7af952c1d8f382cbd91b6159ca2919b3.ppt
- Количество слайдов: 142
 26 -1
26 -1
 26 -2
26 -2
 Lipids u Are constituents of plants or animals that are characterized by their solubility properties u Are insoluble in water, but soluble in nonpolar organic solvents ex ether u The solubility property distinguishes lipids from carbohydrates, proteins and nucleic acids which are not soluble in organic solvents 26 -3
Lipids u Are constituents of plants or animals that are characterized by their solubility properties u Are insoluble in water, but soluble in nonpolar organic solvents ex ether u The solubility property distinguishes lipids from carbohydrates, proteins and nucleic acids which are not soluble in organic solvents 26 -3
 Fats and oils: Triesters of glycerol u Fats and oils are triesters of glycerol and are called triglycerides u When fat or oil is boiled with alkali and acidify the resulting solution a glycerol and a mixture of fatty acid is formed. This reaction is called saponification u Most fatty acids are unbranceh and contain an even number of carbon atoms. u If double bonds are present, they usually have the cis( Z) configuration and are not conjugated. 26 -4
Fats and oils: Triesters of glycerol u Fats and oils are triesters of glycerol and are called triglycerides u When fat or oil is boiled with alkali and acidify the resulting solution a glycerol and a mixture of fatty acid is formed. This reaction is called saponification u Most fatty acids are unbranceh and contain an even number of carbon atoms. u If double bonds are present, they usually have the cis( Z) configuration and are not conjugated. 26 -4
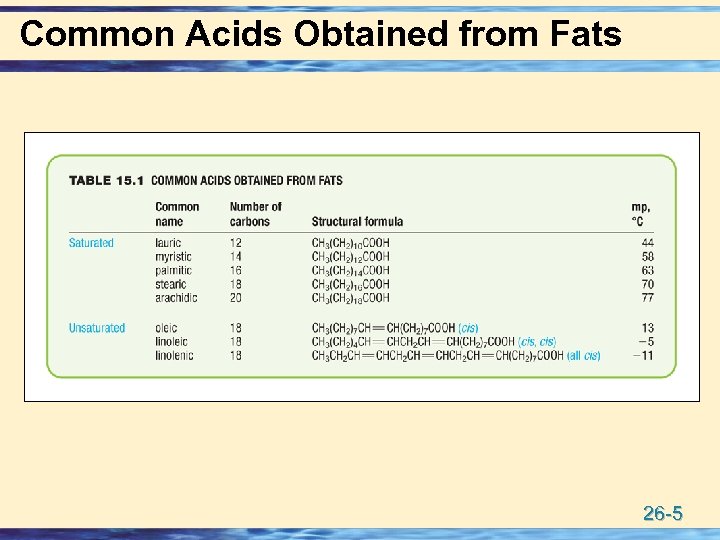 Common Acids Obtained from Fats 26 -5
Common Acids Obtained from Fats 26 -5
 u There are two types of triglycerides: simple triglycerides ( all three fatty acids are identical), and mixed trigycerides u In general, a particular fat or oil consists of a complex mixture of triglycerides. u This is why the composition of a fat or oil is usually expressed in terms of percentage of the various acids obtained from it by saponification. u Some fats and oils give mainly one or two acids, with only minor amounts of others ex: olive oil gives 83% oleic acid, palm oil gives 43 u 5 palmitic acid and 43% oleic acid 26 -6
u There are two types of triglycerides: simple triglycerides ( all three fatty acids are identical), and mixed trigycerides u In general, a particular fat or oil consists of a complex mixture of triglycerides. u This is why the composition of a fat or oil is usually expressed in terms of percentage of the various acids obtained from it by saponification. u Some fats and oils give mainly one or two acids, with only minor amounts of others ex: olive oil gives 83% oleic acid, palm oil gives 43 u 5 palmitic acid and 43% oleic acid 26 -6
 Oils u Oils contain a much higher percentage of unsaturated fatty acids than do fats u In general fats come form animal sources and oils come from vegetable sources, exceptions include fish oils u The more double bonds in a fatty acid portion of the triester, the lower its melting point 26 -7
Oils u Oils contain a much higher percentage of unsaturated fatty acids than do fats u In general fats come form animal sources and oils come from vegetable sources, exceptions include fish oils u The more double bonds in a fatty acid portion of the triester, the lower its melting point 26 -7
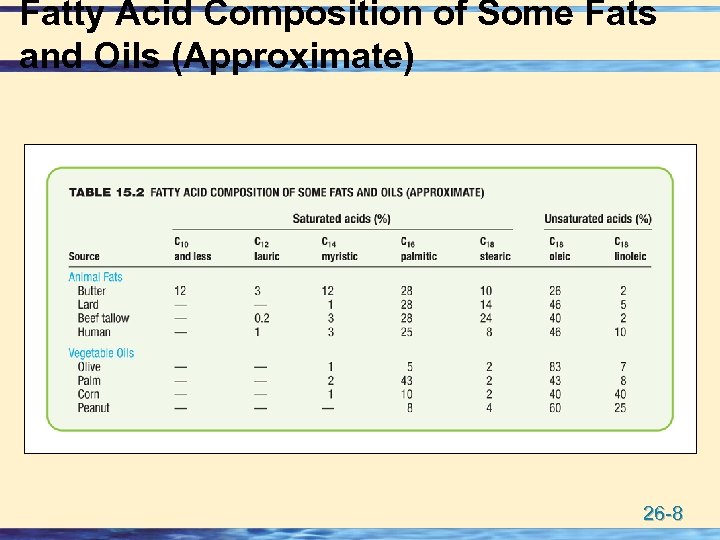 Fatty Acid Composition of Some Fats and Oils (Approximate) 26 -8
Fatty Acid Composition of Some Fats and Oils (Approximate) 26 -8
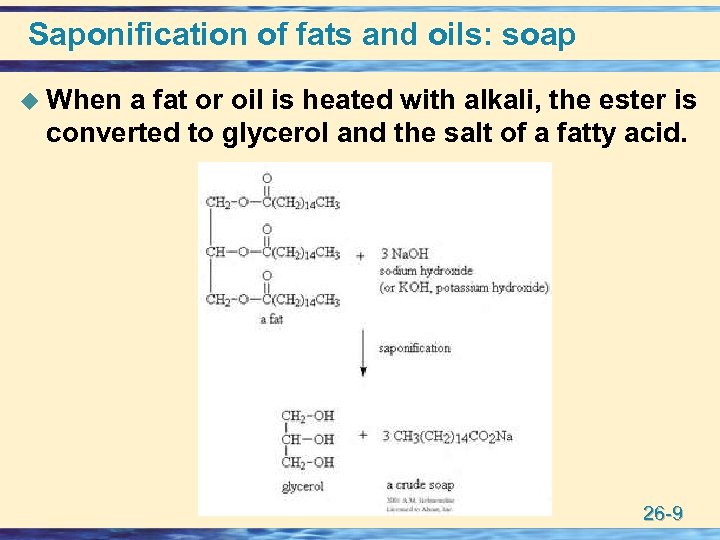 Saponification of fats and oils: soap u When a fat or oil is heated with alkali, the ester is converted to glycerol and the salt of a fatty acid. 26 -9
Saponification of fats and oils: soap u When a fat or oil is heated with alkali, the ester is converted to glycerol and the salt of a fatty acid. 26 -9
 Saponification u The conversion of animal fats into soap by heating with an alkaline is one of the oldest of chemical processes dating back at least 23, 00 years. u As recently as the 16 th and 17 th centuries, soap was still a rather rare substance, used mainly in medicine. u Soap are made by either a batch process or a continuous process 26 -10
Saponification u The conversion of animal fats into soap by heating with an alkaline is one of the oldest of chemical processes dating back at least 23, 00 years. u As recently as the 16 th and 17 th centuries, soap was still a rather rare substance, used mainly in medicine. u Soap are made by either a batch process or a continuous process 26 -10
 batch process u The fat or oil is heated with a slight excess of alkali (Na. OH) in an open kettle. u When saponification is complete, salt is added to precipitate the soap as thick curds. u The water layer, which contains salt, glycerol, and excess alkali, is drawn off, and the glycerol is recovered by distillation. u The crude soap curds, which contain some salt, alkali, and glycerol as impurities are purified by boiling with water and reprecipitating with salt several times. 26 -11
batch process u The fat or oil is heated with a slight excess of alkali (Na. OH) in an open kettle. u When saponification is complete, salt is added to precipitate the soap as thick curds. u The water layer, which contains salt, glycerol, and excess alkali, is drawn off, and the glycerol is recovered by distillation. u The crude soap curds, which contain some salt, alkali, and glycerol as impurities are purified by boiling with water and reprecipitating with salt several times. 26 -11
 u Finally, the curds are boiled with enough water to form a smooth mixture that, on standing gives a homogeneous upper layer of soap ( cheap industrial soaps made this way. ) 26 -12
u Finally, the curds are boiled with enough water to form a smooth mixture that, on standing gives a homogeneous upper layer of soap ( cheap industrial soaps made this way. ) 26 -12
 Continuous Process u More common today. u The fat or oil is hydrolyzed by water at high temperatures and pressures in the presence of a catalyst, usually a zinc soap. u The fat or oil and water are introduced continuously into opposite ends of a larger reactor and the fatty acid and glycerol are removed as formed by distillation. u The acids are then neutralized with an appropriate amount of alkali to make the soap. 26 -13
Continuous Process u More common today. u The fat or oil is hydrolyzed by water at high temperatures and pressures in the presence of a catalyst, usually a zinc soap. u The fat or oil and water are introduced continuously into opposite ends of a larger reactor and the fatty acid and glycerol are removed as formed by distillation. u The acids are then neutralized with an appropriate amount of alkali to make the soap. 26 -13
 u Most dirt on clothing or skin adheres to a thin film of oil. u If the oil film can be removed, the dirt particles can be washed away. u In acting to remove dirt, soap molecules surround and emulsify the droplets of oil or grease. u The lipophilic tail of the soap molecules dissole in the oil and the hydrophilic ends extend out of the oil droplet toward the water. 26 -14
u Most dirt on clothing or skin adheres to a thin film of oil. u If the oil film can be removed, the dirt particles can be washed away. u In acting to remove dirt, soap molecules surround and emulsify the droplets of oil or grease. u The lipophilic tail of the soap molecules dissole in the oil and the hydrophilic ends extend out of the oil droplet toward the water. 26 -14
 Synthetic Detergents u Two problems with ordinary soaps: u 1. being salts of weak acids, soaps give somewhat alkaline solutions in water which can harm certain fabrics. u 2. They form insoluble salts with calcium, magnesium or ferric ions that may be present in hard water…this is responsible for the rings around bathtubs or collars and for the films that dull the look of clothing and hair 26 -15
Synthetic Detergents u Two problems with ordinary soaps: u 1. being salts of weak acids, soaps give somewhat alkaline solutions in water which can harm certain fabrics. u 2. They form insoluble salts with calcium, magnesium or ferric ions that may be present in hard water…this is responsible for the rings around bathtubs or collars and for the films that dull the look of clothing and hair 26 -15
 Synthetic Detergents u Phosphates can also be added to soaps. u Phosphates form soluble complexes with metal ions, thus keeping these ions from forming insoluble salts with the soap. u Another way to eliminate the problems associated with ordinary soaps is to design more effective detergents… these are called syndets u Sodium lauryl sulfate is an excellent detergent u Themost widely used syndets are straight –chain alkylbenzenesulfonates. 26 -16
