3b1b9d38e42a476cf3b91e92f4a0abbe.ppt
- Количество слайдов: 79

A Global Perspective on Emerging Mosquito-Borne Diseases JV Irons / RR Parker Memorial Lecture Laura D. Kramer Wadsworth Center New York State Dept Health Ft. Worth, Texas June 3, 2009

• Defined the concept of EID • Identified factors contributing to disease emergence • Pointed to challenges posed by infectious diseases Emerging Infections: Microbial Threats to Health in the United States. Joshua Lederberg, Robert E. Shope, and Stanley C. Oaks, Jr. , Editors; Committee on Emerging Microbial Threats to Health, Institute of Medicine (1992)

“…the United States has no comprehensive national system for detecting outbreaks of infectious disease. Outbreaks of any disease that is not on CDC's current list of notifiable illnesses may go undetected or may be detected only after an outbreak is well under way. ” “Although many local and regional vectorcontrol programs can effectively combat small and even medium-size outbreaks of vector-borne disease, they are not equipped to deal with outbreaks that are national in scope. ” “The significance of zoonoses in the emergence of human infections cannot be overstated. ” Emerging Infections: Microbial Threats to Health in the United States. Joshua Lederberg, Robert E. Shope, and Stanley C. Oaks, Jr. , Editors; Committee on Emerging Microbial Threats to Health, Institute of Medicine (1992)
Outline • Drivers of emerging / re-emerging diseases • Re/emerging flavivirus – West Nile • Re/emerging alphavirus – Chikungunya Atlantic Monthly, 1997

Re/emerging infectious diseases • Define the concept. EIDs are infections • that have newly appeared in a population, or have existed but are rapidly increasing in incidence or geographic range (Morse 1995) Process – Introduction of agent – Establishment and dissemination
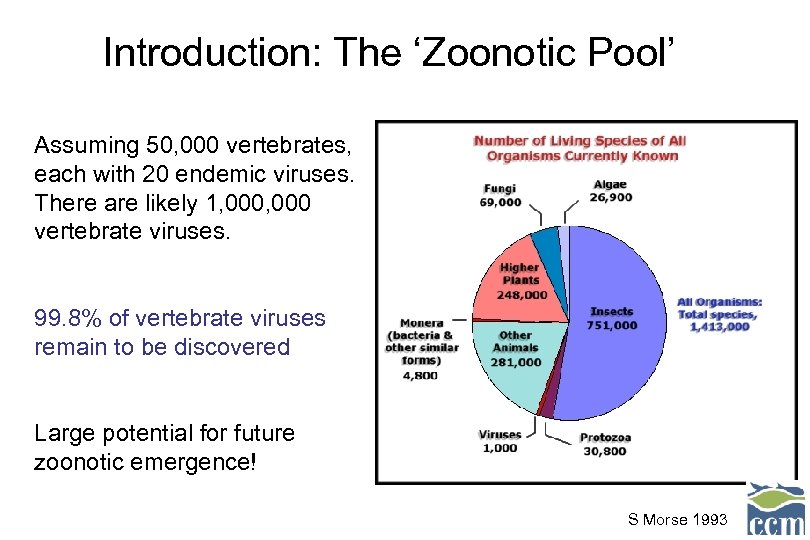
Introduction: The ‘Zoonotic Pool’ Assuming 50, 000 vertebrates, each with 20 endemic viruses. There are likely 1, 000 vertebrate viruses. 99. 8% of vertebrate viruses remain to be discovered Large potential for future zoonotic emergence! S Morse 1993

Question What leads to selection or emergence a new agent?
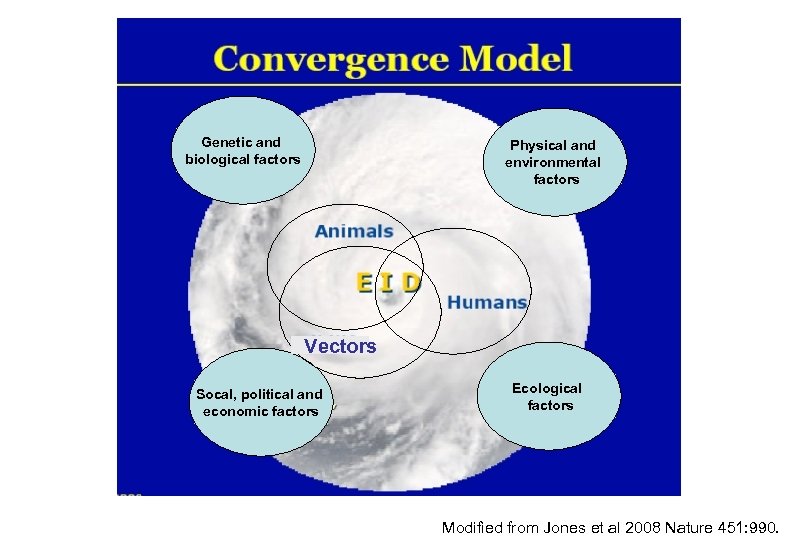
Genetic and biological factors Physical and environmental factors Vectors Socal, political and economic factors Ecological factors Modified from Jones et al 2008 Nature 451: 990.
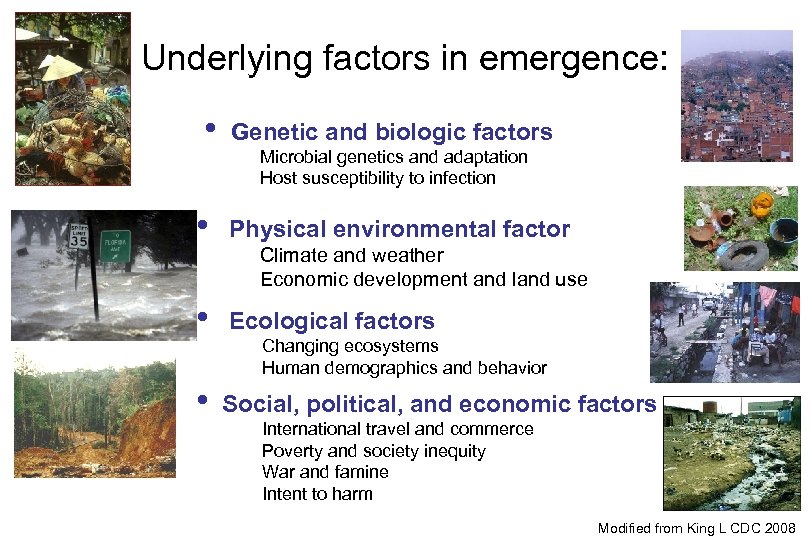
Underlying factors in emergence: • Genetic and biologic factors Microbial genetics and adaptation Host susceptibility to infection • Physical environmental factor • Ecological factors • Social, political, and economic factors Climate and weather Economic development and land use Changing ecosystems Human demographics and behavior International travel and commerce Poverty and society inequity War and famine Intent to harm Modified from King L CDC 2008
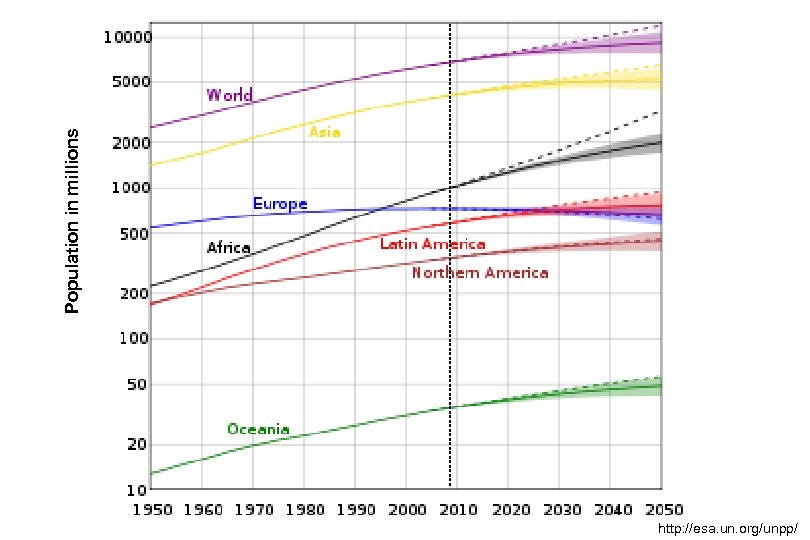
Population in millions http: //esa. un. org/unpp/
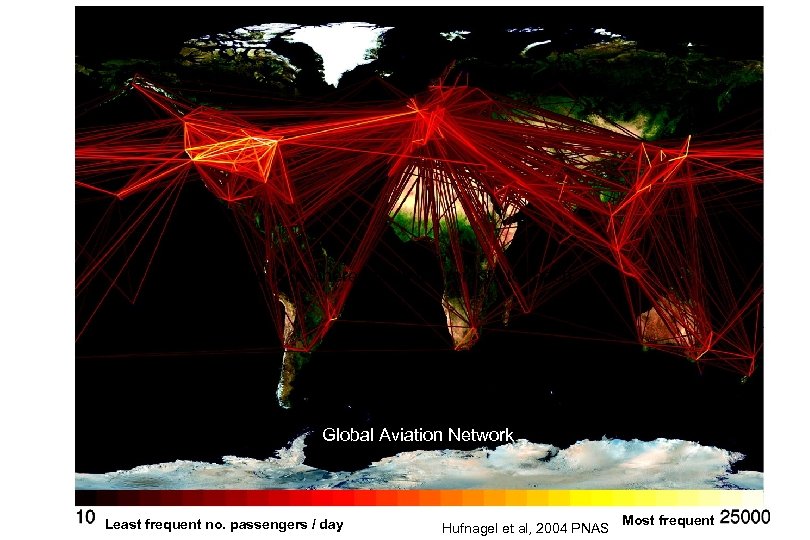
There is nowhere that is too remote to reach Global Aviation Network Least frequent no. passengers / day Hufnagel et al, 2004 PNAS Most frequent
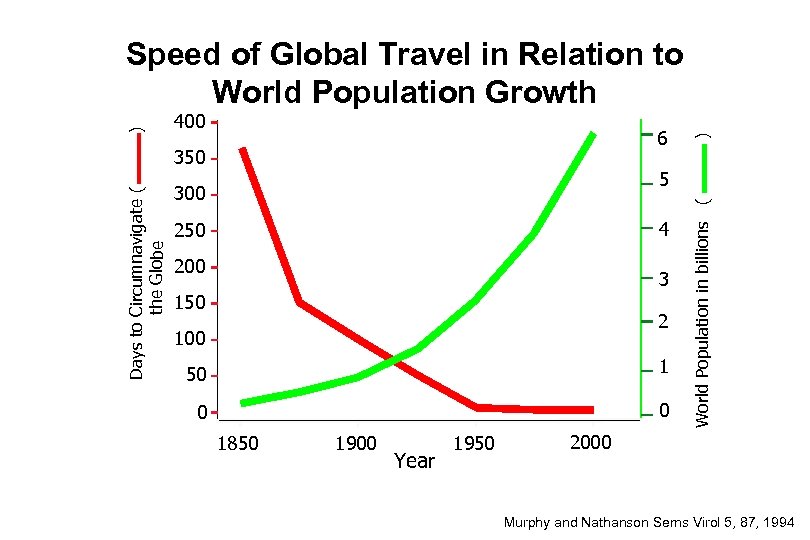
6 Days to Circumnavigate ( the Globe 350 ) 400 5 300 4 250 200 3 150 2 100 50 1 0 0 1850 1900 Year 1950 World Population in billions ( ) Speed of Global Travel in Relation to World Population Growth 2000 Murphy and Nathanson Sems Virol 5, 87, 1994
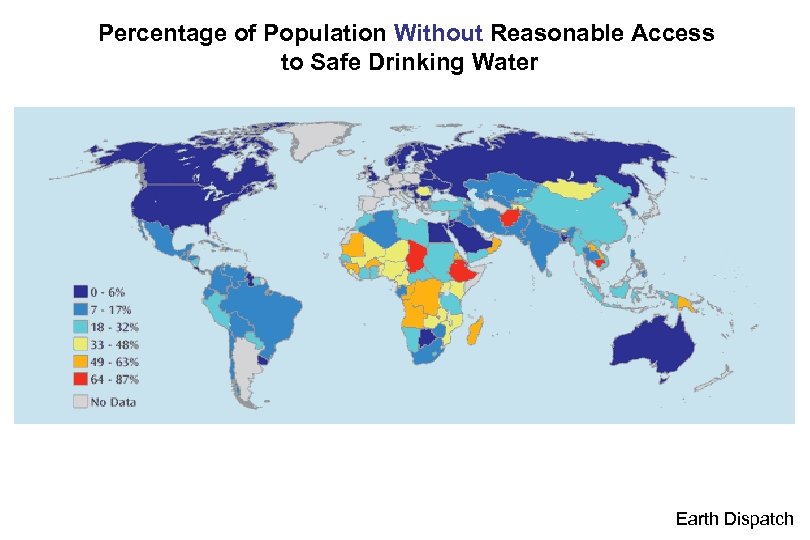
Percentage of Population Without Reasonable Access to Safe Drinking Water Earth Dispatch
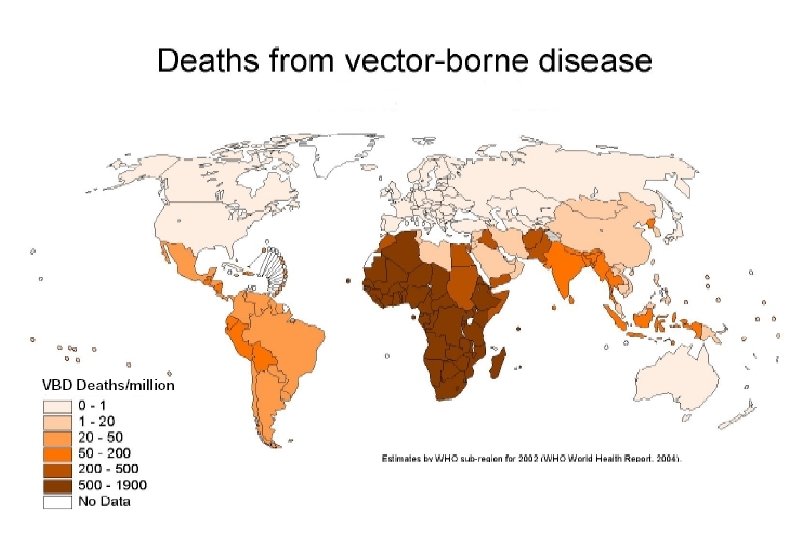

