e0f121b6e775f2784dd80e4970ae38cc.ppt
- Количество слайдов: 34
 6 th China-Japan Joint Nuclear Physics Symposium Shanghai, China, May 17, 2006 Chemical studies of the transactinide elements at JAEA Y. Nagame Advanced Science Research Center Japan Atomic Energy Agency (JAEA)
6 th China-Japan Joint Nuclear Physics Symposium Shanghai, China, May 17, 2006 Chemical studies of the transactinide elements at JAEA Y. Nagame Advanced Science Research Center Japan Atomic Energy Agency (JAEA)
 Periodic table of the elements Z ≥ 104: transactinide elements superheavy elements
Periodic table of the elements Z ≥ 104: transactinide elements superheavy elements
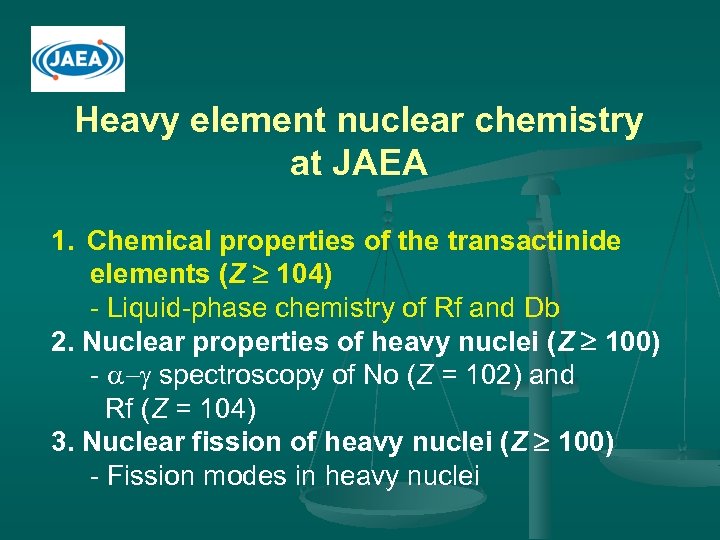 Heavy element nuclear chemistry at JAEA 1. Chemical properties of the transactinide elements (Z 104) - Liquid-phase chemistry of Rf and Db 2. Nuclear properties of heavy nuclei (Z 100) - a-g spectroscopy of No (Z = 102) and Rf (Z = 104) 3. Nuclear fission of heavy nuclei (Z 100) - Fission modes in heavy nuclei
Heavy element nuclear chemistry at JAEA 1. Chemical properties of the transactinide elements (Z 104) - Liquid-phase chemistry of Rf and Db 2. Nuclear properties of heavy nuclei (Z 100) - a-g spectroscopy of No (Z = 102) and Rf (Z = 104) 3. Nuclear fission of heavy nuclei (Z 100) - Fission modes in heavy nuclei
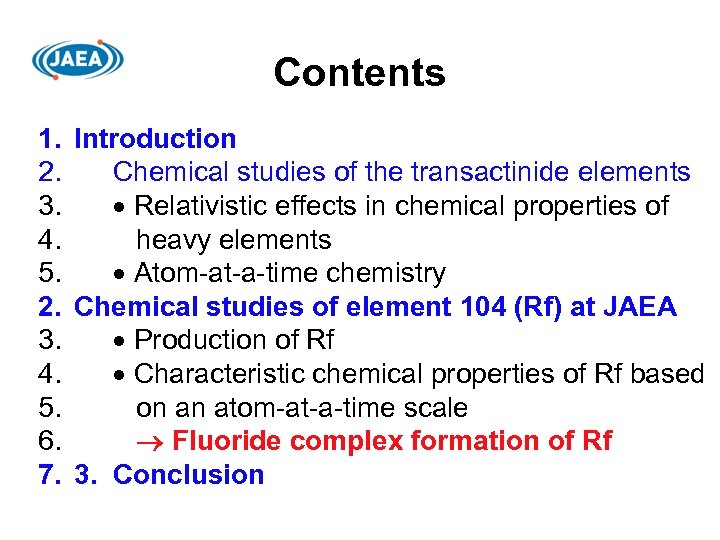 Contents 1. Introduction 2. Chemical studies of the transactinide elements 3. Relativistic effects in chemical properties of 4. heavy elements 5. Atom-at-a-time chemistry 2. Chemical studies of element 104 (Rf) at JAEA 3. Production of Rf 4. Characteristic chemical properties of Rf based 5. on an atom-at-a-time scale 6. Fluoride complex formation of Rf 7. 3. Conclusion
Contents 1. Introduction 2. Chemical studies of the transactinide elements 3. Relativistic effects in chemical properties of 4. heavy elements 5. Atom-at-a-time chemistry 2. Chemical studies of element 104 (Rf) at JAEA 3. Production of Rf 4. Characteristic chemical properties of Rf based 5. on an atom-at-a-time scale 6. Fluoride complex formation of Rf 7. 3. Conclusion
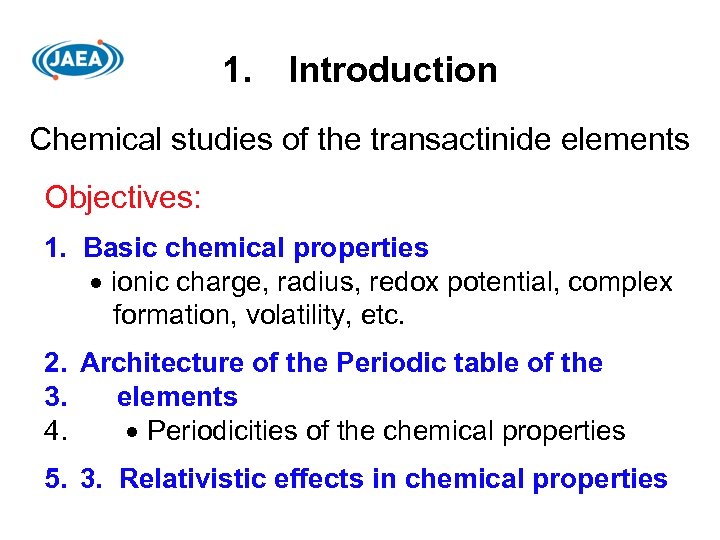 1. Introduction Chemical studies of the transactinide elements Objectives: 1. Basic chemical properties ionic charge, radius, redox potential, complex formation, volatility, etc. 2. Architecture of the Periodic table of the 3. elements 4. Periodicities of the chemical properties 5. 3. Relativistic effects in chemical properties
1. Introduction Chemical studies of the transactinide elements Objectives: 1. Basic chemical properties ionic charge, radius, redox potential, complex formation, volatility, etc. 2. Architecture of the Periodic table of the 3. elements 4. Periodicities of the chemical properties 5. 3. Relativistic effects in chemical properties
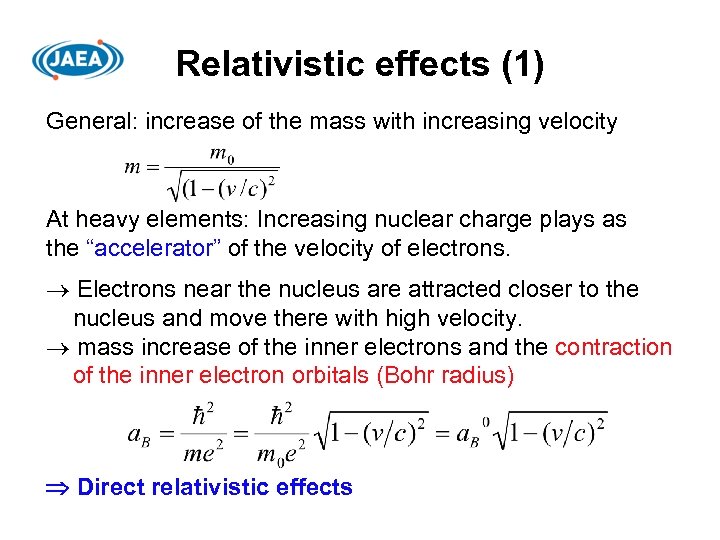 Relativistic effects (1) General: increase of the mass with increasing velocity At heavy elements: Increasing nuclear charge plays as the “accelerator” of the velocity of electrons. Electrons near the nucleus are attracted closer to the nucleus and move there with high velocity. mass increase of the inner electrons and the contraction of the inner electron orbitals (Bohr radius) Direct relativistic effects
Relativistic effects (1) General: increase of the mass with increasing velocity At heavy elements: Increasing nuclear charge plays as the “accelerator” of the velocity of electrons. Electrons near the nucleus are attracted closer to the nucleus and move there with high velocity. mass increase of the inner electrons and the contraction of the inner electron orbitals (Bohr radius) Direct relativistic effects
