f75e5363ab6d643562a168e6286974c4.ppt
- Количество слайдов: 61
 A 25 Gauge View of Prevention: Adult Vaccinations Shobhina Chheda, M. D. , M. P. H Laurel Romer, M. D. Primary Care Conference September 14, 2005
A 25 Gauge View of Prevention: Adult Vaccinations Shobhina Chheda, M. D. , M. P. H Laurel Romer, M. D. Primary Care Conference September 14, 2005
 Learning Objectives 1. Understand epidemiology of vaccine preventable diseases. 2. Review recommendations for common adult vaccinations. 3. Offer vaccination to appropriate patients. 4. Answer patient questions regarding upcoming vaccines.
Learning Objectives 1. Understand epidemiology of vaccine preventable diseases. 2. Review recommendations for common adult vaccinations. 3. Offer vaccination to appropriate patients. 4. Answer patient questions regarding upcoming vaccines.
 Influenza • Epidemiology • Hospitalizations (From 1979/80 to 2000/01) • 54, 000 to 430, 000 per epidemic • 226, 000 influenza-related hospitalizations/year • 63% of these among patients > 65 years old • Deaths • 19, 000 per season from 1976 -1990 • 36, 000 per season from 1990 -1999 MMWR 13 June 2005; 54: 1 -40.
Influenza • Epidemiology • Hospitalizations (From 1979/80 to 2000/01) • 54, 000 to 430, 000 per epidemic • 226, 000 influenza-related hospitalizations/year • 63% of these among patients > 65 years old • Deaths • 19, 000 per season from 1976 -1990 • 36, 000 per season from 1990 -1999 MMWR 13 June 2005; 54: 1 -40.
 Influenza • Virus characteristics – Influenza A • Envelope glycoproteins/Hemagglutinins 1, 2, 3/ Neuraminidases 1, 2 • Antigenic shift (epidemics/pandemics) • Antigenic drift (localized outbreaks) – Influenza B • Antigenic drift
Influenza • Virus characteristics – Influenza A • Envelope glycoproteins/Hemagglutinins 1, 2, 3/ Neuraminidases 1, 2 • Antigenic shift (epidemics/pandemics) • Antigenic drift (localized outbreaks) – Influenza B • Antigenic drift
 Influenza • Vaccine Characteristics – Changes yearly to approximate currently circulating strains of Influenza A/B – Trivalent inactivated influenza vaccine • intramuscular – Trivalent live-attenuated, cold-adapted influenza vaccine • Intranasal – Protection conferred by induction of antibodies (mainly against the hemagglutinin)
Influenza • Vaccine Characteristics – Changes yearly to approximate currently circulating strains of Influenza A/B – Trivalent inactivated influenza vaccine • intramuscular – Trivalent live-attenuated, cold-adapted influenza vaccine • Intranasal – Protection conferred by induction of antibodies (mainly against the hemagglutinin)
 Influenza • Vaccine Efficacy – Greater reduction in serologically confirmed cases than in clinical influenza – Healthy adults ages 14 -65 years: • 68% reduction in serologically confirmed influenza – 48% reduction with intranasal vaccine • 24% reduction in clinical influenza – 13% reduction with intranasal vaccine Demichelli V, et al. Cochrane Database Syst Rev 2001; CD 001269.
Influenza • Vaccine Efficacy – Greater reduction in serologically confirmed cases than in clinical influenza – Healthy adults ages 14 -65 years: • 68% reduction in serologically confirmed influenza – 48% reduction with intranasal vaccine • 24% reduction in clinical influenza – 13% reduction with intranasal vaccine Demichelli V, et al. Cochrane Database Syst Rev 2001; CD 001269.
 Influenza • Vaccine efficacy – Elderly • > 90% of influenza-related deaths occur among those > age 60
Influenza • Vaccine efficacy – Elderly • > 90% of influenza-related deaths occur among those > age 60
 Influenza • Vaccine Recommendations for 2005 -2006 – – – – Persons age > 65 with comorbid conditions Residents of long-term care facilities Persons aged 2 -64 with comorbid conditions Persons age > 65 without comorbid conditions Children age 6 -23 months Pregnant women Health-care personnel who provide direct patient care Household contacts and out-of-home caregivers of children < 6 months CDC
Influenza • Vaccine Recommendations for 2005 -2006 – – – – Persons age > 65 with comorbid conditions Residents of long-term care facilities Persons aged 2 -64 with comorbid conditions Persons age > 65 without comorbid conditions Children age 6 -23 months Pregnant women Health-care personnel who provide direct patient care Household contacts and out-of-home caregivers of children < 6 months CDC
 Pneumococcus • Epidemiology – Illness • 150, 000 - 570, 000 cases per year • 36% of community-acquired pneumonias in adults – Deaths • 6, 000– 12, 000 per year • Case-fatality rate 5%-7%, higher in elderly • Risk highest in – Older adults – Any age with certain underlying chronic diseases CDC/Fishbein
Pneumococcus • Epidemiology – Illness • 150, 000 - 570, 000 cases per year • 36% of community-acquired pneumonias in adults – Deaths • 6, 000– 12, 000 per year • Case-fatality rate 5%-7%, higher in elderly • Risk highest in – Older adults – Any age with certain underlying chronic diseases CDC/Fishbein
 Pneumococcus • Vaccine characteristics – First used in 1945 – First vaccine developed from a capsular polysaccharide – 23 -valent formulation created in 1983 (PPV 23) – Intramuscular injection
Pneumococcus • Vaccine characteristics – First used in 1945 – First vaccine developed from a capsular polysaccharide – 23 -valent formulation created in 1983 (PPV 23) – Intramuscular injection
 Pneumococcus • Efficacy: Cochrane database – Review of all RCT from 1/66 to 6/03 • Combined results fail to show PPV is effective in preventing pneumonia (OR 0. 77) or death (OR 0. 90) – Review of all case-control studies for same interval • Combined results show significant efficacy in preventing invasive pneumococcal disease (OR 0. 47), corresponding to an efficacy of 53% Dear KB, et al. Cochrane Database Syst Rev; 3: 2005.
