4db2d1cdc47e7c134bd6177020602794.ppt
- Количество слайдов: 22
 27. 19 Secondary Structures of Peptides and Proteins
27. 19 Secondary Structures of Peptides and Proteins
 Levels of Protein Structure Primary structure = the amino acid sequence plus disulfide links Secondary structure = conformational relationship between nearest neighbor amino acids a helix pleated b sheet
Levels of Protein Structure Primary structure = the amino acid sequence plus disulfide links Secondary structure = conformational relationship between nearest neighbor amino acids a helix pleated b sheet
 Levels of Protein Structure The a-helix and pleated b sheet are both characterized by: planar geometry of peptide bond anti conformation of main chain hydrogen bonds between N—H and O=C
Levels of Protein Structure The a-helix and pleated b sheet are both characterized by: planar geometry of peptide bond anti conformation of main chain hydrogen bonds between N—H and O=C
 Pleated b Sheet Shown is a b sheet of protein chains composed of alternating glycine and alanine residues. Adjacent chains are antiparallel. Hydrogen bonds between chains. van der Waals forces produce pleated effect.
Pleated b Sheet Shown is a b sheet of protein chains composed of alternating glycine and alanine residues. Adjacent chains are antiparallel. Hydrogen bonds between chains. van der Waals forces produce pleated effect.
 Pleated b Sheet is most commonly seen with amino acids having small side chains (glycine, alanine, serine). 80% of fibroin (main protein in silk) is repeating sequence of —Gly—Ser—Gly—Ala—. b Sheet is flexible, but resists stretching.
Pleated b Sheet is most commonly seen with amino acids having small side chains (glycine, alanine, serine). 80% of fibroin (main protein in silk) is repeating sequence of —Gly—Ser—Gly—Ala—. b Sheet is flexible, but resists stretching.
 a Helix Shown is an a helix of a protein in which all of the amino acids are L-alanine. Helix is right-handed with 3. 6 amino acids per turn. Hydrogen bonds are within a single chain. Protein of muscle (myosin) and wool (a-keratin) contain large regions of a-helix. Chain can be stretched.
a Helix Shown is an a helix of a protein in which all of the amino acids are L-alanine. Helix is right-handed with 3. 6 amino acids per turn. Hydrogen bonds are within a single chain. Protein of muscle (myosin) and wool (a-keratin) contain large regions of a-helix. Chain can be stretched.
 27. 20 Tertiary Structure of Peptides and Proteins
27. 20 Tertiary Structure of Peptides and Proteins
 Tertiary Structure Refers to overall shape (how the chain is folded) Fibrous proteins (hair, tendons, wool) have elongated shapes Globular proteins are approximately spherical most enzymes are globular proteins an example is carboxypeptidase
Tertiary Structure Refers to overall shape (how the chain is folded) Fibrous proteins (hair, tendons, wool) have elongated shapes Globular proteins are approximately spherical most enzymes are globular proteins an example is carboxypeptidase
 Carboxypeptidase is an enzyme that catalyzes the hydrolysis of proteins at their C-terminus. It is a metalloenzyme containing Zn 2+ at its active site. An amino acid with a positively charged side chain (Arg-145) is near the active site.
Carboxypeptidase is an enzyme that catalyzes the hydrolysis of proteins at their C-terminus. It is a metalloenzyme containing Zn 2+ at its active site. An amino acid with a positively charged side chain (Arg-145) is near the active site.