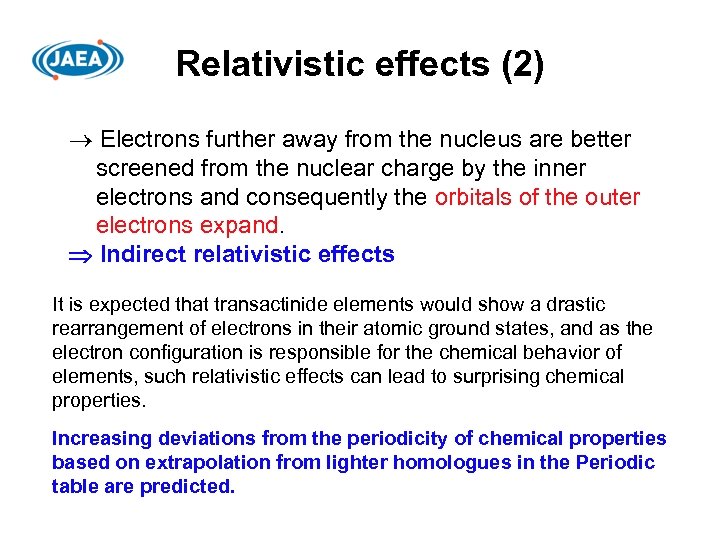 Relativistic effects (2) Electrons further away from the nucleus are better screened from the nuclear charge by the inner electrons and consequently the orbitals of the outer electrons expand. Indirect relativistic effects It is expected that transactinide elements would show a drastic rearrangement of electrons in their atomic ground states, and as the electron configuration is responsible for the chemical behavior of elements, such relativistic effects can lead to surprising chemical properties. Increasing deviations from the periodicity of chemical properties based on extrapolation from lighter homologues in the Periodic table are predicted.
Relativistic effects (2) Electrons further away from the nucleus are better screened from the nuclear charge by the inner electrons and consequently the orbitals of the outer electrons expand. Indirect relativistic effects It is expected that transactinide elements would show a drastic rearrangement of electrons in their atomic ground states, and as the electron configuration is responsible for the chemical behavior of elements, such relativistic effects can lead to surprising chemical properties. Increasing deviations from the periodicity of chemical properties based on extrapolation from lighter homologues in the Periodic table are predicted.
 Atom-at-a-time chemistry The transactinide elements must be produced at accelerators using reactions of heavy-ion beams with heavy target materials. Because of the short half-lives and the low production rates of the transactinide nuclides, each atom produced decays before a new atom is synthesized. Any chemistry to be performed must be done on an "atom-ata-time" basis. Rapid, very efficient and selective chemical procedures are indispensable to isolate desired transactinides. Repetitive experiments
Atom-at-a-time chemistry The transactinide elements must be produced at accelerators using reactions of heavy-ion beams with heavy target materials. Because of the short half-lives and the low production rates of the transactinide nuclides, each atom produced decays before a new atom is synthesized. Any chemistry to be performed must be done on an "atom-ata-time" basis. Rapid, very efficient and selective chemical procedures are indispensable to isolate desired transactinides. Repetitive experiments
 2. Chemical studies of rutherfordium (Rf, Z = 104) at JAEA
2. Chemical studies of rutherfordium (Rf, Z = 104) at JAEA
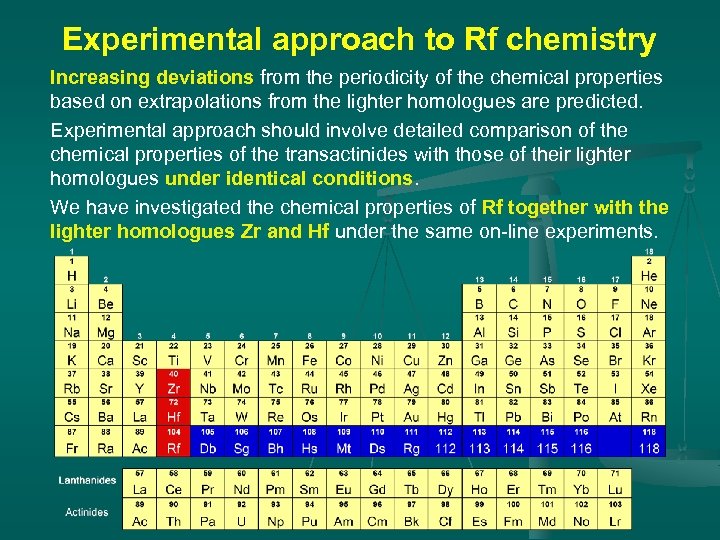 Experimental approach to Rf chemistry Increasing deviations from the periodicity of the chemical properties based on extrapolations from the lighter homologues are predicted. Experimental approach should involve detailed comparison of the chemical properties of the transactinides with those of their lighter homologues under identical conditions. We have investigated the chemical properties of Rf together with the lighter homologues Zr and Hf under the same on-line experiments.
Experimental approach to Rf chemistry Increasing deviations from the periodicity of the chemical properties based on extrapolations from the lighter homologues are predicted. Experimental approach should involve detailed comparison of the chemical properties of the transactinides with those of their lighter homologues under identical conditions. We have investigated the chemical properties of Rf together with the lighter homologues Zr and Hf under the same on-line experiments.