Pneumococcus • Efficacy: Cochrane database – Review of all RCT from 1/66 to 6/03 • Combined results fail to show PPV is effective in preventing pneumonia (OR 0. 77) or death (OR 0. 90) – Review of all case-control studies for same interval • Combined results show significant efficacy in preventing invasive pneumococcal disease (OR 0. 47), corresponding to an efficacy of 53% Dear KB, et al. Cochrane Database Syst Rev; 3: 2005.
 Pneumococcus • Recommendations for use – Adults age 65 or older – Persons age >2 • with chronic disease (similar to influenza) • No spleen • Compromised immunity (HIV, malignancy, chronic renal disease, organ transplant, chemotherapy) – Second dose of vaccine if patient received vaccine > 5 years previously and was < 65 MMWR 2003; 52: 965 MMWR 2002; 51: 931
Pneumococcus • Recommendations for use – Adults age 65 or older – Persons age >2 • with chronic disease (similar to influenza) • No spleen • Compromised immunity (HIV, malignancy, chronic renal disease, organ transplant, chemotherapy) – Second dose of vaccine if patient received vaccine > 5 years previously and was < 65 MMWR 2003; 52: 965 MMWR 2002; 51: 931
 Hepatitis B • Incidence – In endemic areas, ~70% of adult population positive for prior infection • 8 -15% with chronic Hep B – Globally • 2 billion with evidence of prior Hep B infection • 350 million chronic carriers • 1 million deaths annually due to cirrhosis/hepatocellular carcinoma Poland GA and Jacobson RM. NEJM; 351(27): 2004.
Hepatitis B • Incidence – In endemic areas, ~70% of adult population positive for prior infection • 8 -15% with chronic Hep B – Globally • 2 billion with evidence of prior Hep B infection • 350 million chronic carriers • 1 million deaths annually due to cirrhosis/hepatocellular carcinoma Poland GA and Jacobson RM. NEJM; 351(27): 2004.
 Hepatitis B • Viral source – Blood or blood-derived body fluids • Transmission – Percutaneous, mucosal • Sex, injection drug use, mother-child, health care • 100 x more infectious than HIV
Hepatitis B • Viral source – Blood or blood-derived body fluids • Transmission – Percutaneous, mucosal • Sex, injection drug use, mother-child, health care • 100 x more infectious than HIV
 Hepatitis B • Vaccine characteristics – First generation HBV vaccine was plasma derived – Current vaccines are recombinant HBV – Schedule: 3 doses, intramuscular injection • 0, 1 -2 months, 4 -6 months • Combined form with Hepatitis A – Safety • Soreness at injection site (25%) • Fever, malaise, headache, myalgia, joint pain (1 -3%)
Hepatitis B • Vaccine characteristics – First generation HBV vaccine was plasma derived – Current vaccines are recombinant HBV – Schedule: 3 doses, intramuscular injection • 0, 1 -2 months, 4 -6 months • Combined form with Hepatitis A – Safety • Soreness at injection site (25%) • Fever, malaise, headache, myalgia, joint pain (1 -3%)
 Hepatitis B • Efficacy – After completing the 3 dose course of vaccine • 90% of adults have protective serum antibody concentrations • 95% of infants, children and adolescents Mast E, et al. In: Vaccines, 2004. 299 -337.
Hepatitis B • Efficacy – After completing the 3 dose course of vaccine • 90% of adults have protective serum antibody concentrations • 95% of infants, children and adolescents Mast E, et al. In: Vaccines, 2004. 299 -337.
