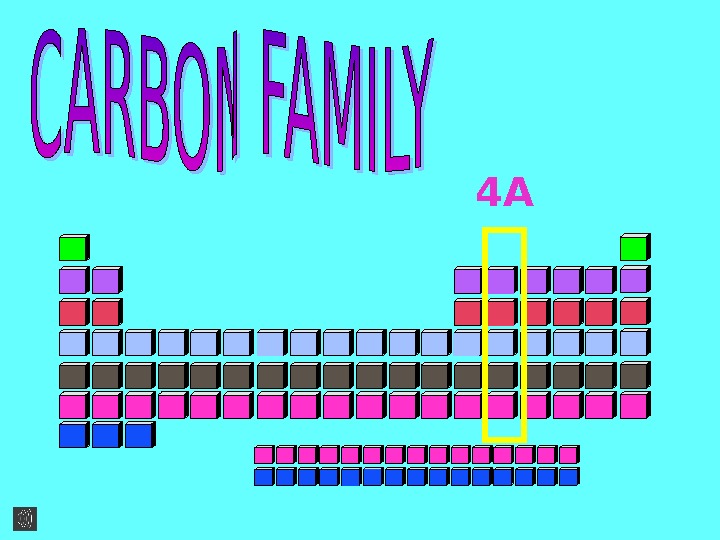
4 A CARBON FAMILY HAS 5





















- Размер: 5.3 Mегабайта
- Количество слайдов: 25
Описание презентации 4 A CARBON FAMILY HAS 5 по слайдам

 CARBON FAMILY HAS 5 MEMBERS. Carbon C * *Silicon Si. G e rm a n iu m * G e *T in S n *Lead Pb
CARBON FAMILY HAS 5 MEMBERS. Carbon C * *Silicon Si. G e rm a n iu m * G e *T in S n *Lead Pb
 Carbon is a nonmetal. Carbon has mainly two allotropes; diamond and graphite. It is usually +4 charged.
Carbon is a nonmetal. Carbon has mainly two allotropes; diamond and graphite. It is usually +4 charged.
 Allotropes Was it night when I was born, Mummy
Allotropes Was it night when I was born, Mummy
 Allotropes Graphite Soft, dark black solid. Good conductor. Used in dry cells, in pencils and as lubricant. Forms sp 2 hybrids. Diamond Formed by transformation of graphite. The hardest and transparent Has very high melting and boiling point. Nonconductor. Forms sp 3 hybrids. Used as an abrasive, as jewellery.
Allotropes Graphite Soft, dark black solid. Good conductor. Used in dry cells, in pencils and as lubricant. Forms sp 2 hybrids. Diamond Formed by transformation of graphite. The hardest and transparent Has very high melting and boiling point. Nonconductor. Forms sp 3 hybrids. Used as an abrasive, as jewellery.
 Carbon is seen in atmosphere as CO 2 , in earth’s crust as carbonate compounds, and minerals. It is the basic element in the living organisms. For example, in the structure of proteins, and carbohydrates.
Carbon is seen in atmosphere as CO 2 , in earth’s crust as carbonate compounds, and minerals. It is the basic element in the living organisms. For example, in the structure of proteins, and carbohydrates.
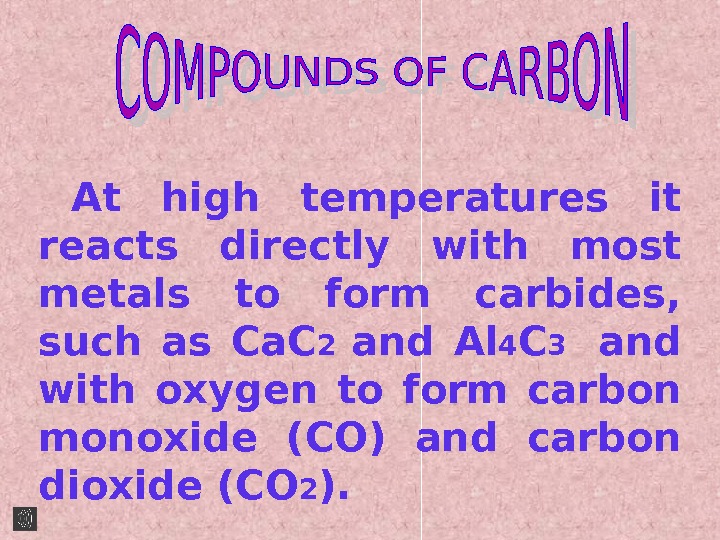
 At high temperatures it reacts directly with most metals to form carbides, such as Ca. C 2 and Al 4 C 3 and with oxygen to form carbon monoxide (CO) and carbon dioxide (CO 2 ).
