df18860db28f125e52e855bf3faadf65.ppt
- Количество слайдов: 25
 4. Chemical Arithmetic chapter 9 Greenhouse Gases (Chapt 8)
4. Chemical Arithmetic chapter 9 Greenhouse Gases (Chapt 8)
 The Law of Conservation of Mass Matter can neither be created nor destroyed in a chemical reaction.
The Law of Conservation of Mass Matter can neither be created nor destroyed in a chemical reaction.
 The Mole • 1 mole of anything contains 6. 02 x 1023 units (Avogadro’s number) • In chemistry 1 mole of anything weighs the same as its molecular weight in grams • So for methane CH 4, atomic weight of C=12 and atomic weight of H=1. • Thus one mole of methane weighs 12+4(1)=16 grams
The Mole • 1 mole of anything contains 6. 02 x 1023 units (Avogadro’s number) • In chemistry 1 mole of anything weighs the same as its molecular weight in grams • So for methane CH 4, atomic weight of C=12 and atomic weight of H=1. • Thus one mole of methane weighs 12+4(1)=16 grams
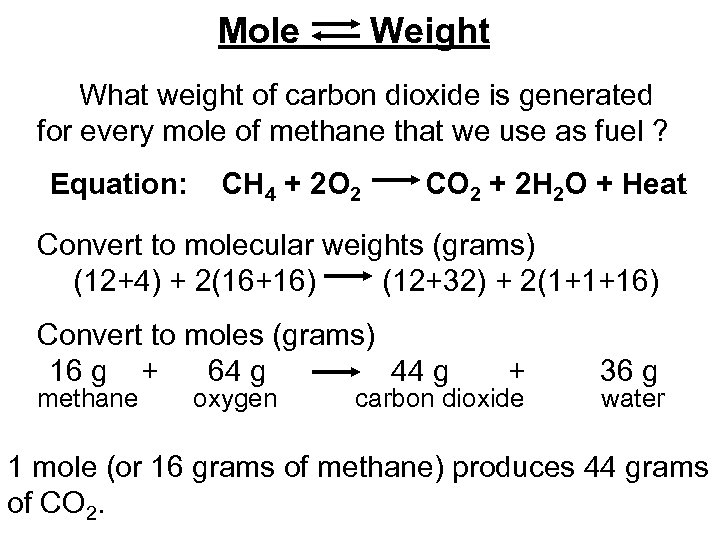 Mole Weight What weight of carbon dioxide is generated for every mole of methane that we use as fuel ? Equation: CH 4 + 2 O 2 CO 2 + 2 H 2 O + Heat Convert to molecular weights (grams) (12+4) + 2(16+16) (12+32) + 2(1+1+16) Convert to moles (grams) 16 g + 64 g 44 g methane oxygen + carbon dioxide 36 g water 1 mole (or 16 grams of methane) produces 44 grams of CO 2.
Mole Weight What weight of carbon dioxide is generated for every mole of methane that we use as fuel ? Equation: CH 4 + 2 O 2 CO 2 + 2 H 2 O + Heat Convert to molecular weights (grams) (12+4) + 2(16+16) (12+32) + 2(1+1+16) Convert to moles (grams) 16 g + 64 g 44 g methane oxygen + carbon dioxide 36 g water 1 mole (or 16 grams of methane) produces 44 grams of CO 2.
 Ramifications for Global Warming • Combustion of carbonaceous fuels produces CO 2 (a greenhouse gas) and heat • Greenhouse effect-glass walls and roof of a greenhouse are transparent to visible light, but opaque to “thermal infrared” • Surfaces inside the greenhouse absorb solar radiation and reradiate thermal IR
Ramifications for Global Warming • Combustion of carbonaceous fuels produces CO 2 (a greenhouse gas) and heat • Greenhouse effect-glass walls and roof of a greenhouse are transparent to visible light, but opaque to “thermal infrared” • Surfaces inside the greenhouse absorb solar radiation and reradiate thermal IR
 On the molecular level • Surfaces then reradiate the heat as long wavelength IR radiation. • Greenhouse gases absorb this IR radiation –causes them to bend & vibrate • H 2 O, CH 4, CO 2 do this , but major air components N 2 (79%) and O 2 (20%) are IR opaque • Collisions between greenhouse gases and air molecules occur and temp of air rises.