 Hepatitis B • Vaccine recommendations – – – All infants Catch-up vaccination Pregnant women Homosexual/bisexual men Multiple sexual partners (4 or more/lifetime) Household contacts of patients with Hep B Injection drug users Healthcare workers Patients on hemodialysis (recipients of frequent transfusion) Patients with chronic illness Immunocompromised patients
Hepatitis B • Vaccine recommendations – – – All infants Catch-up vaccination Pregnant women Homosexual/bisexual men Multiple sexual partners (4 or more/lifetime) Household contacts of patients with Hep B Injection drug users Healthcare workers Patients on hemodialysis (recipients of frequent transfusion) Patients with chronic illness Immunocompromised patients
 Hepatitis A • Significant decrease in incidence with vaccine • Most occurs in community wide epidemics • Higher disease incidence in West and Southwest • Highest incidence in children ages 5 -14 • Children – reservoir
Hepatitis A • Significant decrease in incidence with vaccine • Most occurs in community wide epidemics • Higher disease incidence in West and Southwest • Highest incidence in children ages 5 -14 • Children – reservoir
 Hepatitis A • Transmission: Fecal-oral • 70% children asymptomatic or nonspecific symptoms • > 70% adults have jaundice – Liver failure rare • Chronic infection doesn’t occur
Hepatitis A • Transmission: Fecal-oral • 70% children asymptomatic or nonspecific symptoms • > 70% adults have jaundice – Liver failure rare • Chronic infection doesn’t occur
 Hepatitis A and Hurricane Katrina • No transmission from contaminated water in US since 1980’s • No outbreak seen in other recent hurricane/floods • < 10 cases of hepatitis A in New Orleans in past 3 months CDC
Hepatitis A and Hurricane Katrina • No transmission from contaminated water in US since 1980’s • No outbreak seen in other recent hurricane/floods • < 10 cases of hepatitis A in New Orleans in past 3 months CDC
 Hepatitis A • Inactivated vaccine • 2 brands licensed for children>2 and adults • Different pediatric and adult formulations
Hepatitis A • Inactivated vaccine • 2 brands licensed for children>2 and adults • Different pediatric and adult formulations
 Hepatitis A • 2 doses – 6 months apart – 97% immunogenic with first dose – 100% with second dose- long term immunity • No severe/adverse reactions • Side effects – Soreness/tenderness – 50% – Headache-15% – Malaise-7%
Hepatitis A • 2 doses – 6 months apart – 97% immunogenic with first dose – 100% with second dose- long term immunity • No severe/adverse reactions • Side effects – Soreness/tenderness – 50% – Headache-15% – Malaise-7%
 Hepatitis A • Not routine pediatric immunization • Adult recommendations – – – Certain international travelers Men who have sex with men Illicit drug users Chronic liver disease Persons receiving clotting factor concentrates Persons working with laboratory HAV • Not routinely recommended for healthcare workers
Hepatitis A • Not routine pediatric immunization • Adult recommendations – – – Certain international travelers Men who have sex with men Illicit drug users Chronic liver disease Persons receiving clotting factor concentrates Persons working with laboratory HAV • Not routinely recommended for healthcare workers
 Combined Hepatitis A and B vaccine • FDA approved for > age 18 • Immunogencity/safety similar to single antigen vaccines • Schedule 0, 1, 6 months (same as Hep B) • Total 3 injections instead of 5
Combined Hepatitis A and B vaccine • FDA approved for > age 18 • Immunogencity/safety similar to single antigen vaccines • Schedule 0, 1, 6 months (same as Hep B) • Total 3 injections instead of 5
 Meningococcal disease • 1, 400 -2, 800 cases/yr • Rate 0. 5 -1. 1/100, 000 • College freshman* – 1. 9/100, 000 – Living in dorms 5. 1/100, 000 • Leading cause of bacterial meningitis – Dramatic reductions of Strep Pneumoniae and HIB meningitis from universal vaccination of children *Bruce et al. JAMA 2001 286: 688 -93
Meningococcal disease • 1, 400 -2, 800 cases/yr • Rate 0. 5 -1. 1/100, 000 • College freshman* – 1. 9/100, 000 – Living in dorms 5. 1/100, 000 • Leading cause of bacterial meningitis – Dramatic reductions of Strep Pneumoniae and HIB meningitis from universal vaccination of children *Bruce et al. JAMA 2001 286: 688 -93
 Meningococcal disease • Three clinical forms – Meningitis (49%) – Bacteremia (33%) – Pneumonia (9%) • High case- fatality ratio (10 -14%) • High morbidity – 11 -19% of survivors have sequelae • Transmission: direct contact with large droplet respiratory secretions – 5 -10% carries bacteria
Meningococcal disease • Three clinical forms – Meningitis (49%) – Bacteremia (33%) – Pneumonia (9%) • High case- fatality ratio (10 -14%) • High morbidity – 11 -19% of survivors have sequelae • Transmission: direct contact with large droplet respiratory secretions – 5 -10% carries bacteria
 Meningococcal disease • Disease caused by 5 serogroups worldwide – A, B, C, Y W-135 • United States – B, C, Y • Serogroup B (no vaccine available) – > 50% cases in age <1 – < 25% cases age >11
Meningococcal disease • Disease caused by 5 serogroups worldwide – A, B, C, Y W-135 • United States – B, C, Y • Serogroup B (no vaccine available) – > 50% cases in age <1 – < 25% cases age >11
 Meningococcal vaccines • MCPV 4 – licensed 1981 – Polysaccharide vaccine – Mature B-lymphocyte response, no T-cell stimulation – Not long lasting, no amnestic response • MCV 4 – licensed 2005 for ages 11 -55 – Polysaccharide protein conjugate vaccine – T-cell dependent immune response – Longer lasting and stronger amnestic response
Meningococcal vaccines • MCPV 4 – licensed 1981 – Polysaccharide vaccine – Mature B-lymphocyte response, no T-cell stimulation – Not long lasting, no amnestic response • MCV 4 – licensed 2005 for ages 11 -55 – Polysaccharide protein conjugate vaccine – T-cell dependent immune response – Longer lasting and stronger amnestic response
 Meningococcal disease: MCV 4 use • Universal vaccination – Ages 11 -12 – Adolescents at age 15 if not previously vaccinated • Groups at elevated risk – – – College freshman in dorms Military recruits Certain microbiologists Certain travelers Asplenia/Terminal complement component deficiencies • Single dose IM
Meningococcal disease: MCV 4 use • Universal vaccination – Ages 11 -12 – Adolescents at age 15 if not previously vaccinated • Groups at elevated risk – – – College freshman in dorms Military recruits Certain microbiologists Certain travelers Asplenia/Terminal complement component deficiencies • Single dose IM
 Meningococcal disease: MCV 4 use (continued) • Adverse reactions – Mild injection site pain and tenderness – Brief fever 5% – Severe allergic reaction (<0. 1/100, 000) – Neurological reaction (<0. 1/100, 000)
Meningococcal disease: MCV 4 use (continued) • Adverse reactions – Mild injection site pain and tenderness – Brief fever 5% – Severe allergic reaction (<0. 1/100, 000) – Neurological reaction (<0. 1/100, 000)
 Meningococcal disease: MPSV 4 use • Groups at elevated risk ages 2 -10; >55 • Groups at elevated risk if MSV 4 not available • No longer recommended for routine vaccination • Single dose IM • Adverse reactions similar to MCV 4
Meningococcal disease: MPSV 4 use • Groups at elevated risk ages 2 -10; >55 • Groups at elevated risk if MSV 4 not available • No longer recommended for routine vaccination • Single dose IM • Adverse reactions similar to MCV 4
 Pertussis: Secular Trends • Incidence – – 1940 (Prior to vaccination): 150 cases /100, 000 1960: 8 cases/100, 000 1980 -90: 1 cases/100, 000 (2, 900 cases/yr) 2003: 11, 647 cases Only disease for which universal immunization is recommended that incidence is on the rise !