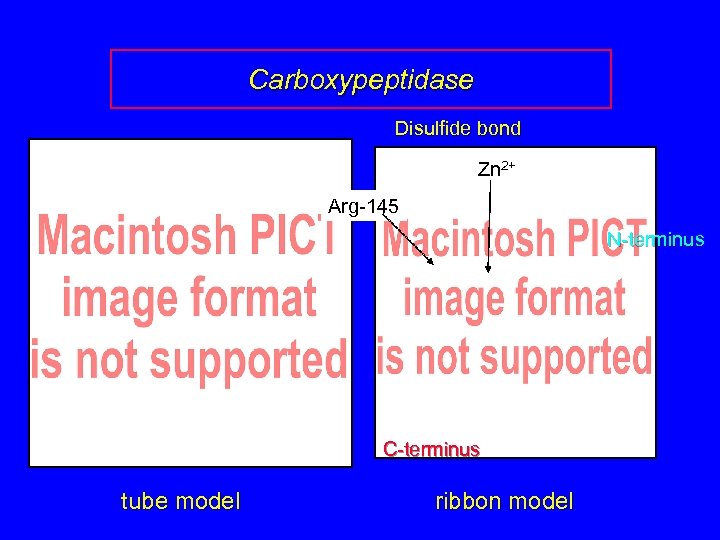 Carboxypeptidase Disulfide bond Zn 2+ Arg-145 N-terminus C-terminus tube model ribbon model
Carboxypeptidase Disulfide bond Zn 2+ Arg-145 N-terminus C-terminus tube model ribbon model
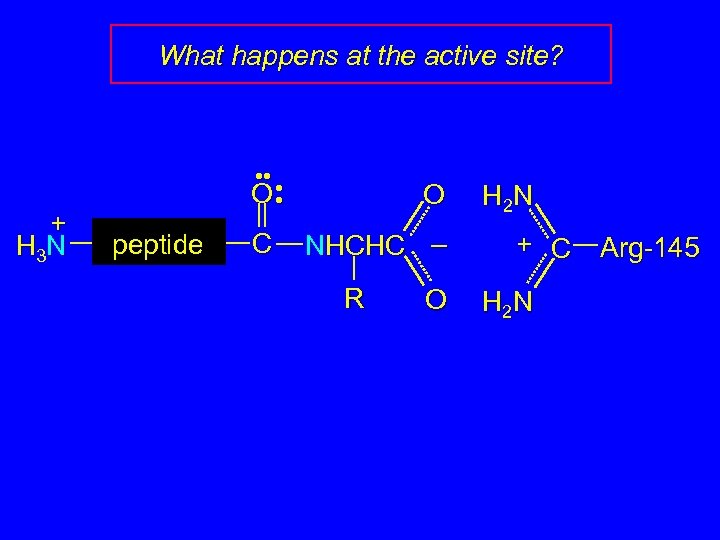 What happens at the active site? + H 3 N • • • O • peptide C O NHCHC – R O H 2 N + C H 2 N Arg-145
What happens at the active site? + H 3 N • • • O • peptide C O NHCHC – R O H 2 N + C H 2 N Arg-145
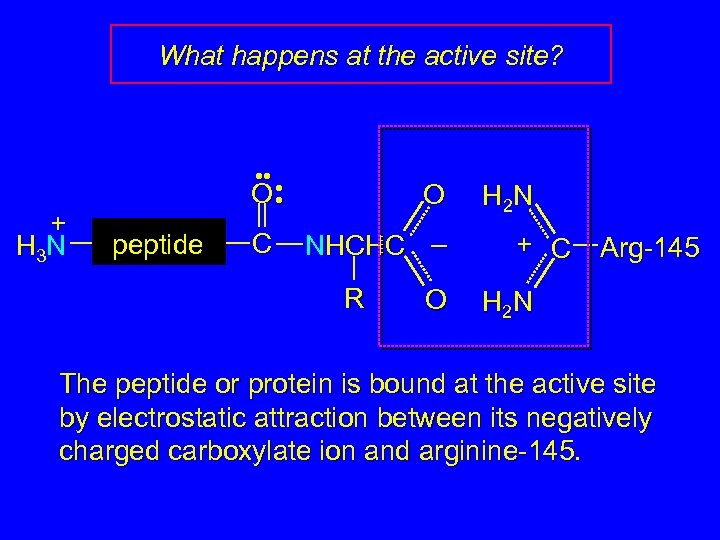 What happens at the active site? + H 3 N • • • O • peptide C O NHCHC – R O H 2 N + C Arg-145 H 2 N The peptide or protein is bound at the active site by electrostatic attraction between its negatively charged carboxylate ion and arginine-145.
What happens at the active site? + H 3 N • • • O • peptide C O NHCHC – R O H 2 N + C Arg-145 H 2 N The peptide or protein is bound at the active site by electrostatic attraction between its negatively charged carboxylate ion and arginine-145.
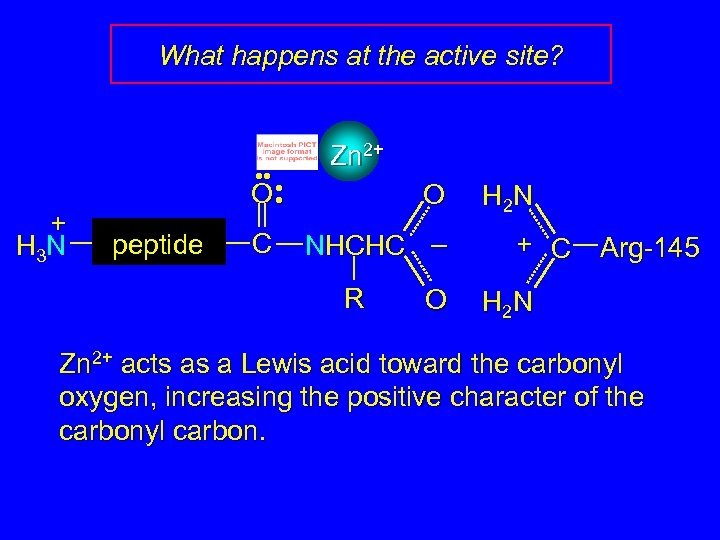 What happens at the active site? + H 3 N • • • O • peptide C Zn 2+ O NHCHC – R O H 2 N + C Arg-145 H 2 N Zn 2+ acts as a Lewis acid toward the carbonyl oxygen, increasing the positive character of the carbonyl carbon.
