c81aba301ef46689ac320337b3215b3d.ppt
- Количество слайдов: 39
 34 th EMWA Conference Challenges of Pediatric Drug Development & Impact of Pediatric Legislation (Plenary Lecture) Dr. med. Klaus Rose, M. D. , M. S. Pediatric Drug Development & More klausrose Consulting klaus. rose@klausrose. net 1
34 th EMWA Conference Challenges of Pediatric Drug Development & Impact of Pediatric Legislation (Plenary Lecture) Dr. med. Klaus Rose, M. D. , M. S. Pediatric Drug Development & More klausrose Consulting klaus. rose@klausrose. net 1
 Conclusions • • • No easy black or white conclusions. No more drug development without considering children Increases cost & complexity of drug development EMA/PDCO: nice vision; limited interest in economic reality; bureaucratic procedures; not all needed PIP skills are science Invested resources could be used better – as is mostly the outcome of complex decision making Reviews 2013/18: opposing proposals will be made Costs/ benefit is difficult to quantify due to confidentiality Law drives child research in some areas; road block in others There will be some future clinical benefit for children It will ensure more work for many groups including medical writers. Background understanding remains essential klaus. rose@klausrose. net 2
Conclusions • • • No easy black or white conclusions. No more drug development without considering children Increases cost & complexity of drug development EMA/PDCO: nice vision; limited interest in economic reality; bureaucratic procedures; not all needed PIP skills are science Invested resources could be used better – as is mostly the outcome of complex decision making Reviews 2013/18: opposing proposals will be made Costs/ benefit is difficult to quantify due to confidentiality Law drives child research in some areas; road block in others There will be some future clinical benefit for children It will ensure more work for many groups including medical writers. Background understanding remains essential klaus. rose@klausrose. net 2
 Why Pediatric Pharmaceutical Legislation? Official Objectives on EMA Website: • Facilitate development of availability of Medicines for Children (Mf. C) from birth to < 18 y • Ensure that Mf. C‘s are of high quality, ethically researched, and authorised appropriately • Improve availability of information on the use of Mf. C • Q: Would such a program have made sense 1950? klaus. rose@klausrose. net 3
Why Pediatric Pharmaceutical Legislation? Official Objectives on EMA Website: • Facilitate development of availability of Medicines for Children (Mf. C) from birth to < 18 y • Ensure that Mf. C‘s are of high quality, ethically researched, and authorised appropriately • Improve availability of information on the use of Mf. C • Q: Would such a program have made sense 1950? klaus. rose@klausrose. net 3
 Why a Legislation on Mf. C? - Klaus’ Tentative Answers • Benefit of pharmaceutical treatment in adults • Scientific progress in clinical pharmacology, pediatric clinical pharmacology & pediatric medicine • General high interest in health • Obvious wealth of Big Pharma • Big Pharma’s reputation • Politicians’ preference: spend somebody else’s money klaus. rose@klausrose. net 4
Why a Legislation on Mf. C? - Klaus’ Tentative Answers • Benefit of pharmaceutical treatment in adults • Scientific progress in clinical pharmacology, pediatric clinical pharmacology & pediatric medicine • General high interest in health • Obvious wealth of Big Pharma • Big Pharma’s reputation • Politicians’ preference: spend somebody else’s money klaus. rose@klausrose. net 4
 Progress in Clinical Pharmacology: Key Publication Kearns, 2003, NEJM • Absorption, distribution, metabolization, excretion in children are different from adults • Maturation is not linear and not in parallel • Variability much higher • Drugs in children often underdosed / overdosed klaus. rose@klausrose. net 5