On the molecular level • Surfaces then reradiate the heat as long wavelength IR radiation. • Greenhouse gases absorb this IR radiation –causes them to bend & vibrate • H 2 O, CH 4, CO 2 do this , but major air components N 2 (79%) and O 2 (20%) are IR opaque • Collisions between greenhouse gases and air molecules occur and temp of air rises.
 In the atmosphere • Same effect-solar radiation is absorbed by the earth (pavement etc). Eventually heat is re-emitted as IR radiation • Then greenhouse gases absorb this IR radiation and transfer the heat to N 2 and O 2 by molecular collisions.
In the atmosphere • Same effect-solar radiation is absorbed by the earth (pavement etc). Eventually heat is re-emitted as IR radiation • Then greenhouse gases absorb this IR radiation and transfer the heat to N 2 and O 2 by molecular collisions.
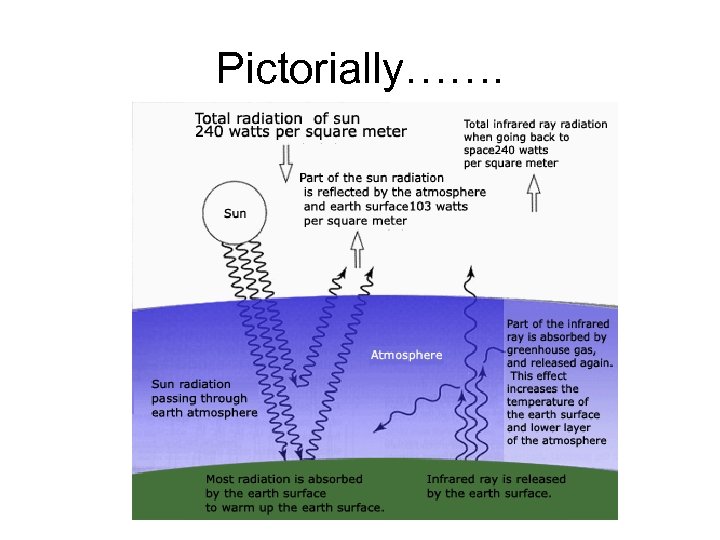 Pictorially…….
Pictorially…….
 Is the greenhouse effect a good thing? • No • Yes
Is the greenhouse effect a good thing? • No • Yes
 Yes! (in general) • Without it, the Earth’s average temp would drop from a present value of ~15 o. C, to 15 o. C • Problem, excess amounts of CO 2, CH 4 do contribute to global warming • But, changes in strength of solar radiation may also be having an effect
Yes! (in general) • Without it, the Earth’s average temp would drop from a present value of ~15 o. C, to 15 o. C • Problem, excess amounts of CO 2, CH 4 do contribute to global warming • But, changes in strength of solar radiation may also be having an effect
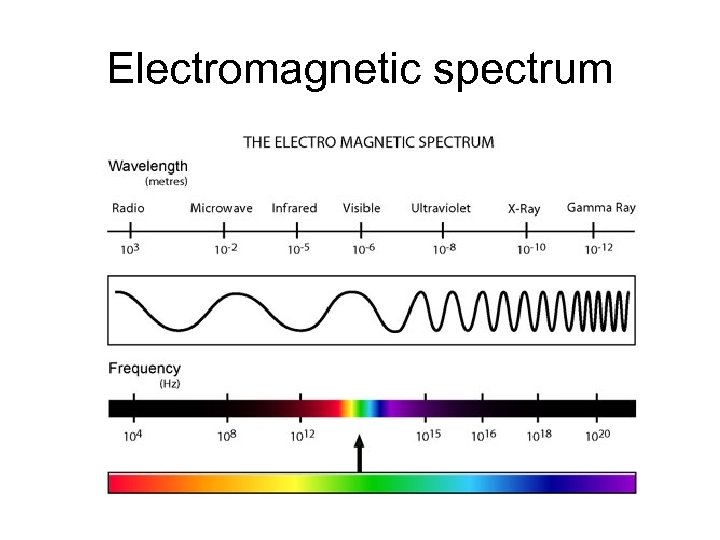 Electromagnetic spectrum
Electromagnetic spectrum
 Wave and particle character • Energy of radiation is quantized (particle) • frequency and wavelength are related (wave) • 2 equations govern all regions (a continuum)
Wave and particle character • Energy of radiation is quantized (particle) • frequency and wavelength are related (wave) • 2 equations govern all regions (a continuum)
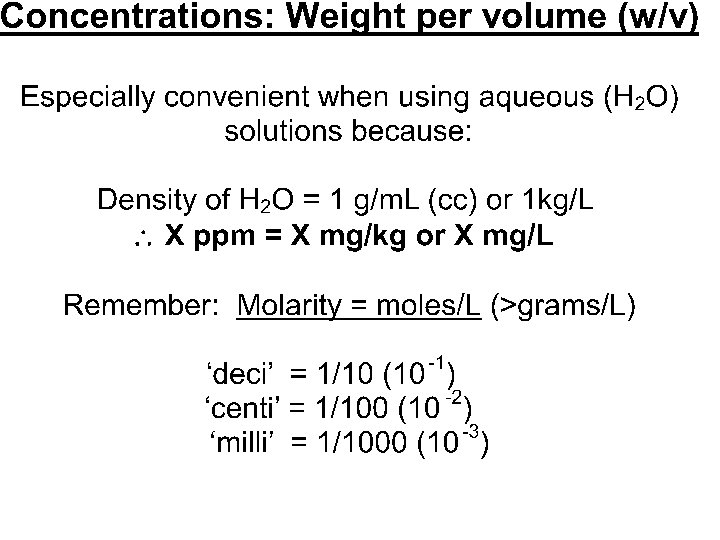