What is an Arbovirus? • • Arthropod-borne Group of viruses spread by arthropods Many are zoonotic Infection spread to incidental hosts that are not essential to the life cycle.
Outline • Drivers in emerging diseases • Re/emerging flaviviruses – West Nile – Dengue – Japanese encephalitis – Yellow fever – Kyassanur Forest • Re/emerging alphavirus – Chikungunya
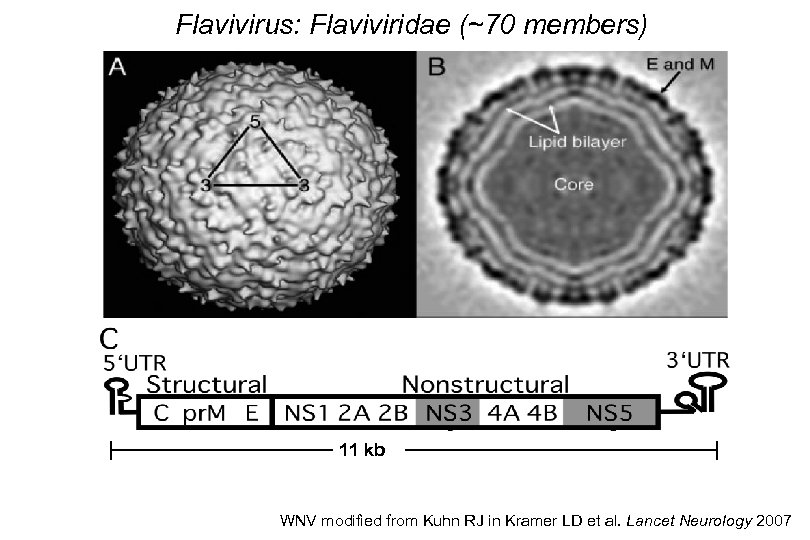
Flavivirus: Flaviviridae (~70 members) 11 kb WNV modified from Kuhn RJ in Kramer LD et al. Lancet Neurology 2007
Flavivirus: Flaviviridae (~70 members) – Human pathogens • Hemorrhagic fevers (flavi=yellow) • Encephalitis • Febrile illness – 3 phylogenetic clusters • No known vector • Tick-borne • Mosquito borne – Japanese encephalitis serocomplex » Includes JEV, SLEV, WNV » Primarily bird viruses » Humans not “amplifying” host – Other serocomplexes include YFV, DENV
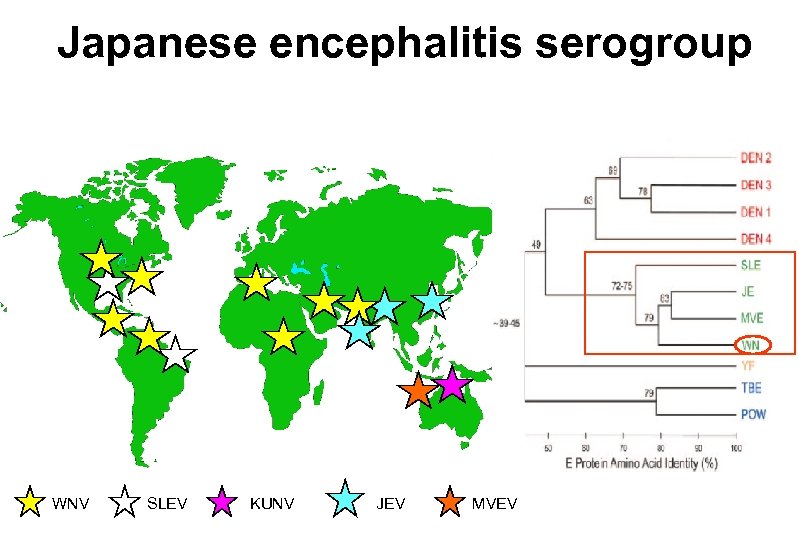
Japanese encephalitis serogroup WNV SLEV KUNV JEV MVEV
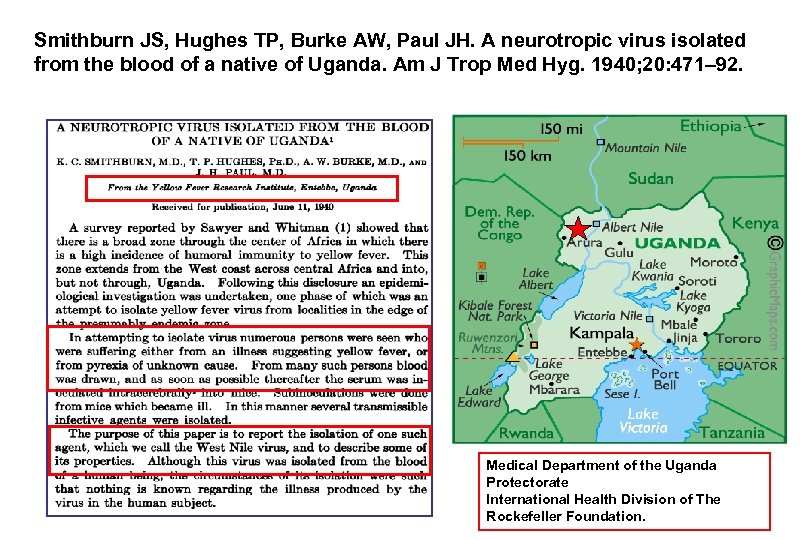
Smithburn JS, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940; 20: 471– 92. Medical Department of the Uganda Protectorate International Health Division of The Rockefeller Foundation.
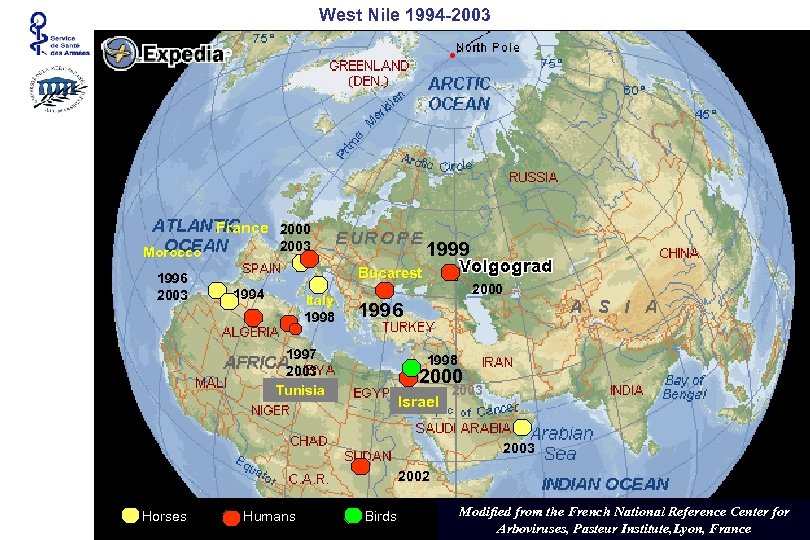
West Nile 1994 -2003 France 2000 1996 2003 1999 2003 Morocco Bucarest 1994 Italy 1998 2000 1996 1997 2003 Tunisia 1998 2000 Israel 2003 2002 Horses Humans Birds Modified from the French National Reference Center for Arboviruses, Pasteur Institute, Lyon, France
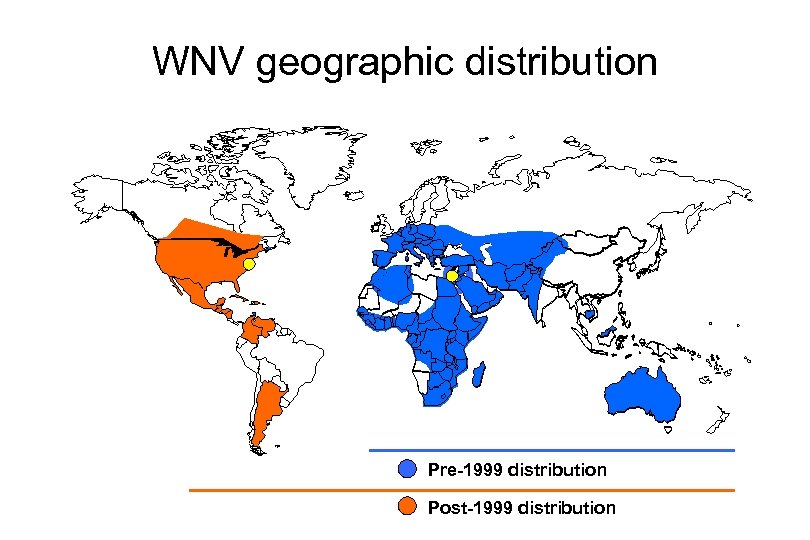
WNV geographic distribution Pre-1999 distribution Post-1999 distribution