Progress in Clinical Pharmacology: Key Publication Kearns, 2003, NEJM • Absorption, distribution, metabolization, excretion in children are different from adults • Maturation is not linear and not in parallel • Variability much higher • Drugs in children often underdosed / overdosed klaus. rose@klausrose. net 5
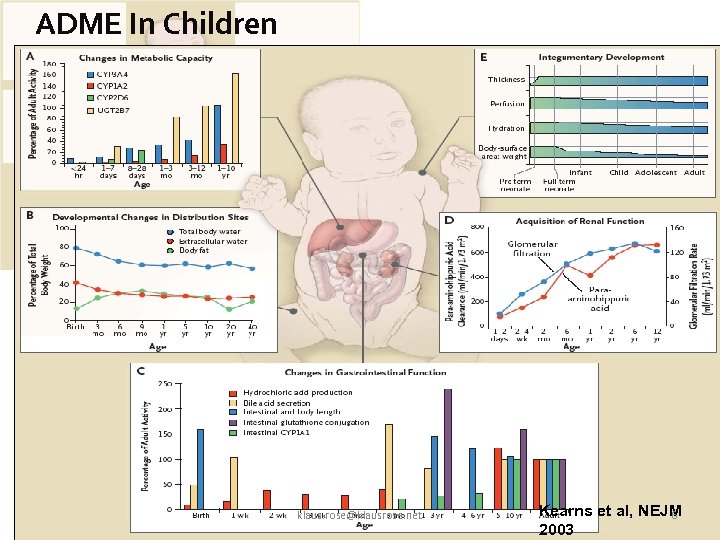 ADME In Children klaus. rose@klausrose. net Kearns et al, NEJM 6 2003
ADME In Children klaus. rose@klausrose. net Kearns et al, NEJM 6 2003
 Iron Lungs For Children With Polio 1950 ies 7
Iron Lungs For Children With Polio 1950 ies 7
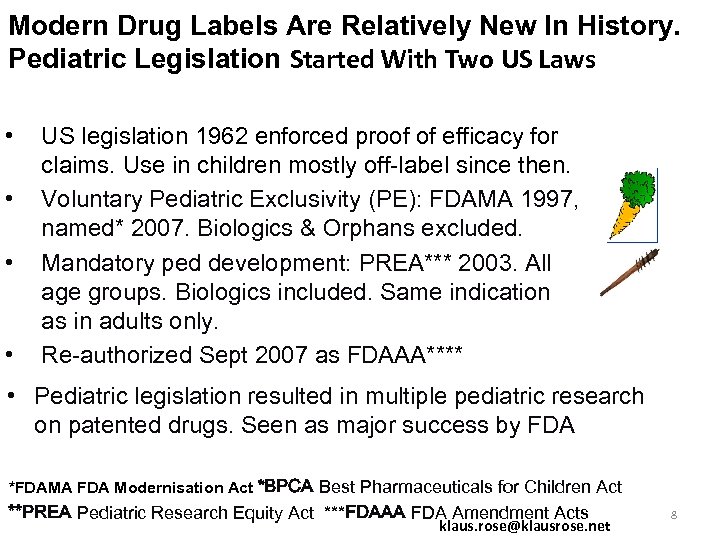 Modern Drug Labels Are Relatively New In History. Pediatric Legislation Started With Two US Laws • • US legislation 1962 enforced proof of efficacy for claims. Use in children mostly off-label since then. Voluntary Pediatric Exclusivity (PE): FDAMA 1997, named* 2007. Biologics & Orphans excluded. Mandatory ped development: PREA*** 2003. All age groups. Biologics included. Same indication as in adults only. Re-authorized Sept 2007 as FDAAA**** • Pediatric legislation resulted in multiple pediatric research on patented drugs. Seen as major success by FDA *FDAMA FDA Modernisation Act *BPCA Best Pharmaceuticals for Children Act **PREA Pediatric Research Equity Act ***FDAAA FDA Amendment Acts klaus. rose@klausrose. net 8
Modern Drug Labels Are Relatively New In History. Pediatric Legislation Started With Two US Laws • • US legislation 1962 enforced proof of efficacy for claims. Use in children mostly off-label since then. Voluntary Pediatric Exclusivity (PE): FDAMA 1997, named* 2007. Biologics & Orphans excluded. Mandatory ped development: PREA*** 2003. All age groups. Biologics included. Same indication as in adults only. Re-authorized Sept 2007 as FDAAA**** • Pediatric legislation resulted in multiple pediatric research on patented drugs. Seen as major success by FDA *FDAMA FDA Modernisation Act *BPCA Best Pharmaceuticals for Children Act **PREA Pediatric Research Equity Act ***FDAAA FDA Amendment Acts klaus. rose@klausrose. net 8
 EU Pediatric Regulation • In force since January 2007 • Combines mandatory development • Pediatric Investigation Plan (PIP) mandatory @ of human PK • PIP must cover all age groups • Ped Committee (PDCO) assesses PIPs, waivers & deferrals • Reward of six months SPC* prolongation • EMA will not validate submission without agreed PIP • PDCO members + alternates (66) represent EU states+CHMP • EMA team: 20 pediatric coordinators with reward *SPC Supplementary Protection Certificate klaus. rose@klausrose. net 9
EU Pediatric Regulation • In force since January 2007 • Combines mandatory development • Pediatric Investigation Plan (PIP) mandatory @ of human PK • PIP must cover all age groups • Ped Committee (PDCO) assesses PIPs, waivers & deferrals • Reward of six months SPC* prolongation • EMA will not validate submission without agreed PIP • PDCO members + alternates (66) represent EU states+CHMP • EMA team: 20 pediatric coordinators with reward *SPC Supplementary Protection Certificate klaus. rose@klausrose. net 9
 Drugs Were Developed For Children Before 1997 – Where There Was A Market • Vaccines: children • Lung Surfactant: preterm newborns • Growth hormone: Dornase-alfa (pulmozyme): Cystic Fibrosis • Iboprufen: pain relief in adults; arterial duct in newborns • Antibiotics • Cough & cold medication: not always beneficial klaus. rose@klausrose. net 10
Drugs Were Developed For Children Before 1997 – Where There Was A Market • Vaccines: children • Lung Surfactant: preterm newborns • Growth hormone: Dornase-alfa (pulmozyme): Cystic Fibrosis • Iboprufen: pain relief in adults; arterial duct in newborns • Antibiotics • Cough & cold medication: not always beneficial klaus. rose@klausrose. net 10