Synthetic Detergents u Phosphates can also be added to soaps. u Phosphates form soluble complexes with metal ions, thus keeping these ions from forming insoluble salts with the soap. u Another way to eliminate the problems associated with ordinary soaps is to design more effective detergents… these are called syndets u Sodium lauryl sulfate is an excellent detergent u Themost widely used syndets are straight –chain alkylbenzenesulfonates. 26 -16
 Phospholipids u Phospholipids are the second most abundant group of naturally occurring lipids. • They are found almost exclusively in plant and animal membranes, which typically consist of 40% -50% phospholipids and 50% - 60% proteins. • The most abundant phospholipids are esters of phosphatidic acid (glycerol esterified with two molecules of fatty acid and one of phosphoric acid). • The three most abundant fatty acids in phosphatidic acids are palmitic acid (16: 0), stearic acid (18: 0), and oleic acid (18: 1). 26 -17
Phospholipids u Phospholipids are the second most abundant group of naturally occurring lipids. • They are found almost exclusively in plant and animal membranes, which typically consist of 40% -50% phospholipids and 50% - 60% proteins. • The most abundant phospholipids are esters of phosphatidic acid (glycerol esterified with two molecules of fatty acid and one of phosphoric acid). • The three most abundant fatty acids in phosphatidic acids are palmitic acid (16: 0), stearic acid (18: 0), and oleic acid (18: 1). 26 -17
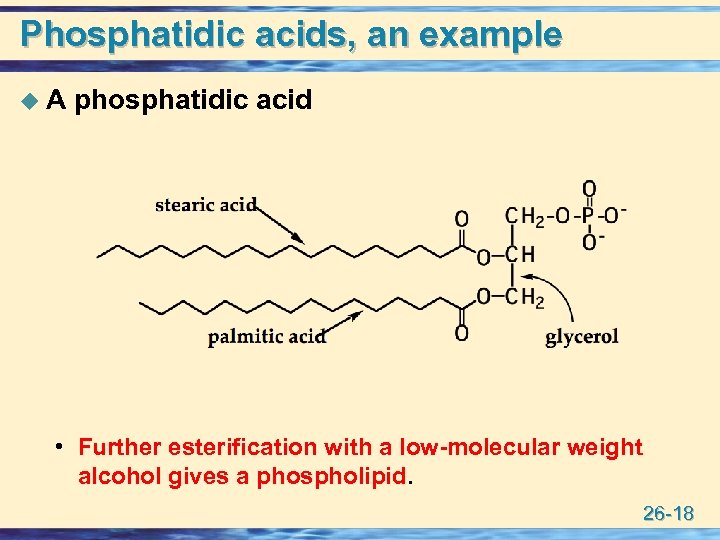 Phosphatidic acids, an example u A phosphatidic acid • Further esterification with a low-molecular weight alcohol gives a phospholipid. 26 -18
Phosphatidic acids, an example u A phosphatidic acid • Further esterification with a low-molecular weight alcohol gives a phospholipid. 26 -18
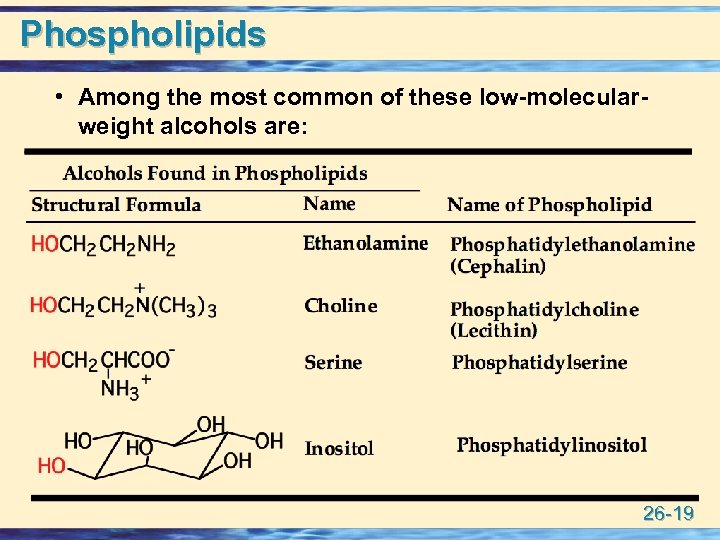 Phospholipids • Among the most common of these low-molecularweight alcohols are: 26 -19
Phospholipids • Among the most common of these low-molecularweight alcohols are: 26 -19
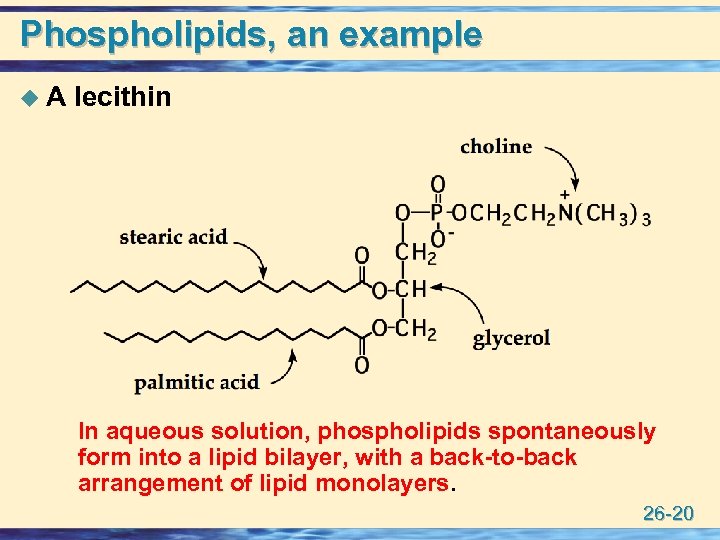 Phospholipids, an example u A lecithin In aqueous solution, phospholipids spontaneously form into a lipid bilayer, with a back-to-back arrangement of lipid monolayers. 26 -20
Phospholipids, an example u A lecithin In aqueous solution, phospholipids spontaneously form into a lipid bilayer, with a back-to-back arrangement of lipid monolayers. 26 -20
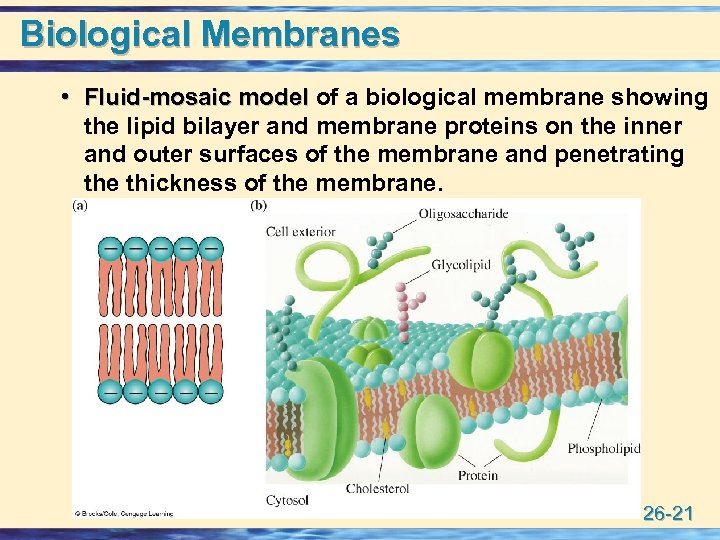 Biological Membranes • Fluid-mosaic model of a biological membrane showing Fluid-mosaic model the lipid bilayer and membrane proteins on the inner and outer surfaces of the membrane and penetrating the thickness of the membrane. 26 -21
Biological Membranes • Fluid-mosaic model of a biological membrane showing Fluid-mosaic model the lipid bilayer and membrane proteins on the inner and outer surfaces of the membrane and penetrating the thickness of the membrane. 26 -21
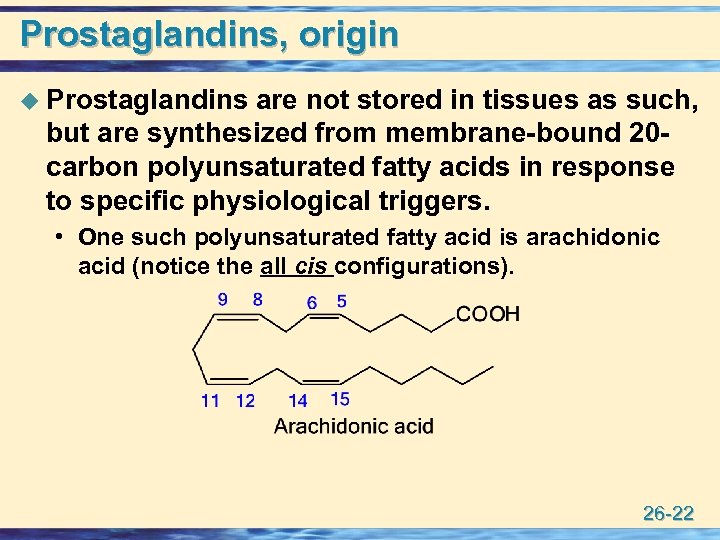 Prostaglandins, origin u Prostaglandins are not stored in tissues as such, but are synthesized from membrane-bound 20 carbon polyunsaturated fatty acids in response to specific physiological triggers. • One such polyunsaturated fatty acid is arachidonic acid (notice the all cis configurations). 26 -22
Prostaglandins, origin u Prostaglandins are not stored in tissues as such, but are synthesized from membrane-bound 20 carbon polyunsaturated fatty acids in response to specific physiological triggers. • One such polyunsaturated fatty acid is arachidonic acid (notice the all cis configurations). 26 -22
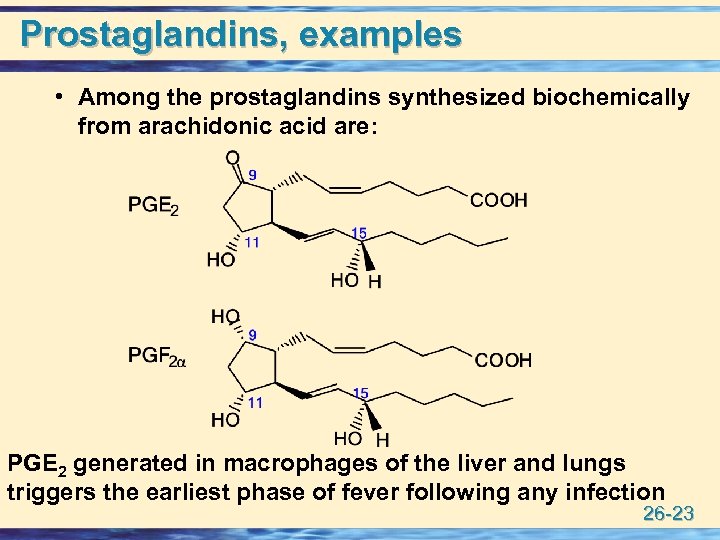 Prostaglandins, examples • Among the prostaglandins synthesized biochemically from arachidonic acid are: PGE 2 generated in macrophages of the liver and lungs triggers the earliest phase of fever following any infection 26 -23
Prostaglandins, examples • Among the prostaglandins synthesized biochemically from arachidonic acid are: PGE 2 generated in macrophages of the liver and lungs triggers the earliest phase of fever following any infection 26 -23
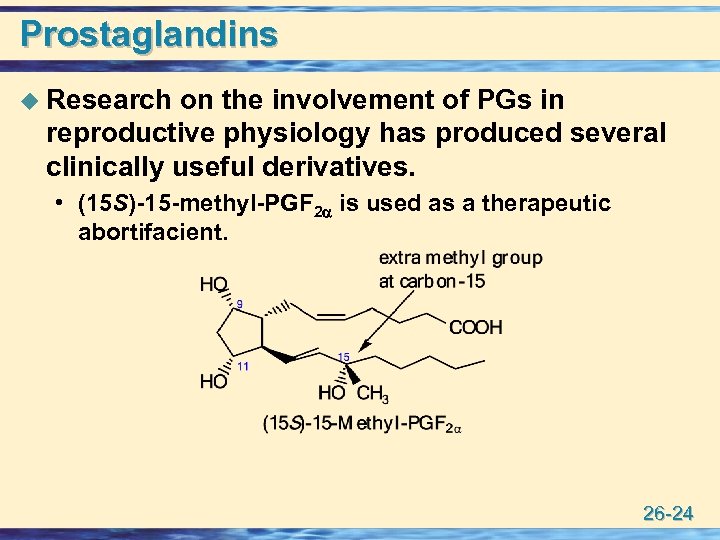 Prostaglandins u Research on the involvement of PGs in reproductive physiology has produced several clinically useful derivatives. • (15 S)-15 -methyl-PGF 2 is used as a therapeutic abortifacient. 26 -24
Prostaglandins u Research on the involvement of PGs in reproductive physiology has produced several clinically useful derivatives. • (15 S)-15 -methyl-PGF 2 is used as a therapeutic abortifacient. 26 -24
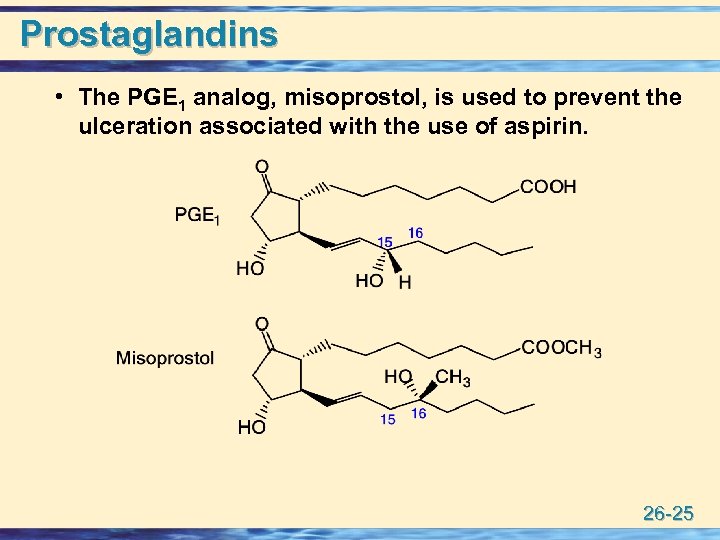 Prostaglandins • The PGE 1 analog, misoprostol, is used to prevent the ulceration associated with the use of aspirin. 26 -25
Prostaglandins • The PGE 1 analog, misoprostol, is used to prevent the ulceration associated with the use of aspirin. 26 -25
 Prostaglandins, Aspirin, and Pain u It was not until recently that the mechanism behind how aspirins work was realized. u Prostaglandins are made in cells by a series of enzyme-catalyzed reactions, with many playing important regulatory roles in digestion, blood circulation and reproduction. u Aspirin inhibits the production of prostaglandins by injured tissue. u This stops the production of prostaglandins resulting in a reduction of inflammation and pain. 26 -26
Prostaglandins, Aspirin, and Pain u It was not until recently that the mechanism behind how aspirins work was realized. u Prostaglandins are made in cells by a series of enzyme-catalyzed reactions, with many playing important regulatory roles in digestion, blood circulation and reproduction. u Aspirin inhibits the production of prostaglandins by injured tissue. u This stops the production of prostaglandins resulting in a reduction of inflammation and pain. 26 -26
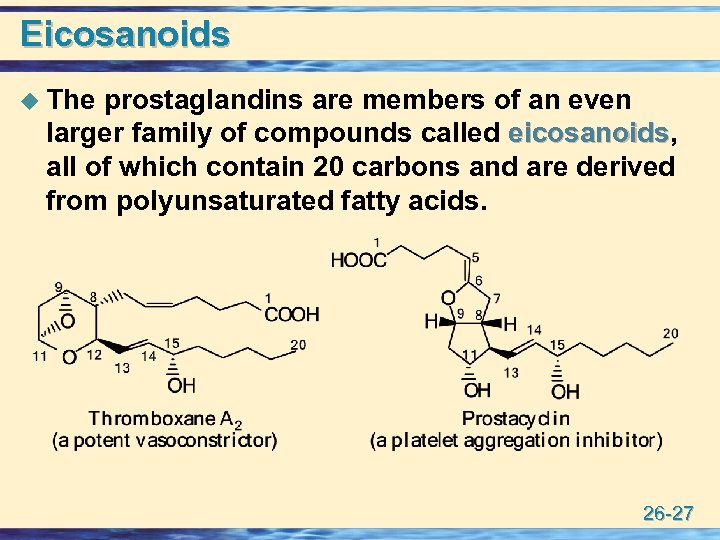 Eicosanoids u The prostaglandins are members of an even larger family of compounds called eicosanoids, eicosanoids all of which contain 20 carbons and are derived from polyunsaturated fatty acids. 26 -27
Eicosanoids u The prostaglandins are members of an even larger family of compounds called eicosanoids, eicosanoids all of which contain 20 carbons and are derived from polyunsaturated fatty acids. 26 -27
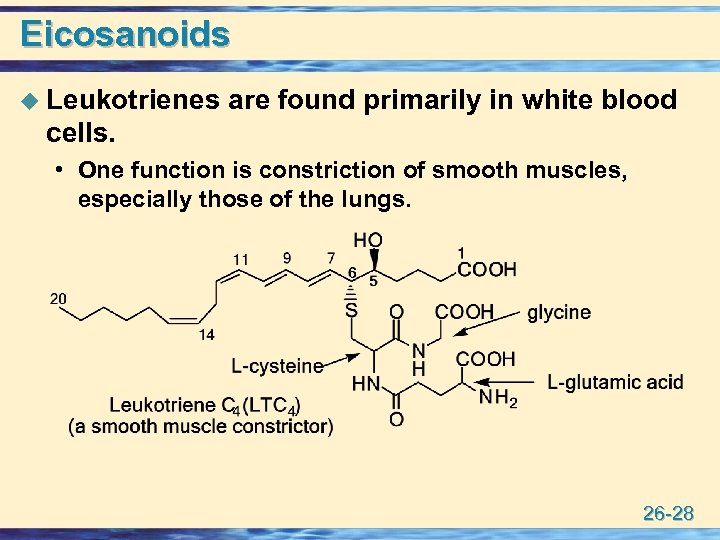 Eicosanoids u Leukotrienes are found primarily in white blood cells. • One function is constriction of smooth muscles, especially those of the lungs. 26 -28
Eicosanoids u Leukotrienes are found primarily in white blood cells. • One function is constriction of smooth muscles, especially those of the lungs. 26 -28
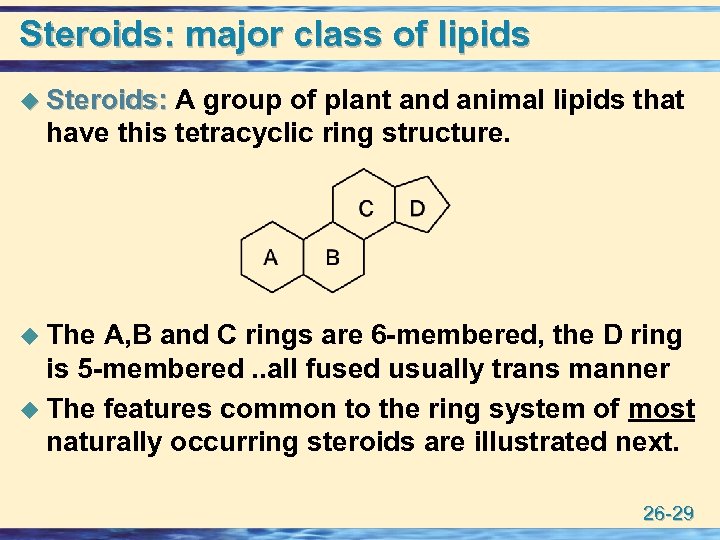 Steroids: major class of lipids u Steroids: A group of plant and animal lipids that Steroids: have this tetracyclic ring structure. u The A, B and C rings are 6 -membered, the D ring is 5 -membered. . all fused usually trans manner u The features common to the ring system of most naturally occurring steroids are illustrated next. 26 -29
Steroids: major class of lipids u Steroids: A group of plant and animal lipids that Steroids: have this tetracyclic ring structure. u The A, B and C rings are 6 -membered, the D ring is 5 -membered. . all fused usually trans manner u The features common to the ring system of most naturally occurring steroids are illustrated next. 26 -29
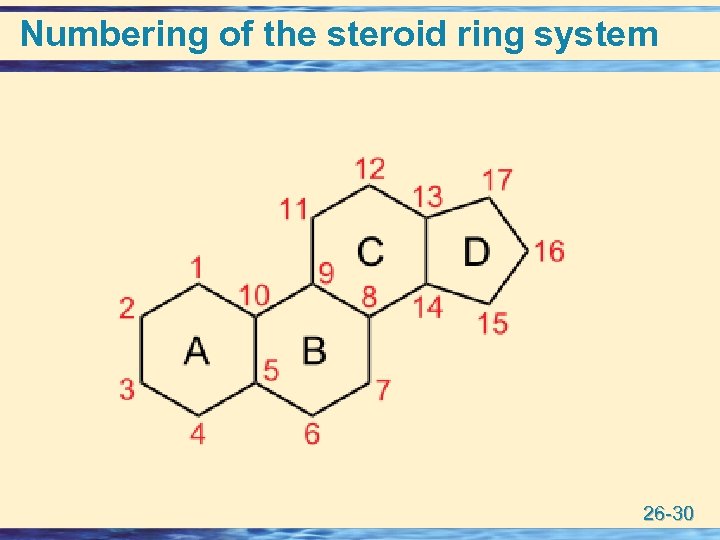 Numbering of the steroid ring system 26 -30
Numbering of the steroid ring system 26 -30
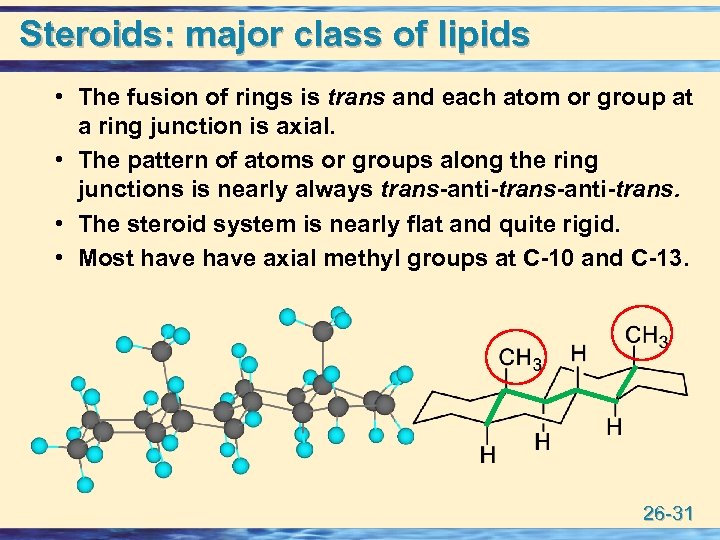 Steroids: major class of lipids • The fusion of rings is trans and each atom or group at a ring junction is axial. • The pattern of atoms or groups along the ring junctions is nearly always trans-anti-trans. • The steroid system is nearly flat and quite rigid. • Most have axial methyl groups at C-10 and C-13. 26 -31
Steroids: major class of lipids • The fusion of rings is trans and each atom or group at a ring junction is axial. • The pattern of atoms or groups along the ring junctions is nearly always trans-anti-trans. • The steroid system is nearly flat and quite rigid. • Most have axial methyl groups at C-10 and C-13. 26 -31
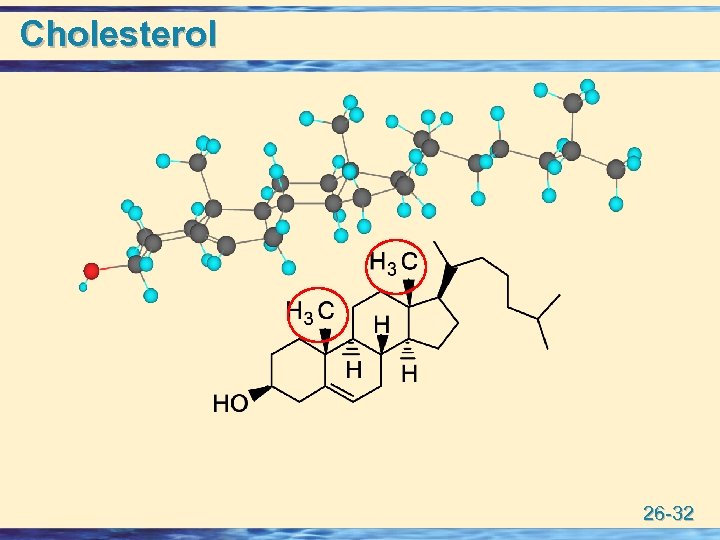 Cholesterol 26 -32
Cholesterol 26 -32
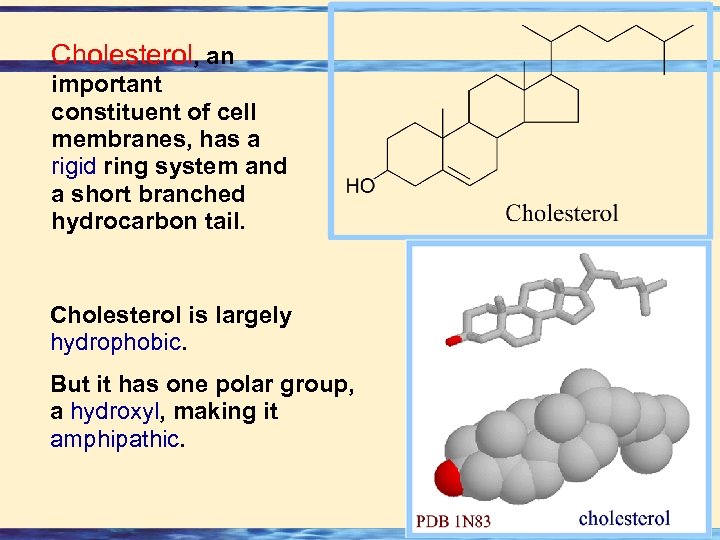 Cholesterol, an important constituent of cell membranes, has a rigid ring system and a short branched hydrocarbon tail. Cholesterol is largely hydrophobic. But it has one polar group, a hydroxyl, making it amphipathic. 26 -33
Cholesterol, an important constituent of cell membranes, has a rigid ring system and a short branched hydrocarbon tail. Cholesterol is largely hydrophobic. But it has one polar group, a hydroxyl, making it amphipathic. 26 -33
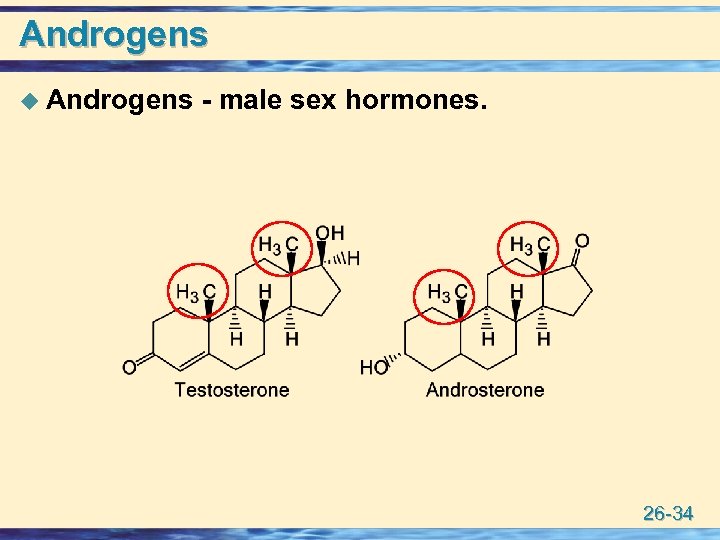 Androgens u Androgens - male sex hormones. 26 -34
