4fa16bf608351cbee4b8b03e72e0909b.ppt
- Количество слайдов: 20
 3 – Chemical Compounds Leaving Certificate Chemistry
3 – Chemical Compounds Leaving Certificate Chemistry
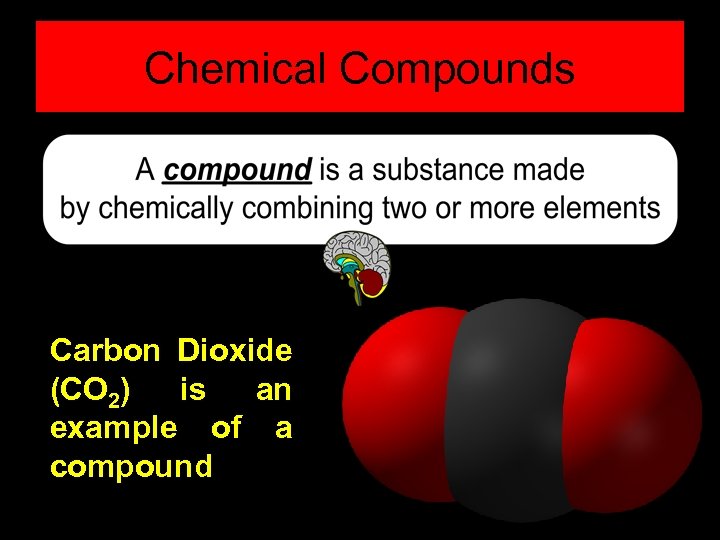 Chemical Compounds Carbon Dioxide (CO 2) is an example of a compound
Chemical Compounds Carbon Dioxide (CO 2) is an example of a compound
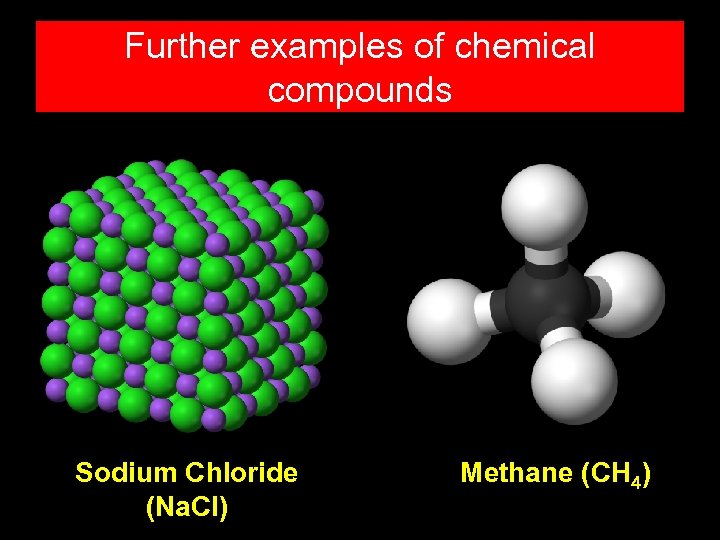 Further examples of chemical compounds Sodium Chloride (Na. Cl) Methane (CH 4)
Further examples of chemical compounds Sodium Chloride (Na. Cl) Methane (CH 4)
 The Noble Gases are very unreactive - They are not found combined with any other element Helium – a full outer shell of electrons Argon – a full outer shell of electrons
The Noble Gases are very unreactive - They are not found combined with any other element Helium – a full outer shell of electrons Argon – a full outer shell of electrons
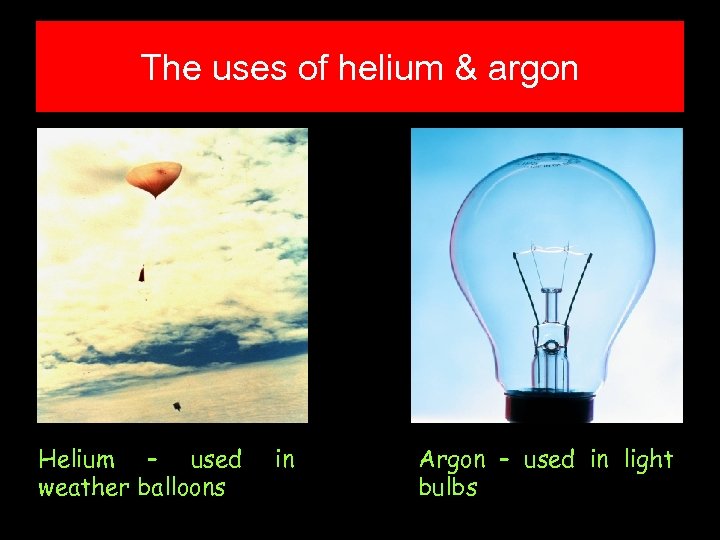 The uses of helium & argon Helium – used weather balloons in Argon – used in light bulbs
The uses of helium & argon Helium – used weather balloons in Argon – used in light bulbs
 Valence
Valence