 Labels in the Past Showcard 1918. 19. Jahrhundert Source: www. wellcomecollection. org klaus. rose@klausrose. net 11
Labels in the Past Showcard 1918. 19. Jahrhundert Source: www. wellcomecollection. org klaus. rose@klausrose. net 11
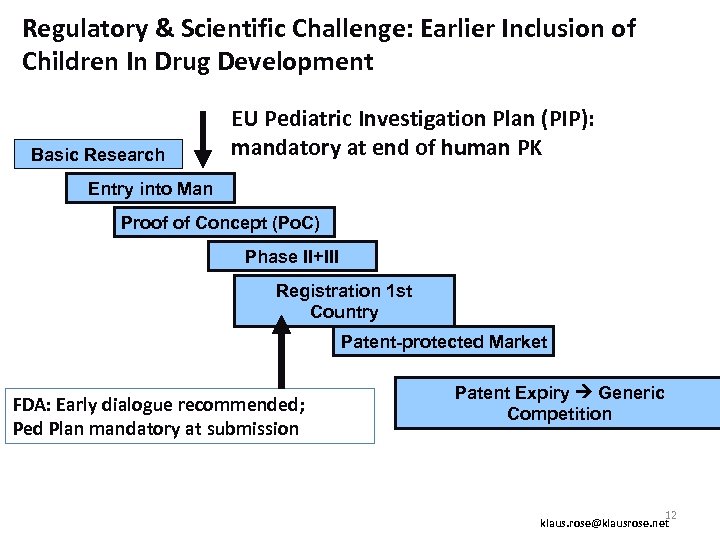 Regulatory & Scientific Challenge: Earlier Inclusion of Children In Drug Development Basic Research EU Pediatric Investigation Plan (PIP): mandatory at end of human PK Entry into Man Proof of Concept (Po. C) Phase II+III Registration 1 st Country Patent-protected Market FDA: Early dialogue recommended; Ped Plan mandatory at submission Patent Expiry Generic Competition 12 klaus. rose@klausrose. net
Regulatory & Scientific Challenge: Earlier Inclusion of Children In Drug Development Basic Research EU Pediatric Investigation Plan (PIP): mandatory at end of human PK Entry into Man Proof of Concept (Po. C) Phase II+III Registration 1 st Country Patent-protected Market FDA: Early dialogue recommended; Ped Plan mandatory at submission Patent Expiry Generic Competition 12 klaus. rose@klausrose. net
 Waivers & Deferrals • Waivers are given for all children or specific age groups • Age classification based on ICH E 11 • Waivers only if drug is probably ineffective/ unsafe; disease not in children; no significant therapeutic benefit • Deferral allows company to perform pediatric measures (studies, technical development etc. ) later • Only concrete measures can be deferred 13
Waivers & Deferrals • Waivers are given for all children or specific age groups • Age classification based on ICH E 11 • Waivers only if drug is probably ineffective/ unsafe; disease not in children; no significant therapeutic benefit • Deferral allows company to perform pediatric measures (studies, technical development etc. ) later • Only concrete measures can be deferred 13
 PIP: When? • Should be submitted in time • Better not too early, and never too late • Too early: potential added workload, need for later modification • Too late: can block submission è There is no perfect recommendation klaus. rose@klausrose. net 14
PIP: When? • Should be submitted in time • Better not too early, and never too late • Too early: potential added workload, need for later modification • Too late: can block submission è There is no perfect recommendation klaus. rose@klausrose. net 14
 Dialogue Partners • Decisions: PDCO • Dialogue: EMA Pediatric coordinator; PDCO rapporteur + peer reviewer • Procedure usually 275 days, rarely less, can be much more • Dialogue primarily with EMA coordinator; clarification TCs with coordinator, PDCO rapporteur & peer reviewer • F 2 F with PDCO at the end of procedure only (Oral Explanation) klaus. rose@klausrose. net 15
Dialogue Partners • Decisions: PDCO • Dialogue: EMA Pediatric coordinator; PDCO rapporteur + peer reviewer • Procedure usually 275 days, rarely less, can be much more • Dialogue primarily with EMA coordinator; clarification TCs with coordinator, PDCO rapporteur & peer reviewer • F 2 F with PDCO at the end of procedure only (Oral Explanation) klaus. rose@klausrose. net 15
 Key People In The PIP Negotiation • EMA pediatric coordinator – focus on procedures, but … • PDCO rapporteur • PDCO peer reviewer • Pre-submission meeting (TC) possible since spring 2011 • Whatever you discuss, final decision by PDCO only klaus. rose@klausrose. net 16
Key People In The PIP Negotiation • EMA pediatric coordinator – focus on procedures, but … • PDCO rapporteur • PDCO peer reviewer • Pre-submission meeting (TC) possible since spring 2011 • Whatever you discuss, final decision by PDCO only klaus. rose@klausrose. net 16
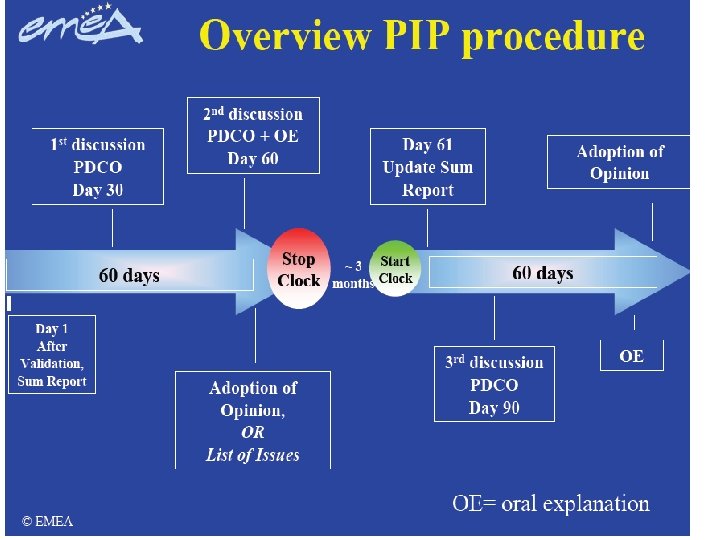 17
17
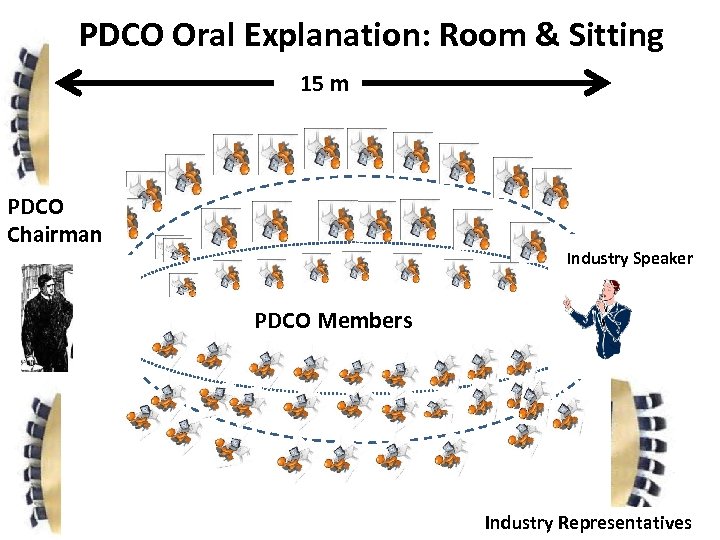 PDCO Oral Explanation: Room & Sitting 15 m PDCO Chairman Industry Speaker PDCO Members 18 klaus. rose@klausrose. net Industry Representatives