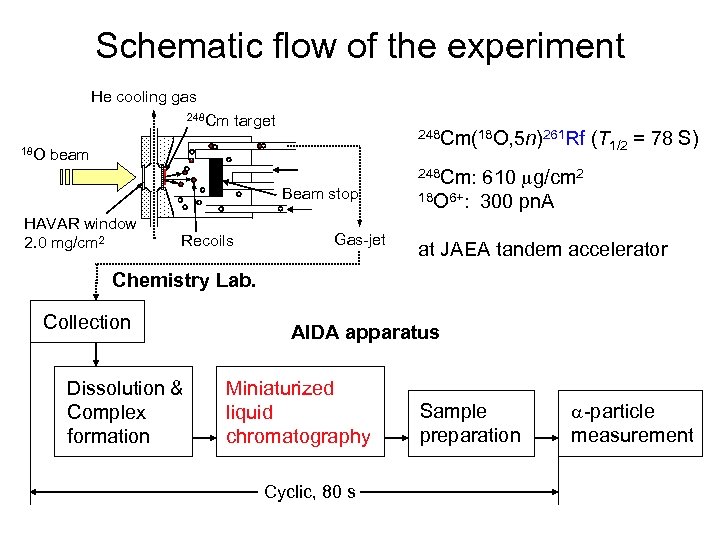 Schematic flow of the experiment He cooling gas 248 Cm 18 O target 248 Cm(18 O, 5 n)261 Rf beam Beam stop HAVAR window 2. 0 mg/cm 2 Recoils Gas-jet (T 1/2 = 78 S) 610 mg/cm 2 18 O 6+: 300 pn. A 248 Cm: at JAEA tandem accelerator Chemistry Lab. Collection Dissolution & Complex formation AIDA apparatus Miniaturized liquid chromatography Cyclic, 80 s Sample preparation a-particle measurement
Schematic flow of the experiment He cooling gas 248 Cm 18 O target 248 Cm(18 O, 5 n)261 Rf beam Beam stop HAVAR window 2. 0 mg/cm 2 Recoils Gas-jet (T 1/2 = 78 S) 610 mg/cm 2 18 O 6+: 300 pn. A 248 Cm: at JAEA tandem accelerator Chemistry Lab. Collection Dissolution & Complex formation AIDA apparatus Miniaturized liquid chromatography Cyclic, 80 s Sample preparation a-particle measurement
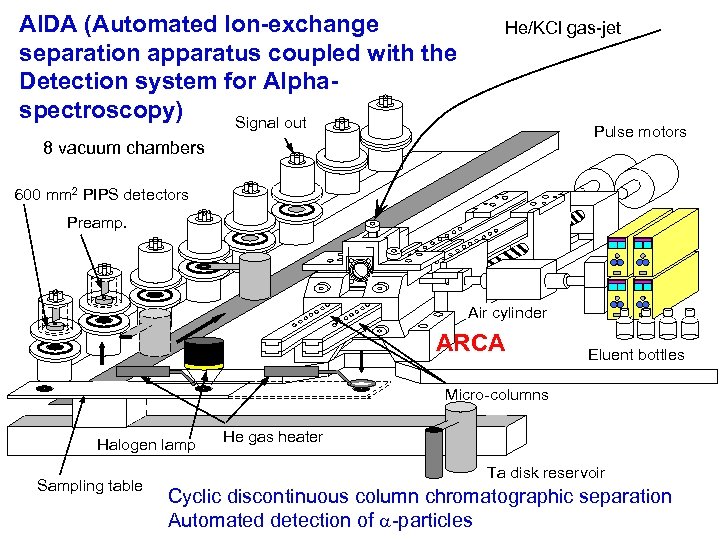 AIDA (Automated Ion-exchange separation apparatus coupled with the Detection system for Alphaspectroscopy) Signal out He/KCl gas-jet Pulse motors 8 vacuum chambers 600 mm 2 PIPS detectors Preamp. Air cylinder ARCA Eluent bottles Micro-columns Halogen lamp Sampling table He gas heater Ta disk reservoir Cyclic discontinuous column chromatographic separation Automated detection of a-particles
AIDA (Automated Ion-exchange separation apparatus coupled with the Detection system for Alphaspectroscopy) Signal out He/KCl gas-jet Pulse motors 8 vacuum chambers 600 mm 2 PIPS detectors Preamp. Air cylinder ARCA Eluent bottles Micro-columns Halogen lamp Sampling table He gas heater Ta disk reservoir Cyclic discontinuous column chromatographic separation Automated detection of a-particles
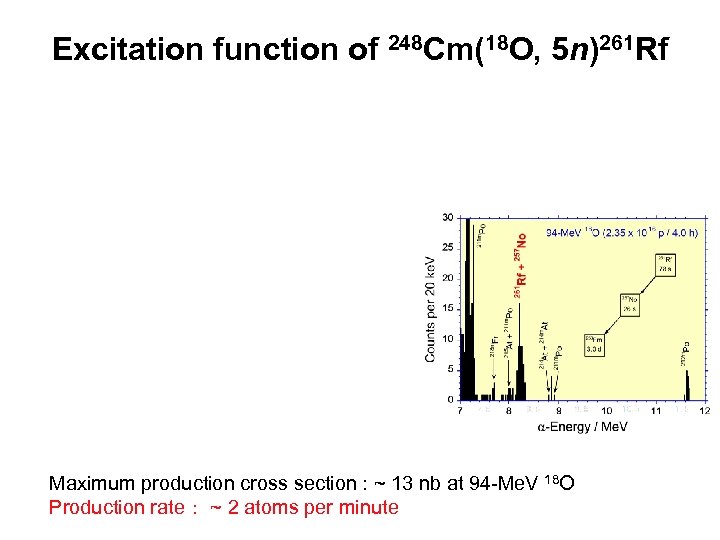 Excitation function of 248 Cm(18 O, 5 n)261 Rf Maximum production cross section : ~ 13 nb at 94 -Me. V 18 O Production rate: ~ 2 atoms per minute
Excitation function of 248 Cm(18 O, 5 n)261 Rf Maximum production cross section : ~ 13 nb at 94 -Me. V 18 O Production rate: ~ 2 atoms per minute
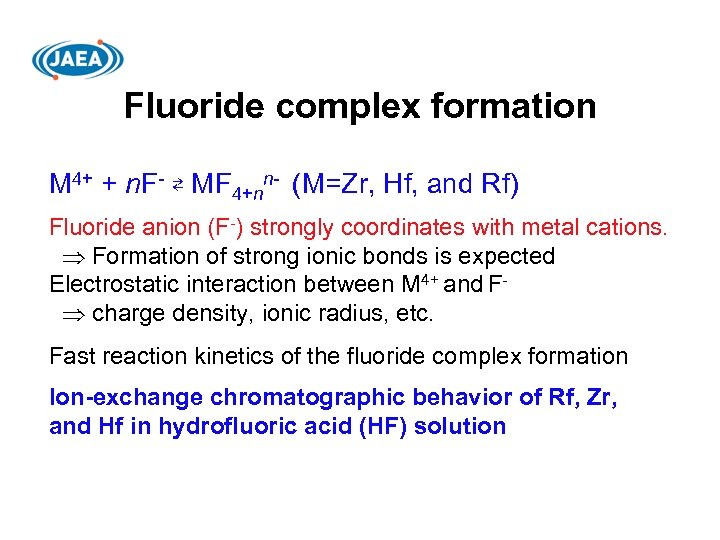 Fluoride complex formation M 4+ + n. F- ⇄ MF 4+nn- (M=Zr, Hf, and Rf) Fluoride anion (F-) strongly coordinates with metal cations. Formation of strong ionic bonds is expected Electrostatic interaction between M 4+ and F charge density, ionic radius, etc. Fast reaction kinetics of the fluoride complex formation Ion-exchange chromatographic behavior of Rf, Zr, and Hf in hydrofluoric acid (HF) solution