 The Octet Rule When elements combine they do so to ensure that the outermost electron of each bonding atom has in its outer shell. 8 electrons There are exceptions: 1. Hydrogen and lithium look to have a full outer shell of 2 electrons. 2. Boron (element no. 5) forms many compounds with 6 electrons in its outer shell.
The Octet Rule When elements combine they do so to ensure that the outermost electron of each bonding atom has in its outer shell. 8 electrons There are exceptions: 1. Hydrogen and lithium look to have a full outer shell of 2 electrons. 2. Boron (element no. 5) forms many compounds with 6 electrons in its outer shell.
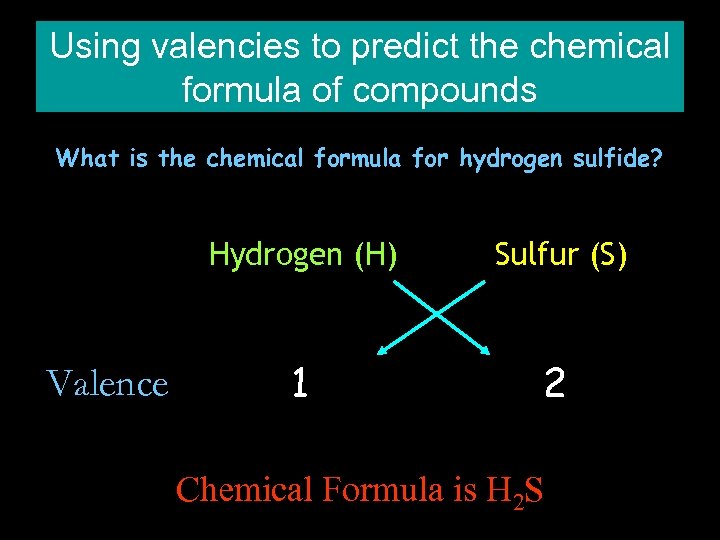 Using valencies to predict the chemical formula of compounds What is the chemical formula for hydrogen sulfide? Hydrogen (H) Valence Sulfur (S) 1 2 Chemical Formula is H 2 S
Using valencies to predict the chemical formula of compounds What is the chemical formula for hydrogen sulfide? Hydrogen (H) Valence Sulfur (S) 1 2 Chemical Formula is H 2 S
 Can you… • Give the definition of a chemical compound? • Give some examples of compounds? • Tell some uses of noble gases • Say what the octet rule states and some exceptions • Define vacancy?
Can you… • Give the definition of a chemical compound? • Give some examples of compounds? • Tell some uses of noble gases • Say what the octet rule states and some exceptions • Define vacancy?