PDCO Oral Explanation: Room & Sitting 15 m PDCO Chairman Industry Speaker PDCO Members 18 klaus. rose@klausrose. net Industry Representatives
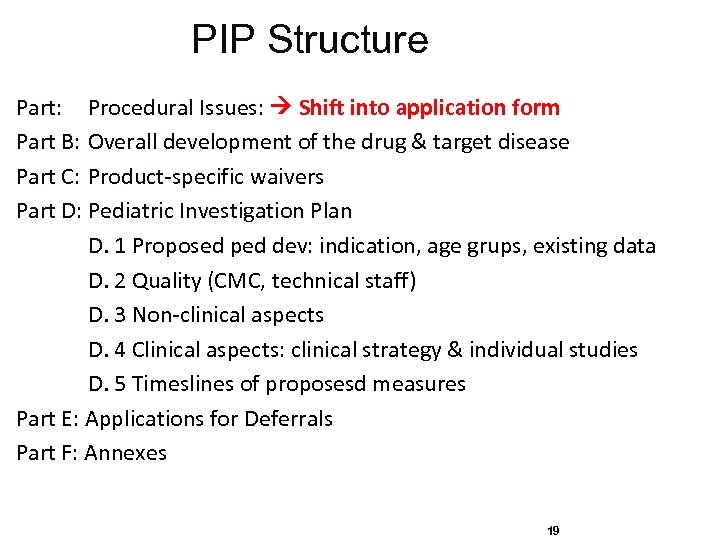 PIP Structure Part: Procedural Issues: Shift into application form Part B: Overall development of the drug & target disease Part C: Product-specific waivers Part D: Pediatric Investigation Plan D. 1 Proposed ped dev: indication, age grups, existing data D. 2 Quality (CMC, technical staff) D. 3 Non-clinical aspects D. 4 Clinical aspects: clinical strategy & individual studies D. 5 Timeslines of proposesd measures Part E: Applications for Deferrals Part F: Annexes 19
PIP Structure Part: Procedural Issues: Shift into application form Part B: Overall development of the drug & target disease Part C: Product-specific waivers Part D: Pediatric Investigation Plan D. 1 Proposed ped dev: indication, age grups, existing data D. 2 Quality (CMC, technical staff) D. 3 Non-clinical aspects D. 4 Clinical aspects: clinical strategy & individual studies D. 5 Timeslines of proposesd measures Part E: Applications for Deferrals Part F: Annexes 19
 EU Pediatric Regulation, EMA Expectations • FDA started with looking for ‘some‘ pediatric data • EMA wants, as far as possible, full pediatric indication(s) • Want the necessary data as soon as possible for marketed drugs and as early as possible for new drugs • Expect each company to be knowledgeable + up to date • EMEA / PDCO style: have a mission; science-driven; tough • Some requests can be perceived as exaggerated • A lot of procedural guidance on the EMA website, including 26 procedural Q&As klaus. rose@klausrose. net 20
EU Pediatric Regulation, EMA Expectations • FDA started with looking for ‘some‘ pediatric data • EMA wants, as far as possible, full pediatric indication(s) • Want the necessary data as soon as possible for marketed drugs and as early as possible for new drugs • Expect each company to be knowledgeable + up to date • EMEA / PDCO style: have a mission; science-driven; tough • Some requests can be perceived as exaggerated • A lot of procedural guidance on the EMA website, including 26 procedural Q&As klaus. rose@klausrose. net 20
 PIP-related And Other Documentation Pre-PIP • Briefing Book for advisory board meeting • Briefing book for scientific advice meeting Peri- & Post-PIP • Request for PIP modification • Request for compliance check • Request for complete waiver Operational in clinical trials • Protocol writing • Informed consent adults & children • Clinical summary, etc. klaus. rose@klausrose. net 21
PIP-related And Other Documentation Pre-PIP • Briefing Book for advisory board meeting • Briefing book for scientific advice meeting Peri- & Post-PIP • Request for PIP modification • Request for compliance check • Request for complete waiver Operational in clinical trials • Protocol writing • Informed consent adults & children • Clinical summary, etc. klaus. rose@klausrose. net 21
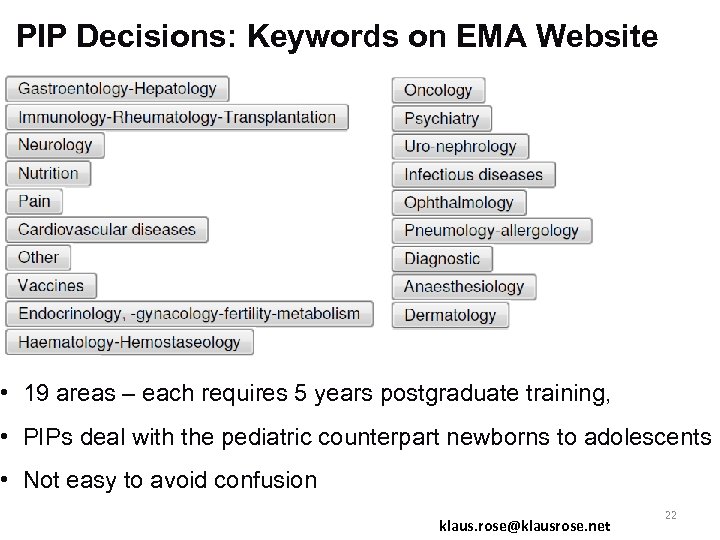 PIP Decisions: Keywords on EMA Website • 19 areas – each requires 5 years postgraduate training, • PIPs deal with the pediatric counterpart newborns to adolescents • Not easy to avoid confusion klaus. rose@klausrose. net 22
PIP Decisions: Keywords on EMA Website • 19 areas – each requires 5 years postgraduate training, • PIPs deal with the pediatric counterpart newborns to adolescents • Not easy to avoid confusion klaus. rose@klausrose. net 22
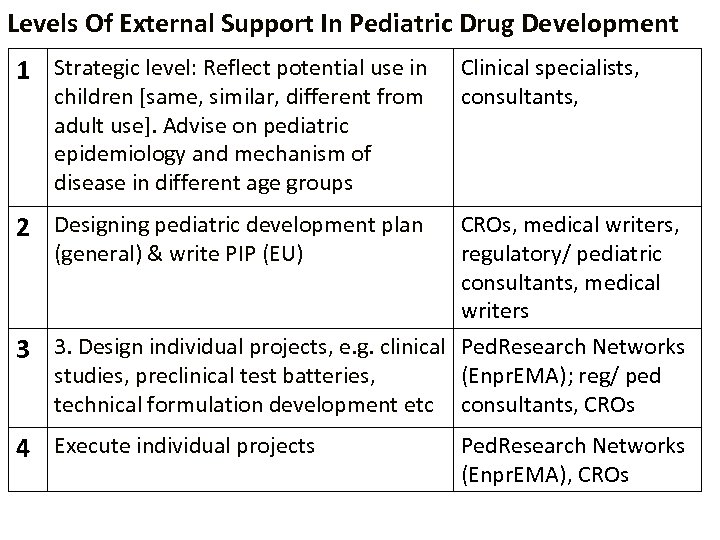 Levels Of External Support In Pediatric Drug Development 1 Strategic level: Reflect potential use in children [same, similar, different from adult use]. Advise on pediatric epidemiology and mechanism of disease in different age groups Clinical specialists, consultants, 2 Designing pediatric development plan 3 CROs, medical writers, (general) & write PIP (EU) regulatory/ pediatric consultants, medical writers 3. Design individual projects, e. g. clinical Ped. Research Networks studies, preclinical test batteries, (Enpr. EMA); reg/ ped technical formulation development etc consultants, CROs 4 Execute individual projects Ped. Research Networks (Enpr. EMA), CROs