 Concentrations A solution is a homogeneous mixture of one substance (solute) dissolved in another(solvent). The solute is present in a smaller proportion than the solvent. The concentration of a solution expresses the quantity of solute(eg. moles or grams) dissolved in a specific quantity of solution (usually stated as a volume). Terms and units used to express concentrations can be: a) imprecise - 'teaspoon per glass' b) approximate (labels for consumer products) - 'percent' c) well-defined - 'molarity'
Concentrations A solution is a homogeneous mixture of one substance (solute) dissolved in another(solvent). The solute is present in a smaller proportion than the solvent. The concentration of a solution expresses the quantity of solute(eg. moles or grams) dissolved in a specific quantity of solution (usually stated as a volume). Terms and units used to express concentrations can be: a) imprecise - 'teaspoon per glass' b) approximate (labels for consumer products) - 'percent' c) well-defined - 'molarity'
 Molarity. The molarity of a solution refers simply to the number of moles of solute per liter of solution. A 1 M (one molar) solution contains one mole of solute in each liter of solution, a 2 M solution contains two moles of solute per liter of solution, etc.
Molarity. The molarity of a solution refers simply to the number of moles of solute per liter of solution. A 1 M (one molar) solution contains one mole of solute in each liter of solution, a 2 M solution contains two moles of solute per liter of solution, etc.
 Percentage Concentrations(parts per 100) Commercial labels often express concentrations of solutes as a percentage (%) of weight or volume. Eg. a typical bottle of table vinegar states the product is "diluted with water to 5% acidity" or contains 5 g of acetic acid in every 100 g of vinegar. A percentage concentration expressing the weight of solute for every 100 units of weight of the solution is a weight / weight percentage, or w/w %. A 3% solution of H 2 O 2 in water makes up the common antiseptic hydrogen peroxide , but 30% H 2 O 2 bleaches hair (don’t mix them up !)