West Nile Virus in North America: Background • Discovered in 1999 in New York City during an outbreak of meningitis and encephalitis in humans and an accompanying epizootic in birds – Emergence during heat wave

West Nile Virus In New York - 1999 NYC - 1795 Yellow Fever Outbreak 730 Deaths



“I love the smell of malathion in the morning”

Buzz City by Barry Blitt The New Yorker Sept. 27, 2000

The Bite of Spring by Peter de Seve The New Yorker April 17, 2000
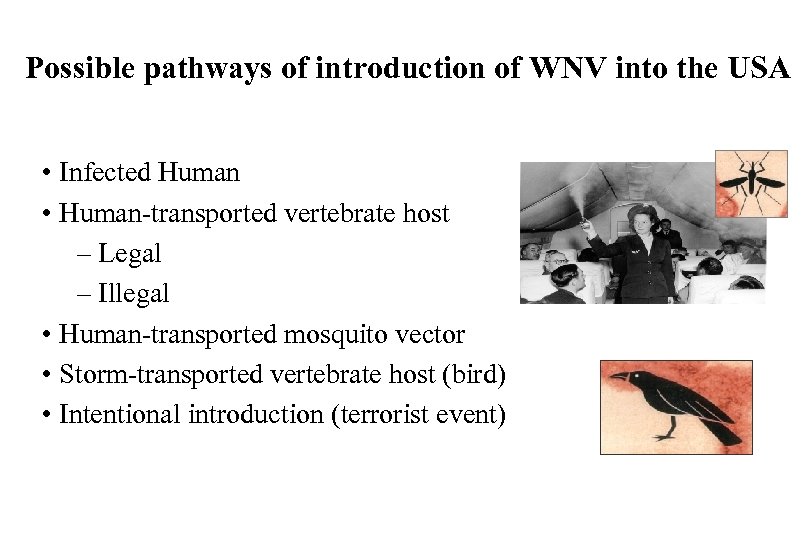
Possible pathways of introduction of WNV into the USA • Infected Human • Human-transported vertebrate host – Legal – Illegal • Human-transported mosquito vector • Storm-transported vertebrate host (bird) • Intentional introduction (terrorist event)
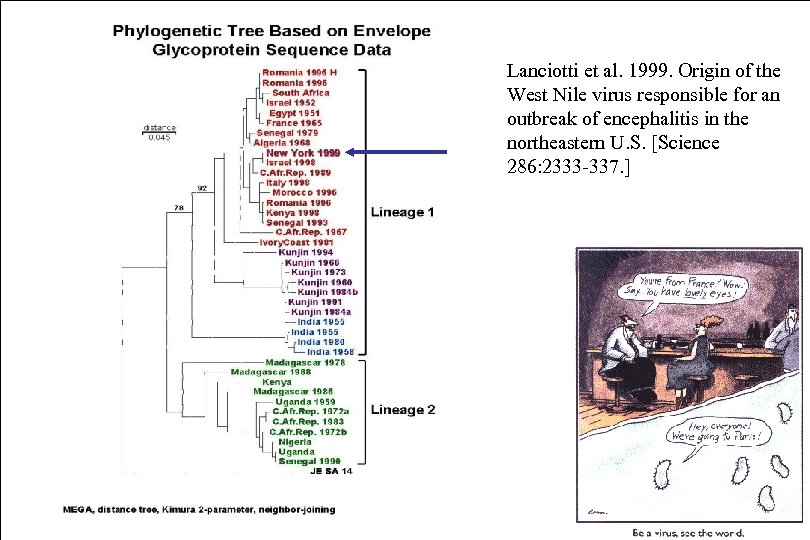
Lanciotti et al. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern U. S. [Science 286: 2333 -337. ]
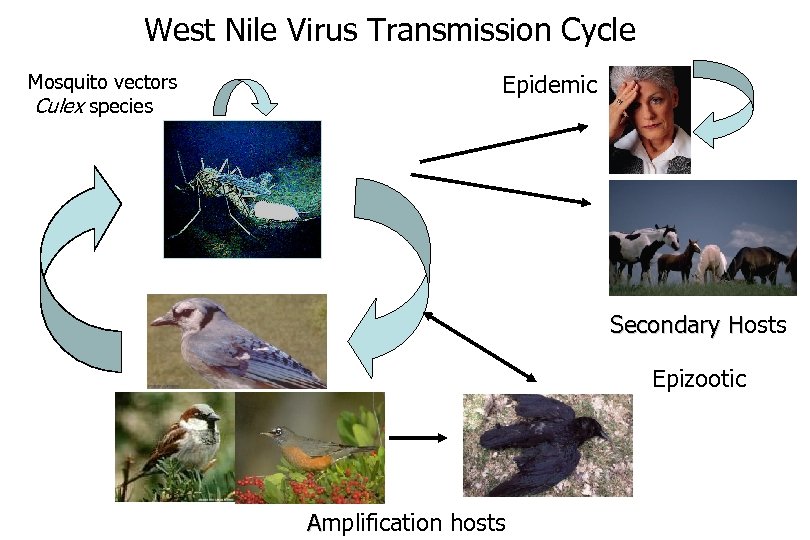
West Nile Virus Transmission Cycle Mosquito vectors Culex species Epidemic Secondary Hosts H Epizootic Amplification hosts