Levels Of External Support In Pediatric Drug Development 1 Strategic level: Reflect potential use in children [same, similar, different from adult use]. Advise on pediatric epidemiology and mechanism of disease in different age groups Clinical specialists, consultants, 2 Designing pediatric development plan 3 CROs, medical writers, (general) & write PIP (EU) regulatory/ pediatric consultants, medical writers 3. Design individual projects, e. g. clinical Ped. Research Networks studies, preclinical test batteries, (Enpr. EMA); reg/ ped technical formulation development etc consultants, CROs 4 Execute individual projects Ped. Research Networks (Enpr. EMA), CROs
 Case Study Coronary Artery Disease (CAD) • Nykomed requested a full waiver for a diagnostic agent for coronary artery disease (CAD), a disease listed on the class waiver list • EMA: condition is “Visualisation of myocardial perfusion for diagnostic purposes”. Myocardial perfusion deficits exists in children (congenital heart defects, coronary anomalies, cardiomyopathies) • Negative opinion 2008 • Applicant took EMA to EU Court of Justice; 1 st instance backed EMA • US originator company negotiated a new PIP with EMA, agreed 2011 • Nykomed continued law suit. EU General Court backed EMA 2011: otherwise it would be too easy for companies to circumvent pediatric development. • klaus. rose@klausrose. net 24
Case Study Coronary Artery Disease (CAD) • Nykomed requested a full waiver for a diagnostic agent for coronary artery disease (CAD), a disease listed on the class waiver list • EMA: condition is “Visualisation of myocardial perfusion for diagnostic purposes”. Myocardial perfusion deficits exists in children (congenital heart defects, coronary anomalies, cardiomyopathies) • Negative opinion 2008 • Applicant took EMA to EU Court of Justice; 1 st instance backed EMA • US originator company negotiated a new PIP with EMA, agreed 2011 • Nykomed continued law suit. EU General Court backed EMA 2011: otherwise it would be too easy for companies to circumvent pediatric development. • klaus. rose@klausrose. net 24
 EMA Decisions • EMA decision of 28 November 2008 on the application for product specific waiver for perflubutane EMEA-000194 -PIP 01 -08 in accordance with Regulation (EC) No 1901/2006 of the European Parliament and of the Council as amended. http: //www. ema. europa. eu/docs/en_GB/document_library/PIP_deci sion/WC 500005753. pdf • EMA decision of 18 May 2011 on the agreement of a paediatric investigation plan and on the granting of a deferral and on the granting of a waiver for perflubutane (EMEA-000194 -PIP 03 -10) http: //www. ema. europa. eu/docs/en_GB/document_library/PIP_deci sion/WC 500107411. pdf klaus. rose@klausrose. net 25
EMA Decisions • EMA decision of 28 November 2008 on the application for product specific waiver for perflubutane EMEA-000194 -PIP 01 -08 in accordance with Regulation (EC) No 1901/2006 of the European Parliament and of the Council as amended. http: //www. ema. europa. eu/docs/en_GB/document_library/PIP_deci sion/WC 500005753. pdf • EMA decision of 18 May 2011 on the agreement of a paediatric investigation plan and on the granting of a deferral and on the granting of a waiver for perflubutane (EMEA-000194 -PIP 03 -10) http: //www. ema. europa. eu/docs/en_GB/document_library/PIP_deci sion/WC 500107411. pdf klaus. rose@klausrose. net 25