What happens at the active site? + H 3 N • • • O • peptide C Zn 2+ O NHCHC – R O H 2 N + C Arg-145 H 2 N Zn 2+ acts as a Lewis acid toward the carbonyl oxygen, increasing the positive character of the carbonyl carbon.
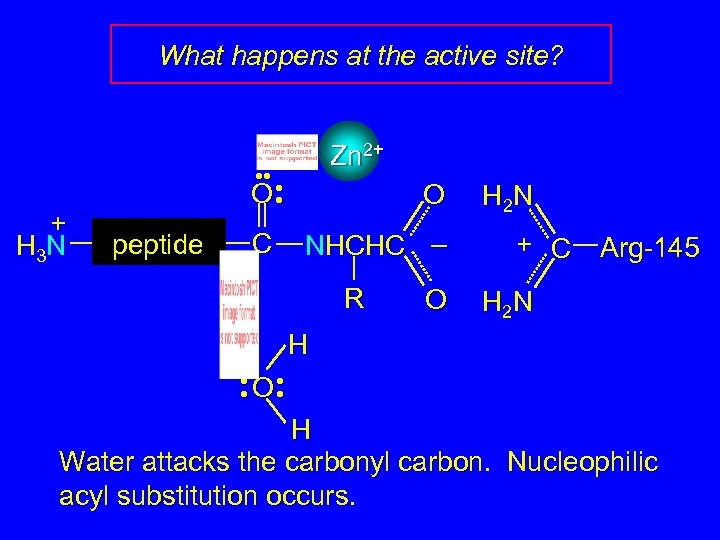 What happens at the active site? + H 3 N Zn 2+ • • • O • peptide C O NHCHC – R O H 2 N + C Arg-145 H 2 N H • O • • • H Water attacks the carbonyl carbon. Nucleophilic acyl substitution occurs.
What happens at the active site? + H 3 N Zn 2+ • • • O • peptide C O NHCHC – R O H 2 N + C Arg-145 H 2 N H • O • • • H Water attacks the carbonyl carbon. Nucleophilic acyl substitution occurs.
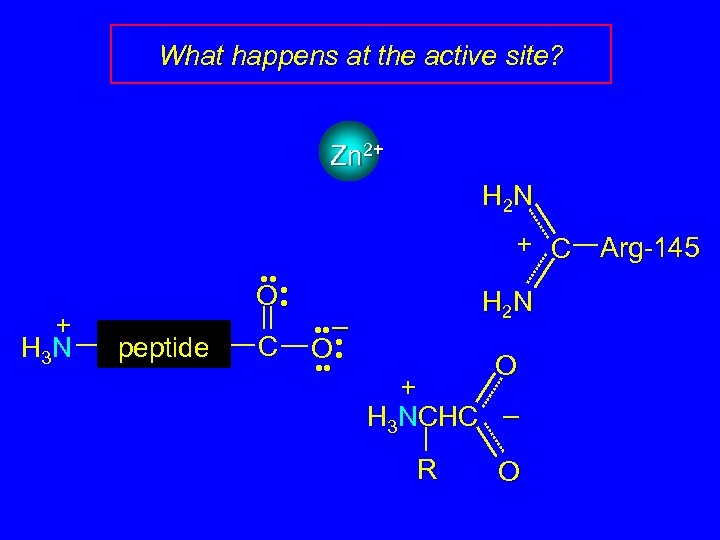 What happens at the active site? Zn 2+ H 2 N + C + H 3 N • • • O • peptide C • • – O • • • • H 2 N O + H 3 NCHC – R O Arg-145
What happens at the active site? Zn 2+ H 2 N + C + H 3 N • • • O • peptide C • • – O • • • • H 2 N O + H 3 NCHC – R O Arg-145
 27. 21 Coenzymes
27. 21 Coenzymes
 Coenzymes The range of chemical reactions that amino acid side chains can participate in is relatively limited. acid-base (transfer and accept protons) nucleophilic acyl substitution Many other biological processes, such as oxidation-reduction, require coenzymes, cofactors, or prosthetic groups in order to occur.