Fluoride complex formation M 4+ + n. F- ⇄ MF 4+nn- (M=Zr, Hf, and Rf) Fluoride anion (F-) strongly coordinates with metal cations. Formation of strong ionic bonds is expected Electrostatic interaction between M 4+ and F charge density, ionic radius, etc. Fast reaction kinetics of the fluoride complex formation Ion-exchange chromatographic behavior of Rf, Zr, and Hf in hydrofluoric acid (HF) solution
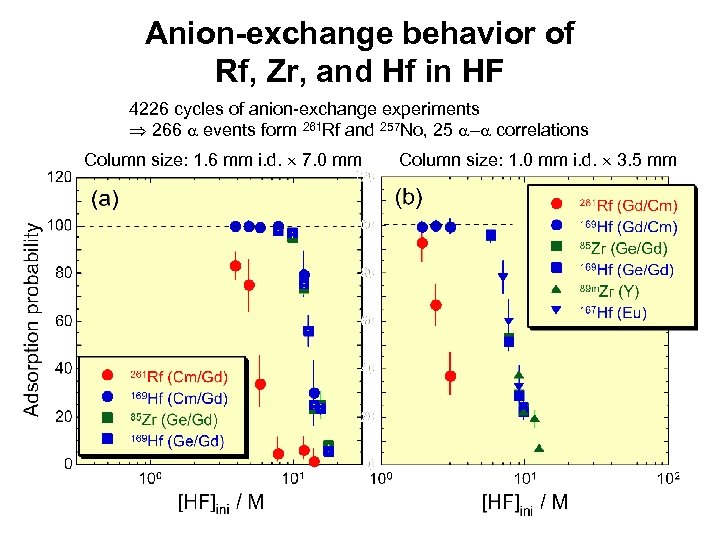 Anion-exchange behavior of Rf, Zr, and Hf in HF 4226 cycles of anion-exchange experiments 266 a events form 261 Rf and 257 No, 25 a-a correlations Column size: 1. 6 mm i. d. 7. 0 mm Column size: 1. 0 mm i. d. 3. 5 mm
Anion-exchange behavior of Rf, Zr, and Hf in HF 4226 cycles of anion-exchange experiments 266 a events form 261 Rf and 257 No, 25 a-a correlations Column size: 1. 6 mm i. d. 7. 0 mm Column size: 1. 0 mm i. d. 3. 5 mm
![Kd vs. [HF 2 -] HF ⇄ H+ + FHF + F- ⇄ HF Kd vs. [HF 2 -] HF ⇄ H+ + FHF + F- ⇄ HF](https://present5.com/presentation/e0f121b6e775f2784dd80e4970ae38cc/image-16.jpg) Kd vs. [HF 2 -] HF ⇄ H+ + FHF + F- ⇄ HF 2 - Rn-MF 4+n + n HF 2 - ⇄ n R-HF 2 + MF 4+nn- (M=Rf, Hf and Zr), R: resin [R-HF 2 ]n [MF 4+n n–] K= [Rn-MF 4+n ] [HF 2–]n log Kd = C - n log[HF 2 -] [M]r [Rn-MF 4+n ] Kd = = [M]aq [MF 4+n n–] = [R-HF 2 ]n [HF 2–]n slope = charge state of the metal complex
Kd vs. [HF 2 -] HF ⇄ H+ + FHF + F- ⇄ HF 2 - Rn-MF 4+n + n HF 2 - ⇄ n R-HF 2 + MF 4+nn- (M=Rf, Hf and Zr), R: resin [R-HF 2 ]n [MF 4+n n–] K= [Rn-MF 4+n ] [HF 2–]n log Kd = C - n log[HF 2 -] [M]r [Rn-MF 4+n ] Kd = = [M]aq [MF 4+n n–] = [R-HF 2 ]n [HF 2–]n slope = charge state of the metal complex
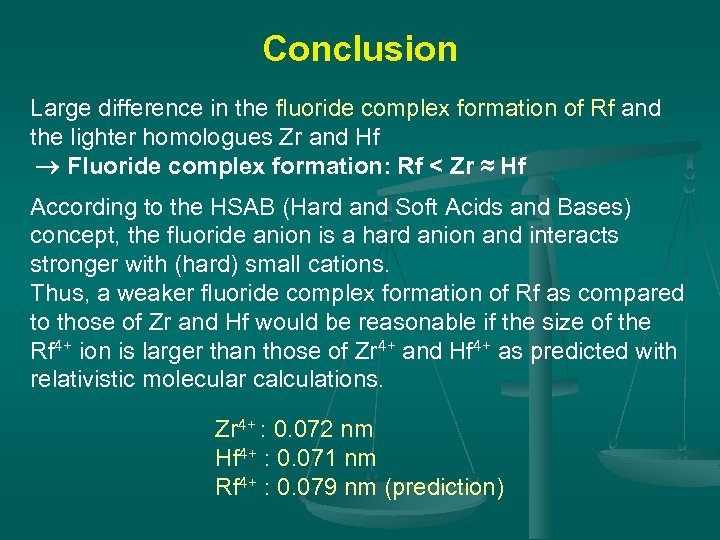 Conclusion Large difference in the fluoride complex formation of Rf and the lighter homologues Zr and Hf Fluoride complex formation: Rf < Zr ≈ Hf According to the HSAB (Hard and Soft Acids and Bases) concept, the fluoride anion is a hard anion and interacts stronger with (hard) small cations. Thus, a weaker fluoride complex formation of Rf as compared to those of Zr and Hf would be reasonable if the size of the Rf 4+ ion is larger than those of Zr 4+ and Hf 4+ as predicted with relativistic molecular calculations. Zr 4+ : 0. 072 nm Hf 4+ : 0. 071 nm Rf 4+ : 0. 079 nm (prediction)
Conclusion Large difference in the fluoride complex formation of Rf and the lighter homologues Zr and Hf Fluoride complex formation: Rf < Zr ≈ Hf According to the HSAB (Hard and Soft Acids and Bases) concept, the fluoride anion is a hard anion and interacts stronger with (hard) small cations. Thus, a weaker fluoride complex formation of Rf as compared to those of Zr and Hf would be reasonable if the size of the Rf 4+ ion is larger than those of Zr 4+ and Hf 4+ as predicted with relativistic molecular calculations. Zr 4+ : 0. 072 nm Hf 4+ : 0. 071 nm Rf 4+ : 0. 079 nm (prediction)