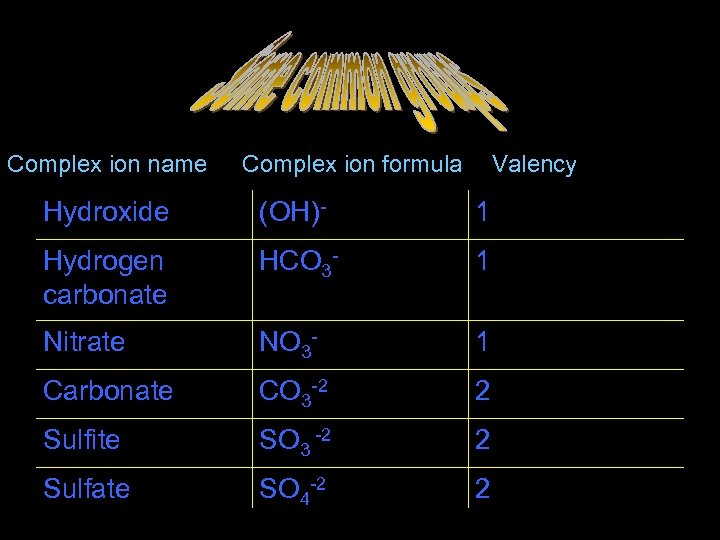 Some common groups Complex ion name Complex ion formula Valency Hydroxide (OH)- 1 Hydrogen carbonate HCO 3 - 1 Nitrate NO 3 - 1 Carbonate CO 3 -2 2 Sulfite SO 3 -2 2 Sulfate SO 4 -2 2
Some common groups Complex ion name Complex ion formula Valency Hydroxide (OH)- 1 Hydrogen carbonate HCO 3 - 1 Nitrate NO 3 - 1 Carbonate CO 3 -2 2 Sulfite SO 3 -2 2 Sulfate SO 4 -2 2
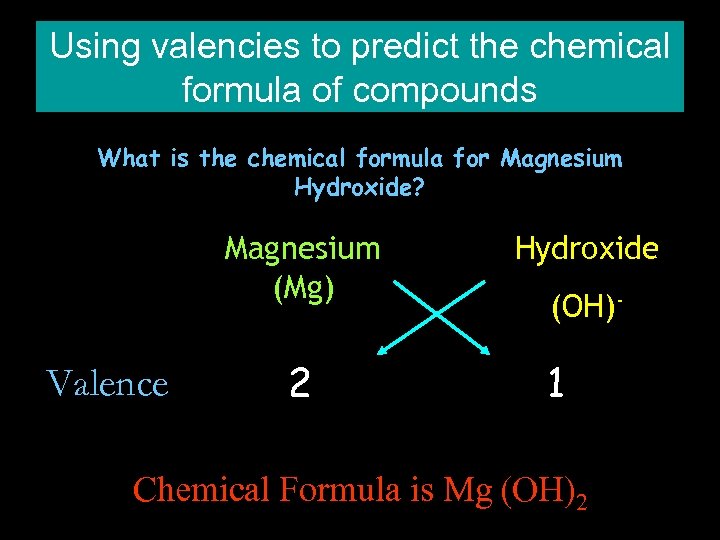 Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Hydroxide? Magnesium (Mg) Valence 2 Hydroxide (OH)- 1 Chemical Formula is Mg (OH)2
Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Hydroxide? Magnesium (Mg) Valence 2 Hydroxide (OH)- 1 Chemical Formula is Mg (OH)2
 Practice Questions Write the formula of each of the following: 1. Aluminium hydroxide 2. Calcium carbonate 3. Magnesium nitrate 4. Sodium hydrogencarbonate 5. Potassium sulfite 6. Aluminium sulfate
Practice Questions Write the formula of each of the following: 1. Aluminium hydroxide 2. Calcium carbonate 3. Magnesium nitrate 4. Sodium hydrogencarbonate 5. Potassium sulfite 6. Aluminium sulfate