Percentage Concentrations(parts per 100) Commercial labels often express concentrations of solutes as a percentage (%) of weight or volume. Eg. a typical bottle of table vinegar states the product is "diluted with water to 5% acidity" or contains 5 g of acetic acid in every 100 g of vinegar. A percentage concentration expressing the weight of solute for every 100 units of weight of the solution is a weight / weight percentage, or w/w %. A 3% solution of H 2 O 2 in water makes up the common antiseptic hydrogen peroxide , but 30% H 2 O 2 bleaches hair (don’t mix them up !)
 Concentrations of Fat in milk • 1% milk contains 1 gram of fat per 100 grams of milk. • Q: How many grams of fat in a 250 m. L carton of 1% milk? • A: Assume density of milk is 1 gram per m. L. (D=M/V). • Thus the weight of fat in each carton is 250/100 =2. 5 grams • Skim milk contains 0% fat –does it have any calories in it? Yes, since all milk contains protein and carbohydrate.
Concentrations of Fat in milk • 1% milk contains 1 gram of fat per 100 grams of milk. • Q: How many grams of fat in a 250 m. L carton of 1% milk? • A: Assume density of milk is 1 gram per m. L. (D=M/V). • Thus the weight of fat in each carton is 250/100 =2. 5 grams • Skim milk contains 0% fat –does it have any calories in it? Yes, since all milk contains protein and carbohydrate.
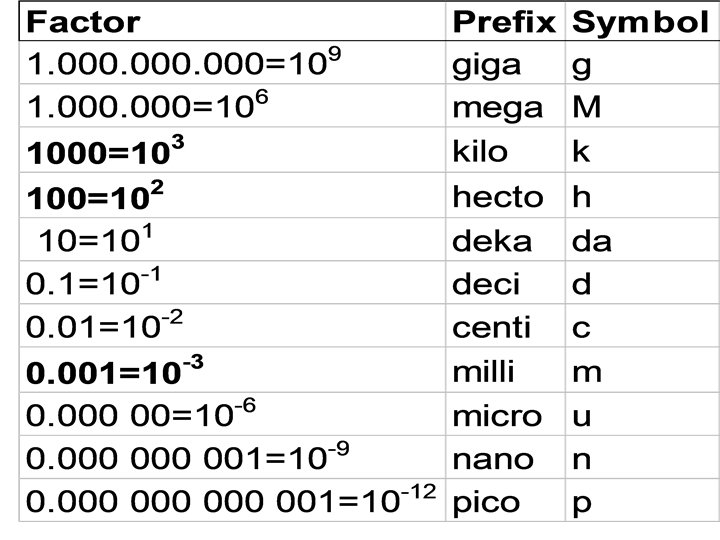
 It is convenient to express exceedingly small concentrations, such as food contaminants and environmental pollutants, as parts per thousand (ppt), parts per million, parts per billion(ppb) One part per million (1 ppm) represents a convenient unit since it is the concentration of one milligram (1/1000 gram) of one substance distributed throughout one kilogram (1000 grams) of another, ie. 1 mg/kg.
It is convenient to express exceedingly small concentrations, such as food contaminants and environmental pollutants, as parts per thousand (ppt), parts per million, parts per billion(ppb) One part per million (1 ppm) represents a convenient unit since it is the concentration of one milligram (1/1000 gram) of one substance distributed throughout one kilogram (1000 grams) of another, ie. 1 mg/kg.