Pertussis: Secular Trends • Incidence – – 1940 (Prior to vaccination): 150 cases /100, 000 1960: 8 cases/100, 000 1980 -90: 1 cases/100, 000 (2, 900 cases/yr) 2003: 11, 647 cases Only disease for which universal immunization is recommended that incidence is on the rise !
 Pertussis: Why the increase? • Increase in reporting vs. actual disease – True burden is at least 10 x > reported • Waning immunity – Less passive Ab transmitted to newborns – Decreased herd immunity – Aging cohort • ? Under-vaccination in childhood
Pertussis: Why the increase? • Increase in reporting vs. actual disease – True burden is at least 10 x > reported • Waning immunity – Less passive Ab transmitted to newborns – Decreased herd immunity – Aging cohort • ? Under-vaccination in childhood
 Pertussis: Important to internists? • Number of cases high in adults – Rate, morbidity and mortality higher in < age 1 • Adults are source of infection for children – 80% secondary attack rate • Wisconsin with high number of cases
Pertussis: Important to internists? • Number of cases high in adults – Rate, morbidity and mortality higher in < age 1 • Adults are source of infection for children – 80% secondary attack rate • Wisconsin with high number of cases
 Pertussis Children • Catarrhal stage 1 -2 weeks • Paroxysmal cough stage 1 -6 weeks • Convalescent stage weeks-months
Pertussis Children • Catarrhal stage 1 -2 weeks • Paroxysmal cough stage 1 -6 weeks • Convalescent stage weeks-months
 Pertussis Adults • Accounts for 7% of all cough illnesses per year • Mild disease – No phases – Persistent cough >21 d • Often not diagnosed/treated until after maximum transmission
Pertussis Adults • Accounts for 7% of all cough illnesses per year • Mild disease – No phases – Persistent cough >21 d • Often not diagnosed/treated until after maximum transmission
 Pertussis • • * Aerobic gram negative rod Attaches to cilia Local tissue damage Decreased ability to clear secretions Challenging to diagnose – – Gold standard-culture (Low sensitivity) PCR (Sensitivity highly age dependent) DFA (now rarely used) Serology (not practical)
Pertussis • • * Aerobic gram negative rod Attaches to cilia Local tissue damage Decreased ability to clear secretions Challenging to diagnose – – Gold standard-culture (Low sensitivity) PCR (Sensitivity highly age dependent) DFA (now rarely used) Serology (not practical)
 Pertussis: Vaccine • Acellular (DTa. P) – Licensed 1996 for primary series – Replaced whole- cell vaccine for children – More effective and fewer side effects • Purified subunit vaccine – Varies between 2 and 4 subunit components
Pertussis: Vaccine • Acellular (DTa. P) – Licensed 1996 for primary series – Replaced whole- cell vaccine for children – More effective and fewer side effects • Purified subunit vaccine – Varies between 2 and 4 subunit components
 Immunogenicity and Safety Study • Prospective, randomized, double blinded trial comparing safety and efficacy of d. T and DTa. P • 4480 participants enrolled – Ages 11 -64 Results: • Elicited robust immune responses to all antigens • No differences observed in side effects in 2 vaccines groups Pinchicherio et al. JAMA 2005: 293(24) 3003
Immunogenicity and Safety Study • Prospective, randomized, double blinded trial comparing safety and efficacy of d. T and DTa. P • 4480 participants enrolled – Ages 11 -64 Results: • Elicited robust immune responses to all antigens • No differences observed in side effects in 2 vaccines groups Pinchicherio et al. JAMA 2005: 293(24) 3003