WNV Surveillance, United States, 1999 -2008*: Summary of Mosquito and Dead Bird Data • 64 WNV-positive mosquito species reported – Culex species account for >98% of the total reported 317 WNV-positive dead bird spp. reported 2006: American crows and blue jay accounted for 62% of the dead bird reported A Hitchcock, The Birds * Reported as of 3/2009
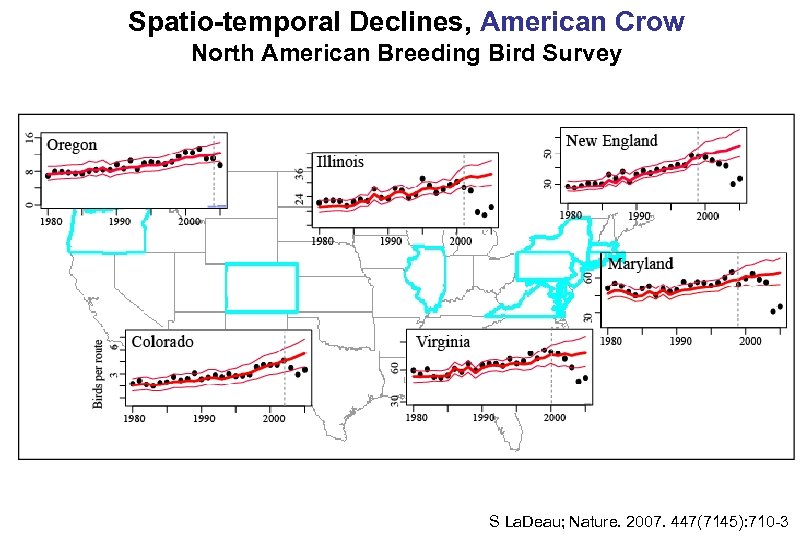
Spatio-temporal Declines, American Crow North American Breeding Bird Survey S La. Deau; Nature. 2007. 447(7145): 710 -3
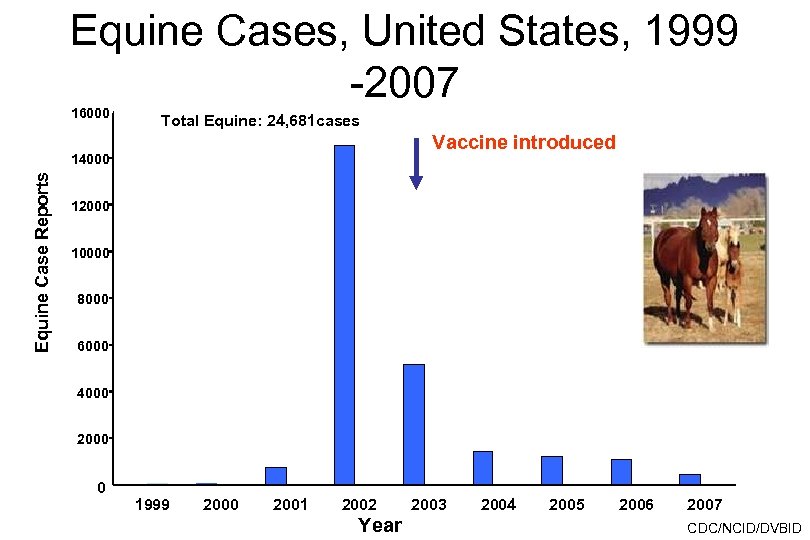
Equine Cases, United States, 1999 -2007 16000 Total Equine: 24, 681 cases Vaccine introduced Equine Case Reports 14000 12000 10000 8000 6000 4000 2000 0 1999 2000 2001 2002 Year 2003 2004 2005 2006 2007 CDC/NCID/DVBID

WN disease
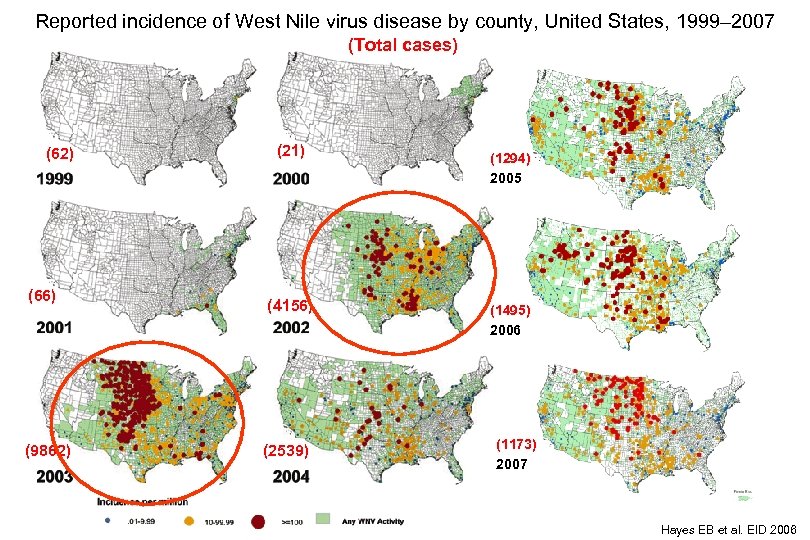
Reported incidence of West Nile virus disease by county, United States, 1999– 2007 (Total cases) (62) (66) (9862) (21) (4156) (2539) (1294) 2005 (1495) 2006 (1173) 2007 Hayes EB et al. EID 2006
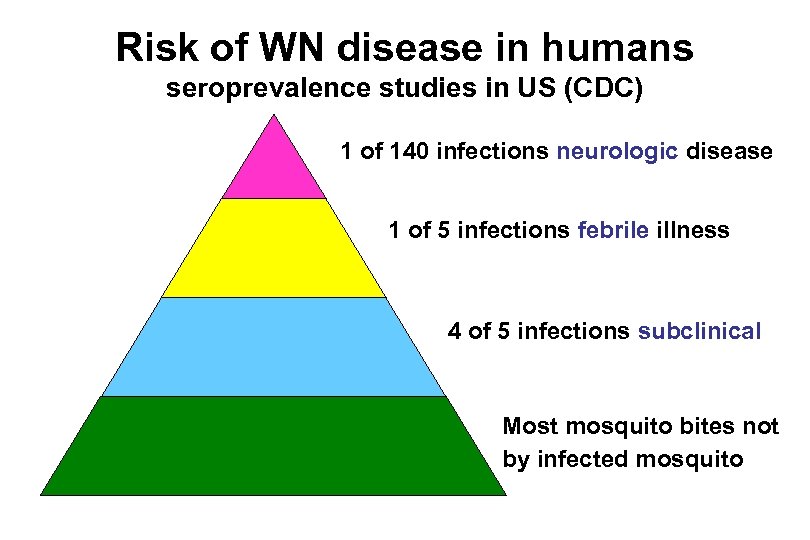
Risk of WN disease in humans seroprevalence studies in US (CDC) 1 of 140 infections neurologic disease 1 of 5 infections febrile illness 4 of 5 infections subclinical Most mosquito bites not by infected mosquito
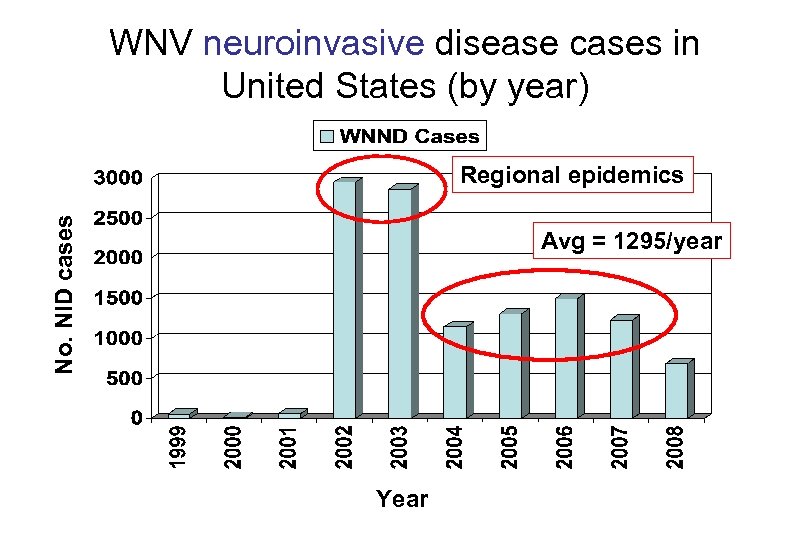
WNV neuroinvasive disease cases in United States (by year) No. NID cases Regional epidemics Avg = 1295/year Year
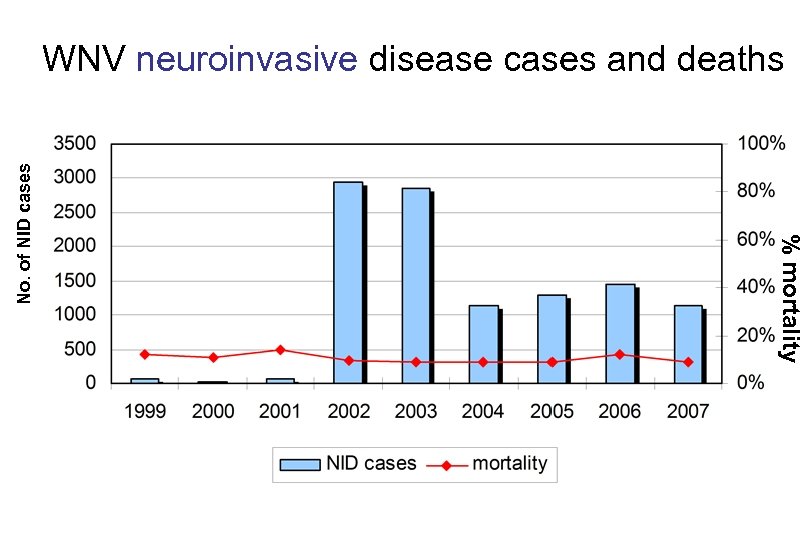
% mortality No. of NID cases WNV neuroinvasive disease cases and deaths