Coenzymes The range of chemical reactions that amino acid side chains can participate in is relatively limited. acid-base (transfer and accept protons) nucleophilic acyl substitution Many other biological processes, such as oxidation-reduction, require coenzymes, cofactors, or prosthetic groups in order to occur.
 Coenzymes NADH, coenzyme A and coenzyme B 12 are examples of coenzymes. Heme is another example.
Coenzymes NADH, coenzyme A and coenzyme B 12 are examples of coenzymes. Heme is another example.
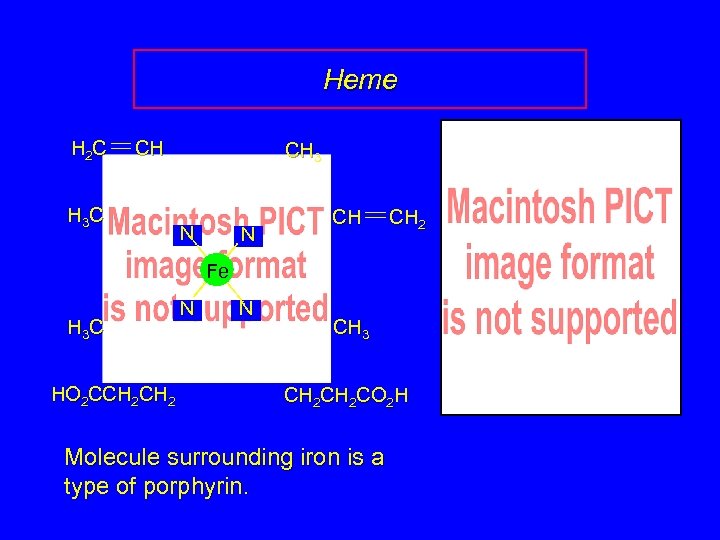 Heme H 2 C CH H 3 C CH 3 N N CH CH 2 Fe H 3 C HO 2 CCH 2 N N CH 3 CH 2 CO 2 H Molecule surrounding iron is a type of porphyrin.
Heme H 2 C CH H 3 C CH 3 N N CH CH 2 Fe H 3 C HO 2 CCH 2 N N CH 3 CH 2 CO 2 H Molecule surrounding iron is a type of porphyrin.
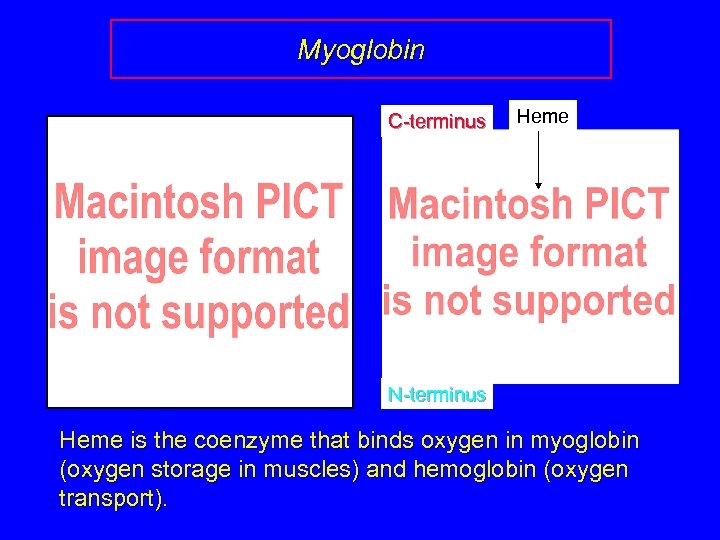 Myoglobin C-terminus Heme N-terminus Heme is the coenzyme that binds oxygen in myoglobin (oxygen storage in muscles) and hemoglobin (oxygen transport).
Myoglobin C-terminus Heme N-terminus Heme is the coenzyme that binds oxygen in myoglobin (oxygen storage in muscles) and hemoglobin (oxygen transport).
 27. 22 Protein Quaternary Structure: Hemoglobin
27. 22 Protein Quaternary Structure: Hemoglobin
 Protein Quaternary Structure Some proteins are assemblies of two or more chains. The way in which these chains are organized is called the quaternary structure. Hemoglobin, for example, consists of 4 subunits. There are 2 a chains (identical) and 2 b chains (also identical). Each subunit contains one heme and each protein is about the size of myoglobin.
Protein Quaternary Structure Some proteins are assemblies of two or more chains. The way in which these chains are organized is called the quaternary structure. Hemoglobin, for example, consists of 4 subunits. There are 2 a chains (identical) and 2 b chains (also identical). Each subunit contains one heme and each protein is about the size of myoglobin.