Androgens u Androgens - male sex hormones. 26 -34
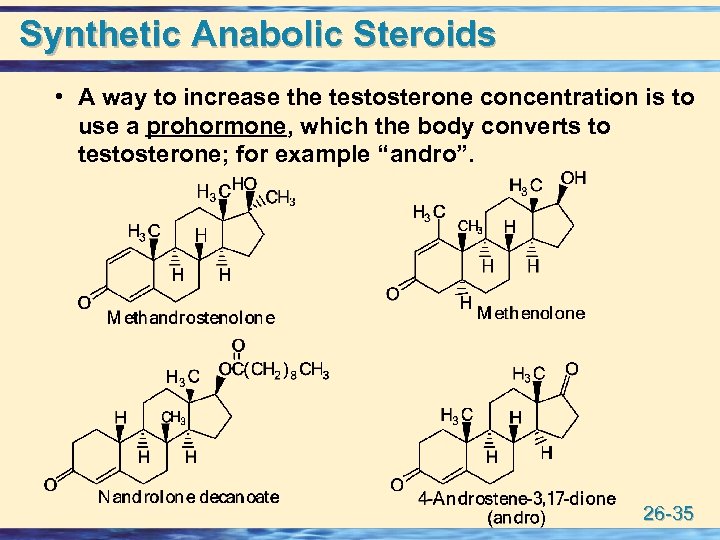 Synthetic Anabolic Steroids • A way to increase the testosterone concentration is to use a prohormone, which the body converts to testosterone; for example “andro”. 26 -35
Synthetic Anabolic Steroids • A way to increase the testosterone concentration is to use a prohormone, which the body converts to testosterone; for example “andro”. 26 -35
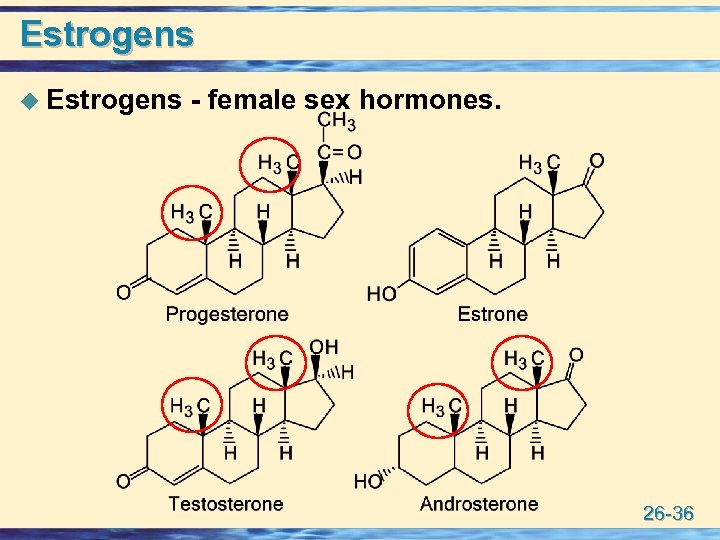 Estrogens u Estrogens - female sex hormones. 26 -36
Estrogens u Estrogens - female sex hormones. 26 -36
 Fat-Soluble Vitamins u Vitamins are divided into two broad classes on the basis of their solubility: • Those that are fat soluble, and hence classified as lipids. • Those that are water soluble. u The fat-soluble vitamins include A, D, E, and K. 26 -37
Fat-Soluble Vitamins u Vitamins are divided into two broad classes on the basis of their solubility: • Those that are fat soluble, and hence classified as lipids. • Those that are water soluble. u The fat-soluble vitamins include A, D, E, and K. 26 -37
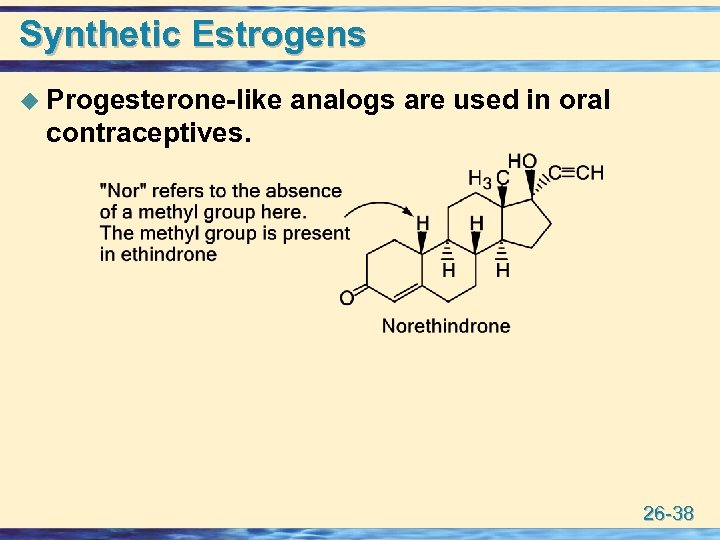 Synthetic Estrogens u Progesterone-like analogs are used in oral contraceptives. 26 -38
Synthetic Estrogens u Progesterone-like analogs are used in oral contraceptives. 26 -38
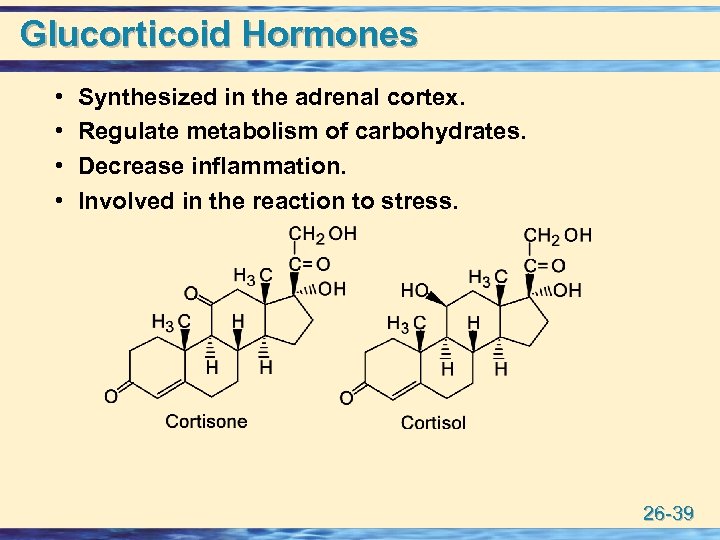 Glucorticoid Hormones • • Synthesized in the adrenal cortex. Regulate metabolism of carbohydrates. Decrease inflammation. Involved in the reaction to stress. 26 -39
Glucorticoid Hormones • • Synthesized in the adrenal cortex. Regulate metabolism of carbohydrates. Decrease inflammation. Involved in the reaction to stress. 26 -39
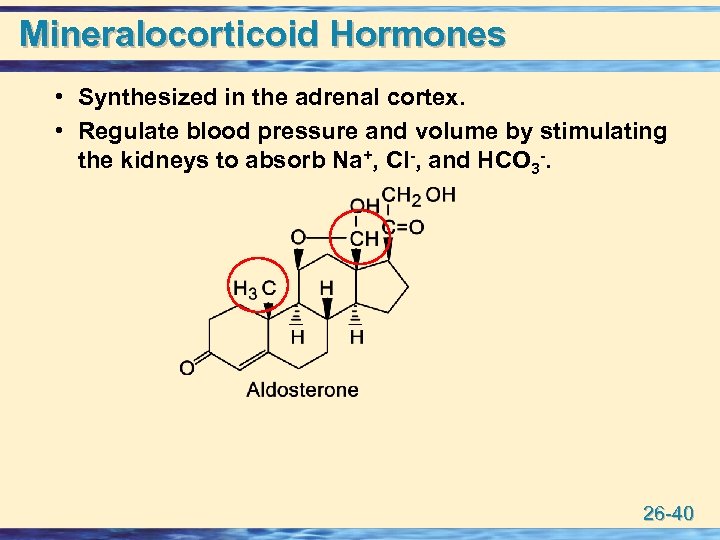 Mineralocorticoid Hormones • Synthesized in the adrenal cortex. • Regulate blood pressure and volume by stimulating the kidneys to absorb Na+, Cl-, and HCO 3 -. 26 -40
Mineralocorticoid Hormones • Synthesized in the adrenal cortex. • Regulate blood pressure and volume by stimulating the kidneys to absorb Na+, Cl-, and HCO 3 -. 26 -40
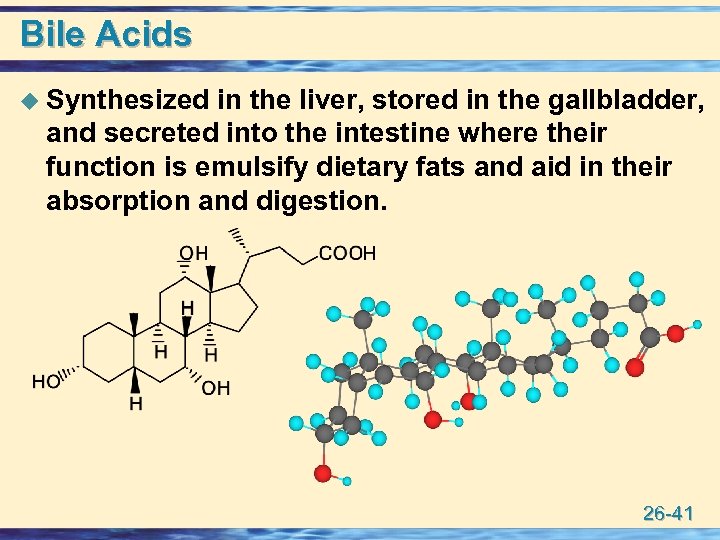 Bile Acids u Synthesized in the liver, stored in the gallbladder, and secreted into the intestine where their function is emulsify dietary fats and aid in their absorption and digestion. 26 -41
Bile Acids u Synthesized in the liver, stored in the gallbladder, and secreted into the intestine where their function is emulsify dietary fats and aid in their absorption and digestion. 26 -41
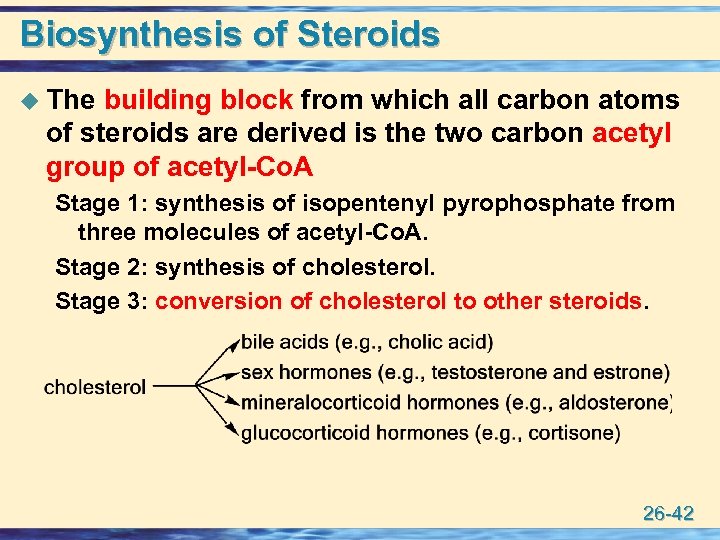 Biosynthesis of Steroids u The building block from which all carbon atoms of steroids are derived is the two carbon acetyl group of acetyl-Co. A Stage 1: synthesis of isopentenyl pyrophosphate from three molecules of acetyl-Co. A. Stage 2: synthesis of cholesterol. Stage 3: conversion of cholesterol to other steroids. 26 -42
Biosynthesis of Steroids u The building block from which all carbon atoms of steroids are derived is the two carbon acetyl group of acetyl-Co. A Stage 1: synthesis of isopentenyl pyrophosphate from three molecules of acetyl-Co. A. Stage 2: synthesis of cholesterol. Stage 3: conversion of cholesterol to other steroids. 26 -42
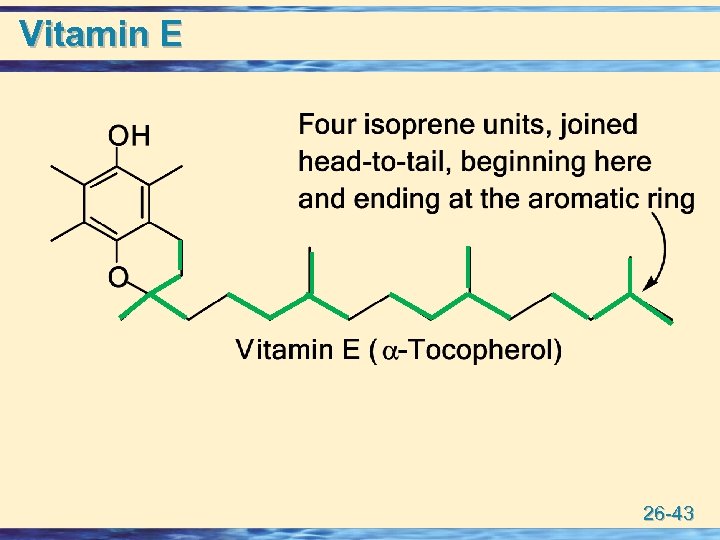 Vitamin E 26 -43
Vitamin E 26 -43
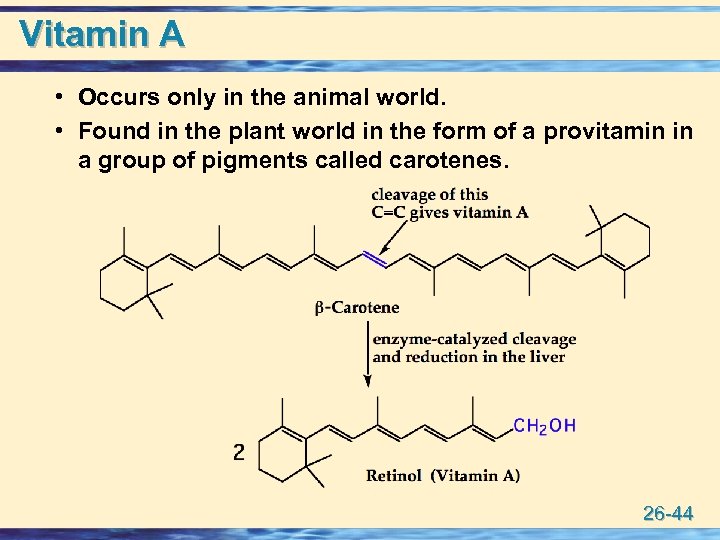 Vitamin A • Occurs only in the animal world. • Found in the plant world in the form of a provitamin in a group of pigments called carotenes. 26 -44
Vitamin A • Occurs only in the animal world. • Found in the plant world in the form of a provitamin in a group of pigments called carotenes. 26 -44
 Vitamin A u The best understood role of Vitamin A is its participation in the visual cycle in rod cells. • the active molecule is retinal (vitamin A aldehyde), which forms an imine with an -NH 2 group of the protein opsin to form the visual pigment called rhodopsin. • the primary chemical event of vision in rod cells is absorption of light by rhodopsin followed by isomerization of the 11 -cis double bond to the 11 -trans configuration. 26 -45
Vitamin A u The best understood role of Vitamin A is its participation in the visual cycle in rod cells. • the active molecule is retinal (vitamin A aldehyde), which forms an imine with an -NH 2 group of the protein opsin to form the visual pigment called rhodopsin. • the primary chemical event of vision in rod cells is absorption of light by rhodopsin followed by isomerization of the 11 -cis double bond to the 11 -trans configuration. 26 -45
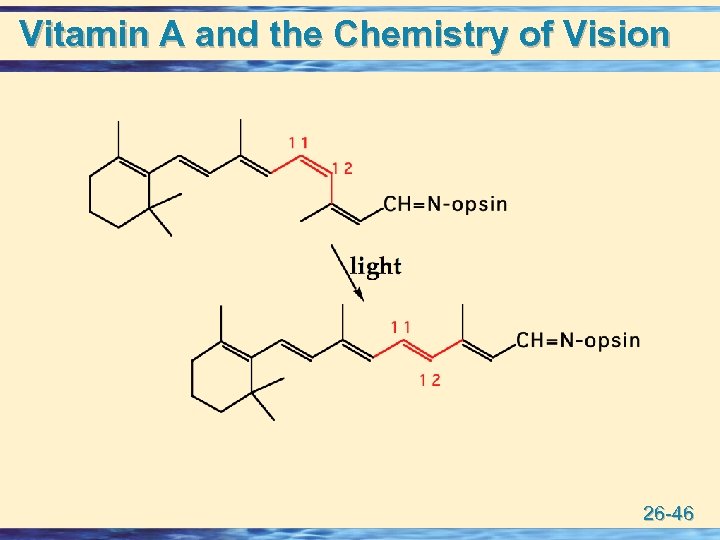 Vitamin A and the Chemistry of Vision 26 -46
Vitamin A and the Chemistry of Vision 26 -46
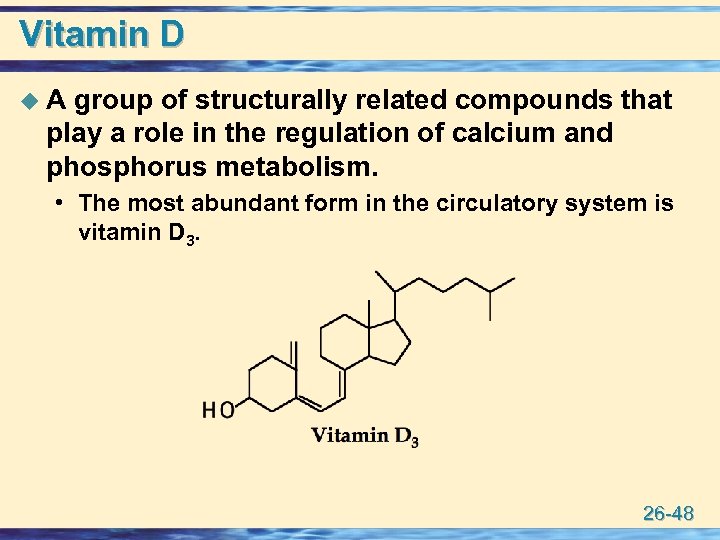 Vitamin D u A group of structurally related compounds that play a role in the regulation of calcium and phosphorus metabolism. • The most abundant form in the circulatory system is vitamin D 3. 26 -48
Vitamin D u A group of structurally related compounds that play a role in the regulation of calcium and phosphorus metabolism. • The most abundant form in the circulatory system is vitamin D 3. 26 -48
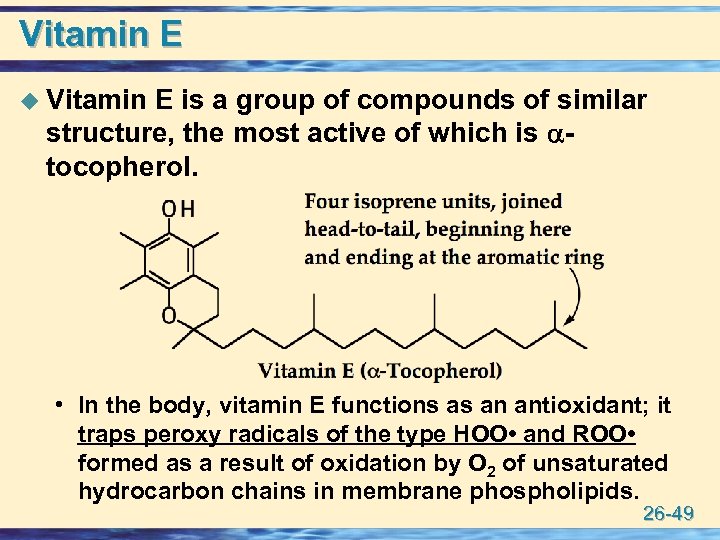 Vitamin E u Vitamin E is a group of compounds of similar structure, the most active of which is tocopherol. • In the body, vitamin E functions as an antioxidant; it traps peroxy radicals of the type HOO • and ROO • formed as a result of oxidation by O 2 of unsaturated hydrocarbon chains in membrane phospholipids. 26 -49
Vitamin E u Vitamin E is a group of compounds of similar structure, the most active of which is tocopherol. • In the body, vitamin E functions as an antioxidant; it traps peroxy radicals of the type HOO • and ROO • formed as a result of oxidation by O 2 of unsaturated hydrocarbon chains in membrane phospholipids. 26 -49
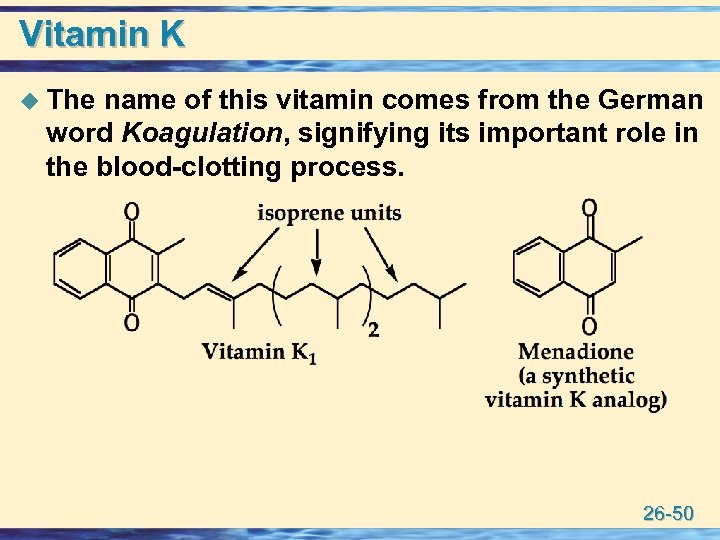 Vitamin K u The name of this vitamin comes from the German word Koagulation, signifying its important role in the blood-clotting process. 26 -50
Vitamin K u The name of this vitamin comes from the German word Koagulation, signifying its important role in the blood-clotting process. 26 -50
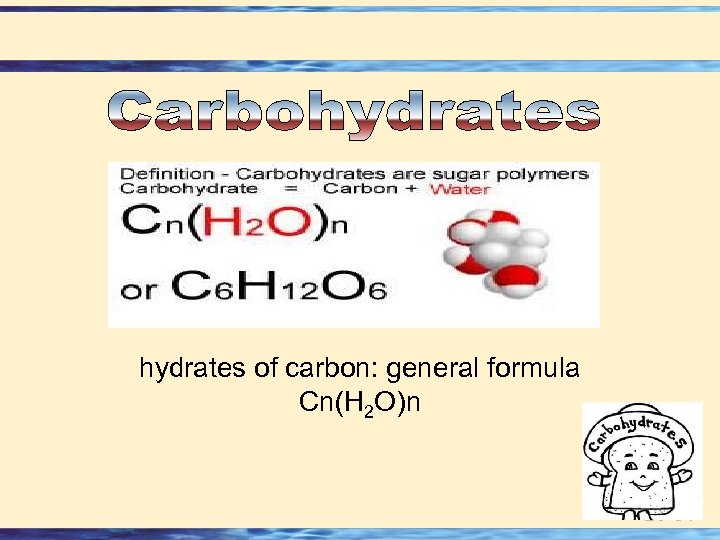 hydrates of carbon: general formula Cn(H 2 O)n 26 -51
hydrates of carbon: general formula Cn(H 2 O)n 26 -51
 u Carbohydrates are abundant in nature: they are high energy biomolecules u The provide structural rigidity for organisms u The polymer backbone on which DNA and RNA are assembled u CARBOHYDRATES ARE NOT TRUE HYDRATES u THEY ARE POLYHYDROXYL ALDEHIDES OR KETONES 26 -52
u Carbohydrates are abundant in nature: they are high energy biomolecules u The provide structural rigidity for organisms u The polymer backbone on which DNA and RNA are assembled u CARBOHYDRATES ARE NOT TRUE HYDRATES u THEY ARE POLYHYDROXYL ALDEHIDES OR KETONES 26 -52
 Classification of Carbohydrates. I. Number of carbohydrate units monosaccharides: one carbohydrate unit (simple carbohydrates) disaccharides: two carbohydrate units (complex carbohydrates) trisaccharides: three carbohydrate units polysaccharides: many carbohydrate units 26 -53
Classification of Carbohydrates. I. Number of carbohydrate units monosaccharides: one carbohydrate unit (simple carbohydrates) disaccharides: two carbohydrate units (complex carbohydrates) trisaccharides: three carbohydrate units polysaccharides: many carbohydrate units 26 -53
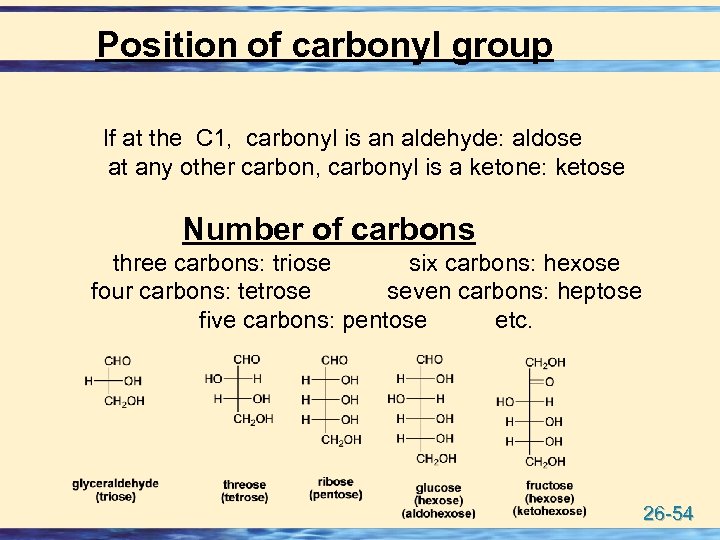 Position of carbonyl group If at the C 1, carbonyl is an aldehyde: aldose at any other carbon, carbonyl is a ketone: ketose Number of carbons three carbons: triose six carbons: hexose four carbons: tetrose seven carbons: heptose five carbons: pentose etc. 26 -54
Position of carbonyl group If at the C 1, carbonyl is an aldehyde: aldose at any other carbon, carbonyl is a ketone: ketose Number of carbons three carbons: triose six carbons: hexose four carbons: tetrose seven carbons: heptose five carbons: pentose etc. 26 -54
 Fischer Projections and the D-L Notation. It is a representation of a three-dimensional molecule as a flat structure. u Tetrahedral carbons are represented by two crossed lines: u 26 -55
Fischer Projections and the D-L Notation. It is a representation of a three-dimensional molecule as a flat structure. u Tetrahedral carbons are represented by two crossed lines: u 26 -55
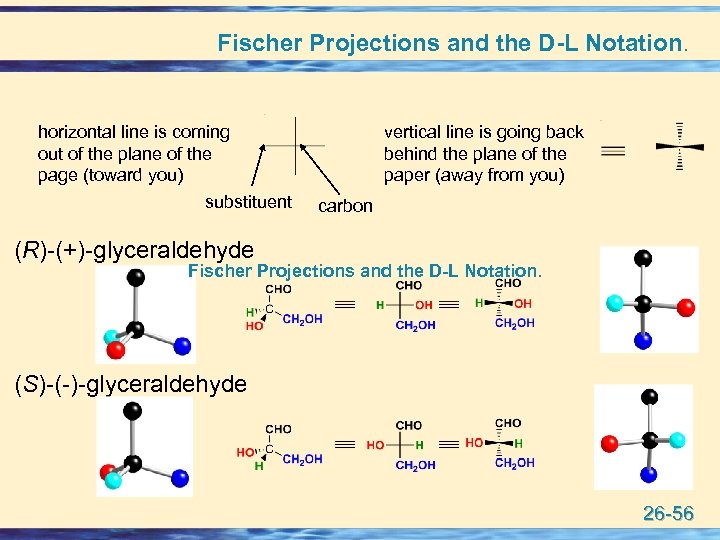 Fischer Projections and the D-L Notation. horizontal line is coming out of the plane of the page (toward you) substituent vertical line is going back behind the plane of the paper (away from you) carbon (R)-(+)-glyceraldehyde Fischer Projections and the D-L Notation. (S)-(-)-glyceraldehyde 26 -56