Reported WNND Cases and WNV Deaths in Humans, United States, 1999 -2008* Year Encephalitis/ Non. Total Meningitis 11, 820 WNND Cases Encephalitis/ AFP (includes some Meningitis AFP) x 140 infections/WNND 19992002 3, 088 2003 2, 866 Total WNND Deaths -- -- 3, 088 303 -- -- 2, 866 WNV 1, 142 Widespread and Pervasive 2004 6 33 1, 148 2005 1, 294 in Environment 82 15 1, 309 264 2006 177 2008 Total ~ 1. 65 Million Infections 1, 459 36 101 1, 495 Produced Widespread and 1, 227 1, 217 10 63 665 Pervasive Impact 1 21 687 11, 616 * Reported as of 11/04/2008 68 300 11, 820 100 119 124 44 1, 114
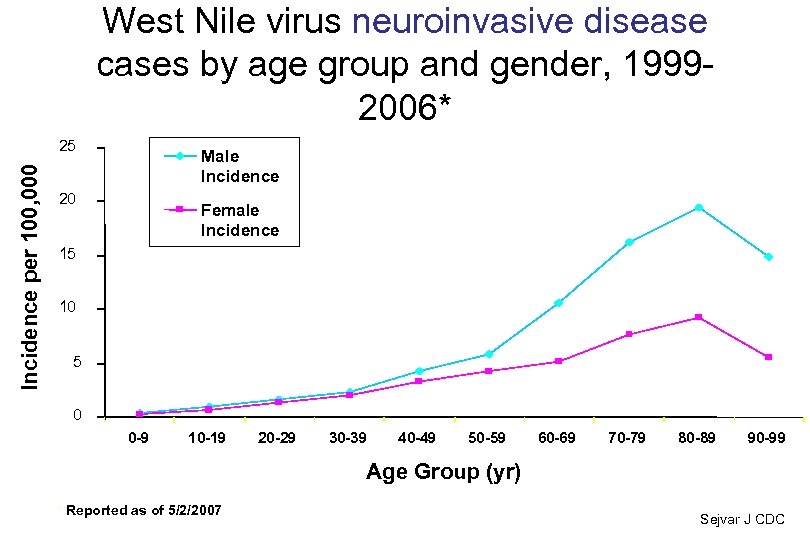
West Nile virus neuroinvasive disease cases by age group and gender, 19992006* Incidence per 100, 000 25 Male Incidence 20 Female Incidence 15 10 5 0 0 -9 10 -19 20 -29 30 -39 40 -49 50 -59 60 -69 70 -79 80 -89 90 -99 Age Group (yr) * Reported as of 5/2/2007 Sejvar J CDC
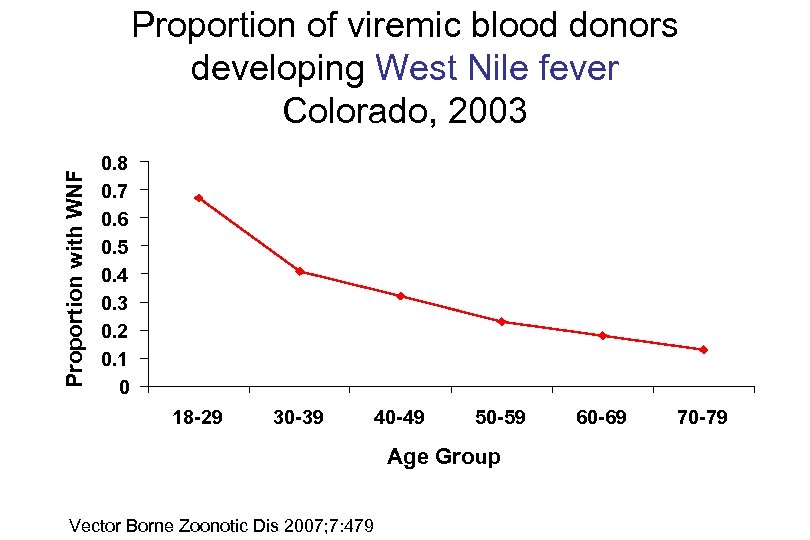
Proportion with WNF Proportion of viremic blood donors developing West Nile fever Colorado, 2003 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 18 -29 30 -39 40 -49 50 -59 Age Group Vector Borne Zoonotic Dis 2007; 7: 479 60 -69 70 -79
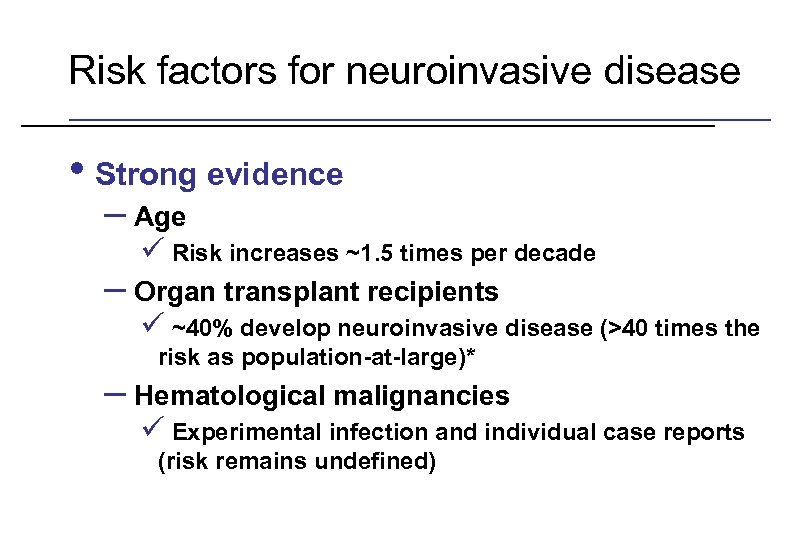
Risk factors for neuroinvasive disease • Strong evidence – Age ü Risk increases ~1. 5 times per decade – Organ transplant recipients ü ~40% develop neuroinvasive disease (>40 times the risk as population-at-large)* – Hematological malignancies ü Experimental infection and individual case reports (risk remains undefined) * Kumar et al. Am J Transplant 2004; 4: 1883 -8

Risk factors for neuroinvasive disease • Weaker evidence – Diabetes – Hypertension – Alcohol abuse – Chronic renal disease – Cardiovascular disease
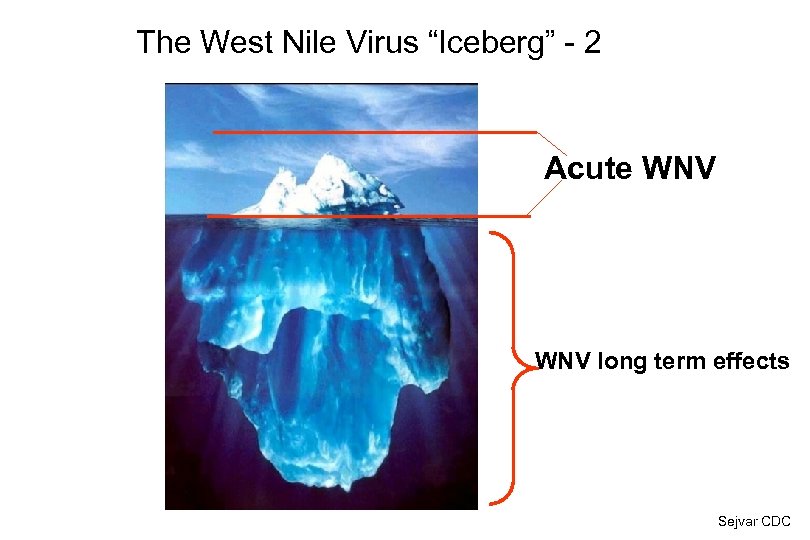
The West Nile Virus “Iceberg” - 2 Acute WNV long term effects Sejvar CDC
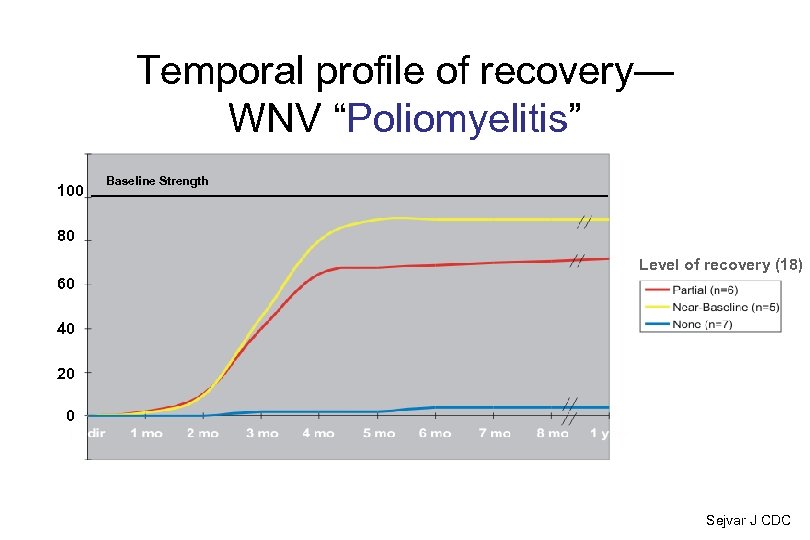
Temporal profile of recovery— WNV “Poliomyelitis” 100 Baseline Strength 80 Level of recovery (18) 60 40 20 0 Sejvar J CDC
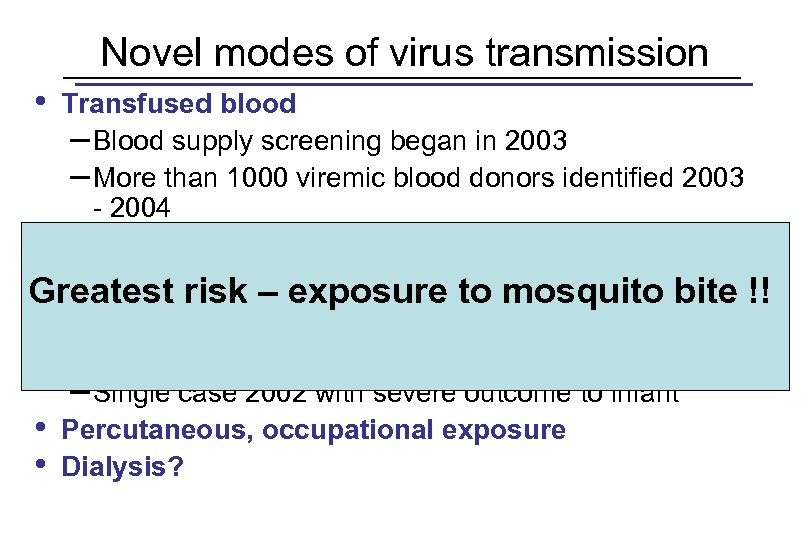
Novel modes of virus transmission • Transfused blood – Blood supply screening began in 2003 – More than 1000 viremic blood donors identified 2003 - 2004 • Transplanted organs • Breast milk Greatest risk – exposure to mosquito bite !! – One case, infant asymptomatic • Transplacental transmission – Single case 2002 with severe outcome to infant • Percutaneous, occupational exposure • Dialysis?