At high temperatures it reacts directly with most metals to form carbides, such as Ca. C 2 and Al 4 C 3 and with oxygen to form carbon monoxide (CO) and carbon dioxide (CO 2 ).
 Carbon has two oxides; CO and CO 2 . Incomplete combustion produces carbon monoxide. It is very dangerous and suffocates when in taken. It is bonded to hemoglobin and carried to tissues and causes deadness.
Carbon has two oxides; CO and CO 2 . Incomplete combustion produces carbon monoxide. It is very dangerous and suffocates when in taken. It is bonded to hemoglobin and carried to tissues and causes deadness.
 Complete combustion produces carbon dioxide. It forms a layer around the atmosphere which allows the sunlight in but prevents going out as in a greenhouse . So the climate changes and becomes hot and hot. This is called Greenhouse effect.
Complete combustion produces carbon dioxide. It forms a layer around the atmosphere which allows the sunlight in but prevents going out as in a greenhouse . So the climate changes and becomes hot and hot. This is called Greenhouse effect.
 CO 2 is an acidic oxide and so it is used in production of some gaseous drinks. And also used to put out the fire. CO 2 is the basic material in producing of baking soda Na. HCO 3. Solid CO 2 is known as dry ice.
CO 2 is an acidic oxide and so it is used in production of some gaseous drinks. And also used to put out the fire. CO 2 is the basic material in producing of baking soda Na. HCO 3. Solid CO 2 is known as dry ice.
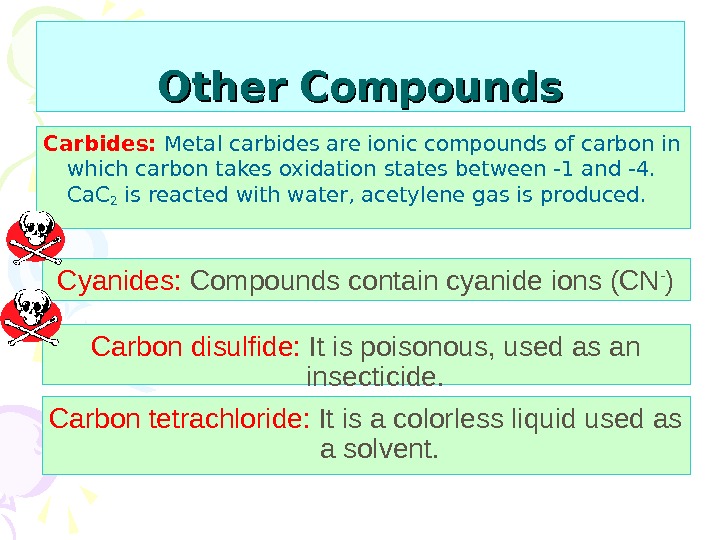
 Other Compounds Carbides: Metal carbides are ionic compounds of carbon in which carbon takes oxidation states between -1 and -4. Ca. C 2 is reacted with water, acetylene gas is produced. Cyanides: Compounds contain cyanide ions (CN — ) Carbon disulfide: It is poisonous, used as an insecticide. Carbon tetrachloride: It is a colorless liquid used as a solvent.
Other Compounds Carbides: Metal carbides are ionic compounds of carbon in which carbon takes oxidation states between -1 and -4. Ca. C 2 is reacted with water, acetylene gas is produced. Cyanides: Compounds contain cyanide ions (CN — ) Carbon disulfide: It is poisonous, used as an insecticide. Carbon tetrachloride: It is a colorless liquid used as a solvent.
 Carbon has one important radioactive isotope ; carbon-14. It is used in the radiocarbon dating to estimate the age of fossils and other organic materials. Coal, wood coal, coke and soot are the other shapes of carbon.
Carbon has one important radioactive isotope ; carbon-14. It is used in the radiocarbon dating to estimate the age of fossils and other organic materials. Coal, wood coal, coke and soot are the other shapes of carbon.
 Some uses of carbon 14 C is used in archeology dating. Used in gas masks to absorb pollutants and poisonous gases. Bleaching of sugar. Printers and photocopy machines as toner. Improves wear resistance of tires. Diamond is used in jewelry and to cut hard substances. Used to produce dye.
Some uses of carbon 14 C is used in archeology dating. Used in gas masks to absorb pollutants and poisonous gases. Bleaching of sugar. Printers and photocopy machines as toner. Improves wear resistance of tires. Diamond is used in jewelry and to cut hard substances. Used to produce dye.