 Immunogenicity and Safety Study • Prospective, randomized, double blinded trial comparing safety and efficacy of d. T and DTa. P • 4480 participants enrolled – Ages 11 -64 – 39 US centers • Results – Elicited robust immune responses to all antigens Pinchicherio et al. JAMA 2005: 293(24) 3003
Immunogenicity and Safety Study • Prospective, randomized, double blinded trial comparing safety and efficacy of d. T and DTa. P • 4480 participants enrolled – Ages 11 -64 – 39 US centers • Results – Elicited robust immune responses to all antigens Pinchicherio et al. JAMA 2005: 293(24) 3003
 Pertussis: Bottom line • DTa. P – 2 vaccines licensed 2005 by FDA – Adcel* ages 11 to 65 – Boostrix ages 11 -19 • ACIP recommendations/most cost effective – Ages 11 -12 give DTa. P instead of d. T – Ages 11 -18 give DTa. P even if d. T given • 5 year interval recommended * Used in JAMA study
Pertussis: Bottom line • DTa. P – 2 vaccines licensed 2005 by FDA – Adcel* ages 11 to 65 – Boostrix ages 11 -19 • ACIP recommendations/most cost effective – Ages 11 -12 give DTa. P instead of d. T – Ages 11 -18 give DTa. P even if d. T given • 5 year interval recommended * Used in JAMA study
 Pertussis: Bottom line (cont. ) Watch for ACIP recommendations for older adults – Universal (using DTa. P instead of d. T for all) vs. “High risk-groups” (health care workers, those in contact with infants) – Economic issue
Pertussis: Bottom line (cont. ) Watch for ACIP recommendations for older adults – Universal (using DTa. P instead of d. T for all) vs. “High risk-groups” (health care workers, those in contact with infants) – Economic issue
 Hurricane Katrina: Evacuees in crowded settings • Influenza -all > 6 months – < 8 years old need 2 doses • Hepatitis A -all > 6 months • Varicella, MMR, d. T (DTa. P) , meningococcal, pneumococcus – Usual recommendations
Hurricane Katrina: Evacuees in crowded settings • Influenza -all > 6 months – < 8 years old need 2 doses • Hepatitis A -all > 6 months • Varicella, MMR, d. T (DTa. P) , meningococcal, pneumococcus – Usual recommendations
 Hurricane Katrina • Evacuees not in crowded settings – Usual recommendations • Responders – d. T and Hepatitis B
Hurricane Katrina • Evacuees not in crowded settings – Usual recommendations • Responders – d. T and Hepatitis B
 Varicella • Vaccine recommendations – Who: • Age >18 lacking history of chicken pox or documentation of prior vaccination – Schedule: • 2 doses • 0, 4 -8 weeks – Characteristics: • Oka/Merck VZV vaccine – 1350 plaque-forming units • IM injection
Varicella • Vaccine recommendations – Who: • Age >18 lacking history of chicken pox or documentation of prior vaccination – Schedule: • 2 doses • 0, 4 -8 weeks – Characteristics: • Oka/Merck VZV vaccine – 1350 plaque-forming units • IM injection
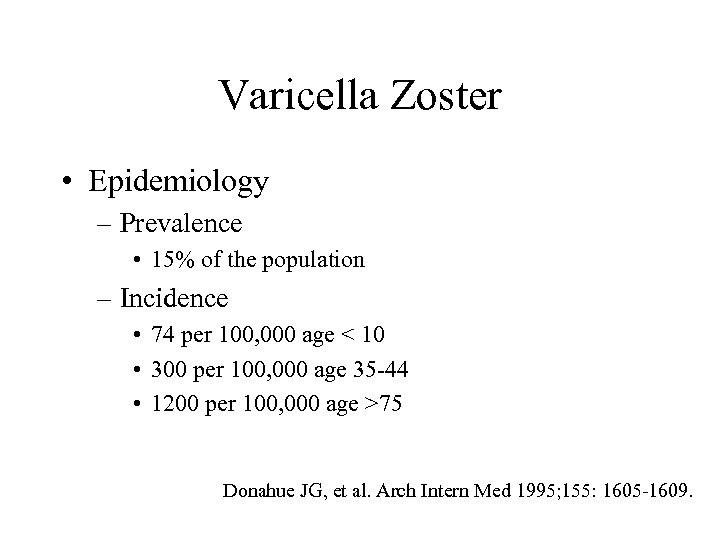 Varicella Zoster • Epidemiology – Prevalence • 15% of the population – Incidence • 74 per 100, 000 age < 10 • 300 per 100, 000 age 35 -44 • 1200 per 100, 000 age >75 Donahue JG, et al. Arch Intern Med 1995; 155: 1605 -1609.
Varicella Zoster • Epidemiology – Prevalence • 15% of the population – Incidence • 74 per 100, 000 age < 10 • 300 per 100, 000 age 35 -44 • 1200 per 100, 000 age >75 Donahue JG, et al. Arch Intern Med 1995; 155: 1605 -1609.
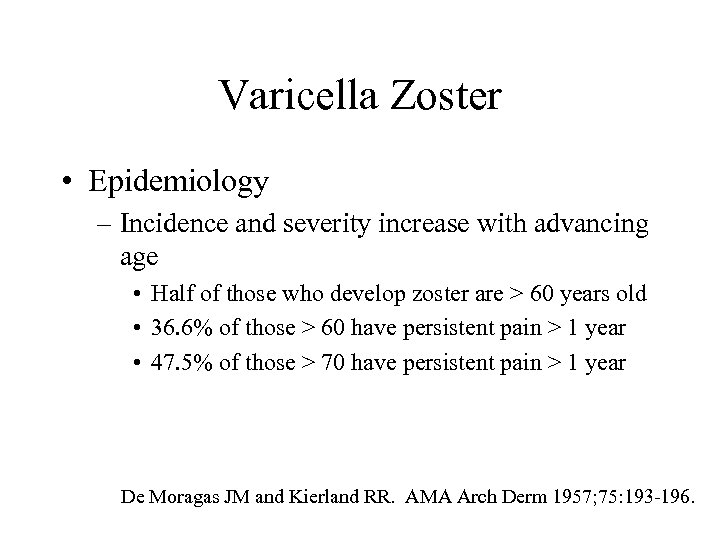 Varicella Zoster • Epidemiology – Incidence and severity increase with advancing age • Half of those who develop zoster are > 60 years old • 36. 6% of those > 60 have persistent pain > 1 year • 47. 5% of those > 70 have persistent pain > 1 year De Moragas JM and Kierland RR. AMA Arch Derm 1957; 75: 193 -196.