Fischer Projections and the D-L Notation. horizontal line is coming out of the plane of the page (toward you) substituent vertical line is going back behind the plane of the paper (away from you) carbon (R)-(+)-glyceraldehyde Fischer Projections and the D-L Notation. (S)-(-)-glyceraldehyde 26 -56
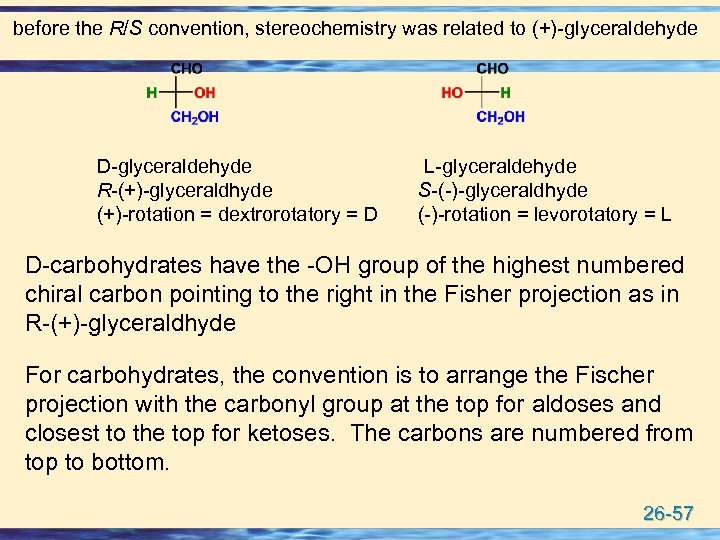 before the R/S convention, stereochemistry was related to (+)-glyceraldehyde D-glyceraldehyde R-(+)-glyceraldhyde (+)-rotation = dextrorotatory = D L-glyceraldehyde S-(-)-glyceraldhyde (-)-rotation = levorotatory = L D-carbohydrates have the -OH group of the highest numbered chiral carbon pointing to the right in the Fisher projection as in R-(+)-glyceraldhyde For carbohydrates, the convention is to arrange the Fischer projection with the carbonyl group at the top for aldoses and closest to the top for ketoses. The carbons are numbered from top to bottom. 26 -57
before the R/S convention, stereochemistry was related to (+)-glyceraldehyde D-glyceraldehyde R-(+)-glyceraldhyde (+)-rotation = dextrorotatory = D L-glyceraldehyde S-(-)-glyceraldhyde (-)-rotation = levorotatory = L D-carbohydrates have the -OH group of the highest numbered chiral carbon pointing to the right in the Fisher projection as in R-(+)-glyceraldhyde For carbohydrates, the convention is to arrange the Fischer projection with the carbonyl group at the top for aldoses and closest to the top for ketoses. The carbons are numbered from top to bottom. 26 -57
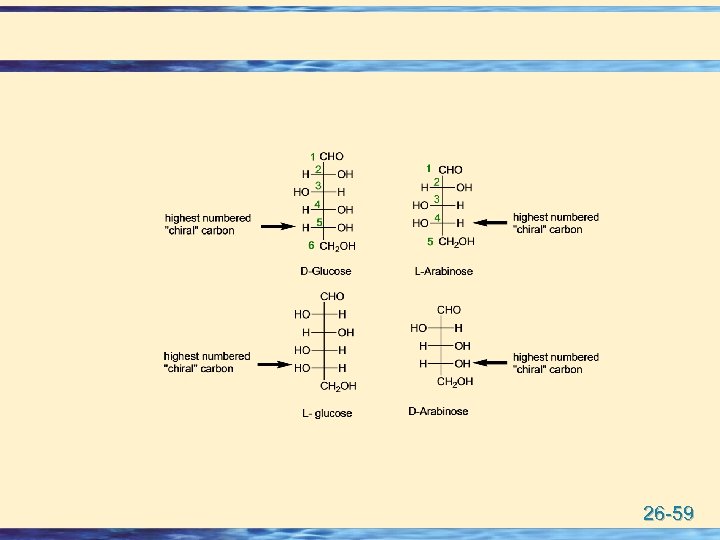
 u Carbohydrates are designated as D- or L- according to the stereochemistry of the highest numbered chiral carbon of the Fischer projection. u If the hydroxyl group of the highest numbered chiral carbon is pointing to the right, the sugar is designated as D (Dextro: Latin for on the right side). u If the hydroxyl group is pointing to the left, the sugar is designated as L (Levo: Latin for on the left side). u Most naturally occurring carbohydrates are of the Dconfiguration. 26 -58
u Carbohydrates are designated as D- or L- according to the stereochemistry of the highest numbered chiral carbon of the Fischer projection. u If the hydroxyl group of the highest numbered chiral carbon is pointing to the right, the sugar is designated as D (Dextro: Latin for on the right side). u If the hydroxyl group is pointing to the left, the sugar is designated as L (Levo: Latin for on the left side). u Most naturally occurring carbohydrates are of the Dconfiguration. 26 -58
 26 -59
26 -59
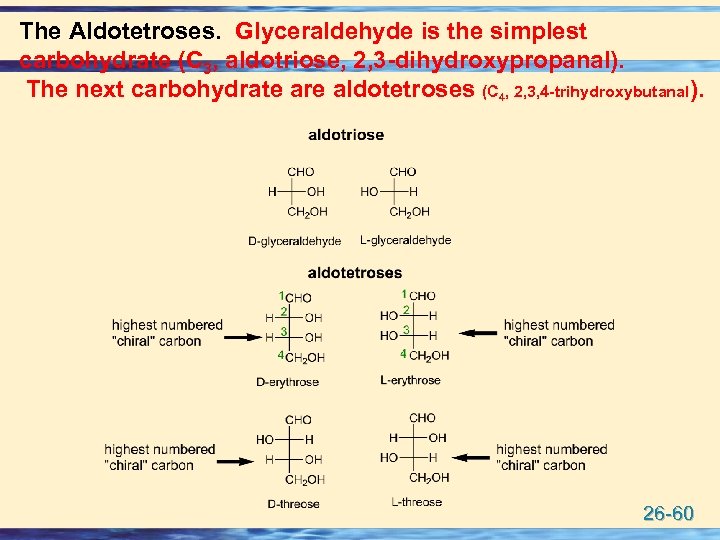 The Aldotetroses. Glyceraldehyde is the simplest carbohydrate (C 3, aldotriose, 2, 3 -dihydroxypropanal). The next carbohydrate are aldotetroses (C 4, 2, 3, 4 -trihydroxybutanal). 26 -60
The Aldotetroses. Glyceraldehyde is the simplest carbohydrate (C 3, aldotriose, 2, 3 -dihydroxypropanal). The next carbohydrate are aldotetroses (C 4, 2, 3, 4 -trihydroxybutanal). 26 -60
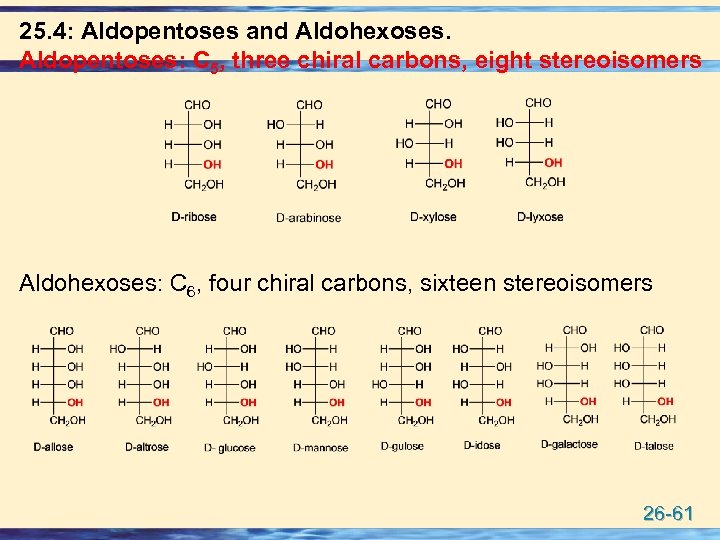 25. 4: Aldopentoses and Aldohexoses. Aldopentoses: C 5, three chiral carbons, eight stereoisomers Aldohexoses: C 6, four chiral carbons, sixteen stereoisomers 26 -61
25. 4: Aldopentoses and Aldohexoses. Aldopentoses: C 5, three chiral carbons, eight stereoisomers Aldohexoses: C 6, four chiral carbons, sixteen stereoisomers 26 -61
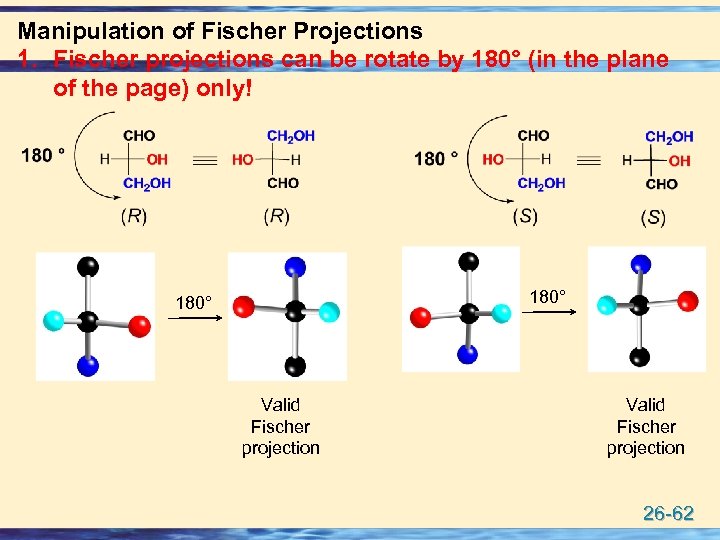 Manipulation of Fischer Projections 1. Fischer projections can be rotate by 180° (in the plane of the page) only! 180° Valid Fischer projection 26 -62
Manipulation of Fischer Projections 1. Fischer projections can be rotate by 180° (in the plane of the page) only! 180° Valid Fischer projection 26 -62
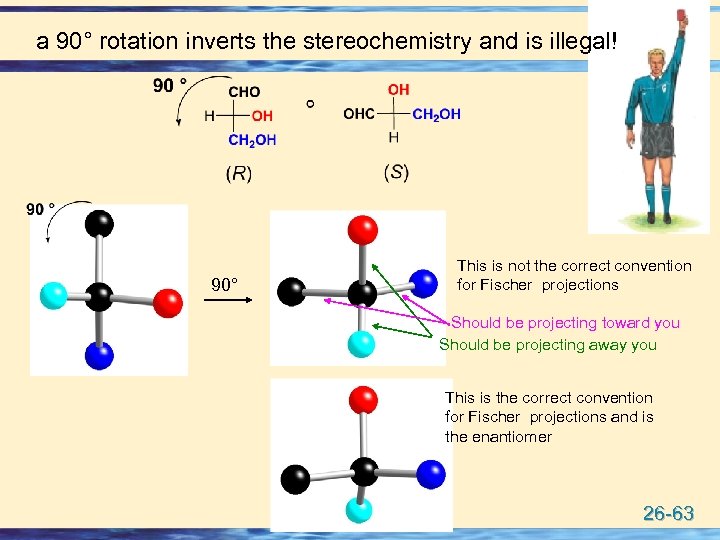 a 90° rotation inverts the stereochemistry and is illegal! 90° This is not the correct convention for Fischer projections Should be projecting toward you Should be projecting away you This is the correct convention for Fischer projections and is the enantiomer 26 -63
a 90° rotation inverts the stereochemistry and is illegal! 90° This is not the correct convention for Fischer projections Should be projecting toward you Should be projecting away you This is the correct convention for Fischer projections and is the enantiomer 26 -63
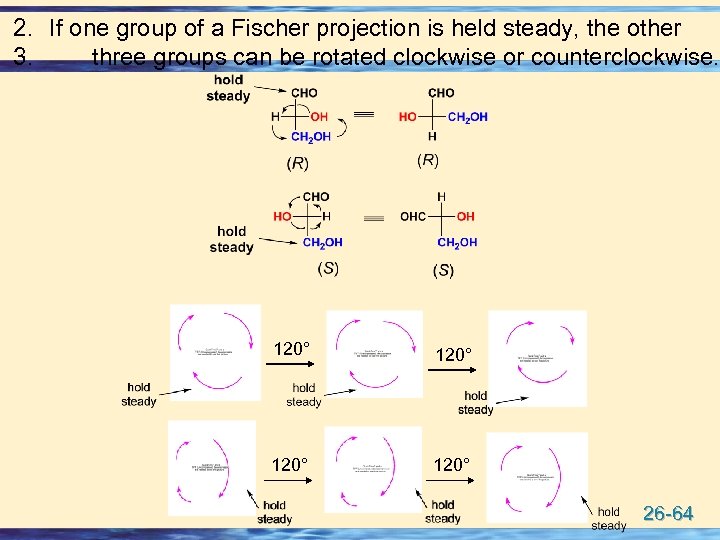 2. If one group of a Fischer projection is held steady, the other 3. three groups can be rotated clockwise or counterclockwise. 120° 26 -64
2. If one group of a Fischer projection is held steady, the other 3. three groups can be rotated clockwise or counterclockwise. 120° 26 -64
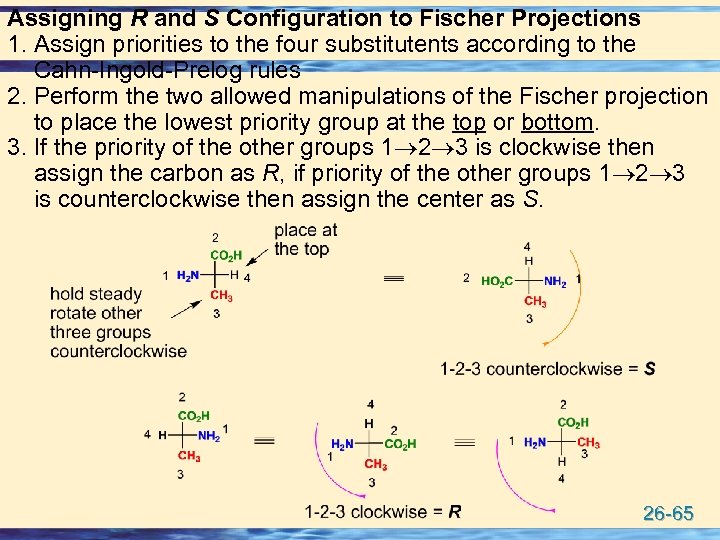 Assigning R and S Configuration to Fischer Projections 1. Assign priorities to the four substitutents according to the Cahn-Ingold-Prelog rules 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group at the top or bottom. 3. If the priority of the other groups 1 2 3 is clockwise then assign the carbon as R, if priority of the other groups 1 2 3 is counterclockwise then assign the center as S. 26 -65
Assigning R and S Configuration to Fischer Projections 1. Assign priorities to the four substitutents according to the Cahn-Ingold-Prelog rules 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group at the top or bottom. 3. If the priority of the other groups 1 2 3 is clockwise then assign the carbon as R, if priority of the other groups 1 2 3 is counterclockwise then assign the center as S. 26 -65
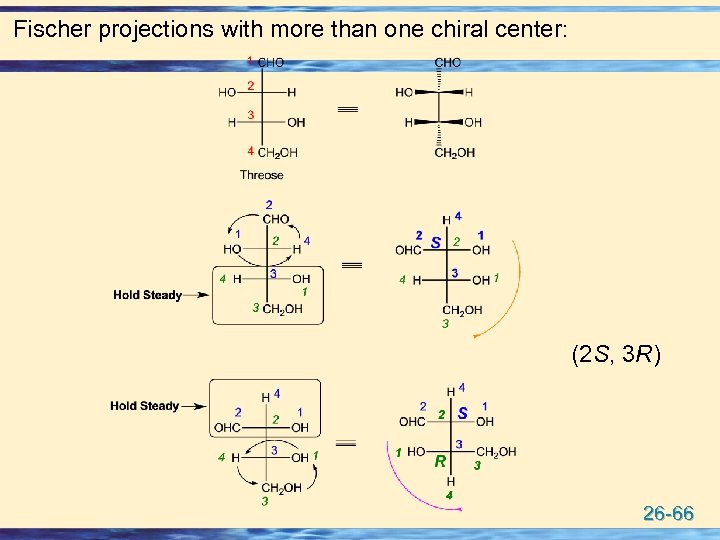 Fischer projections with more than one chiral center: (2 S, 3 R) 26 -66
Fischer projections with more than one chiral center: (2 S, 3 R) 26 -66
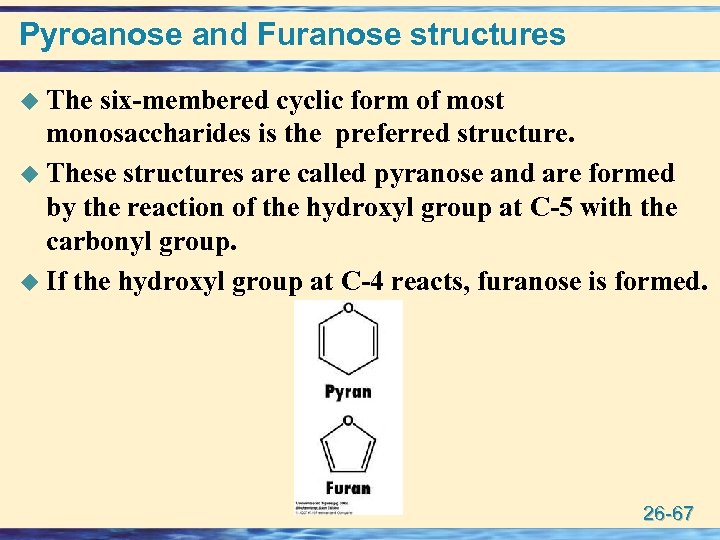 Pyroanose and Furanose structures u The six-membered cyclic form of most monosaccharides is the preferred structure. u These structures are called pyranose and are formed by the reaction of the hydroxyl group at C-5 with the carbonyl group. u If the hydroxyl group at C-4 reacts, furanose is formed. 26 -67
Pyroanose and Furanose structures u The six-membered cyclic form of most monosaccharides is the preferred structure. u These structures are called pyranose and are formed by the reaction of the hydroxyl group at C-5 with the carbonyl group. u If the hydroxyl group at C-4 reacts, furanose is formed. 26 -67
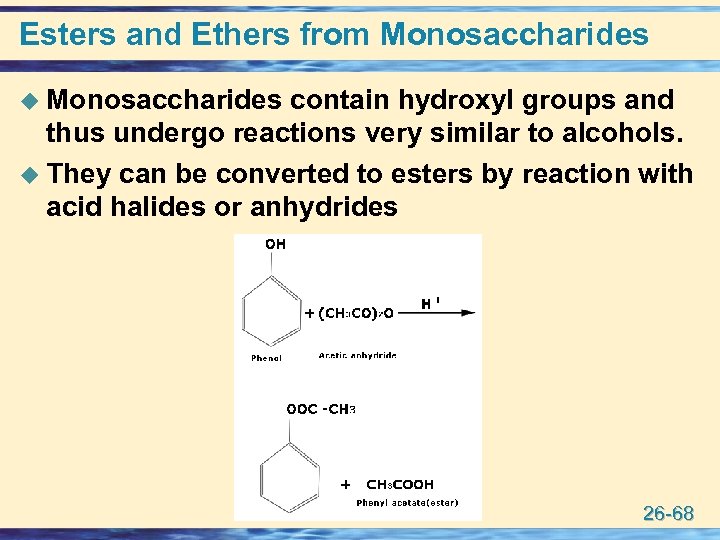 Esters and Ethers from Monosaccharides u Monosaccharides contain hydroxyl groups and thus undergo reactions very similar to alcohols. u They can be converted to esters by reaction with acid halides or anhydrides 26 -68
Esters and Ethers from Monosaccharides u Monosaccharides contain hydroxyl groups and thus undergo reactions very similar to alcohols. u They can be converted to esters by reaction with acid halides or anhydrides 26 -68
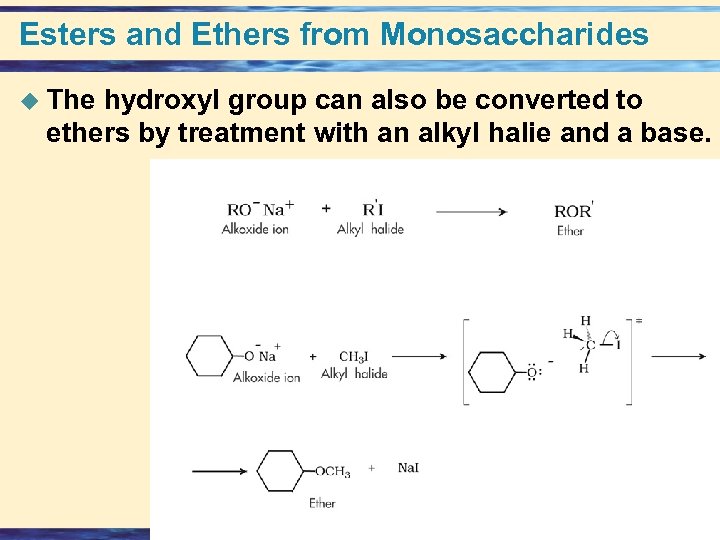 Esters and Ethers from Monosaccharides u The hydroxyl group can also be converted to ethers by treatment with an alkyl halie and a base. 26 -69
Esters and Ethers from Monosaccharides u The hydroxyl group can also be converted to ethers by treatment with an alkyl halie and a base. 26 -69
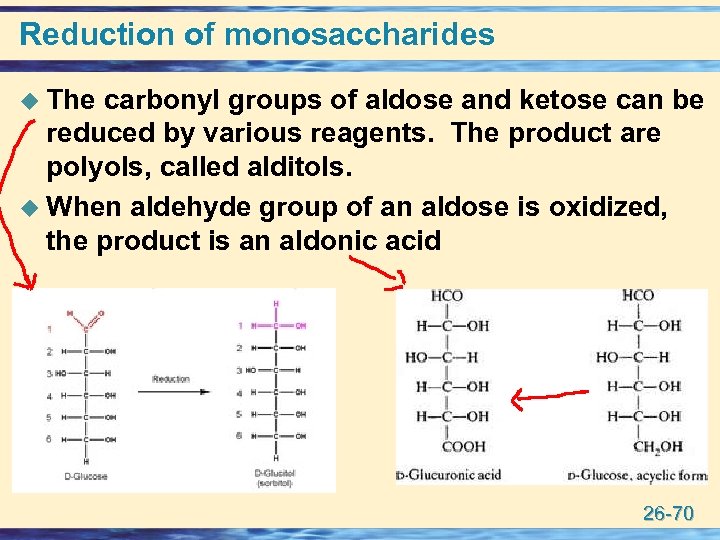 Reduction of monosaccharides u The carbonyl groups of aldose and ketose can be reduced by various reagents. The product are polyols, called alditols. u When aldehyde group of an aldose is oxidized, the product is an aldonic acid 26 -70
Reduction of monosaccharides u The carbonyl groups of aldose and ketose can be reduced by various reagents. The product are polyols, called alditols. u When aldehyde group of an aldose is oxidized, the product is an aldonic acid 26 -70
 Definitions u A polyol is an alcohol containing multiple hydroxyl groups u An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. u Thus, their general chemical formula is HOOC(CHOH)n-CH 2 OH. 26 -71
Definitions u A polyol is an alcohol containing multiple hydroxyl groups u An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. u Thus, their general chemical formula is HOOC(CHOH)n-CH 2 OH. 26 -71
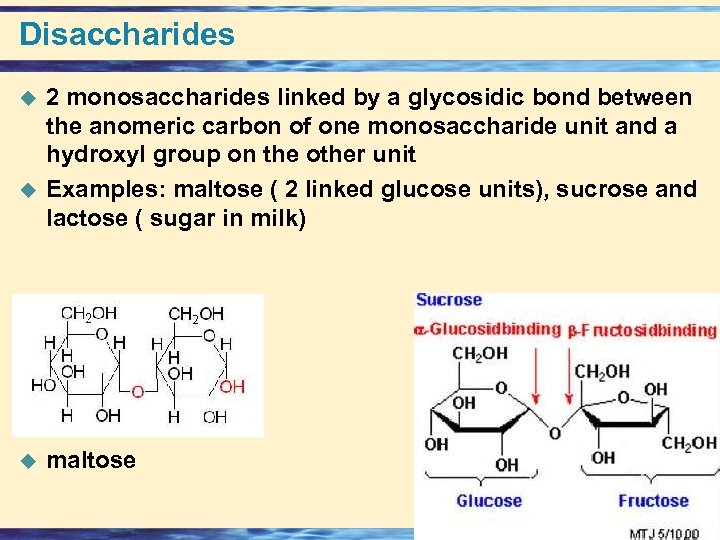 Disaccharides u u u 2 monosaccharides linked by a glycosidic bond between the anomeric carbon of one monosaccharide unit and a hydroxyl group on the other unit Examples: maltose ( 2 linked glucose units), sucrose and lactose ( sugar in milk) maltose 26 -72
Disaccharides u u u 2 monosaccharides linked by a glycosidic bond between the anomeric carbon of one monosaccharide unit and a hydroxyl group on the other unit Examples: maltose ( 2 linked glucose units), sucrose and lactose ( sugar in milk) maltose 26 -72
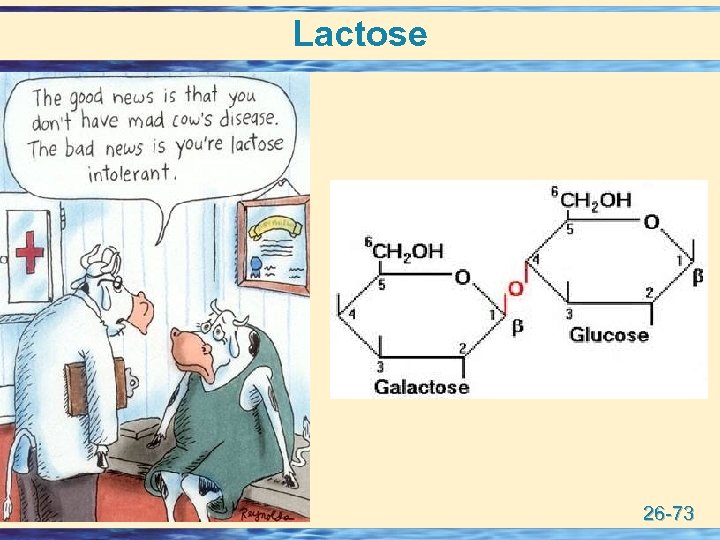 Lactose 26 -73
Lactose 26 -73
 Sweetness and Sweetners u Many synthetic sweeteners are known including saccharine ( discovered in 1879) u Saccharin is 300 x sweeter than sucrose u In 1981 Aspartame was approved by the U. S. Food and Drug Administration u Structurally, aspartame is the methyl ester of a dipeptide of two amino acids that occur naturally in proteins( aspartic acid and phenylalanine ) 26 -74