 Acknowledgement JAERI - M. Asai, M. Hirata, S. Ichikawa, T. Ichikawa, Y. Ishii, I. Nishinaka, T. K. Sato, H. Tome, A. Toyoshima, K. Tsukada, and T. Yaita RIKEN - H. Haba Osaka Univ. - H. Hasegawa, Y. Kitamoto, K. Matsuo, D. Saika, W. Sato, A. Shinohara, and Y. Tani Niigata Univ. - S. Goto, T. Hirai, H. Kudo, M. Ito, S. Ono, and J. Saito Tokyo Metropolitan Univ. - H. Nakahara and Y. Oura Univ. Tsukuba - K. Akiyama and K. Sueki Kanazawa Univ. - H. Kikunaga, N. Kinoshita, and A. Yokoyama Univ. Tokushima - M. Sakama GSI - W. Brüchle, V. Pershina, and M. Schädel Univ. Mainz - J. V. Kratz
Acknowledgement JAERI - M. Asai, M. Hirata, S. Ichikawa, T. Ichikawa, Y. Ishii, I. Nishinaka, T. K. Sato, H. Tome, A. Toyoshima, K. Tsukada, and T. Yaita RIKEN - H. Haba Osaka Univ. - H. Hasegawa, Y. Kitamoto, K. Matsuo, D. Saika, W. Sato, A. Shinohara, and Y. Tani Niigata Univ. - S. Goto, T. Hirai, H. Kudo, M. Ito, S. Ono, and J. Saito Tokyo Metropolitan Univ. - H. Nakahara and Y. Oura Univ. Tsukuba - K. Akiyama and K. Sueki Kanazawa Univ. - H. Kikunaga, N. Kinoshita, and A. Yokoyama Univ. Tokushima - M. Sakama GSI - W. Brüchle, V. Pershina, and M. Schädel Univ. Mainz - J. V. Kratz
![Kd vs. [NO 3]- in HF/HNO 3 Zr, Hf: slope = -2 [MF 6]2 Kd vs. [NO 3]- in HF/HNO 3 Zr, Hf: slope = -2 [MF 6]2](https://present5.com/presentation/e0f121b6e775f2784dd80e4970ae38cc/image-19.jpg) Kd vs. [NO 3]- in HF/HNO 3 Zr, Hf: slope = -2 [MF 6]2 - (M=Zr, Hf) HF ⇄ H+ + F(HF + F- ⇄ HF 2 -) HNO 3 ⇄ H+ + NO 3 - [F-] = 3 x 10 -3 M Rf: slope = -2 [Rf. F 6]2 closed (on-line) open (off-line) log Kd = C - n log[NO 3 -] Rn-MF 4+n + n NO 3 - ⇄ n R-NO 3 + MF 4+nn- : n = -2
Kd vs. [NO 3]- in HF/HNO 3 Zr, Hf: slope = -2 [MF 6]2 - (M=Zr, Hf) HF ⇄ H+ + F(HF + F- ⇄ HF 2 -) HNO 3 ⇄ H+ + NO 3 - [F-] = 3 x 10 -3 M Rf: slope = -2 [Rf. F 6]2 closed (on-line) open (off-line) log Kd = C - n log[NO 3 -] Rn-MF 4+n + n NO 3 - ⇄ n R-NO 3 + MF 4+nn- : n = -2
![Kd vs. [F-] in HF/HNO 3 MF 5 - MF 62 - Rf. F Kd vs. [F-] in HF/HNO 3 MF 5 - MF 62 - Rf. F](https://present5.com/presentation/e0f121b6e775f2784dd80e4970ae38cc/image-20.jpg) Kd vs. [F-] in HF/HNO 3 MF 5 - MF 62 - Rf. F 5 - Rf. F 62 - Rf (on-line) Zr (off-line) Hf (off-line) HF 2 counter ion 3 x 10 -3 M Formation of [MF 6]2 -: Zr Hf > Rf
Kd vs. [F-] in HF/HNO 3 MF 5 - MF 62 - Rf. F 5 - Rf. F 62 - Rf (on-line) Zr (off-line) Hf (off-line) HF 2 counter ion 3 x 10 -3 M Formation of [MF 6]2 -: Zr Hf > Rf
 Energy levels of the valence ns and (n-1)d electrons rel: relativistic nr: non-relativistic
Energy levels of the valence ns and (n-1)d electrons rel: relativistic nr: non-relativistic