Varicella Zoster • Epidemiology – Incidence and severity increase with advancing age • Half of those who develop zoster are > 60 years old • 36. 6% of those > 60 have persistent pain > 1 year • 47. 5% of those > 70 have persistent pain > 1 year De Moragas JM and Kierland RR. AMA Arch Derm 1957; 75: 193 -196.
 Varicella Zoster • Clinical Features – Unilateral radicular pain and vesicular rash usually limited to a single dermatome – Results from reactivation of latent VZV within the sensory ganglia
Varicella Zoster • Clinical Features – Unilateral radicular pain and vesicular rash usually limited to a single dermatome – Results from reactivation of latent VZV within the sensory ganglia
 Varicella Zoster • Vaccine administration: – Live attenuated VZV vaccine • 18, 700 to 60, 000 plaque-forming units of virus – (1350 p-f units in VZV vaccine for children) – Higher dosage necessary to elicit a significant increase in cell-mediated immunity to VZV among older adults – One subcutaneous injection
Varicella Zoster • Vaccine administration: – Live attenuated VZV vaccine • 18, 700 to 60, 000 plaque-forming units of virus – (1350 p-f units in VZV vaccine for children) – Higher dosage necessary to elicit a significant increase in cell-mediated immunity to VZV among older adults – One subcutaneous injection
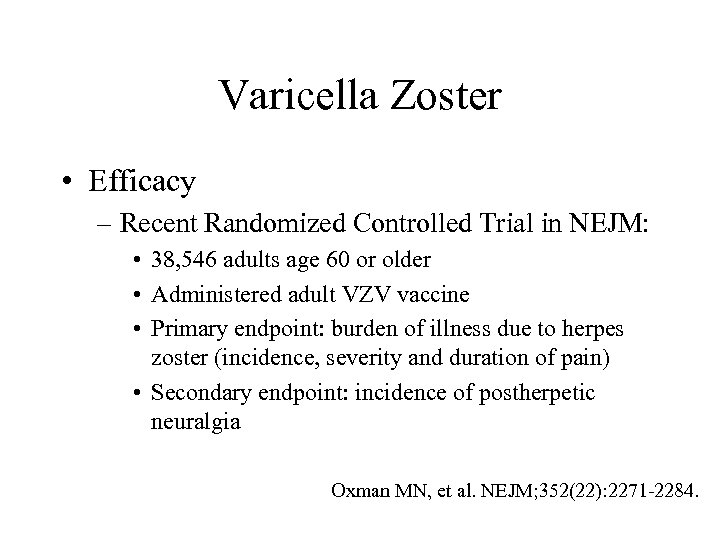 Varicella Zoster • Efficacy – Recent Randomized Controlled Trial in NEJM: • 38, 546 adults age 60 or older • Administered adult VZV vaccine • Primary endpoint: burden of illness due to herpes zoster (incidence, severity and duration of pain) • Secondary endpoint: incidence of postherpetic neuralgia Oxman MN, et al. NEJM; 352(22): 2271 -2284.
Varicella Zoster • Efficacy – Recent Randomized Controlled Trial in NEJM: • 38, 546 adults age 60 or older • Administered adult VZV vaccine • Primary endpoint: burden of illness due to herpes zoster (incidence, severity and duration of pain) • Secondary endpoint: incidence of postherpetic neuralgia Oxman MN, et al. NEJM; 352(22): 2271 -2284.
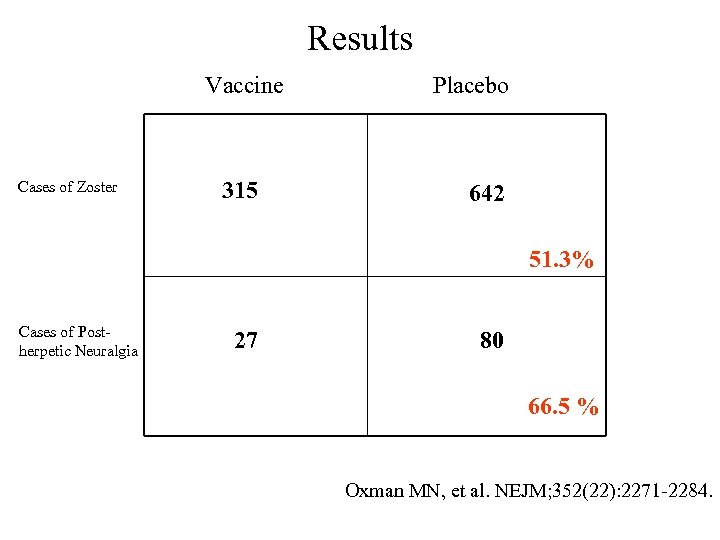 Results Vaccine Cases of Zoster 315 Placebo 642 51. 3% Cases of Postherpetic Neuralgia 27 80 66. 5 % Oxman MN, et al. NEJM; 352(22): 2271 -2284.