Sweetness and Sweetners u Many synthetic sweeteners are known including saccharine ( discovered in 1879) u Saccharin is 300 x sweeter than sucrose u In 1981 Aspartame was approved by the U. S. Food and Drug Administration u Structurally, aspartame is the methyl ester of a dipeptide of two amino acids that occur naturally in proteins( aspartic acid and phenylalanine ) 26 -74
 'SUGAR FREE' on the label; DO NOT EVEN THINK ABOUT IT!I u 'In the keynote address by the EPA, it was announced that in the United States in 2001 there is an epidemic of multiple sclerosis and systemic lupus. u It was difficult to determine exactly what toxin was causing this to be rampant. u 26 -75
'SUGAR FREE' on the label; DO NOT EVEN THINK ABOUT IT!I u 'In the keynote address by the EPA, it was announced that in the United States in 2001 there is an epidemic of multiple sclerosis and systemic lupus. u It was difficult to determine exactly what toxin was causing this to be rampant. u 26 -75
 ASPARTAME u marketed as'Nutra Sweet, ' 'Equal, ' and 'Spoonful u I will explain why Aspartame is so dangerous: u When the temperature of this sweetener exceeds 86 degrees F, the wood alcohol in ASPARTAME converts to formaldehyde and then to formic acid, which in turn causes metabolic acidosis. u Formic acid is the poison found in the sting of fire ants. u The methanol toxicity mimics, among other conditions, multiple sclerosis and systemic lupus. 26 -76
ASPARTAME u marketed as'Nutra Sweet, ' 'Equal, ' and 'Spoonful u I will explain why Aspartame is so dangerous: u When the temperature of this sweetener exceeds 86 degrees F, the wood alcohol in ASPARTAME converts to formaldehyde and then to formic acid, which in turn causes metabolic acidosis. u Formic acid is the poison found in the sting of fire ants. u The methanol toxicity mimics, among other conditions, multiple sclerosis and systemic lupus. 26 -76
 u Many people were being diagnosed in error. Although multiple sclerosis is not a death sentence, Methanol toxicity is! u Systemic lupus has become almost as rampant as multiple sclerosis, especially with Diet Coke and Diet Pepsi drinkers. The victim usually does not know that the Aspartame is the culprit. He or she continues its use; irritating the lupus to such a degree that it may become a life-threatening condition. We have seen patients with systemic lupus become asymptotic, once taken off diet sodas. u u u 26 -77
u Many people were being diagnosed in error. Although multiple sclerosis is not a death sentence, Methanol toxicity is! u Systemic lupus has become almost as rampant as multiple sclerosis, especially with Diet Coke and Diet Pepsi drinkers. The victim usually does not know that the Aspartame is the culprit. He or she continues its use; irritating the lupus to such a degree that it may become a life-threatening condition. We have seen patients with systemic lupus become asymptotic, once taken off diet sodas. u u u 26 -77
 Amino Acids, Peptides and Proteins 26 -78
Amino Acids, Peptides and Proteins 26 -78
 Proteins u Are naturally occurring polymers composed of amino acid units joined together by amides (peptide bonds) u Peptides are oligomers of amino acids that play an important role in many biological processes 26 -79
Proteins u Are naturally occurring polymers composed of amino acid units joined together by amides (peptide bonds) u Peptides are oligomers of amino acids that play an important role in many biological processes 26 -79
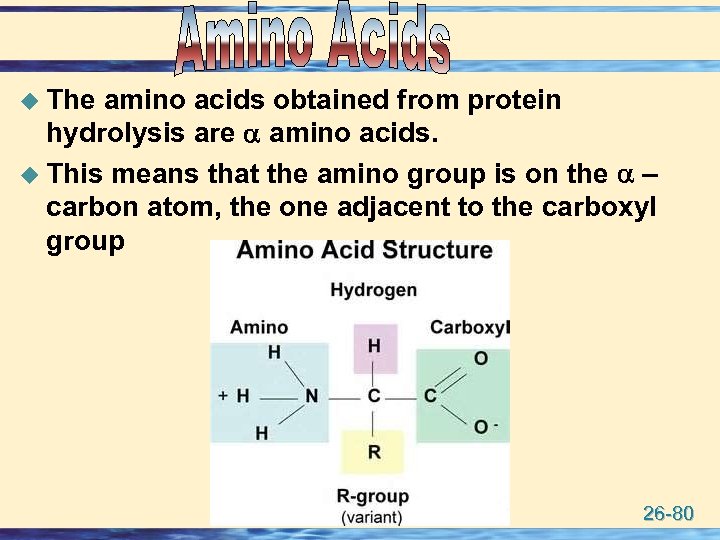 u The amino acids obtained from protein hydrolysis are amino acids. u This means that the amino group is on the – carbon atom, the one adjacent to the carboxyl group 26 -80
u The amino acids obtained from protein hydrolysis are amino acids. u This means that the amino group is on the – carbon atom, the one adjacent to the carboxyl group 26 -80
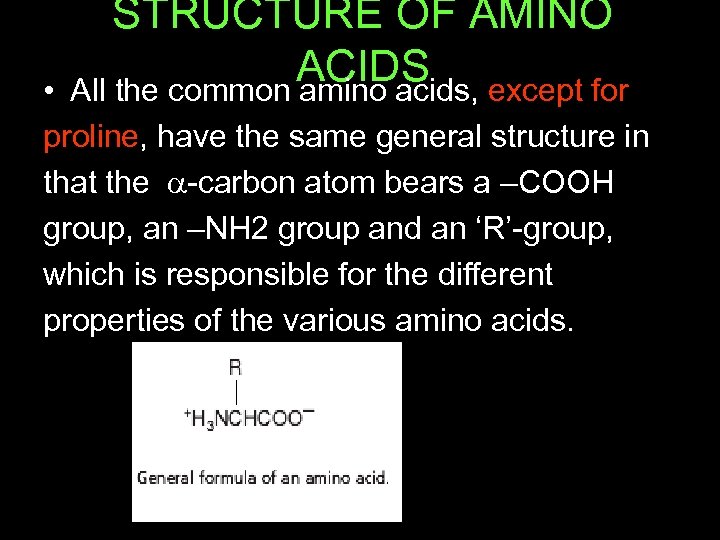 STRUCTURE OF AMINO ACIDS except for All the common amino acids, • proline, have the same general structure in that the -carbon atom bears a –COOH group, an –NH 2 group and an ‘R’-group, which is responsible for the different properties of the various amino acids.
STRUCTURE OF AMINO ACIDS except for All the common amino acids, • proline, have the same general structure in that the -carbon atom bears a –COOH group, an –NH 2 group and an ‘R’-group, which is responsible for the different properties of the various amino acids.
 Amino acids, peptides and proteins The fundamental component of a protein is the polypeptide chain composed of amino acid residues; Twenty different residues are involved in protein synthesis. These residues might be modified after the synthesis of the polypeptide chain.
Amino acids, peptides and proteins The fundamental component of a protein is the polypeptide chain composed of amino acid residues; Twenty different residues are involved in protein synthesis. These residues might be modified after the synthesis of the polypeptide chain.
 • The other components of proteins are called prosthetic groups. • The structure of the amino acids and their characteristic property as amphoteric molecules is described, followed by a description of asymmetry and chirality.
• The other components of proteins are called prosthetic groups. • The structure of the amino acids and their characteristic property as amphoteric molecules is described, followed by a description of asymmetry and chirality.
 • The ionic properties of proteins are important in such interactions and in their electrophoretic separation. • Proteins can also be separated on the basis of their size. • The tertiary structure of proteins can be destroyed by denaturation. • Finally, it is shown that even small peptides can possess biological activity, for example as hormones and transmitters.
• The ionic properties of proteins are important in such interactions and in their electrophoretic separation. • Proteins can also be separated on the basis of their size. • The tertiary structure of proteins can be destroyed by denaturation. • Finally, it is shown that even small peptides can possess biological activity, for example as hormones and transmitters.
 • • • ASYMMETRY IN BIOCHEMISTRY The α- carbon is optically active in all amino acids other than glycine. The two possible isomers are termed D and L. All naturally occurring amino acids found in proteins are of the L-configuration Chirality is derived from the Greek word cheir for ‘hand’– the left and right hands are mirror images of each other. Such asymmetry in molecular structure is of great importance in biochemistry.
• • • ASYMMETRY IN BIOCHEMISTRY The α- carbon is optically active in all amino acids other than glycine. The two possible isomers are termed D and L. All naturally occurring amino acids found in proteins are of the L-configuration Chirality is derived from the Greek word cheir for ‘hand’– the left and right hands are mirror images of each other. Such asymmetry in molecular structure is of great importance in biochemistry.
 • A chiral molecule possesses at least one asymmetric centre, such as a carbon atom, to which are joined four groups that are different from each other. • The amino acid alanine can exist in two forms, denoted D-alanine and L-alanine,
• A chiral molecule possesses at least one asymmetric centre, such as a carbon atom, to which are joined four groups that are different from each other. • The amino acid alanine can exist in two forms, denoted D-alanine and L-alanine,
 R AND S CONVENTION • A chiral centre can be denoted R or S. The method for ascribing the R or S designation to a centre is as follows: • • List the functional groups in order of their priority assigned by convention. The order for some bio chemically important groups is –SH (highest), –OH, –NH 2, –COOH, –CHO, –CH 3, –H (lowest). Then orientate the molecule so that the group of lowest priority points away from the observer. If the order of priority (high to low) of the remaining groups is clock wise, the centre is R. If the order or priority is anticlockwise, the centre is S. Thus the -carbon of L-alanine has the S configuration.
R AND S CONVENTION • A chiral centre can be denoted R or S. The method for ascribing the R or S designation to a centre is as follows: • • List the functional groups in order of their priority assigned by convention. The order for some bio chemically important groups is –SH (highest), –OH, –NH 2, –COOH, –CHO, –CH 3, –H (lowest). Then orientate the molecule so that the group of lowest priority points away from the observer. If the order of priority (high to low) of the remaining groups is clock wise, the centre is R. If the order or priority is anticlockwise, the centre is S. Thus the -carbon of L-alanine has the S configuration.