• • • Conclusions 1 – North America Rapid spread across USA (<4 years to Pacific Coast) • Bird migration and random bird movements Many possible important avian hosts and competent mosquito vectors (unprecedented infection prevalence) Significant impact on wildlife and domestic animals. Persistent seasonal outbreaks. Incidence varies regionally. High infection incidence in humans has led to unusual modes of transmission. Age and immunosuppression highly significant risk factors for neuroinvasive disease. Role of other risk factors unclear, but possibly important.
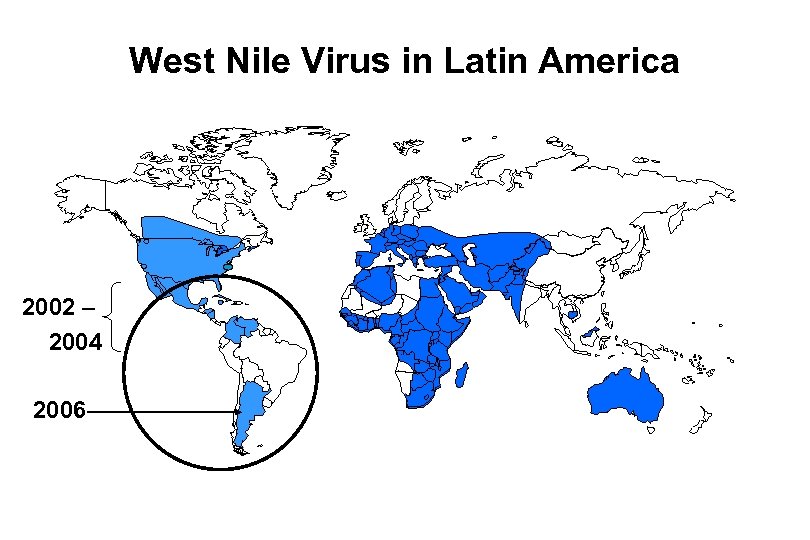
West Nile Virus in Latin America 2002 – 2004 2006

Little evidence of human and animal disease in Latin America • Less virulent virus circulating? • Poor surveillance? • Serological cross reactivity with other flaviviruses? • Previous exposure to other circulating flaviviruses modulating disease expression? • Other causes?

West Nile Virus Puerto Rico, 2007 • • • Sentinel chicken surveillance: up to 50% chickens seroconverted per week for over two months 3 viremic human blood donors 7 sick horses; 1 death Active human surveillance: only one human with West Nile fever; no neuroinvasive disease WNV isolated from chickens and Culex mosquitoes Strain identical to that circulating in United States

Future issues West Nile virus is endemic in the western hemisphere • Vaccines successful for • • • equines but need to weigh cost effectiveness for humans Therapeutics / antivirals Long term sequelae Control and risk prediction

Pools at foreclosed homes raise West Nile threat in Dallas County 10: 38 PM CDT on Friday, May 22, 2009 By THEODORE KIM / The Dallas Morning News tkim@dallasnews. com braceforimpactnow. blogspot. com

Outline • Drivers of emerging diseases • Re/emerging flavivirus – West Nile • Re/emerging alphavirus – Chikungunya Atlantic Monthly, 1997
Togaviridae: Alphaviruses Genome: Single stranded, positive sense RNA 5’ capped , 3’ polyadenylated Cytoplasmic replication Structural proeins encoded at 3’ end in subgenomic message Insect transmitted 3 disease patterns: Arthropathy (Sindbis, Ross River, Chikungunya) Systemic febrile illness (Semliki forest, VEE) Encephalitis (EEE, WEE, VEE)