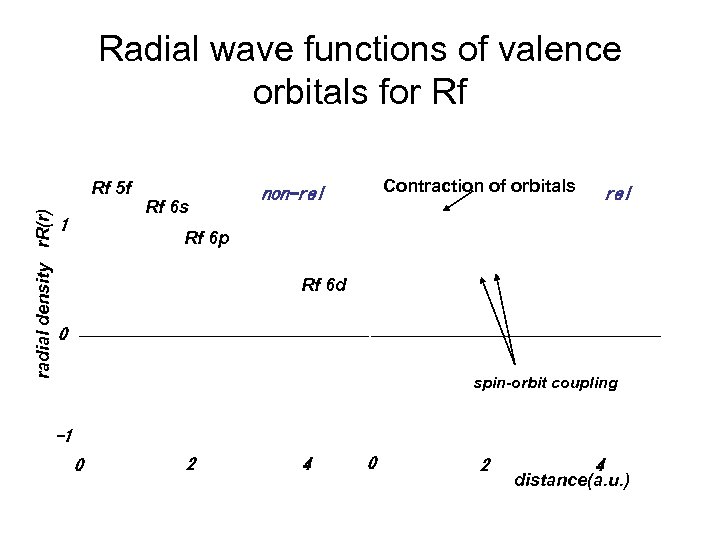 Radial wave functions of valence orbitals for Rf J radial density r. R(r) Rf 5 f 1 Rf 6 s A E R I Contraction of orbitals non-rel Rf 6 p Rf 6 d 0 spin-orbit coupling -1 0 2 4 distance(a. u. )
Radial wave functions of valence orbitals for Rf J radial density r. R(r) Rf 5 f 1 Rf 6 s A E R I Contraction of orbitals non-rel Rf 6 p Rf 6 d 0 spin-orbit coupling -1 0 2 4 distance(a. u. )
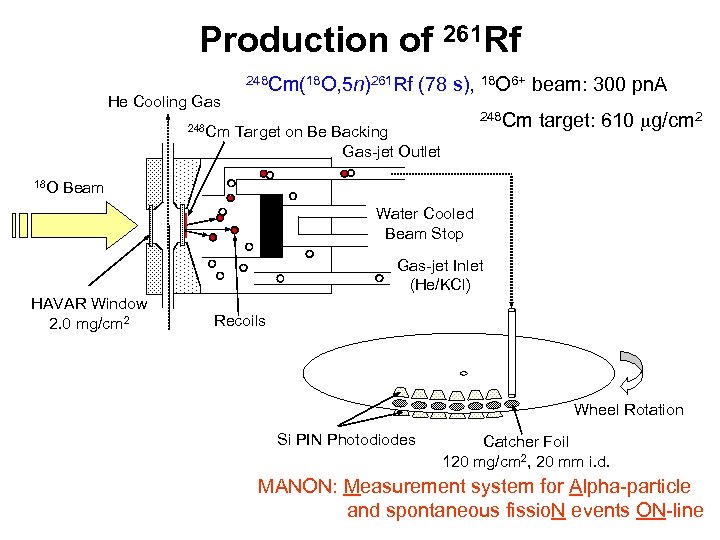 Production of 261 Rf He Cooling Gas 248 Cm 18 O 248 Cm(18 O, 5 n)261 Rf (78 s), 18 O 6+ beam: 300 pn. A 248 Cm Target on Be Backing Gas-jet Outlet target: 610 mg/cm 2 Beam Water Cooled Beam Stop Gas-jet Inlet (He/KCl) HAVAR Window 2. 0 mg/cm 2 Recoils Wheel Rotation Si PIN Photodiodes Catcher Foil 120 mg/cm 2, 20 mm i. d. MANON: Measurement system for Alpha-particle and spontaneous fissio. N events ON-line
Production of 261 Rf He Cooling Gas 248 Cm 18 O 248 Cm(18 O, 5 n)261 Rf (78 s), 18 O 6+ beam: 300 pn. A 248 Cm Target on Be Backing Gas-jet Outlet target: 610 mg/cm 2 Beam Water Cooled Beam Stop Gas-jet Inlet (He/KCl) HAVAR Window 2. 0 mg/cm 2 Recoils Wheel Rotation Si PIN Photodiodes Catcher Foil 120 mg/cm 2, 20 mm i. d. MANON: Measurement system for Alpha-particle and spontaneous fissio. N events ON-line
 Production rates of transactinide nuclides used for chemistry study
Production rates of transactinide nuclides used for chemistry study
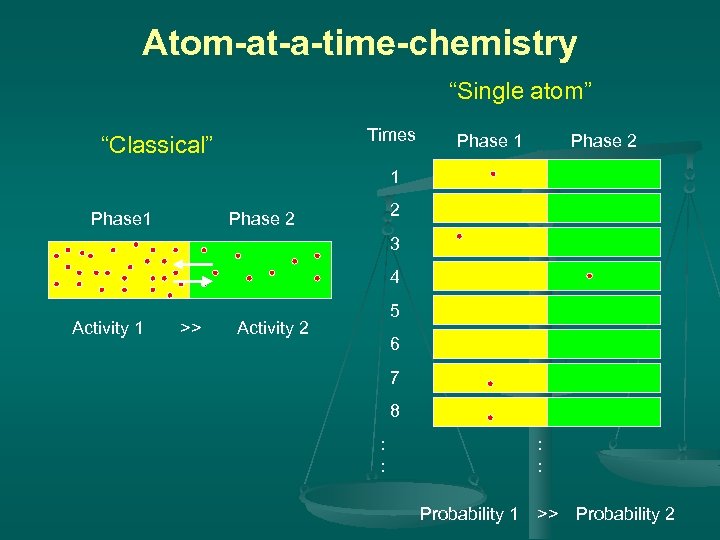 Atom-at-a-time-chemistry “Single atom” Times “Classical” Phase 1 Phase 2 2 3 4 Activity 1 >> Activity 2 5 6 7 8 : : : : Probability 1 >> Probability 2
Atom-at-a-time-chemistry “Single atom” Times “Classical” Phase 1 Phase 2 2 3 4 Activity 1 >> Activity 2 5 6 7 8 : : : : Probability 1 >> Probability 2
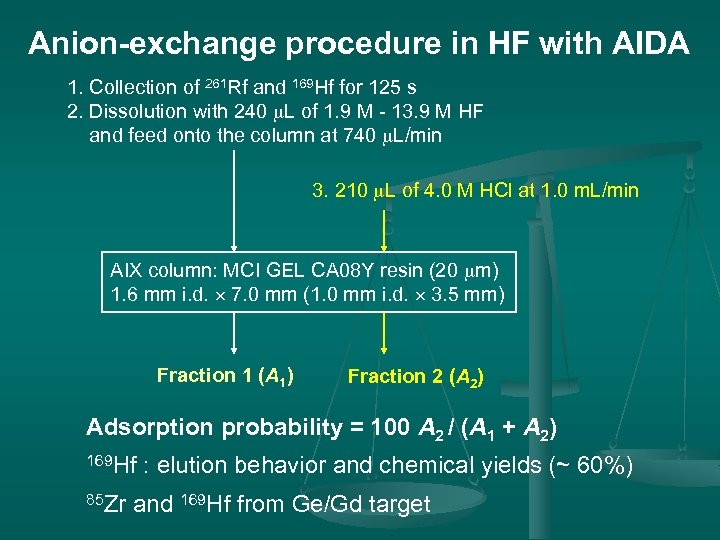 Anion-exchange procedure in HF with AIDA 1. Collection of 261 Rf and 169 Hf for 125 s 2. Dissolution with 240 m. L of 1. 9 M - 13. 9 M HF and feed onto the column at 740 m. L/min 3. 210 m. L of 4. 0 M HCl at 1. 0 m. L/min AIX column: MCI GEL CA 08 Y resin (20 mm) 1. 6 mm i. d. 7. 0 mm (1. 0 mm i. d. 3. 5 mm) Fraction 1 (A 1) Fraction 2 (A 2) Adsorption probability = 100 A 2 / (A 1 + A 2) 169 Hf 85 Zr : elution behavior and chemical yields (~ 60%) and 169 Hf from Ge/Gd target
Anion-exchange procedure in HF with AIDA 1. Collection of 261 Rf and 169 Hf for 125 s 2. Dissolution with 240 m. L of 1. 9 M - 13. 9 M HF and feed onto the column at 740 m. L/min 3. 210 m. L of 4. 0 M HCl at 1. 0 m. L/min AIX column: MCI GEL CA 08 Y resin (20 mm) 1. 6 mm i. d. 7. 0 mm (1. 0 mm i. d. 3. 5 mm) Fraction 1 (A 1) Fraction 2 (A 2) Adsorption probability = 100 A 2 / (A 1 + A 2) 169 Hf 85 Zr : elution behavior and chemical yields (~ 60%) and 169 Hf from Ge/Gd target