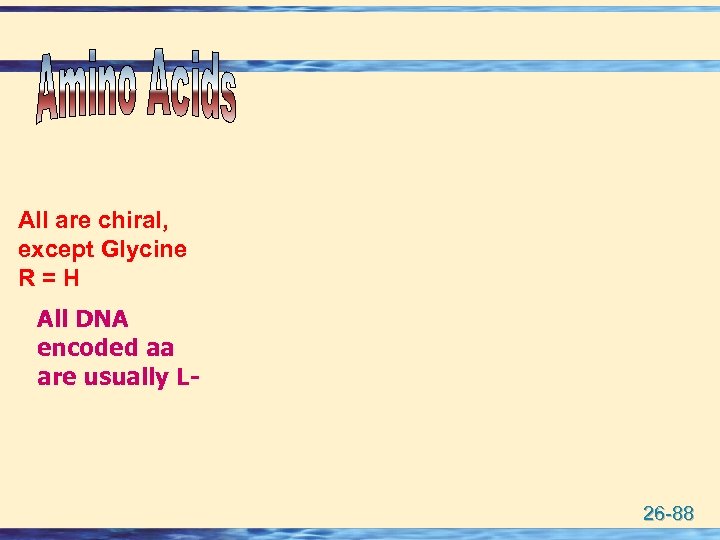 All are chiral, except Glycine R = H All DNA encoded aa are usually L- 26 -88
All are chiral, except Glycine R = H All DNA encoded aa are usually L- 26 -88
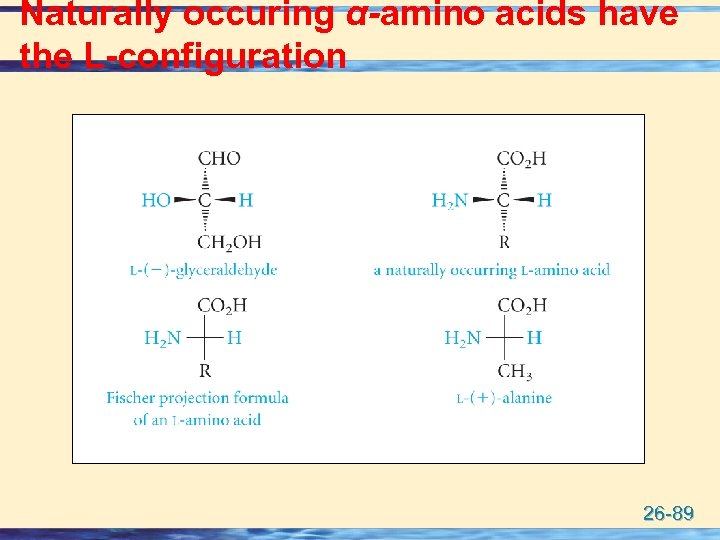 Naturally occuring α-amino acids have the L-configuration 26 -89
Naturally occuring α-amino acids have the L-configuration 26 -89
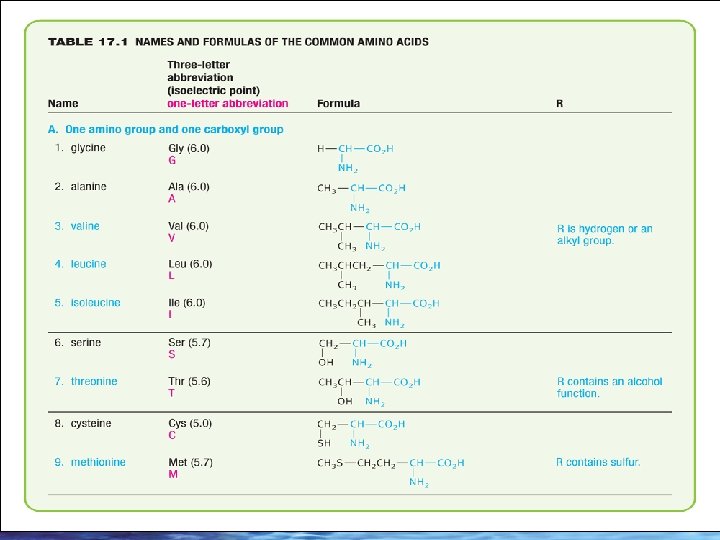 26 -90
26 -90
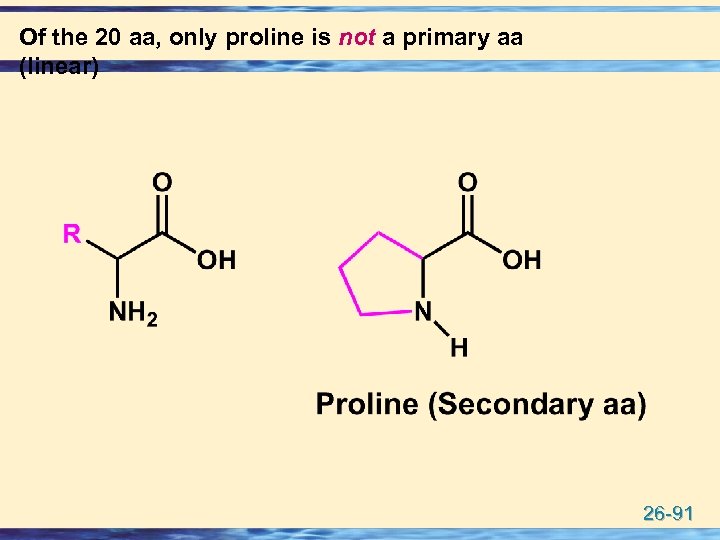 Of the 20 aa, only proline is not a primary aa (linear) 26 -91
Of the 20 aa, only proline is not a primary aa (linear) 26 -91
 IONIC PROPERTIES OF AMINO ACIDS • amino acids have amphoteric properties that allow their separation by electrophoresis at p. H 6. 0, in which the amino acids move along a medium (paper) under the force of an applied electric field. • The separation is based on the charge differences of the amino acids and proteins.
IONIC PROPERTIES OF AMINO ACIDS • amino acids have amphoteric properties that allow their separation by electrophoresis at p. H 6. 0, in which the amino acids move along a medium (paper) under the force of an applied electric field. • The separation is based on the charge differences of the amino acids and proteins.
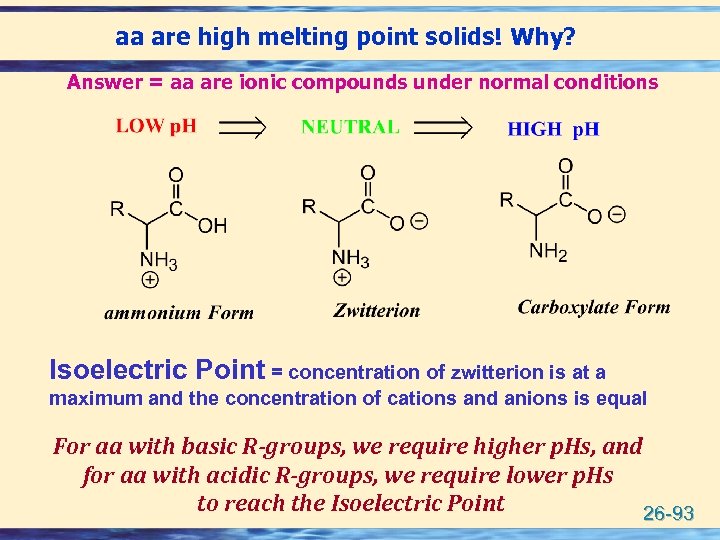 aa are high melting point solids! Why? Answer = aa are ionic compounds under normal conditions Isoelectric Point = concentration of zwitterion is at a maximum and the concentration of cations and anions is equal For aa with basic R-groups, we require higher p. Hs, and for aa with acidic R-groups, we require lower p. Hs to reach the Isoelectric Point 26 -93
aa are high melting point solids! Why? Answer = aa are ionic compounds under normal conditions Isoelectric Point = concentration of zwitterion is at a maximum and the concentration of cations and anions is equal For aa with basic R-groups, we require higher p. Hs, and for aa with acidic R-groups, we require lower p. Hs to reach the Isoelectric Point 26 -93
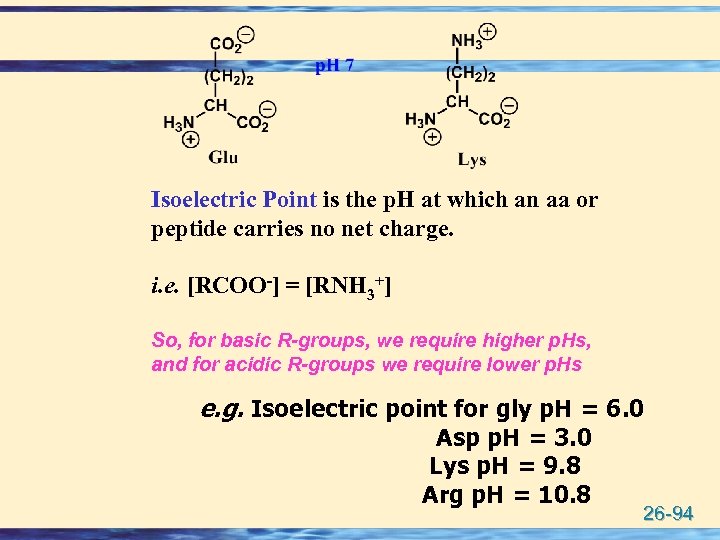 Isoelectric Point is the p. H at which an aa or peptide carries no net charge. i. e. [RCOO-] = [RNH 3+] So, for basic R-groups, we require higher p. Hs, and for acidic R-groups we require lower p. Hs e. g. Isoelectric point for gly p. H = 6. 0 Asp p. H = 3. 0 Lys p. H = 9. 8 Arg p. H = 10. 8 26 -94
Isoelectric Point is the p. H at which an aa or peptide carries no net charge. i. e. [RCOO-] = [RNH 3+] So, for basic R-groups, we require higher p. Hs, and for acidic R-groups we require lower p. Hs e. g. Isoelectric point for gly p. H = 6. 0 Asp p. H = 3. 0 Lys p. H = 9. 8 Arg p. H = 10. 8 26 -94
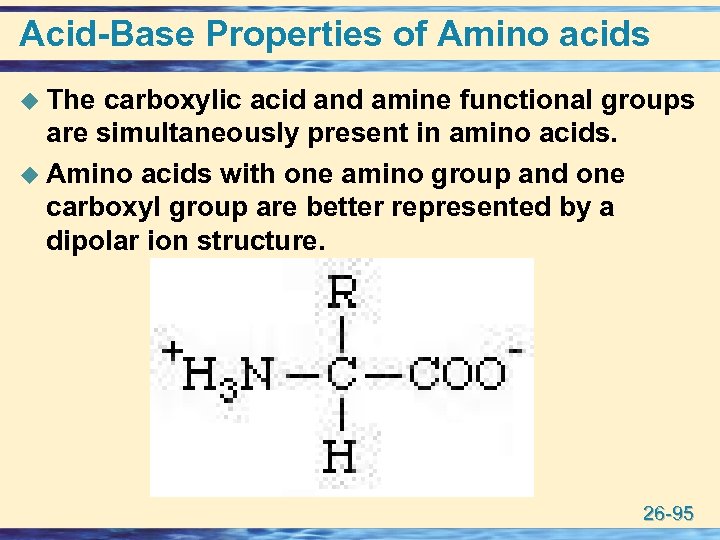 Acid-Base Properties of Amino acids u The carboxylic acid and amine functional groups are simultaneously present in amino acids. u Amino acids with one amino group and one carboxyl group are better represented by a dipolar ion structure. 26 -95
Acid-Base Properties of Amino acids u The carboxylic acid and amine functional groups are simultaneously present in amino acids. u Amino acids with one amino group and one carboxyl group are better represented by a dipolar ion structure. 26 -95
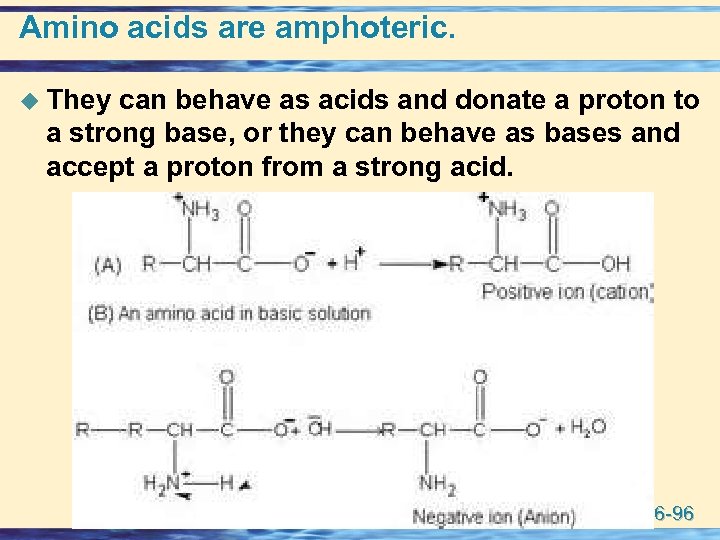 Amino acids are amphoteric. u They can behave as acids and donate a proton to a strong base, or they can behave as bases and accept a proton from a strong acid. 26 -96
Amino acids are amphoteric. u They can behave as acids and donate a proton to a strong base, or they can behave as bases and accept a proton from a strong acid. 26 -96
 Formation of an amino acid u The Strecker amino-acid synthesis, devised by Adolph Strecker, is a series of chemical reactions that synthesize an amino acid from an aldehyde or ketone u The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino-acid 26 -97
Formation of an amino acid u The Strecker amino-acid synthesis, devised by Adolph Strecker, is a series of chemical reactions that synthesize an amino acid from an aldehyde or ketone u The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino-acid 26 -97
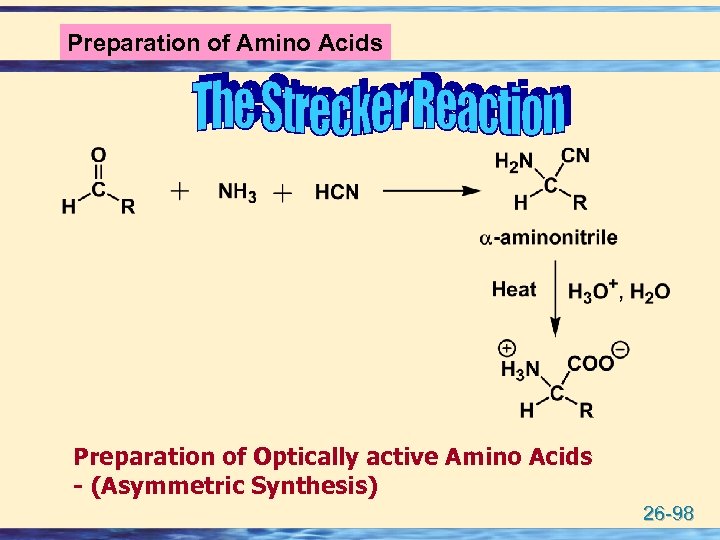 Preparation of Amino Acids Preparation of Optically active Amino Acids - (Asymmetric Synthesis) 26 -98
Preparation of Amino Acids Preparation of Optically active Amino Acids - (Asymmetric Synthesis) 26 -98
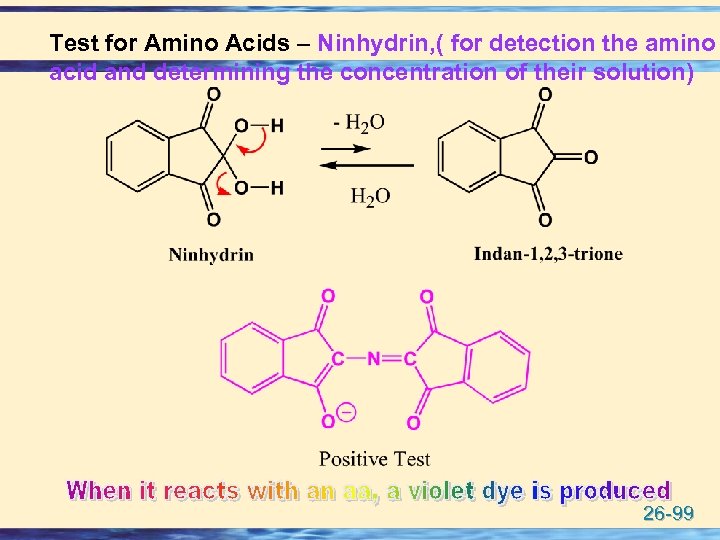 Test for Amino Acids – Ninhydrin, ( for detection the amino acid and determining the concentration of their solution) 26 -99
Test for Amino Acids – Ninhydrin, ( for detection the amino acid and determining the concentration of their solution) 26 -99
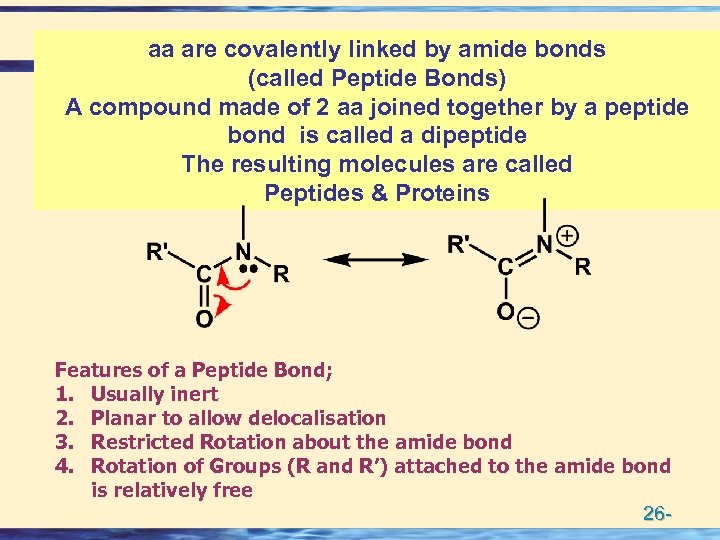 aa are covalently linked by amide bonds (called Peptide Bonds) A compound made of 2 aa joined together by a peptide bond is called a dipeptide The resulting molecules are called Peptides & Proteins Features of a Peptide Bond; 1. Usually inert 2. Planar to allow delocalisation 3. Restricted Rotation about the amide bond 4. Rotation of Groups (R and R’) attached to the amide bond is relatively free 26 -
aa are covalently linked by amide bonds (called Peptide Bonds) A compound made of 2 aa joined together by a peptide bond is called a dipeptide The resulting molecules are called Peptides & Proteins Features of a Peptide Bond; 1. Usually inert 2. Planar to allow delocalisation 3. Restricted Rotation about the amide bond 4. Rotation of Groups (R and R’) attached to the amide bond is relatively free 26 -
 aa that are part of a peptide or protein are referred to as residues. Peptides are made up of about 50 residues, and do not possess a well-defined 3 D-structure Proteins are larger molecules that usually contain at least 50 residues, and sometimes 1000. The most important feature of proteins is that they possess well-defined 3 D-structure. Primary Structure is the order (or sequence) of amino acid residues Peptides are always written and named with the amino terminus on the left and the carboxy terminus on the right 26 -
aa that are part of a peptide or protein are referred to as residues. Peptides are made up of about 50 residues, and do not possess a well-defined 3 D-structure Proteins are larger molecules that usually contain at least 50 residues, and sometimes 1000. The most important feature of proteins is that they possess well-defined 3 D-structure. Primary Structure is the order (or sequence) of amino acid residues Peptides are always written and named with the amino terminus on the left and the carboxy terminus on the right 26 -
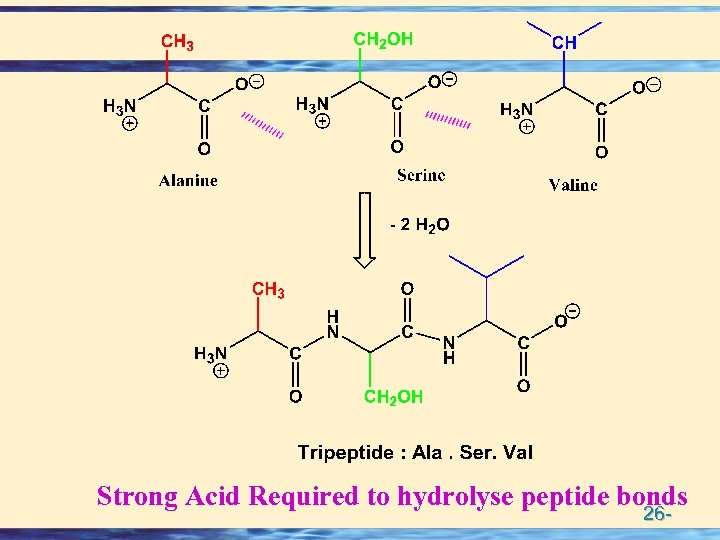 Strong Acid Required to hydrolyse peptide bonds 26 -
Strong Acid Required to hydrolyse peptide bonds 26 -
 u Since peptides and proteins consist of amino acids held together by amide bond, they can be hydrolyzed to their amino acid components. u This hydolysis is accomplished by heating the peptide or protein with 6 M HCl at 110 C for 24 hrs. u Analysis will help in the identity of each amino acid present. 26 -