Results Vaccine Cases of Zoster 315 Placebo 642 51. 3% Cases of Postherpetic Neuralgia 27 80 66. 5 % Oxman MN, et al. NEJM; 352(22): 2271 -2284.
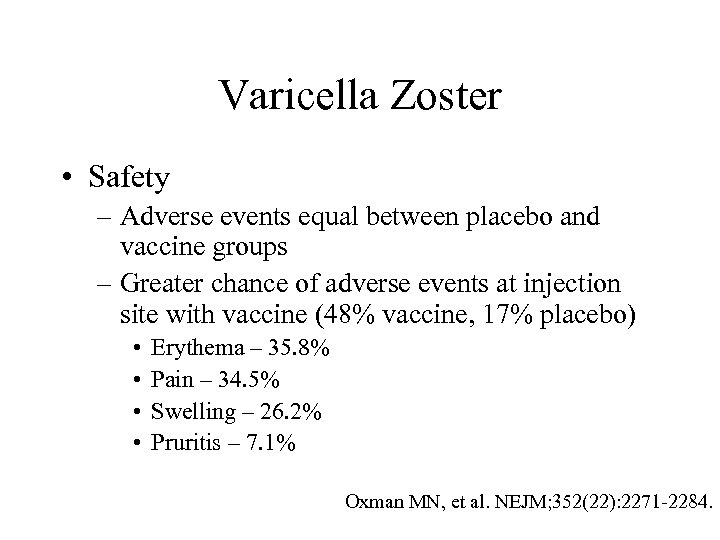 Varicella Zoster • Safety – Adverse events equal between placebo and vaccine groups – Greater chance of adverse events at injection site with vaccine (48% vaccine, 17% placebo) • • Erythema – 35. 8% Pain – 34. 5% Swelling – 26. 2% Pruritis – 7. 1% Oxman MN, et al. NEJM; 352(22): 2271 -2284.
Varicella Zoster • Safety – Adverse events equal between placebo and vaccine groups – Greater chance of adverse events at injection site with vaccine (48% vaccine, 17% placebo) • • Erythema – 35. 8% Pain – 34. 5% Swelling – 26. 2% Pruritis – 7. 1% Oxman MN, et al. NEJM; 352(22): 2271 -2284.
 Varicella Zoster • Vaccine recommendations – Pending FDA approval • Submitted in April 2005 – No current recommendations to use existing Varivax for prevention of Zoster – Unclear whether the higher dosage vaccine is necessary for zoster prevention
Varicella Zoster • Vaccine recommendations – Pending FDA approval • Submitted in April 2005 – No current recommendations to use existing Varivax for prevention of Zoster – Unclear whether the higher dosage vaccine is necessary for zoster prevention
 Human Papillomavirus Cervical cancer • United states – 10, 000+ cases – 3700 deaths • In women worldwide… – Third most common cancer – Most common cause of cancer death
Human Papillomavirus Cervical cancer • United states – 10, 000+ cases – 3700 deaths • In women worldwide… – Third most common cancer – Most common cause of cancer death
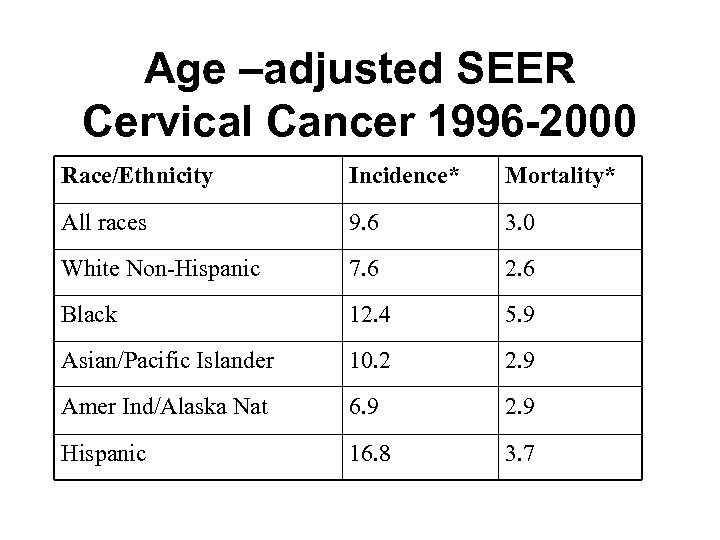 Age –adjusted SEER Cervical Cancer 1996 -2000 Race/Ethnicity Incidence* Mortality* All races 9. 6 3. 0 White Non-Hispanic 7. 6 2. 6 Black 12. 4 5. 9 Asian/Pacific Islander 10. 2 2. 9 Amer Ind/Alaska Nat 6. 9 2. 9 Hispanic 16. 8 3. 7
Age –adjusted SEER Cervical Cancer 1996 -2000 Race/Ethnicity Incidence* Mortality* All races 9. 6 3. 0 White Non-Hispanic 7. 6 2. 6 Black 12. 4 5. 9 Asian/Pacific Islander 10. 2 2. 9 Amer Ind/Alaska Nat 6. 9 2. 9 Hispanic 16. 8 3. 7
 On the horizon: HPV vaccine • Two vaccines in phase 3 trials – Cervarix- bivalent • HPV 16 and 18 (cervical and anogenital cancer) • women ages 15 -25 – Gardasil- quadrivalent • • HPV 16 and 18 HPV 6 and 11 (anogenital warts) Men and women ages 15 -25 Possibly submit FDA request October 2005