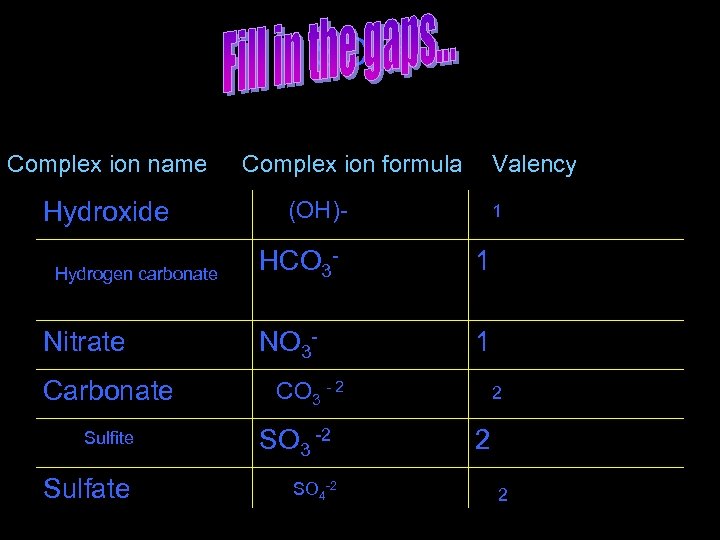 SO 4 -2 Complex ion name Hydroxide Hydrogen carbonate Nitrate Carbonate Complex ion formula Valency (OH)- 1 HCO 3 - 1 NO 3 - 1 CO 3 - 2 Sulfite SO 3 -2 Sulfate SO 4 -2 2
SO 4 -2 Complex ion name Hydroxide Hydrogen carbonate Nitrate Carbonate Complex ion formula Valency (OH)- 1 HCO 3 - 1 NO 3 - 1 CO 3 - 2 Sulfite SO 3 -2 Sulfate SO 4 -2 2
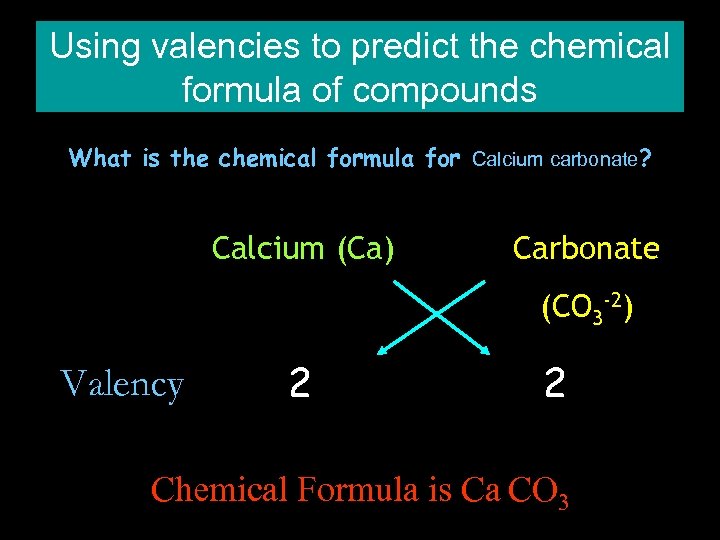 Using valencies to predict the chemical formula of compounds What is the chemical formula for Calcium carbonate? Calcium (Ca) Carbonate (CO 3 -2) Valency 2 2 Chemical Formula is Ca CO 3
Using valencies to predict the chemical formula of compounds What is the chemical formula for Calcium carbonate? Calcium (Ca) Carbonate (CO 3 -2) Valency 2 2 Chemical Formula is Ca CO 3
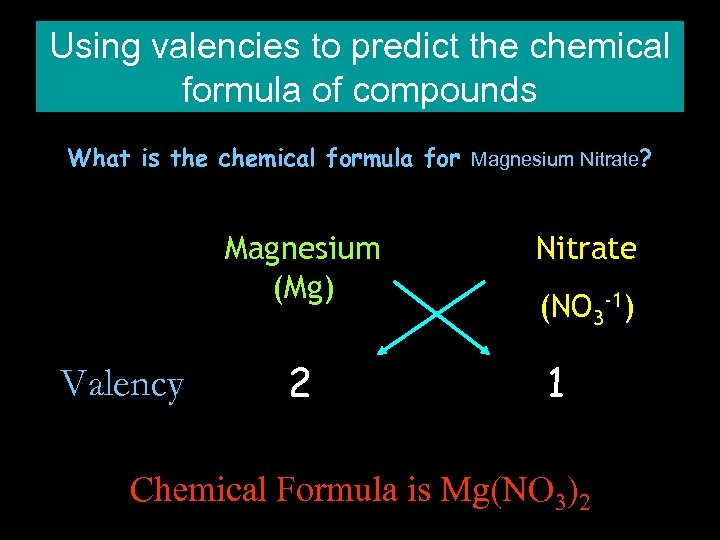 Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Nitrate? Magnesium (Mg) Valency 2 Nitrate (NO 3 -1) 1 Chemical Formula is Mg(NO 3)2
Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Nitrate? Magnesium (Mg) Valency 2 Nitrate (NO 3 -1) 1 Chemical Formula is Mg(NO 3)2