u Since peptides and proteins consist of amino acids held together by amide bond, they can be hydrolyzed to their amino acid components. u This hydolysis is accomplished by heating the peptide or protein with 6 M HCl at 110 C for 24 hrs. u Analysis will help in the identity of each amino acid present. 26 -
 Sanger Method u Fredrick Sanger devised a method for sequencing peptides based on the observations that the N-terminal amino acid differs from all others in the chain by having a free amino group. u Sanger’s reagent is : u 2, 4 -dinitrofluorobenzene, which reacts with the NH 2 group of the amino acids and peptides to give yellow 2, 4 -dinitrophenyl (DNP) derivatives. 26 -
Sanger Method u Fredrick Sanger devised a method for sequencing peptides based on the observations that the N-terminal amino acid differs from all others in the chain by having a free amino group. u Sanger’s reagent is : u 2, 4 -dinitrofluorobenzene, which reacts with the NH 2 group of the amino acids and peptides to give yellow 2, 4 -dinitrophenyl (DNP) derivatives. 26 -
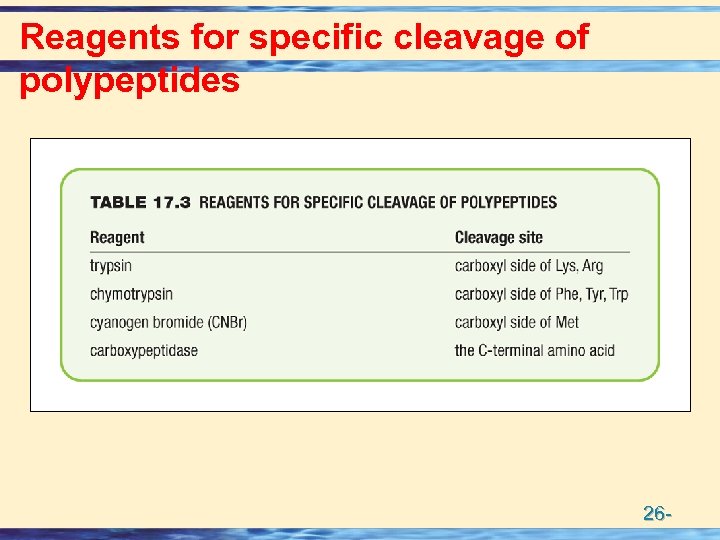 Reagents for specific cleavage of polypeptides 26 -
Reagents for specific cleavage of polypeptides 26 -
 Prof. Linus Pauling Dr. Frederick Sanger, Prof. R. B. Merrifield Nobel Prize for Chemistry 1984 1958 and 1980 Automated Peptide Synthesis 26 Peptide sequencing
Prof. Linus Pauling Dr. Frederick Sanger, Prof. R. B. Merrifield Nobel Prize for Chemistry 1984 1958 and 1980 Automated Peptide Synthesis 26 Peptide sequencing
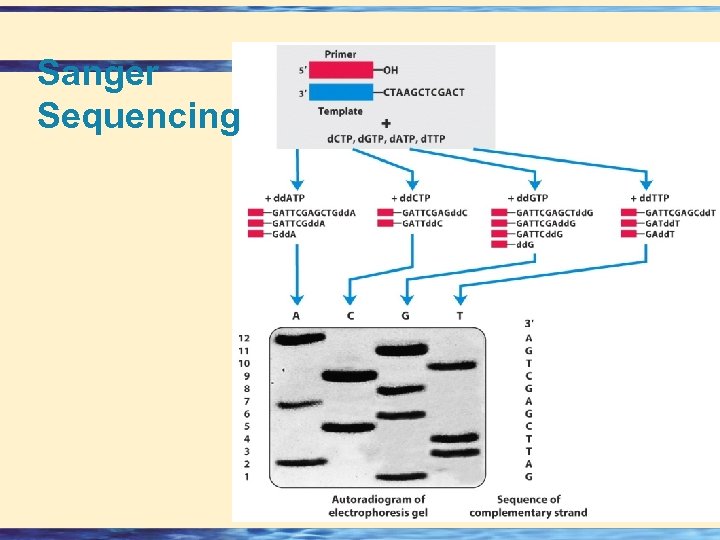 Sanger Sequencing 26 -
Sanger Sequencing 26 -
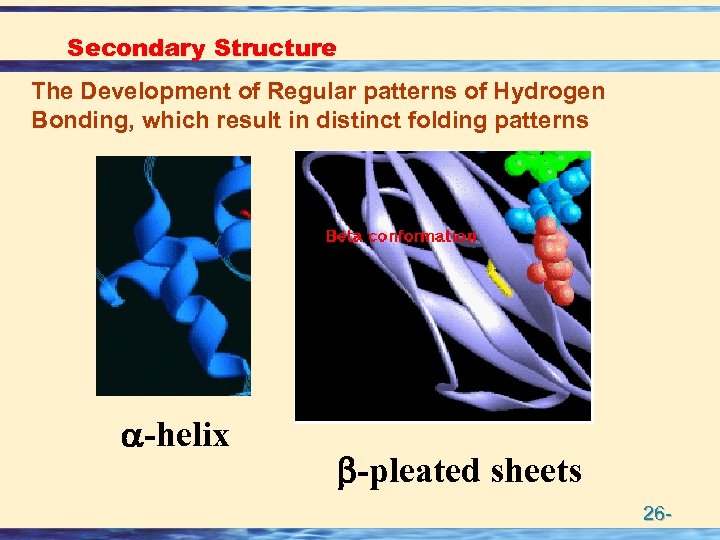 Secondary Structure The Development of Regular patterns of Hydrogen Bonding, which result in distinct folding patterns -helix -pleated sheets 26 -
Secondary Structure The Development of Regular patterns of Hydrogen Bonding, which result in distinct folding patterns -helix -pleated sheets 26 -
 Tertiary Structure This is the 3 D structure resulting from further regular folding of the polypeptide chains using H-bonding, Van der Waals, disulfide bonds and electrostatic forces – Often detected by X-ray crystallographic methods Globular Proteins – “Spherical Shape” , include Insulin, Hemoglobin, Enzymes, Antibodies ---polar hydrophilic groups are aimed outwards towards water, whereas non-polar “greasy” hydrophobic hydrocarbon portions cluster inside the molecule, so protecting them from the hostile aqueous environment ----- Soluble Proteins Fibrous Proteins – “Long thin fibres” , include Hair, wool, skin, nails – less folded ----- e. g. keratin - the -helix strands are wound into a “superhelix”. The superhelix makes one complete turn for each 35 turns of the -helix. 26 -
Tertiary Structure This is the 3 D structure resulting from further regular folding of the polypeptide chains using H-bonding, Van der Waals, disulfide bonds and electrostatic forces – Often detected by X-ray crystallographic methods Globular Proteins – “Spherical Shape” , include Insulin, Hemoglobin, Enzymes, Antibodies ---polar hydrophilic groups are aimed outwards towards water, whereas non-polar “greasy” hydrophobic hydrocarbon portions cluster inside the molecule, so protecting them from the hostile aqueous environment ----- Soluble Proteins Fibrous Proteins – “Long thin fibres” , include Hair, wool, skin, nails – less folded ----- e. g. keratin - the -helix strands are wound into a “superhelix”. The superhelix makes one complete turn for each 35 turns of the -helix. 26 -
 In globular proteins this tertiary structure or macromolecular shape determines biological properties Bays or pockets in proteins are called Active Sites Enzymes are Stereospecific and possess Geometric Specificity The range of compounds that an enzyme excepts varies from a particular functional group to a specific compound Emil Fischer formulated the lock-and-key mechanism for enzymes All reactions which occur in living cells are mediated by enzymes and are catalysed by 106 -108 Some enzymes may require the presence of a Cofactor. This may be a metal atom, which is essential for its redox activity. Others may require the presence of an organic molecule, such as NAD+, called a Coenzyme. If the Cofactor is permanently bound to the enzyme, it is called a Prosthetic Group. 26 -
In globular proteins this tertiary structure or macromolecular shape determines biological properties Bays or pockets in proteins are called Active Sites Enzymes are Stereospecific and possess Geometric Specificity The range of compounds that an enzyme excepts varies from a particular functional group to a specific compound Emil Fischer formulated the lock-and-key mechanism for enzymes All reactions which occur in living cells are mediated by enzymes and are catalysed by 106 -108 Some enzymes may require the presence of a Cofactor. This may be a metal atom, which is essential for its redox activity. Others may require the presence of an organic molecule, such as NAD+, called a Coenzyme. If the Cofactor is permanently bound to the enzyme, it is called a Prosthetic Group. 26 -
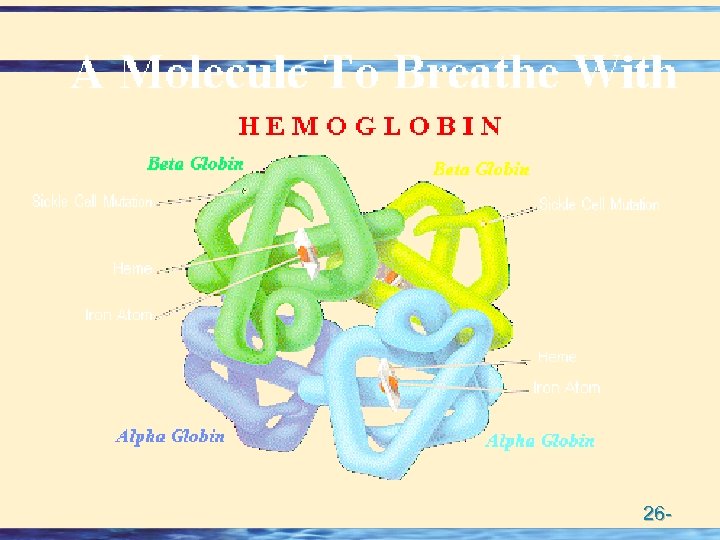
 For a protein composed of a single polypeptide molecule, tertiary structure is the highest level of structure that is attained Myoglobin and hemoglobin were the first proteins to be successfully subjected to completely successful X-rays analysis by J. C. Kendrew and Max Perutz (Nobel Prize for Chemistry 1962) Quaternary Structure When multiple sub-units are held together in aggregates by Van der Waals and electrostatic forces (not covalent bonds) Hemoglobin is tetrameric myglobin For example, Hemoglobin has four heme units, the protein globin surrounds the heme – Takes the shape of a giant tetrahedron – Two identical and globins. The and chains are very similar but distinguishable in both primary structure and folding 26 -
For a protein composed of a single polypeptide molecule, tertiary structure is the highest level of structure that is attained Myoglobin and hemoglobin were the first proteins to be successfully subjected to completely successful X-rays analysis by J. C. Kendrew and Max Perutz (Nobel Prize for Chemistry 1962) Quaternary Structure When multiple sub-units are held together in aggregates by Van der Waals and electrostatic forces (not covalent bonds) Hemoglobin is tetrameric myglobin For example, Hemoglobin has four heme units, the protein globin surrounds the heme – Takes the shape of a giant tetrahedron – Two identical and globins. The and chains are very similar but distinguishable in both primary structure and folding 26 -
 26 -
26 -
 26 -
26 -
 26 -
26 -
 Nucleotides and Nucleic Acid Nucleic acids: are linear chainlike macromolecules that were first isolated from cell nuclei. 26 -
Nucleotides and Nucleic Acid Nucleic acids: are linear chainlike macromolecules that were first isolated from cell nuclei. 26 -
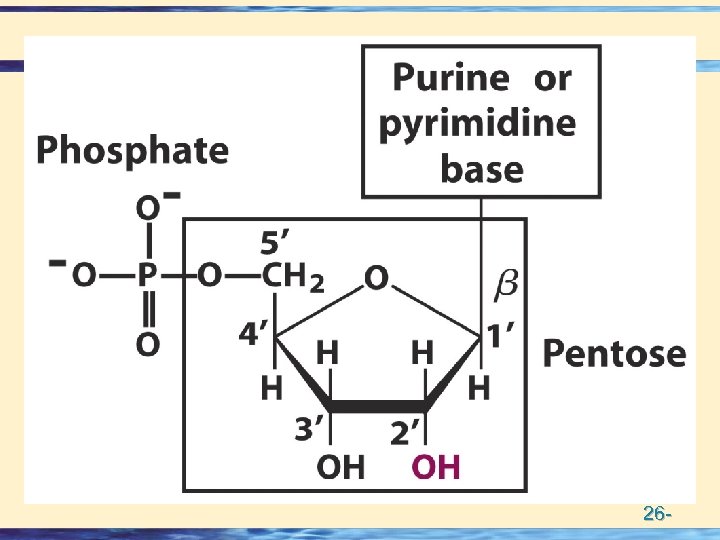