On the horizon: HPV vaccine • Two vaccines in phase 3 trials – Cervarix- bivalent • HPV 16 and 18 (cervical and anogenital cancer) • women ages 15 -25 – Gardasil- quadrivalent • • HPV 16 and 18 HPV 6 and 11 (anogenital warts) Men and women ages 15 -25 Possibly submit FDA request October 2005
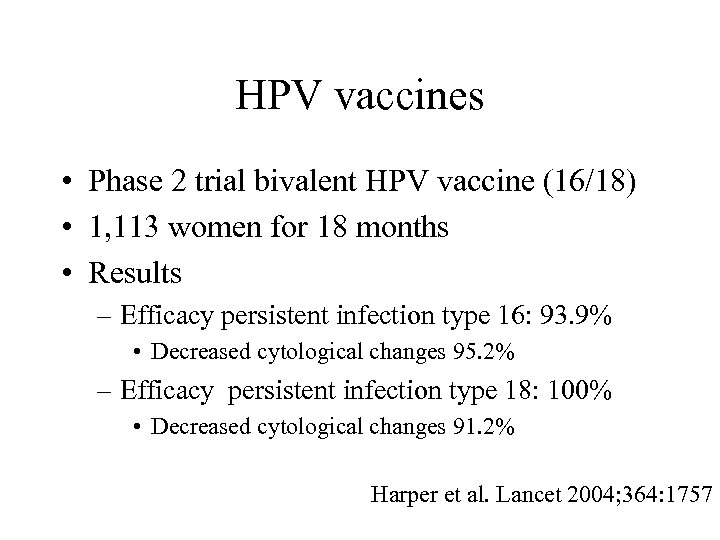 HPV vaccines • Phase 2 trial bivalent HPV vaccine (16/18) • 1, 113 women for 18 months • Results – Efficacy persistent infection type 16: 93. 9% • Decreased cytological changes 95. 2% – Efficacy persistent infection type 18: 100% • Decreased cytological changes 91. 2% Harper et al. Lancet 2004; 364: 1757
HPV vaccines • Phase 2 trial bivalent HPV vaccine (16/18) • 1, 113 women for 18 months • Results – Efficacy persistent infection type 16: 93. 9% • Decreased cytological changes 95. 2% – Efficacy persistent infection type 18: 100% • Decreased cytological changes 91. 2% Harper et al. Lancet 2004; 364: 1757
 HPV vaccines • Phase 2 trial quadrivalent HPV vaccine (16/18/6/11) • 552 women • Results – Persistent infection, cervical atypia or external genital lesions was decreased by 90% compared to control group Villa et al. Lancet Onc 2005; 6: 5: 271
HPV vaccines • Phase 2 trial quadrivalent HPV vaccine (16/18/6/11) • 552 women • Results – Persistent infection, cervical atypia or external genital lesions was decreased by 90% compared to control group Villa et al. Lancet Onc 2005; 6: 5: 271
 HPV vaccine • Likely primary preventive efforts will target pre-adolescent (age 11) – Age 15 - median age of sexual activity in US • Both vaccines require 3 doses in 6 months
HPV vaccine • Likely primary preventive efforts will target pre-adolescent (age 11) – Age 15 - median age of sexual activity in US • Both vaccines require 3 doses in 6 months
 On the horizon: HIV vaccine • February 2003 failed Phase 3 trial • Currently two phase 2 trials are underway – Not primary prevention – Prevent/limit viral replication and delay disease progression
On the horizon: HIV vaccine • February 2003 failed Phase 3 trial • Currently two phase 2 trials are underway – Not primary prevention – Prevent/limit viral replication and delay disease progression
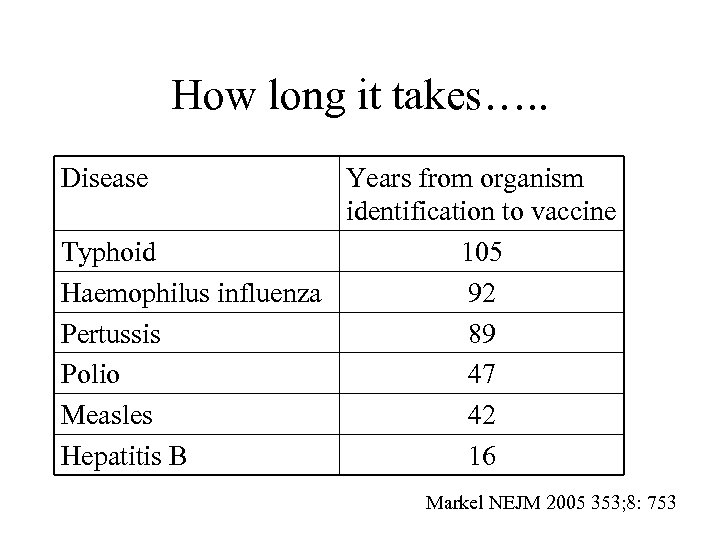 How long it takes…. . Disease Years from organism identification to vaccine Typhoid 105 Haemophilus influenza 92 Pertussis 89 Polio 47 Measles 42 Hepatitis B 16 Markel NEJM 2005 353; 8: 753
How long it takes…. . Disease Years from organism identification to vaccine Typhoid 105 Haemophilus influenza 92 Pertussis 89 Polio 47 Measles 42 Hepatitis B 16 Markel NEJM 2005 353; 8: 753


