes will enable a multimodal neural interface. References: [1] L Chang, PY Li, L Zhao, T Hoang, and E Meng. 3 rd IEEE International Conference on NEMS, Sanya, China, 921 -926 (2008). [2] AR Vancha, S Govindaraju, KVL Parsa, M Jasti, M Gonzalez. Garcia, RP Ballestero. BMC Biotechnol. , 4, 23 (2004). [3] LA Greene, JM Aletta, A Rukenstein, SH Green. Method. Enzymol. , 147, 207 -216 (1987). [4] KR Gee, KA Brown, WNU Chen, J Bishop-Stewart, D Gray, I Johnson. Cell Calcium, 27(2), 97 -106 (2000). [5] KC Appell, DS Barefoot. Biochem. J. , 263, 11 -18 (1989). [6] P Stetson, PASP 99, 191 (1987). Acknowledgments: This work was supported by NSF CAREER grant number EEC-0547544. G. M. -O. is supported by a USC Provost Fellowship. G. M-O. wishes to thank the members of the Biomedical Microsystems Lab at the University of Southern California, especially Mei Li Nickles for her contribution to the fabrication of the device packaging.