Chikungunya In Swahili, “chikungunya” : “ that which contorts or bends “up” Disease: High fever (103 -104 F) Rash Severe incapacitating arthritis/arthralgia – Generalized – Usually acute Hemorrhagic manifestations have been reported Rarely fatal G. Pialoux et al. , 2007, Lancet Infect Dis A. M. Powers and C. H. Logue, 2007 J Gen Virol,
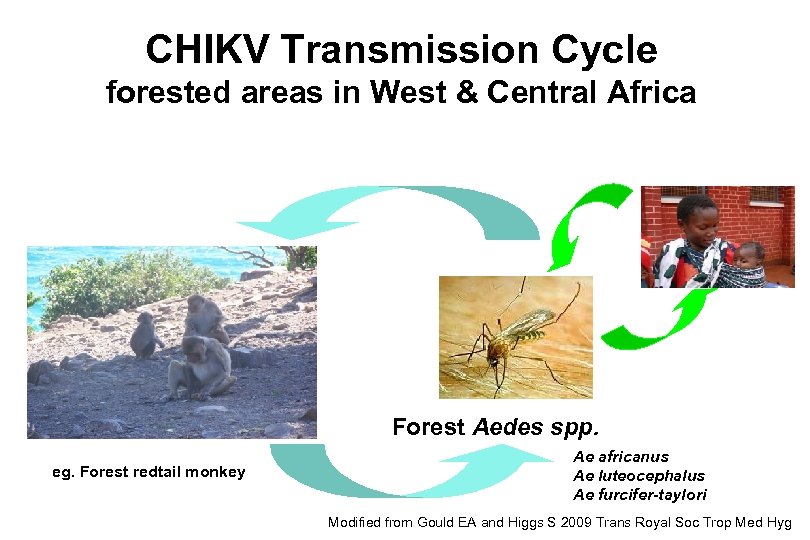
CHIKV Transmission Cycle forested areas in West & Central Africa Forest Aedes spp. eg. Forest redtail monkey Ae africanus Ae luteocephalus Ae furcifer-taylori Modified from Gould EA and Higgs S 2009 Trans Royal Soc Trop Med Hyg
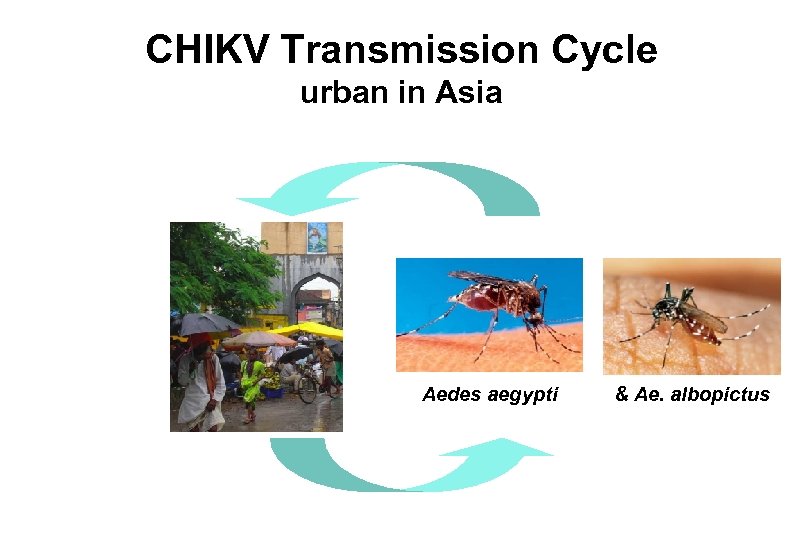
CHIKV Transmission Cycle urban in Asia Aedes aegypti & Ae. albopictus
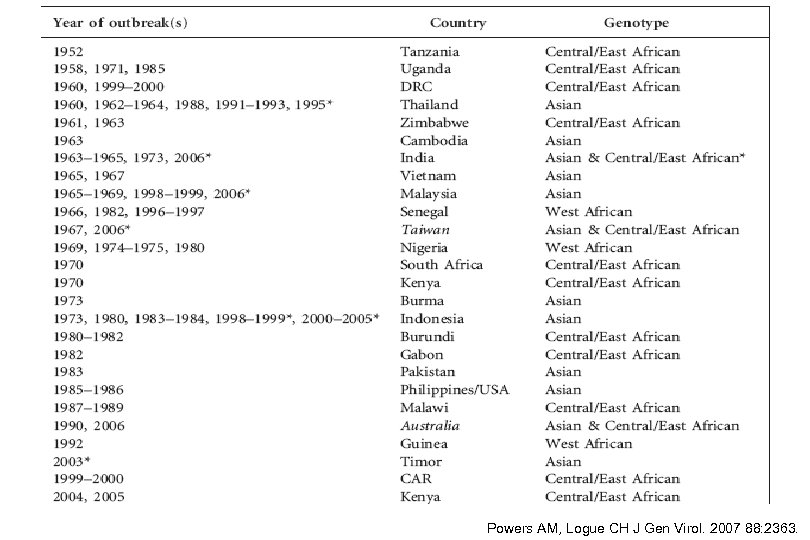
Powers AM, Logue CH J Gen Virol. 2007 88: 2363.
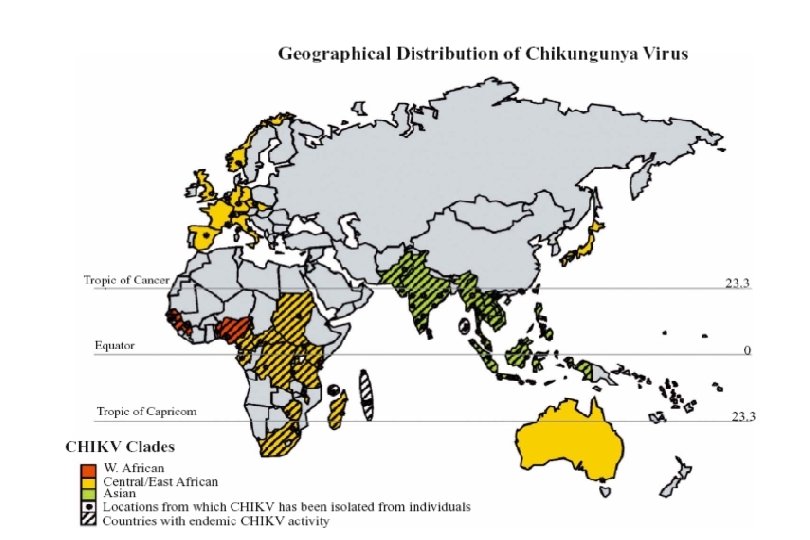
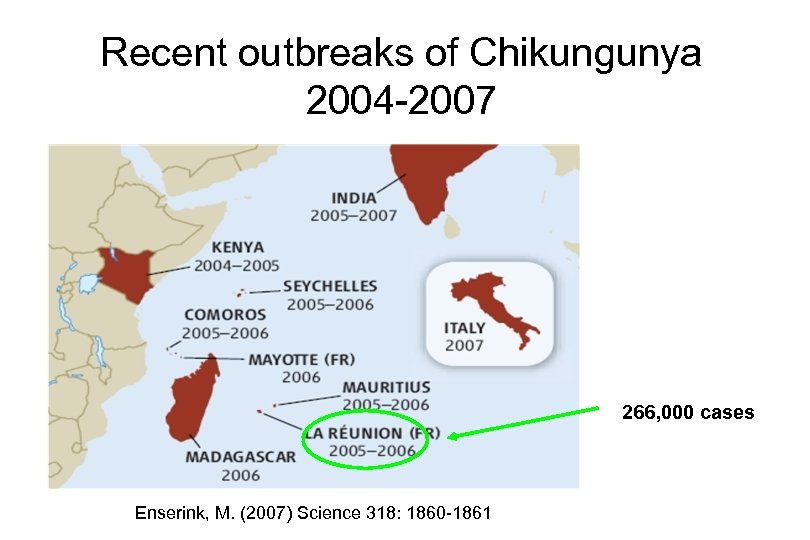
Recent outbreaks of Chikungunya 2004 -2007 266, 000 cases Enserink, M. (2007) Science 318: 1860 -1861
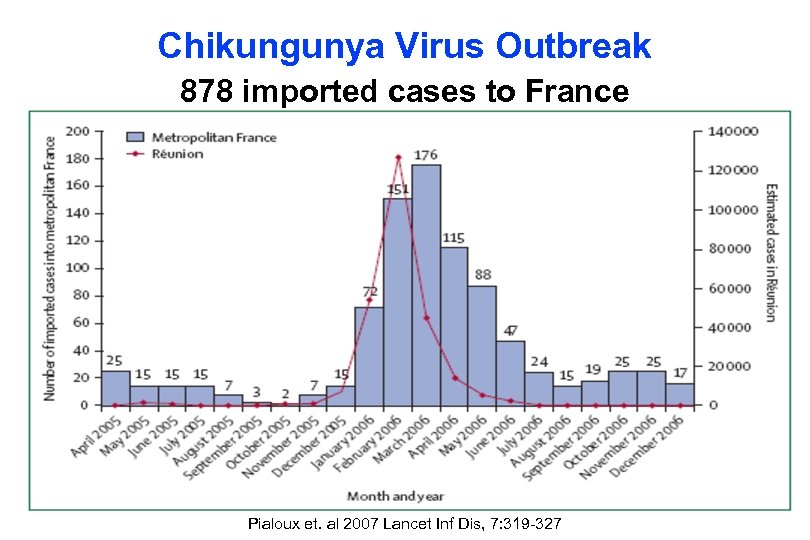
Chikungunya Virus Outbreak 878 imported cases to France Pialoux et. al 2007 Lancet Inf Dis, 7: 319 -327
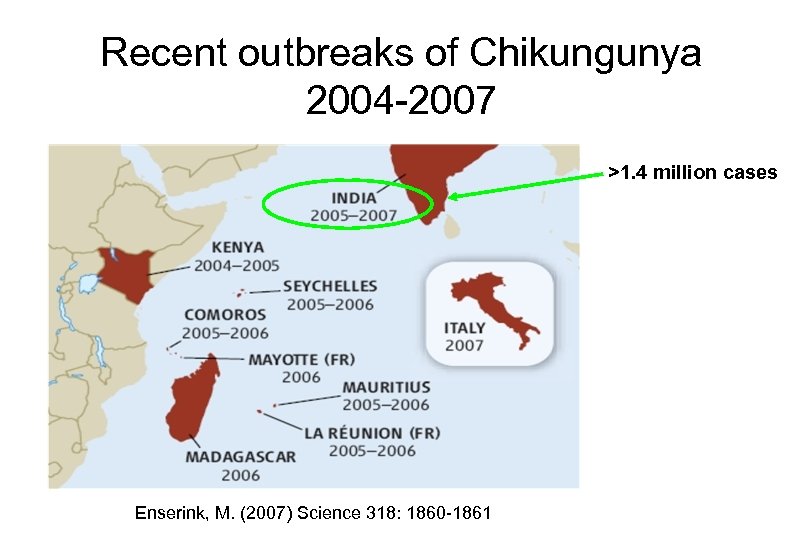
Recent outbreaks of Chikungunya 2004 -2007 >1. 4 million cases Enserink, M. (2007) Science 318: 1860 -1861
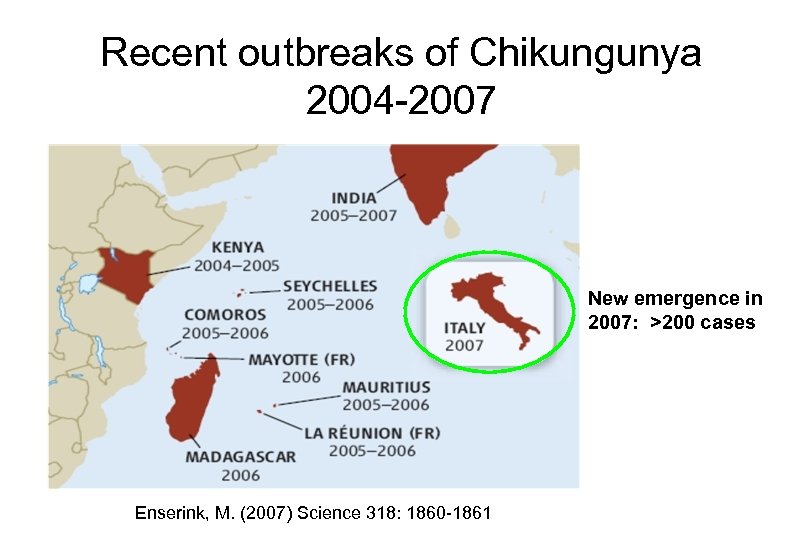
Recent outbreaks of Chikungunya 2004 -2007 New emergence in 2007: >200 cases Enserink, M. (2007) Science 318: 1860 -1861
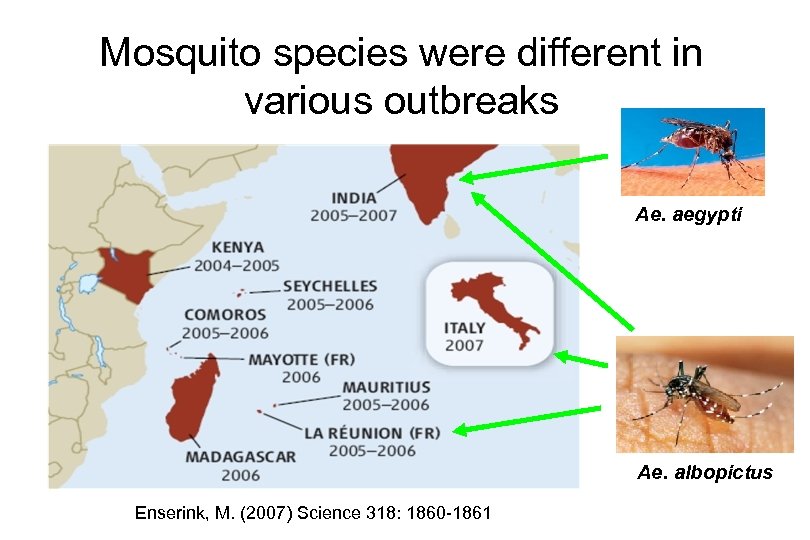
Mosquito species were different in various outbreaks Ae. aegypti Ae. albopictus Enserink, M. (2007) Science 318: 1860 -1861
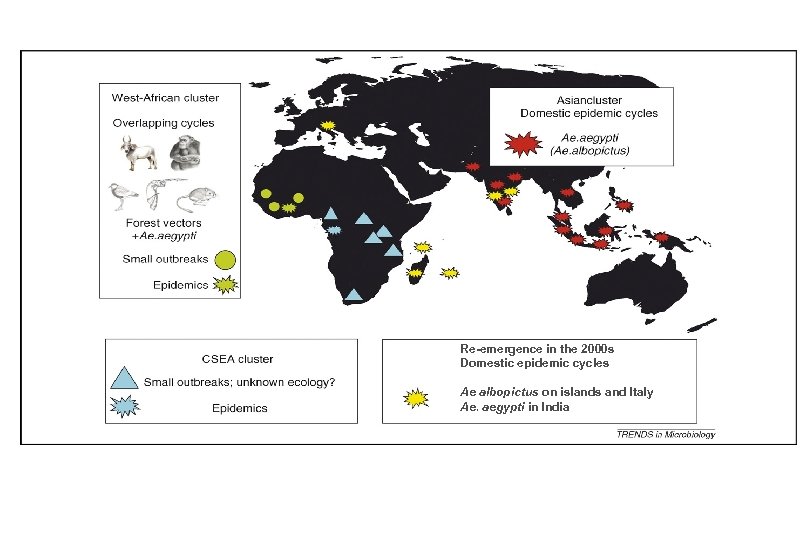
Re-emergence in the 2000 s Domestic epidemic cycles Ae albopictus on islands and Italy Ae. aegypti in India