 EU Court of Justice Decisions • Order of the President of the Court of First Instance of 24 April 2009 – Nycomed Danmark v EMEA (Case T-52/09 R). http: //curia. europa. eu/juris/document. jsf? text=&docid=7 3453&page. Index=0&doclang=EN&mode=lst&dir=&occ=first&part=1 &cid=327397 • Judgment Of The General Court (Third Chamber) 14 December 2011. http: //curia. europa. eu/juris/document. jsf? text=&docid=1 16583&page. Index=0&doclang=EN&mode=doc&dir=&occ=first&part= 1&cid=234507 klaus. rose@klausrose. net 26
EU Court of Justice Decisions • Order of the President of the Court of First Instance of 24 April 2009 – Nycomed Danmark v EMEA (Case T-52/09 R). http: //curia. europa. eu/juris/document. jsf? text=&docid=7 3453&page. Index=0&doclang=EN&mode=lst&dir=&occ=first&part=1 &cid=327397 • Judgment Of The General Court (Third Chamber) 14 December 2011. http: //curia. europa. eu/juris/document. jsf? text=&docid=1 16583&page. Index=0&doclang=EN&mode=doc&dir=&occ=first&part= 1&cid=234507 klaus. rose@klausrose. net 26
 EU Court of Justice - Consequences • Have strengthened considerably legal EMA/PDCO position • For a new PIP, companies now know minimal requirements Example rules: • Don’t propose a waiver because the disease is rare • Know the gray zone between rare & ultra-rare: juvenile melanoma with 1. 7/100’ 000 in 15 -19 y olds is pediatric disease; ovarian cancer in the same age group with 1. 4/100’ 000 is not • Never argue that a requested measure is too expensive klaus. rose@klausrose. net 27
EU Court of Justice - Consequences • Have strengthened considerably legal EMA/PDCO position • For a new PIP, companies now know minimal requirements Example rules: • Don’t propose a waiver because the disease is rare • Know the gray zone between rare & ultra-rare: juvenile melanoma with 1. 7/100’ 000 in 15 -19 y olds is pediatric disease; ovarian cancer in the same age group with 1. 4/100’ 000 is not • Never argue that a requested measure is too expensive klaus. rose@klausrose. net 27
 EMA Assessment – 2 Key Documents: • Olski T, Lampus S, Gherarducci G, Saint Raymond S: Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol (2011) 67: 245– 252 • Report to the European Commission On companies and products that have benefited from any of the rewards and incentives in the Paediatric Regulation and on the companies that have failed to comply with any of the obligations in this Regulation, covering the year 2010. 3 rd May 2011. http: //www. ema. europa. eu/docs/en_GB/document_library/Report/ 2011/05/WC 500106262. pdf klaus. rose@klausrose. net 28
EMA Assessment – 2 Key Documents: • Olski T, Lampus S, Gherarducci G, Saint Raymond S: Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol (2011) 67: 245– 252 • Report to the European Commission On companies and products that have benefited from any of the rewards and incentives in the Paediatric Regulation and on the companies that have failed to comply with any of the obligations in this Regulation, covering the year 2010. 3 rd May 2011. http: //www. ema. europa. eu/docs/en_GB/document_library/Report/ 2011/05/WC 500106262. pdf klaus. rose@klausrose. net 28
 EMA-EFPIA Info Day 2011: EFPIA Conclusions* • Impact on R&D and resources - Additional PDCO requests on submitted PIPs - PIP withdrawals/ abandoned programs: wasted resources - PIP regulatory procedure is resource intensive - Initial submission plus downstream modifications • To be considered in context - Pediatric trials are more expensive than adults - R&D budgets are defined - Global project viability may be at greater risk by increase of costs *www. efpia. org 29
EMA-EFPIA Info Day 2011: EFPIA Conclusions* • Impact on R&D and resources - Additional PDCO requests on submitted PIPs - PIP withdrawals/ abandoned programs: wasted resources - PIP regulatory procedure is resource intensive - Initial submission plus downstream modifications • To be considered in context - Pediatric trials are more expensive than adults - R&D budgets are defined - Global project viability may be at greater risk by increase of costs *www. efpia. org 29