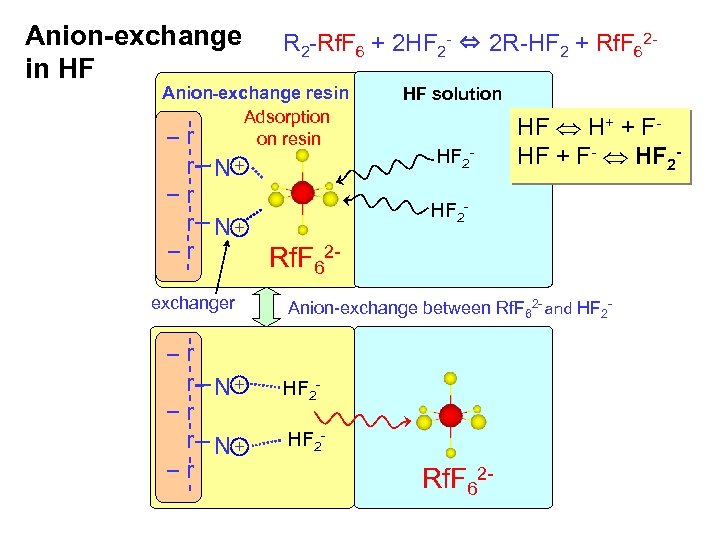 Anion-exchange in HF R 2 -Rf. F 6 + 2 HF 2 - ⇔ 2 R-HF 2 + Rf. F 62 - Anion-exchange resin Adsorption resin r r r N+ r Rf. F 62 N+ exchanger r r N+ r HF solution HF 2 - HF H+ + FHF + F- HF 2 - Anion-exchange between Rf. F 62 - and HF 2 - Rf. F 62 -
Anion-exchange in HF R 2 -Rf. F 6 + 2 HF 2 - ⇔ 2 R-HF 2 + Rf. F 62 - Anion-exchange resin Adsorption resin r r r N+ r Rf. F 62 N+ exchanger r r N+ r HF solution HF 2 - HF H+ + FHF + F- HF 2 - Anion-exchange between Rf. F 62 - and HF 2 - Rf. F 62 -
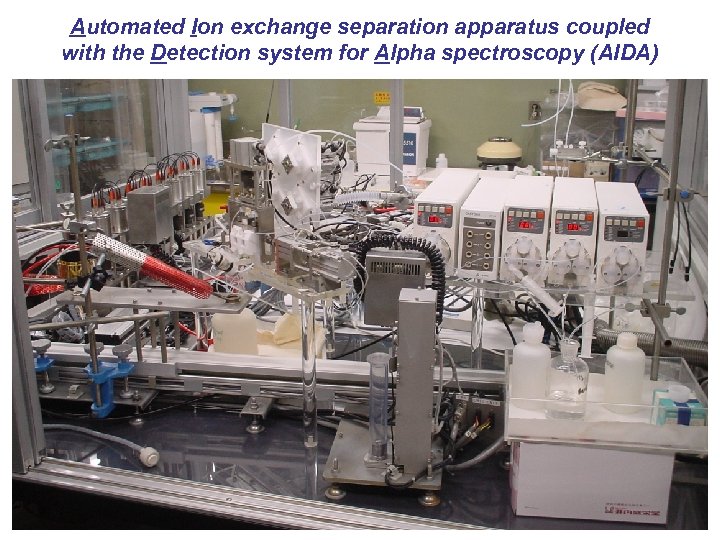 Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA)
Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA)
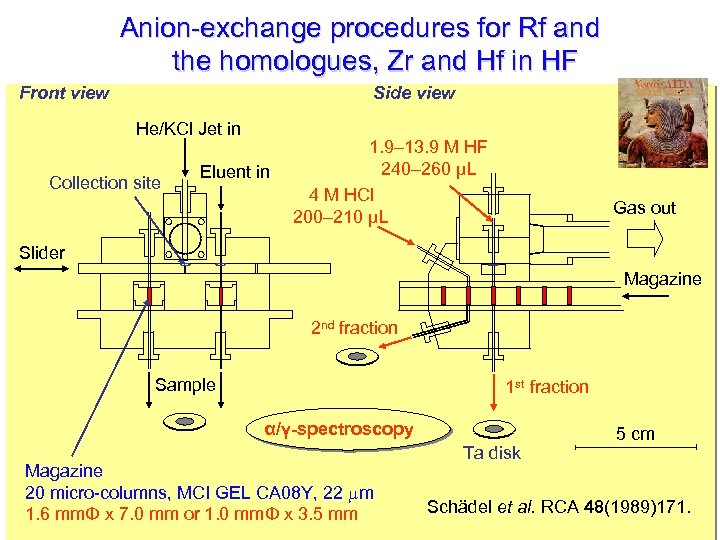 Anion-exchange procedures for Rf and the homologues, Zr and Hf in HF Front view Side view AIDAM HF 1. 9– 13. 9 He/KCl Jet in Collection site 240– 260 μL Eluent in 4 M HCl 200– 210 μL Gas out Slider Magazine 2 nd fraction Sample 1 st fraction α/γ-spectroscopy Magazine 20 micro-columns, MCI GEL CA 08 Y, 22 mm 1. 6 mmΦ x 7. 0 mm or 1. 0 mmΦ x 3. 5 mm Ta disk 5 cm Schädel et al. RCA 48(1989)171.
Anion-exchange procedures for Rf and the homologues, Zr and Hf in HF Front view Side view AIDAM HF 1. 9– 13. 9 He/KCl Jet in Collection site 240– 260 μL Eluent in 4 M HCl 200– 210 μL Gas out Slider Magazine 2 nd fraction Sample 1 st fraction α/γ-spectroscopy Magazine 20 micro-columns, MCI GEL CA 08 Y, 22 mm 1. 6 mmΦ x 7. 0 mm or 1. 0 mmΦ x 3. 5 mm Ta disk 5 cm Schädel et al. RCA 48(1989)171.
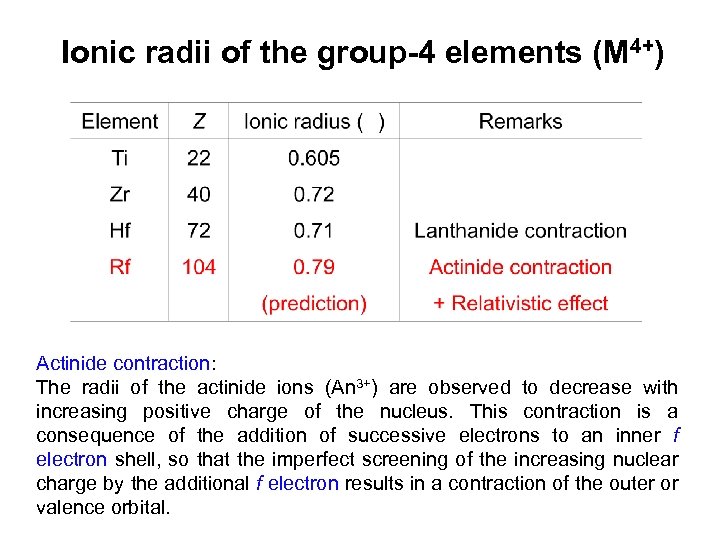 Ionic radii of the group-4 elements (M 4+) Actinide contraction: The radii of the actinide ions (An 3+) are observed to decrease with increasing positive charge of the nucleus. This contraction is a consequence of the addition of successive electrons to an inner f electron shell, so that the imperfect screening of the increasing nuclear charge by the additional f electron results in a contraction of the outer or valence orbital.
Ionic radii of the group-4 elements (M 4+) Actinide contraction: The radii of the actinide ions (An 3+) are observed to decrease with increasing positive charge of the nucleus. This contraction is a consequence of the addition of successive electrons to an inner f electron shell, so that the imperfect screening of the increasing nuclear charge by the additional f electron results in a contraction of the outer or valence orbital.