Mosquito-borne virus hits 20, 000 HEALTH MINISTRY ISSUES ALERT OVER CHIKUNGUNYA DISEASE By: APIRADEE TREERUTKUARKUL Bangkok Post 05/24/09

Charrel et al. 2007. N Engl J Med 356; 8
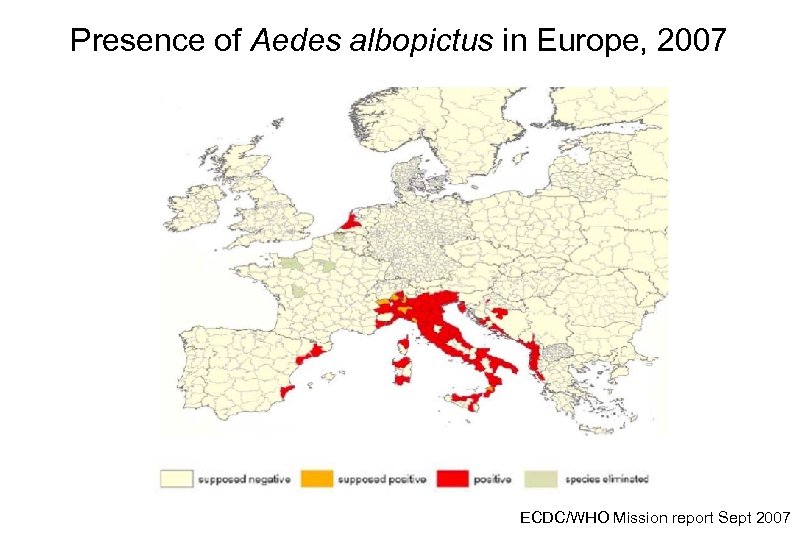
Presence of Aedes albopictus in Europe, 2007 ECDC/WHO Mission report Sept 2007
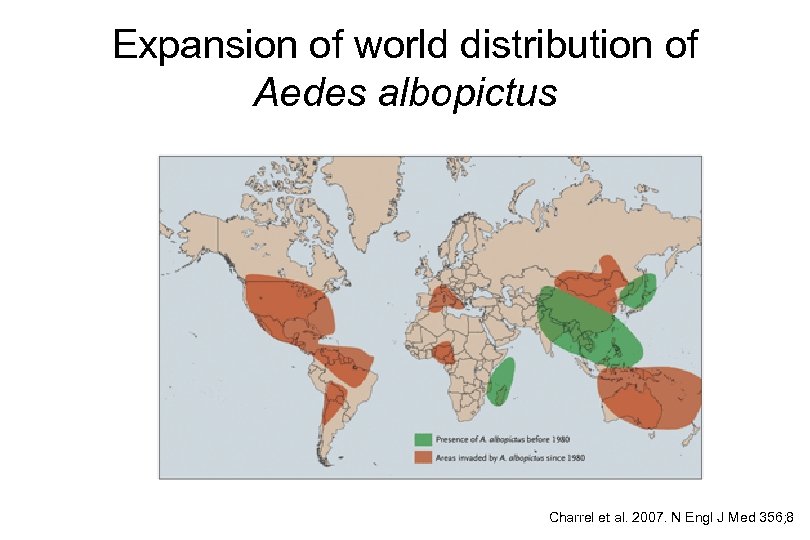
Expansion of world distribution of Aedes albopictus Charrel et al. 2007. N Engl J Med 356; 8

Factors involved in re-emergence of Chikungunya virus • • Biologic and genetic – Non-immune population – Adaptation of virus to new mosquito: Ae. albopictus Ecologic conditions – – – Standing water due to droughts Warm European summer Mosquito abundance Social, economic, political – – – International travel Previous introduction of Ae. albopictus into Reunion Island & Italy Delayed identification and control of initial outbreaks Physical environment – Stored water/atificial breeding sites Modified from Chretien JP, Linthicum KJ. Lancet. 2007

Can Chikungunya virus emerge in US? • 37 imported cases in 2005 -2006 è No autochthonous transmission so far • Components of the transmission cycle? è Climate in southern states è Humans è Monkeys X è Mosquitoes ? ?
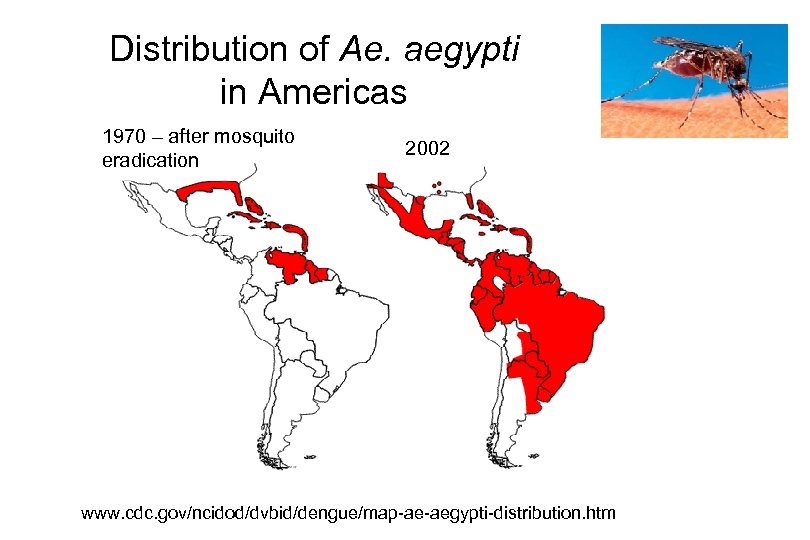
Distribution of Ae. aegypti in Americas 1970 – after mosquito eradication 2002 www. cdc. gov/ncidod/dvbid/dengue/map-ae-aegypti-distribution. htm
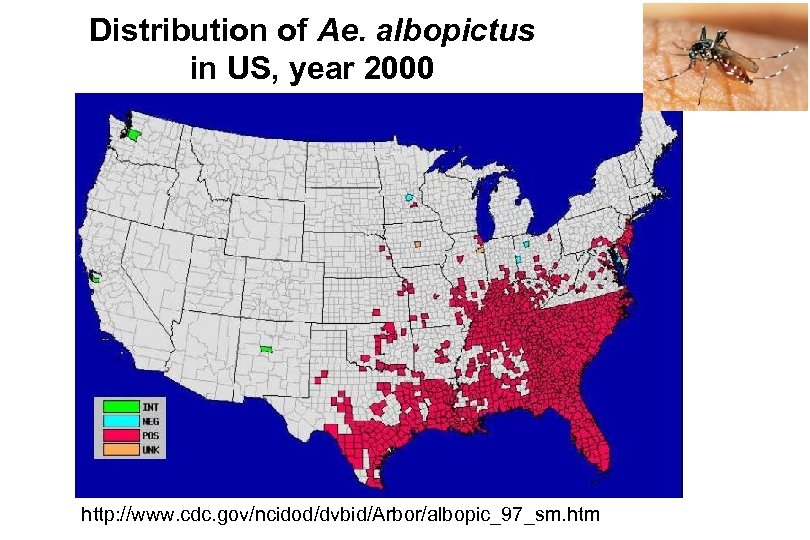
Distribution of Ae. albopictus in US, year 2000 http: //www. cdc. gov/ncidod/dvbid/Arbor/albopic_97_sm. htm

Can Chikungunya virus emerge in US? • 37 imported cases in 2005 -2006 • Components of the transmission cycle? è Climate in southern states è Humans è Monkeys X è Mosquitoes • Human behavior ? ? ?

Can we predict the next new emerging zoonosis? “In general, there is no way to predict when or where the next important new zoonotic pathogen will emerge or what its ultimate importance might be. ” F. A. Murphy, ICEID 1998 Emerg. Infect. Dis. 1998 4: 429 -435

Malaria, yellow fever, dengue, West Nile virus, chikungunya, WHAT’S NEXT?

Thank you! QUESTIONS? ? ? ?
3b1b9d38e42a476cf3b91e92f4a0abbe.ppt