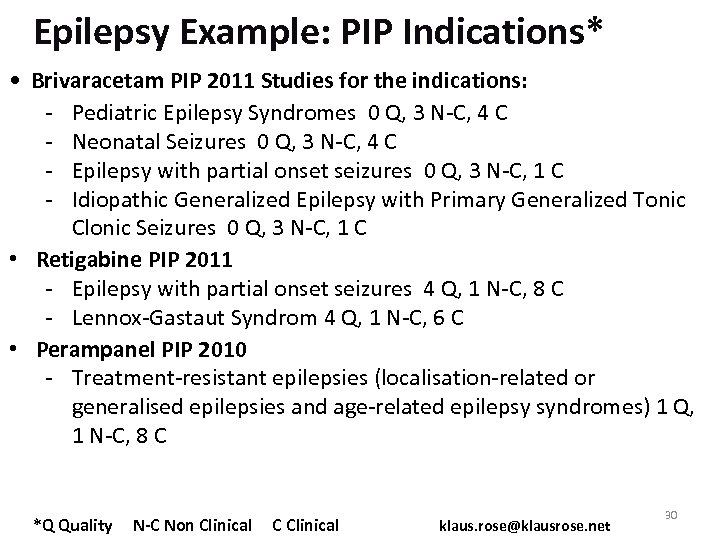 Epilepsy Example: PIP Indications* • Brivaracetam PIP 2011 Studies for the indications: - Pediatric Epilepsy Syndromes 0 Q, 3 N-C, 4 C - Neonatal Seizures 0 Q, 3 N-C, 4 C - Epilepsy with partial onset seizures 0 Q, 3 N-C, 1 C - Idiopathic Generalized Epilepsy with Primary Generalized Tonic Clonic Seizures 0 Q, 3 N-C, 1 C • Retigabine PIP 2011 - Epilepsy with partial onset seizures 4 Q, 1 N-C, 8 C - Lennox-Gastaut Syndrom 4 Q, 1 N-C, 6 C • Perampanel PIP 2010 - Treatment-resistant epilepsies (localisation-related or generalised epilepsies and age-related epilepsy syndromes) 1 Q, 1 N-C, 8 C *Q Quality N-C Non Clinical C Clinical klaus. rose@klausrose. net 30
Epilepsy Example: PIP Indications* • Brivaracetam PIP 2011 Studies for the indications: - Pediatric Epilepsy Syndromes 0 Q, 3 N-C, 4 C - Neonatal Seizures 0 Q, 3 N-C, 4 C - Epilepsy with partial onset seizures 0 Q, 3 N-C, 1 C - Idiopathic Generalized Epilepsy with Primary Generalized Tonic Clonic Seizures 0 Q, 3 N-C, 1 C • Retigabine PIP 2011 - Epilepsy with partial onset seizures 4 Q, 1 N-C, 8 C - Lennox-Gastaut Syndrom 4 Q, 1 N-C, 6 C • Perampanel PIP 2010 - Treatment-resistant epilepsies (localisation-related or generalised epilepsies and age-related epilepsy syndromes) 1 Q, 1 N-C, 8 C *Q Quality N-C Non Clinical C Clinical klaus. rose@klausrose. net 30
 Thoughts • US pediatric legislation was introduced when pharma industry peaked in size, output & productivity (or had passed it zenith) • EU: 10 years later: - Changed framework of drug development: Output down and requirements up - Silent assumptions: Flow of new products & budgets are unlimited, pushing drug developers is noble & justified - Desire: anticipate any future pediatric use ASAP • As individuals, PDCO members /EMA coordinators are fair • But we talk about structures here that include misconceptions, group dynamics & politics • Nobody is against pediatric legislation – is that good or bad? klaus. rose@klausrose. net 31
Thoughts • US pediatric legislation was introduced when pharma industry peaked in size, output & productivity (or had passed it zenith) • EU: 10 years later: - Changed framework of drug development: Output down and requirements up - Silent assumptions: Flow of new products & budgets are unlimited, pushing drug developers is noble & justified - Desire: anticipate any future pediatric use ASAP • As individuals, PDCO members /EMA coordinators are fair • But we talk about structures here that include misconceptions, group dynamics & politics • Nobody is against pediatric legislation – is that good or bad? klaus. rose@klausrose. net 31
 More Throughts • Epilepsy PIPs discourage further R&D. • Companies in late development had to comply • Others will avoid areas of heavy PDCO requests • Light at The End of The Tunnel? – EMA report 2011 emphasizes need for penalties – EMA admits request for too many details and works on reducing them – Revision of ped regulation in 2018 – Different sides will propose different modifications klaus. rose@klausrose. net 32
More Throughts • Epilepsy PIPs discourage further R&D. • Companies in late development had to comply • Others will avoid areas of heavy PDCO requests • Light at The End of The Tunnel? – EMA report 2011 emphasizes need for penalties – EMA admits request for too many details and works on reducing them – Revision of ped regulation in 2018 – Different sides will propose different modifications klaus. rose@klausrose. net 32
 Better Medicines for Children or Better Use of Adult Medicines in Children? • EU & US pediatric pharmaceutical legislation tries to close a gap - in the use of existing adult drugs in children • There are few companies that develop drugs for children • Such an industry could exist. Children don’t vote or pay. Adults would have to decide to spend more for children • There are many rare diseases – but somebody must pay • Today, not even a straw facilitating intake of antibiotics is reimbursed in Germany – formulation was abandoned • Two issues: (1) Additional pediatric requests for adult drugs, (2) ‘better medicines for children’ - with many meanings klaus. rose@klausrose. net 33