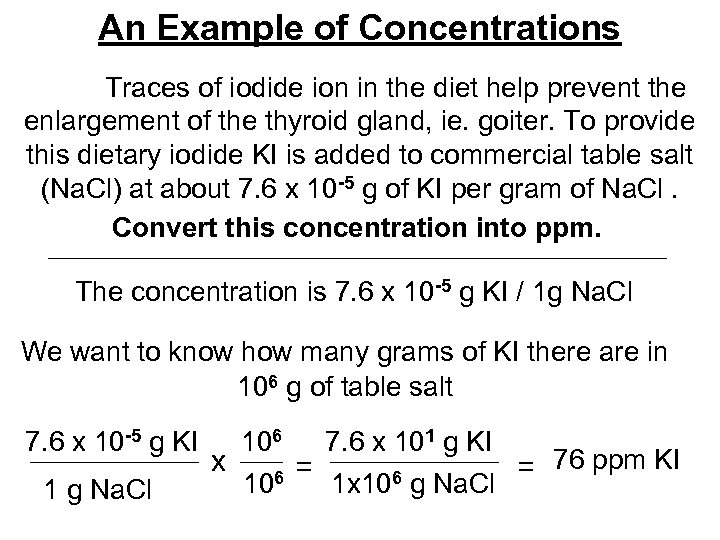 An Example of Concentrations Traces of iodide ion in the diet help prevent the enlargement of the thyroid gland, ie. goiter. To provide this dietary iodide KI is added to commercial table salt (Na. Cl) at about 7. 6 x 10 -5 g of KI per gram of Na. Cl. Convert this concentration into ppm. The concentration is 7. 6 x 10 -5 g KI / 1 g Na. Cl We want to know how many grams of KI there are in 106 g of table salt 7. 6 x 10 -5 g KI 1 g Na. Cl 106 7. 6 x 101 g KI x = = 76 ppm KI 106 1 x 106 g Na. Cl
An Example of Concentrations Traces of iodide ion in the diet help prevent the enlargement of the thyroid gland, ie. goiter. To provide this dietary iodide KI is added to commercial table salt (Na. Cl) at about 7. 6 x 10 -5 g of KI per gram of Na. Cl. Convert this concentration into ppm. The concentration is 7. 6 x 10 -5 g KI / 1 g Na. Cl We want to know how many grams of KI there are in 106 g of table salt 7. 6 x 10 -5 g KI 1 g Na. Cl 106 7. 6 x 101 g KI x = = 76 ppm KI 106 1 x 106 g Na. Cl
 Homeopathic Dilutions Homeopathic medicine is based on ‘similarity’ between the disease and symptoms ‘induced’ by the medicine. Extreme dilutions are common and the cure is induced (supposedly) by a ‘memory effect’. The mode of action is unknown but the treatments are effective for some people.
Homeopathic Dilutions Homeopathic medicine is based on ‘similarity’ between the disease and symptoms ‘induced’ by the medicine. Extreme dilutions are common and the cure is induced (supposedly) by a ‘memory effect’. The mode of action is unknown but the treatments are effective for some people.
 Each dilution is 1/100, ie. 10 -2. Six successive dil’ns (10 -12) are usual, 12 dilutions (10 -24) are common and even 30 dilutions (10 -60!) are used. If a 1 M sol’n contains 6 x 1023 molecules (approx. 1 x 1024) A 12 times diluted sol’n contains 1024 x 10 -24 = 1 molecule in 1 Liter! If you buy 10 m. L of this sol’n what are your chances of getting that single molecule ?
Each dilution is 1/100, ie. 10 -2. Six successive dil’ns (10 -12) are usual, 12 dilutions (10 -24) are common and even 30 dilutions (10 -60!) are used. If a 1 M sol’n contains 6 x 1023 molecules (approx. 1 x 1024) A 12 times diluted sol’n contains 1024 x 10 -24 = 1 molecule in 1 Liter! If you buy 10 m. L of this sol’n what are your chances of getting that single molecule ?
 Would you buy it? Odds are 10/1000=. 01 or 1 % chance of getting a single molecule of the medicine in your sample.
Would you buy it? Odds are 10/1000=. 01 or 1 % chance of getting a single molecule of the medicine in your sample.
 Homeopathic Medicines
Homeopathic Medicines
 Fancy Names in Homeopathy • “Kalium Muriaticum” 5 CH means 5 dilutions of a factor of 100 each. • So 10 -10 M concentration of KCl (present in normal table salt) • Muriatic acid is 31% HCl in water • Kalium is Latin for potassium (K in Periodic Table)
Fancy Names in Homeopathy • “Kalium Muriaticum” 5 CH means 5 dilutions of a factor of 100 each. • So 10 -10 M concentration of KCl (present in normal table salt) • Muriatic acid is 31% HCl in water • Kalium is Latin for potassium (K in Periodic Table)