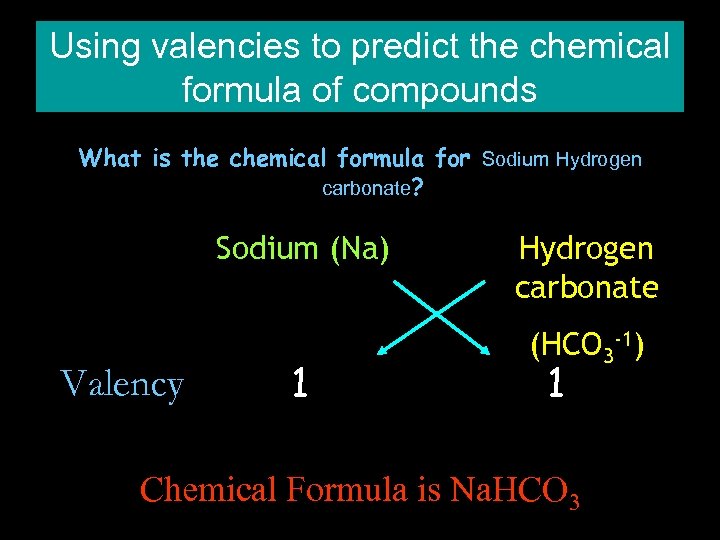 Using valencies to predict the chemical formula of compounds What is the chemical formula for Sodium Hydrogen carbonate? Sodium (Na) Valency 1 Hydrogen carbonate (HCO 3 -1) 1 Chemical Formula is Na. HCO 3
Using valencies to predict the chemical formula of compounds What is the chemical formula for Sodium Hydrogen carbonate? Sodium (Na) Valency 1 Hydrogen carbonate (HCO 3 -1) 1 Chemical Formula is Na. HCO 3
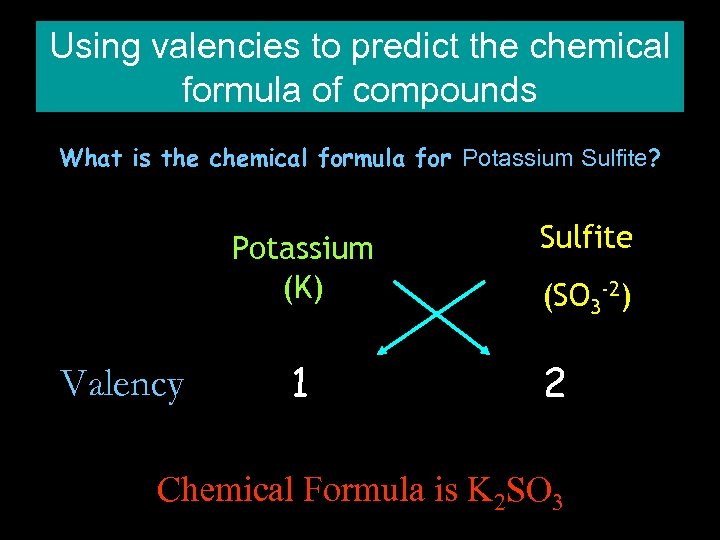 Using valencies to predict the chemical formula of compounds What is the chemical formula for Potassium Sulfite? Potassium (K) Valency 1 Sulfite (SO 3 -2) 2 Chemical Formula is K 2 SO 3
Using valencies to predict the chemical formula of compounds What is the chemical formula for Potassium Sulfite? Potassium (K) Valency 1 Sulfite (SO 3 -2) 2 Chemical Formula is K 2 SO 3
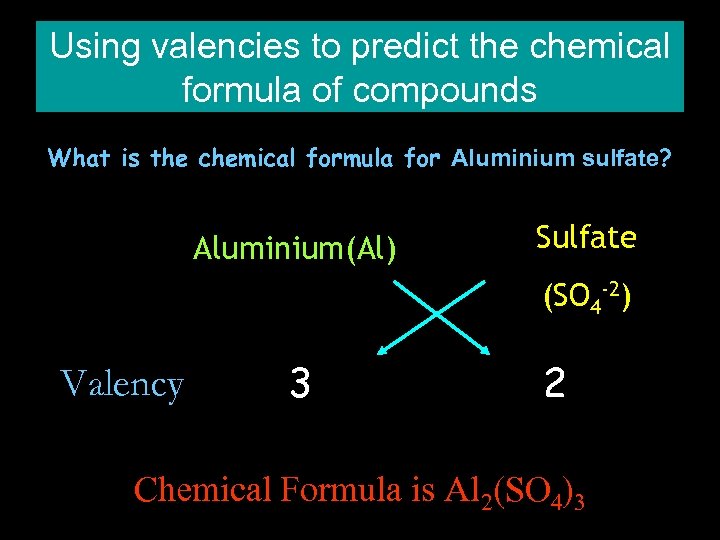 Using valencies to predict the chemical formula of compounds What is the chemical formula for Aluminium sulfate? Aluminium(Al) Sulfate (SO 4 -2) Valency 3 2 Chemical Formula is Al 2(SO 4)3