 Carbon
Carbon
 Silicon is the most important element of mineral world. It has a metallic appearance and known as very hard and brittle, dark-gray solid.
Silicon is the most important element of mineral world. It has a metallic appearance and known as very hard and brittle, dark-gray solid.
 Physical properties of silicon likes to metalloids. But it’s chemical properties likes to nonmetals. It is found in the form of compounds as silicon dioxide and complex silicates that consists of 95% of rocks. Silicon dioxide, Si. O 2 is known as sand quartz.
Physical properties of silicon likes to metalloids. But it’s chemical properties likes to nonmetals. It is found in the form of compounds as silicon dioxide and complex silicates that consists of 95% of rocks. Silicon dioxide, Si. O 2 is known as sand quartz.
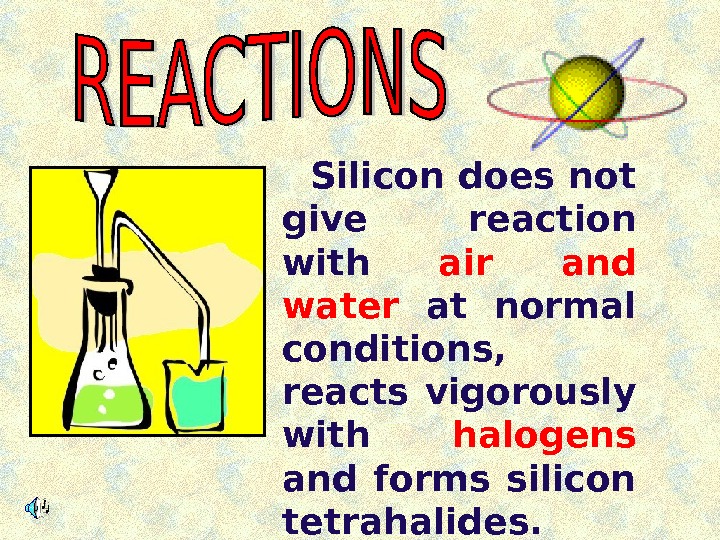
 Silicon does not give reaction with air and water at normal conditions, reacts vigorously with halogens and forms silicon tetrahalides.
Silicon does not give reaction with air and water at normal conditions, reacts vigorously with halogens and forms silicon tetrahalides.
 Silicon is attacked by hot and strong bases. Silicon does not react with most acids under normal condition but is dissolved by hydrofluoric acid, HF.
Silicon is attacked by hot and strong bases. Silicon does not react with most acids under normal condition but is dissolved by hydrofluoric acid, HF.
 Silicon is a semiconductor. So, it is used to make microchips for electronics (e. g. computers), Silicon is used in the steel industry as a constituent of silicon-steel alloys.
Silicon is a semiconductor. So, it is used to make microchips for electronics (e. g. computers), Silicon is used in the steel industry as a constituent of silicon-steel alloys.
 Silica and silicates are used in the manufacture of glass, glazes, enamels, cement, and porcelain, and have important individual applications. Oxide of silicon is known as silica; Si. O 2.
Silica and silicates are used in the manufacture of glass, glazes, enamels, cement, and porcelain, and have important individual applications. Oxide of silicon is known as silica; Si. O 2.
 It has as a structure just like that of diamond That’s why, Si. O 2 is very hard as diamond. Si. O 2 minerals are used in production of souvenir. If it contains Mn. O or Ti. O 2 in its structure , it is called as amethyst or sapphire respectively.
It has as a structure just like that of diamond That’s why, Si. O 2 is very hard as diamond. Si. O 2 minerals are used in production of souvenir. If it contains Mn. O or Ti. O 2 in its structure , it is called as amethyst or sapphire respectively.
 Metallic element that has been used by people since ancient times. It is used as a protective coating for copper vessels, used in the tin cans, and similar articles.
Metallic element that has been used by people since ancient times. It is used as a protective coating for copper vessels, used in the tin cans, and similar articles.
 Tin is important in the production of the common alloys; Bronze (tin and copper), Solder (tin and lead), Type metal (tin, lead, and antimony) It is also used as an alloy with titanium in the aerospace industry and as an ingredient in some insecticides.
Tin is important in the production of the common alloys; Bronze (tin and copper), Solder (tin and lead), Type metal (tin, lead, and antimony) It is also used as an alloy with titanium in the aerospace industry and as an ingredient in some insecticides.
 Tin Sn
Tin Sn
 SAVE THE ENVIRONMENT !
SAVE THE ENVIRONMENT !