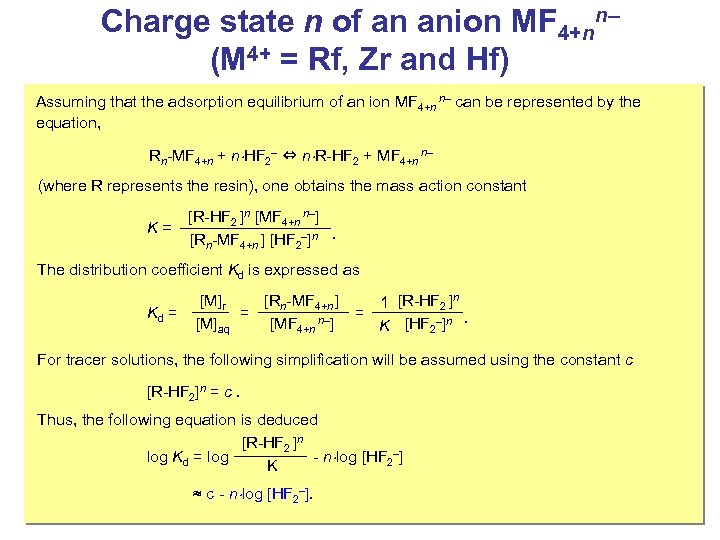 Charge state n of an anion MF 4+nn– (M 4+ = Rf, Zr and Hf) Assuming that the adsorption equilibrium of an ion MF 4+n n– can be represented by the equation, Rn-MF 4+n + n HF 2– ⇔ n R-HF 2 + MF 4+n n– (where R represents the resin), one obtains the mass action constant K= [R-HF 2 ]n [MF 4+n n–] [Rn-MF 4+n ] [HF 2–]n . The distribution coefficient Kd is expressed as Kd = [M]r [Rn-MF 4+n ] 1 [R-HF 2 ]n = = [M]aq [MF 4+n n–] K [HF 2–]n. For tracer solutions, the following simplification will be assumed using the constant c [R-HF 2]n = c. Thus, the following equation is deduced [R-HF 2 ]n log Kd = log - n log [HF 2–] K ≈ c - n log [HF 2–].
Charge state n of an anion MF 4+nn– (M 4+ = Rf, Zr and Hf) Assuming that the adsorption equilibrium of an ion MF 4+n n– can be represented by the equation, Rn-MF 4+n + n HF 2– ⇔ n R-HF 2 + MF 4+n n– (where R represents the resin), one obtains the mass action constant K= [R-HF 2 ]n [MF 4+n n–] [Rn-MF 4+n ] [HF 2–]n . The distribution coefficient Kd is expressed as Kd = [M]r [Rn-MF 4+n ] 1 [R-HF 2 ]n = = [M]aq [MF 4+n n–] K [HF 2–]n. For tracer solutions, the following simplification will be assumed using the constant c [R-HF 2]n = c. Thus, the following equation is deduced [R-HF 2 ]n log Kd = log - n log [HF 2–] K ≈ c - n log [HF 2–].
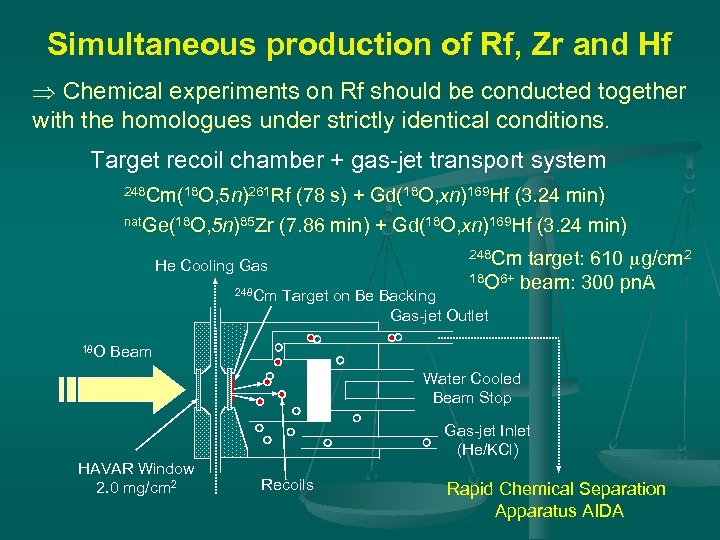 Simultaneous production of Rf, Zr and Hf Chemical experiments on Rf should be conducted together with the homologues under strictly identical conditions. Target recoil chamber + gas-jet transport system 248 Cm(18 O, 5 n)261 Rf (78 s) + Gd(18 O, xn)169 Hf (3. 24 min) nat. Ge(18 O, 5 n)85 Zr (7. 86 min) + Gd(18 O, xn)169 Hf (3. 24 min) 248 Cm 18 O target: 610 mg/cm 2 18 O 6+ beam: 300 pn. A 248 Cm He Cooling Gas Target on Be Backing Gas-jet Outlet Beam Water Cooled Beam Stop Gas-jet Inlet (He/KCl) HAVAR Window 2. 0 mg/cm 2 Recoils Rapid Chemical Separation Apparatus AIDA
Simultaneous production of Rf, Zr and Hf Chemical experiments on Rf should be conducted together with the homologues under strictly identical conditions. Target recoil chamber + gas-jet transport system 248 Cm(18 O, 5 n)261 Rf (78 s) + Gd(18 O, xn)169 Hf (3. 24 min) nat. Ge(18 O, 5 n)85 Zr (7. 86 min) + Gd(18 O, xn)169 Hf (3. 24 min) 248 Cm 18 O target: 610 mg/cm 2 18 O 6+ beam: 300 pn. A 248 Cm He Cooling Gas Target on Be Backing Gas-jet Outlet Beam Water Cooled Beam Stop Gas-jet Inlet (He/KCl) HAVAR Window 2. 0 mg/cm 2 Recoils Rapid Chemical Separation Apparatus AIDA
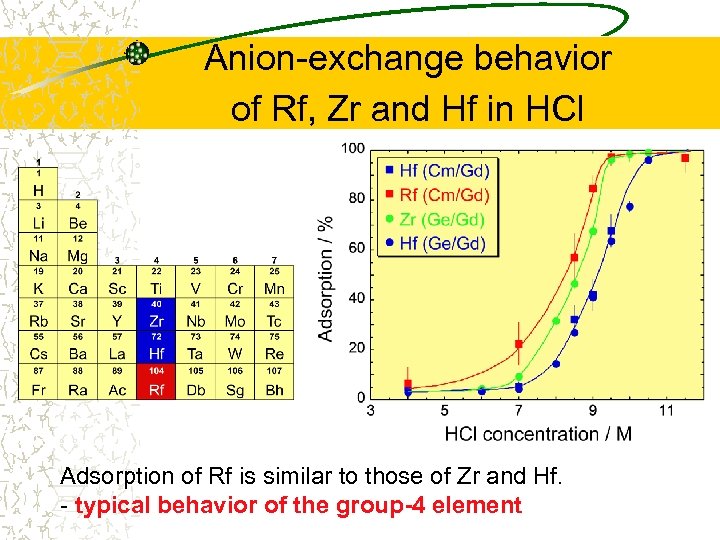 Anion-exchange behavior of Rf, Zr and Hf in HCl Adsorption of Rf is similar to those of Zr and Hf. - typical behavior of the group-4 element
Anion-exchange behavior of Rf, Zr and Hf in HCl Adsorption of Rf is similar to those of Zr and Hf. - typical behavior of the group-4 element
 Upper part of the chart of nuclides
Upper part of the chart of nuclides