Better Medicines for Children or Better Use of Adult Medicines in Children? • EU & US pediatric pharmaceutical legislation tries to close a gap - in the use of existing adult drugs in children • There are few companies that develop drugs for children • Such an industry could exist. Children don’t vote or pay. Adults would have to decide to spend more for children • There are many rare diseases – but somebody must pay • Today, not even a straw facilitating intake of antibiotics is reimbursed in Germany – formulation was abandoned • Two issues: (1) Additional pediatric requests for adult drugs, (2) ‘better medicines for children’ - with many meanings klaus. rose@klausrose. net 33
 JUST ANNOUNCED ! Joint DIA/ EFGCP/ EMA Paediatric Forum 2012 The EU paediatric regulation in its 6 th year: From Learning to Adapting 26 & 27 September 2012 London, UK Programme Committee: Gesine Bejeuhr, Vf. A (Association of Researchbased Pharmaceutical Companies, Germany) Irja Lutsar, PDCO member for Estonia Cecile Ollivier, EMA, London, UK Thorsten Olski, EMA, London, UK Klaus Rose, klausrose Consulting, Switzerland Thomas Severin, Novartis, Switzerland Organised by : In partnership with :
JUST ANNOUNCED ! Joint DIA/ EFGCP/ EMA Paediatric Forum 2012 The EU paediatric regulation in its 6 th year: From Learning to Adapting 26 & 27 September 2012 London, UK Programme Committee: Gesine Bejeuhr, Vf. A (Association of Researchbased Pharmaceutical Companies, Germany) Irja Lutsar, PDCO member for Estonia Cecile Ollivier, EMA, London, UK Thorsten Olski, EMA, London, UK Klaus Rose, klausrose Consulting, Switzerland Thomas Severin, Novartis, Switzerland Organised by : In partnership with :
 Conclusions • • • No easy black or white conclusions. No more drug development without considering children Increases cost & complexity of drug development EMA/PDCO: nice vision; limited interest in economic reality; bureaucratic procedures; not all needed PIP skills are science Invested resources could be used better – as is mostly the outcome of complex decision making Reviews 2013/18: opposing proposals will be made Costs/ benefit is difficult to quantify due to confidentiality Law drives child research in some areas; road block in others There will be some future clinical benefit for children It will ensure more work for many groups including medical writers. Background understanding remains essential klaus. rose@klausrose. net 35
Conclusions • • • No easy black or white conclusions. No more drug development without considering children Increases cost & complexity of drug development EMA/PDCO: nice vision; limited interest in economic reality; bureaucratic procedures; not all needed PIP skills are science Invested resources could be used better – as is mostly the outcome of complex decision making Reviews 2013/18: opposing proposals will be made Costs/ benefit is difficult to quantify due to confidentiality Law drives child research in some areas; road block in others There will be some future clinical benefit for children It will ensure more work for many groups including medical writers. Background understanding remains essential klaus. rose@klausrose. net 35
 Thank You For Your Attention! klaus. rose@klausrose. net 36
Thank You For Your Attention! klaus. rose@klausrose. net 36
 Back-Ups klaus. rose@klausrose. net 37
Back-Ups klaus. rose@klausrose. net 37
 klaus. rose@klausrose. net 38
klaus. rose@klausrose. net 38
 Released May 2010 klaus. rose@klausrose. net 39
Released May 2010 klaus. rose@klausrose. net 39