Using valencies to predict the chemical formula of compounds What is the chemical formula for Aluminium sulfate? Aluminium(Al) Sulfate (SO 4 -2) Valency 3 2 Chemical Formula is Al 2(SO 4)3
 • The transition metals can have more than one valency number • Examples : Iron, Copper, Chromium and Manganese
• The transition metals can have more than one valency number • Examples : Iron, Copper, Chromium and Manganese
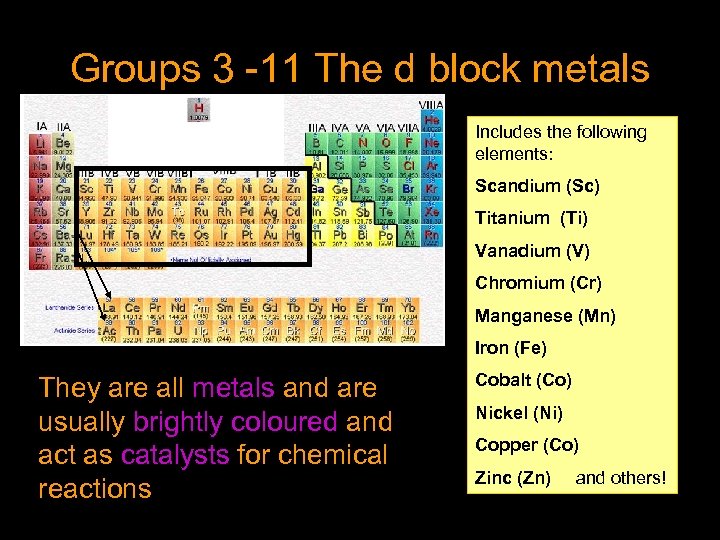 Groups 3 -11 The d block metals Includes the following elements: Scandium (Sc) Titanium (Ti) Vanadium (V) Chromium (Cr) Manganese (Mn) Iron (Fe) They are all metals and are usually brightly coloured and act as catalysts for chemical reactions Cobalt (Co) Nickel (Ni) Copper (Co) Zinc (Zn) and others!
Groups 3 -11 The d block metals Includes the following elements: Scandium (Sc) Titanium (Ti) Vanadium (V) Chromium (Cr) Manganese (Mn) Iron (Fe) They are all metals and are usually brightly coloured and act as catalysts for chemical reactions Cobalt (Co) Nickel (Ni) Copper (Co) Zinc (Zn) and others!