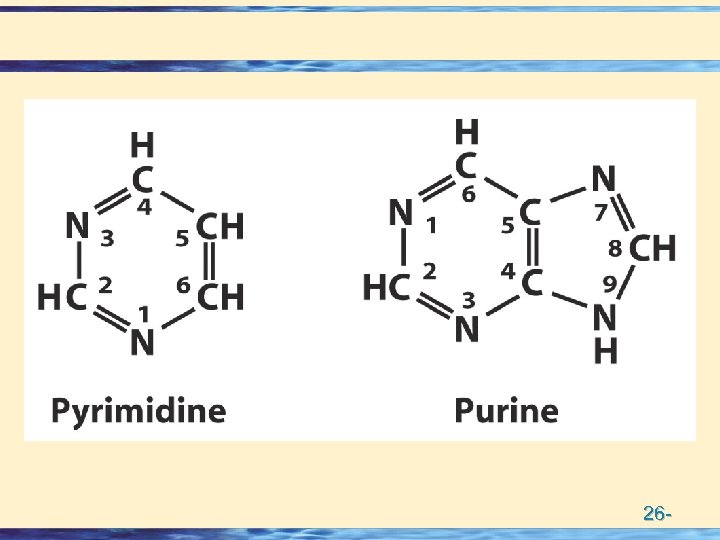
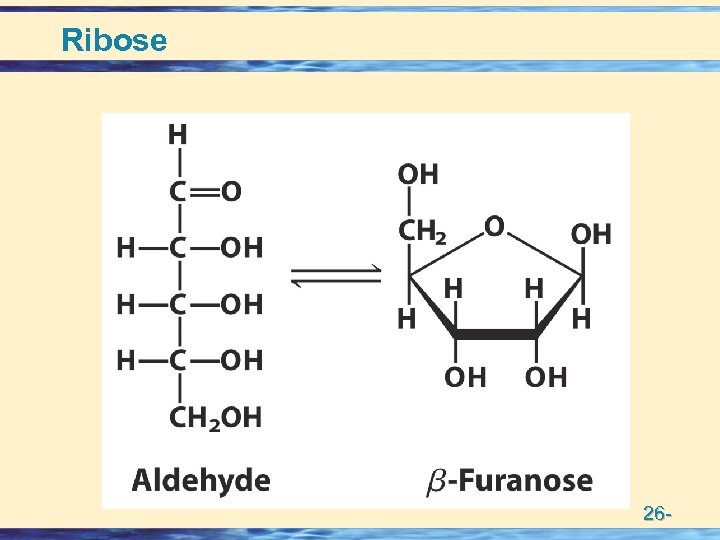
 Building Blocks of Nucleic acids • Nucleotides = Base + Sugar + Phosphate • Nucleosides = Base + Sugar • Nitrogen Bases • Purines (5 + 6 membered rings) – numbering – Adenine Guanine • Pyrimidines (6 membered ring) – numbering – Thymine Cytosine Uracil • Pentose Sugars (numbering) • – Ribose • – Deoxy Ribose 26 -
Building Blocks of Nucleic acids • Nucleotides = Base + Sugar + Phosphate • Nucleosides = Base + Sugar • Nitrogen Bases • Purines (5 + 6 membered rings) – numbering – Adenine Guanine • Pyrimidines (6 membered ring) – numbering – Thymine Cytosine Uracil • Pentose Sugars (numbering) • – Ribose • – Deoxy Ribose 26 -
 26 -
26 -
 26 -
26 -
 Ribose 26 -
Ribose 26 -
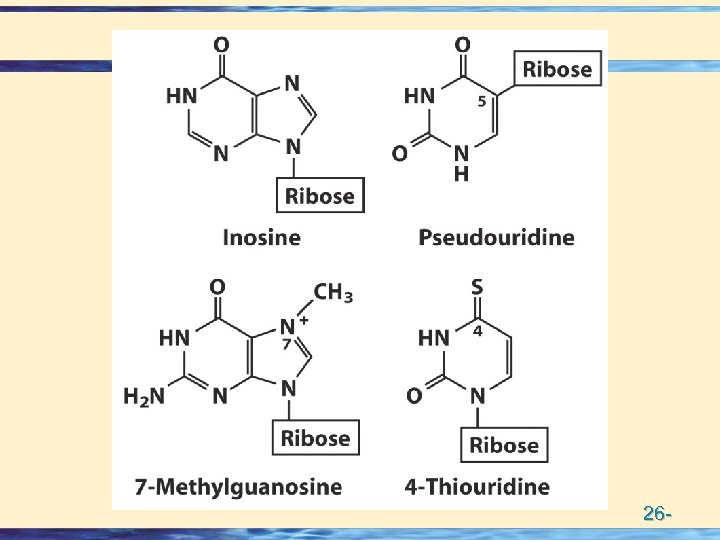
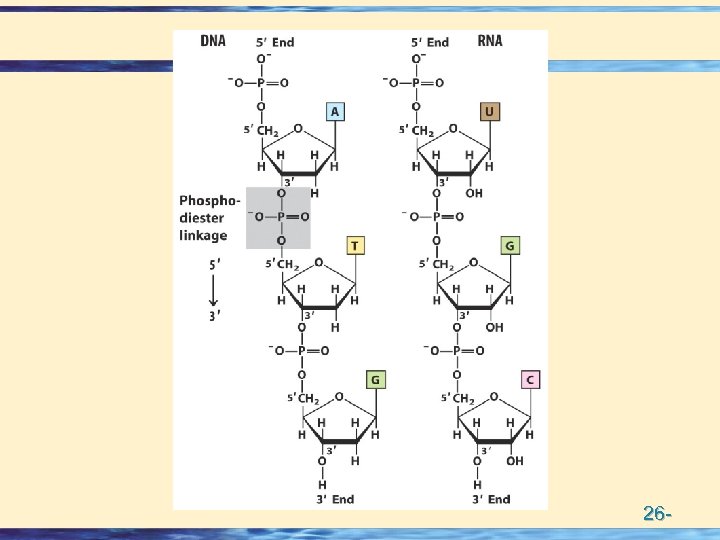
 Bases • DNA A, G, C, T • RNA A, G, C, U • Modified bases • Methylation in DNA • Lots of Mods in RNA 26 -
Bases • DNA A, G, C, T • RNA A, G, C, U • Modified bases • Methylation in DNA • Lots of Mods in RNA 26 -
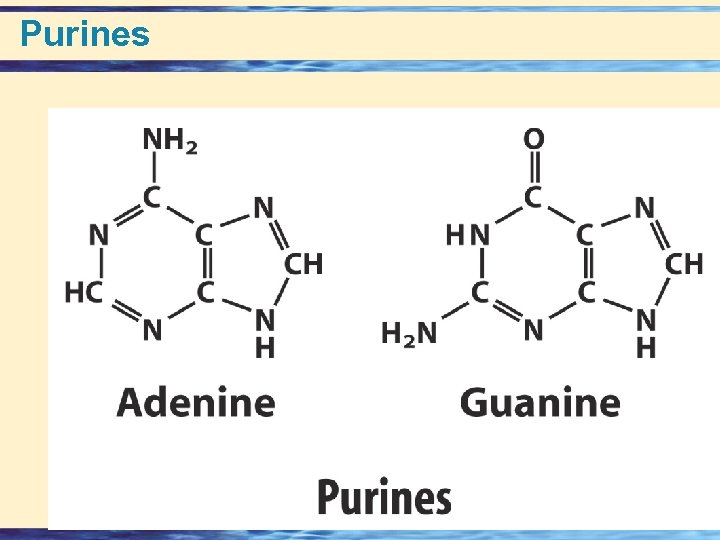 Purines 26 -
Purines 26 -
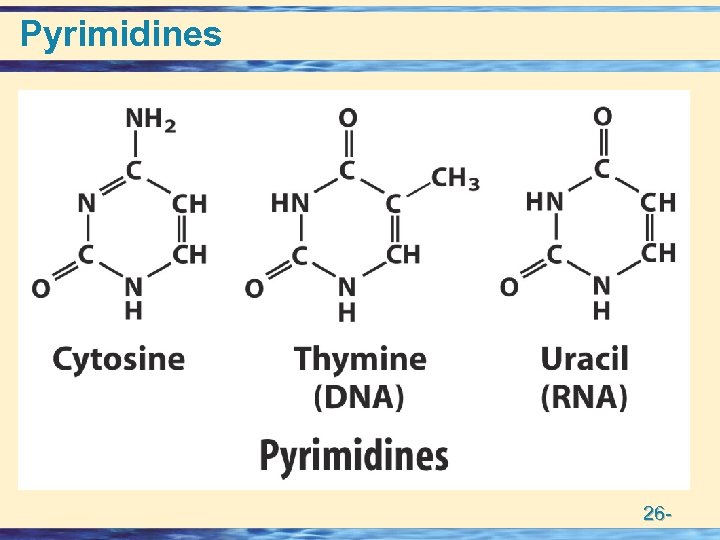 Pyrimidines 26 -
Pyrimidines 26 -
 26 -
26 -
 26 -
26 -
 26 -
26 -
 Structures: DNA and RNA • DNA contains genetic Information • Distinctive base composition foretells base pairing patterns • Double helical structures • Local structures • m. RNAs - little structure • Stable RNAs - complex structures 26 -
Structures: DNA and RNA • DNA contains genetic Information • Distinctive base composition foretells base pairing patterns • Double helical structures • Local structures • m. RNAs - little structure • Stable RNAs - complex structures 26 -
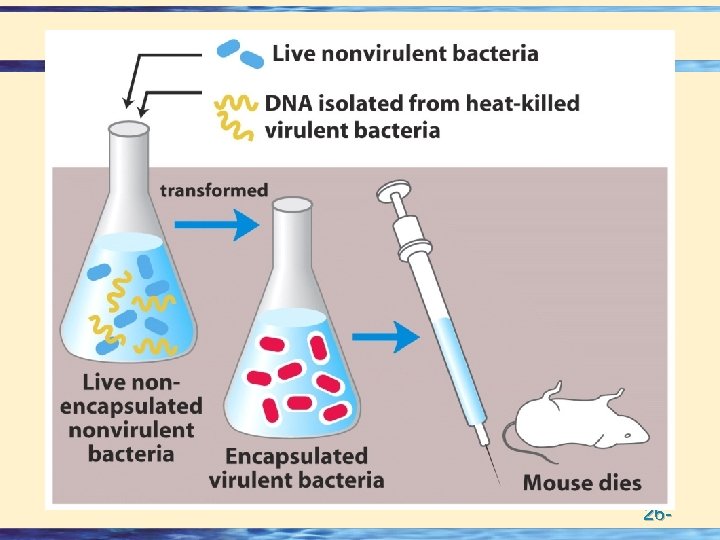
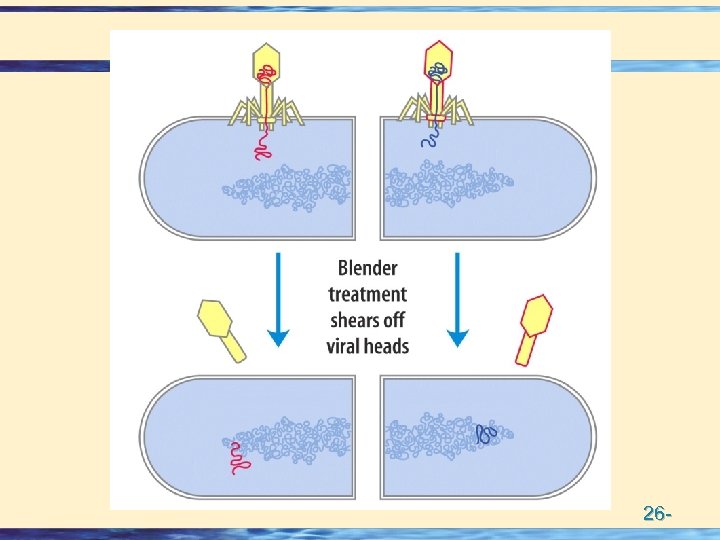
 DNA contains genetic Information • Purified DNA can "transform" Bacteria • Avery, Mac. Leod & Mc. Carty transferred the virulence trait to pneumococci • The genetic material contains 32 P (DNA) and not 35 S (protein – C, M) • Hershey and Chase grew bacteriophage on either 32 P or 35 S • Bacteriophage infection resulted in transfer of 32 P and not 35 S 26 -
DNA contains genetic Information • Purified DNA can "transform" Bacteria • Avery, Mac. Leod & Mc. Carty transferred the virulence trait to pneumococci • The genetic material contains 32 P (DNA) and not 35 S (protein – C, M) • Hershey and Chase grew bacteriophage on either 32 P or 35 S • Bacteriophage infection resulted in transfer of 32 P and not 35 S 26 -
 26 -
26 -
 26 -
26 -
 Distinctive Base composition foretell base pairing patterns • Hydrolysis of DNA and analysis of base composition • Same for different individuals of a given species • Same over time • Same in different tissues • %A = %T and %G = %C (Chargaff's Rules) • Amino acid compositions vary under all three conditions • No quantitative relationships in AA composition 26 -
Distinctive Base composition foretell base pairing patterns • Hydrolysis of DNA and analysis of base composition • Same for different individuals of a given species • Same over time • Same in different tissues • %A = %T and %G = %C (Chargaff's Rules) • Amino acid compositions vary under all three conditions • No quantitative relationships in AA composition 26 -
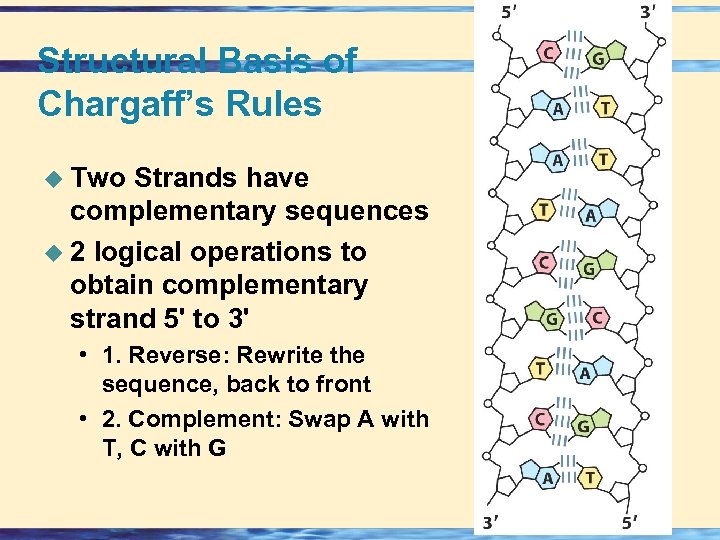 Structural Basis of Chargaff’s Rules u Two Strands have complementary sequences u 2 logical operations to obtain complementary strand 5' to 3' • 1. Reverse: Rewrite the sequence, back to front • 2. Complement: Swap A with T, C with G 26 -
Structural Basis of Chargaff’s Rules u Two Strands have complementary sequences u 2 logical operations to obtain complementary strand 5' to 3' • 1. Reverse: Rewrite the sequence, back to front • 2. Complement: Swap A with T, C with G 26 -
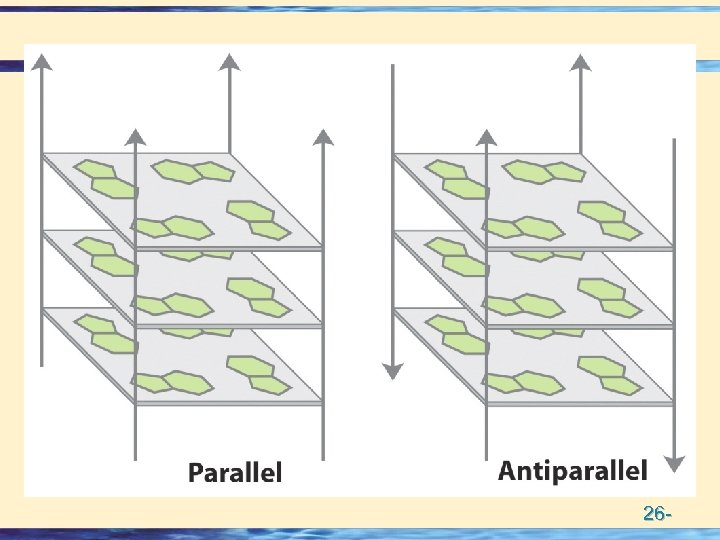
 Double helical structures • Potentially Right or Left Handed • Actually Mostly Right Handed • Potentially Parallel or Anti-parallel • Actually anti-parallel 26 -
Double helical structures • Potentially Right or Left Handed • Actually Mostly Right Handed • Potentially Parallel or Anti-parallel • Actually anti-parallel 26 -
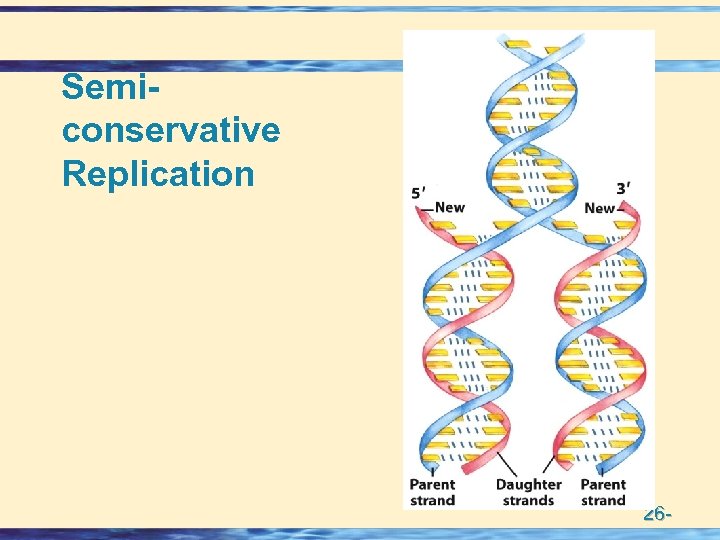 Semiconservative Replication 26 -
Semiconservative Replication 26 -
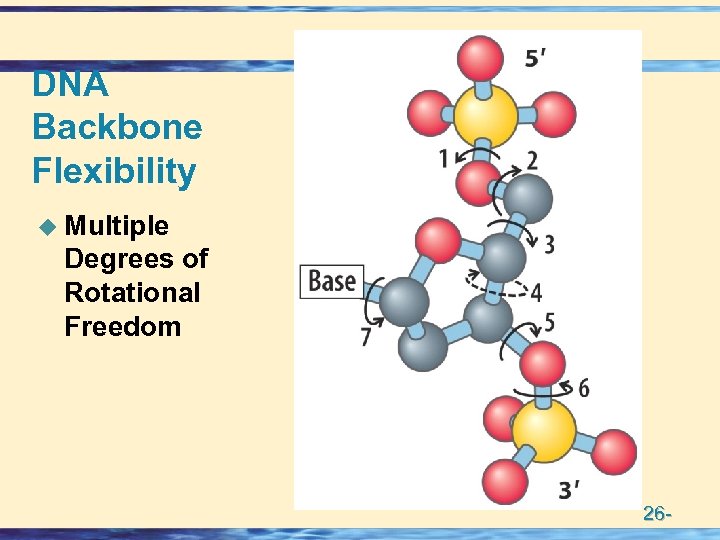 DNA Backbone Flexibility u Multiple Degrees of Rotational Freedom 26 -
DNA Backbone Flexibility u Multiple Degrees of Rotational Freedom 26 -
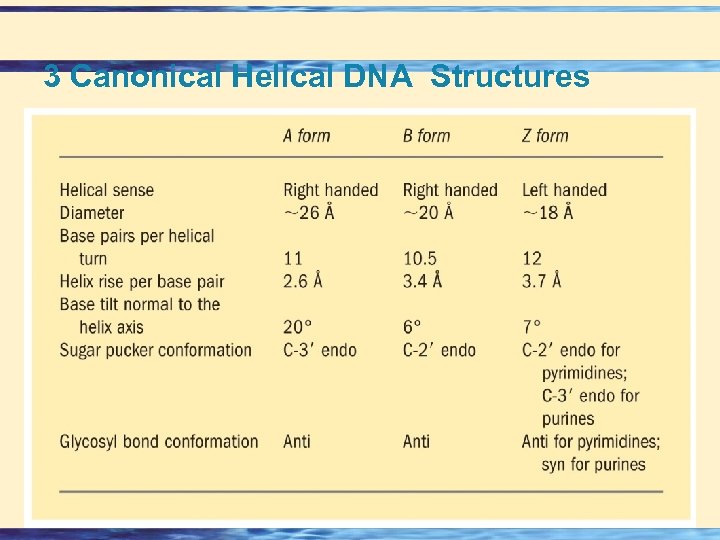 3 Canonical Helical DNA Structures 26 -
3 Canonical Helical DNA Structures 26 -
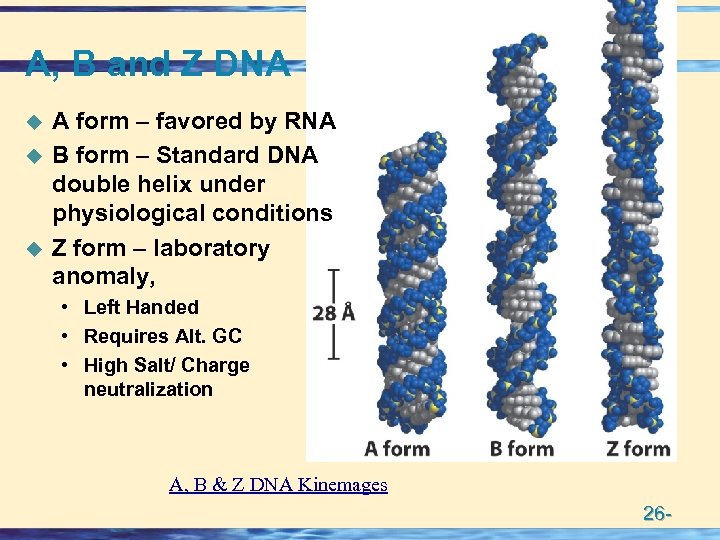 A, B and Z DNA u u u A form – favored by RNA B form – Standard DNA double helix under physiological conditions Z form – laboratory anomaly, • Left Handed • Requires Alt. GC • High Salt/ Charge neutralization A, B & Z DNA Kinemages 26 -
A, B and Z DNA u u u A form – favored by RNA B form – Standard DNA double helix under physiological conditions Z form – laboratory anomaly, • Left Handed • Requires Alt. GC • High Salt/ Charge neutralization A, B & Z DNA Kinemages 26 -
 26 -
26 -
 Messenger RNAs u Contain protein coding information • • u ATG start codon to UAA, UAG, UGA Stop Codon A cistron is the unit of RNA that encodes one polypeptide chain Prokaryotic m. RNAs are poly-cistronic Eukaryotic m. RNAs are mono-cistronic Base pairing/3 D structure is the exception • Can be used to regulate RNA stability termination, RNA editng, RNA splicing 26 -
Messenger RNAs u Contain protein coding information • • u ATG start codon to UAA, UAG, UGA Stop Codon A cistron is the unit of RNA that encodes one polypeptide chain Prokaryotic m. RNAs are poly-cistronic Eukaryotic m. RNAs are mono-cistronic Base pairing/3 D structure is the exception • Can be used to regulate RNA stability termination, RNA editng, RNA splicing 26 -
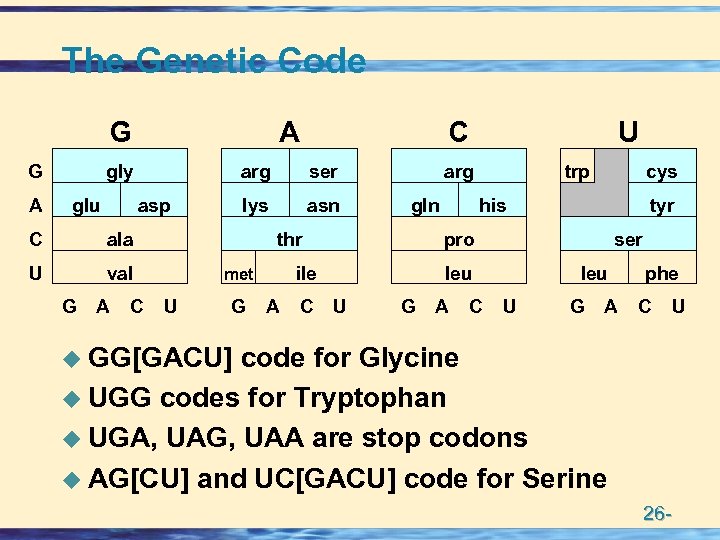 The Genetic Code G G A A gly glu arg asp C val ser lys asn ala U G A C C arg gln thr U G A C trp his G A C tyr ser leu U cys pro ile met U leu U G A phe C U u GG[GACU] code for Glycine u UGG codes for Tryptophan u UGA, UAG, UAA are stop codons u AG[CU] and UC[GACU] code for Serine 26 -
The Genetic Code G G A A gly glu arg asp C val ser lys asn ala U G A C C arg gln thr U G A C trp his G A C tyr ser leu U cys pro ile met U leu U G A phe C U u GG[GACU] code for Glycine u UGG codes for Tryptophan u UGA, UAG, UAA are stop codons u AG[CU] and UC[GACU] code for Serine 26 -
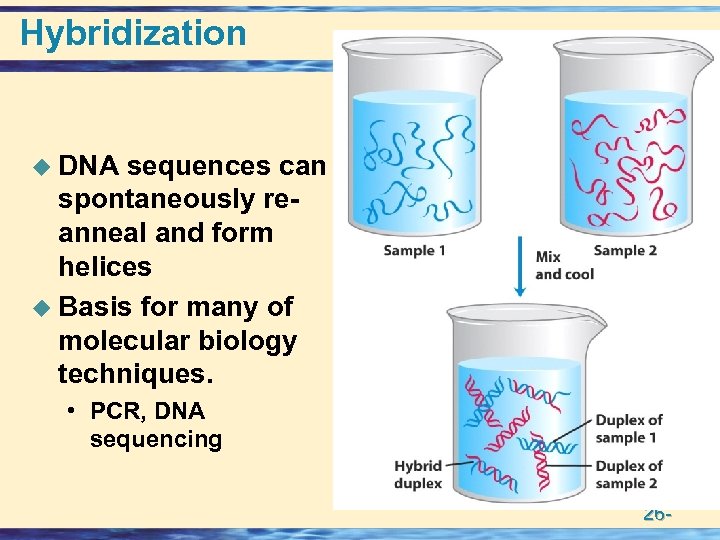 Hybridization u DNA sequences can spontaneously reanneal and form helices u Basis for many of molecular biology techniques. • PCR, DNA sequencing 26 -
Hybridization u DNA sequences can spontaneously reanneal and form helices u Basis for many of molecular biology techniques. • PCR, DNA sequencing 26 -
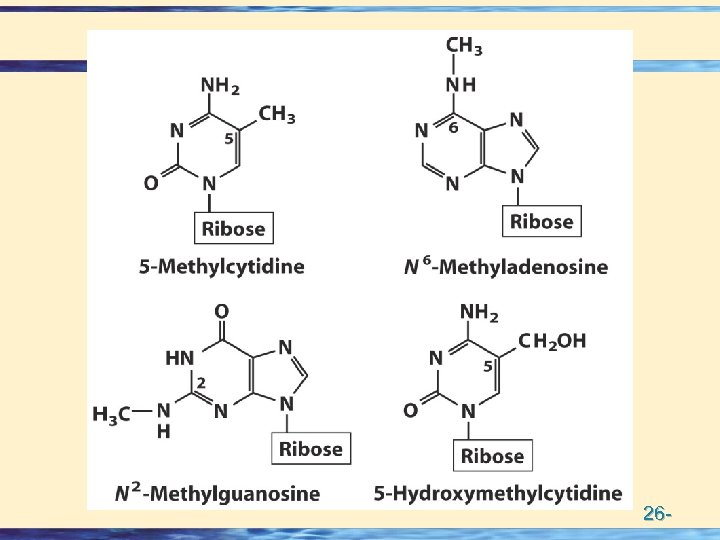
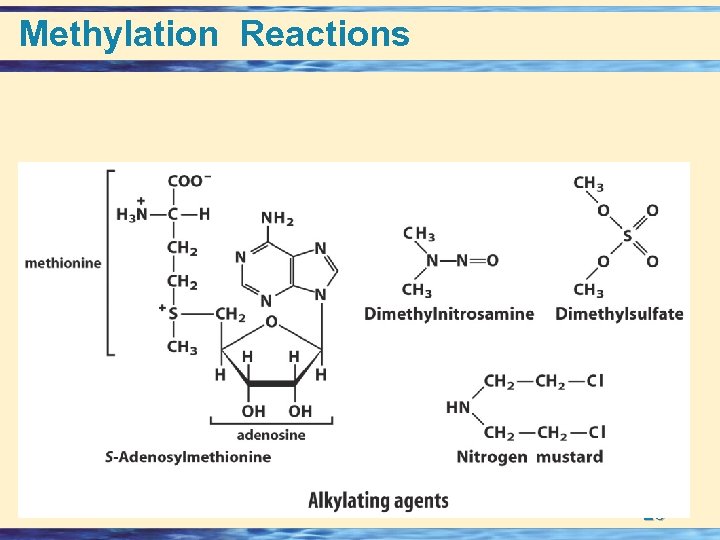 Methylation Reactions 26 -
Methylation Reactions 26 -
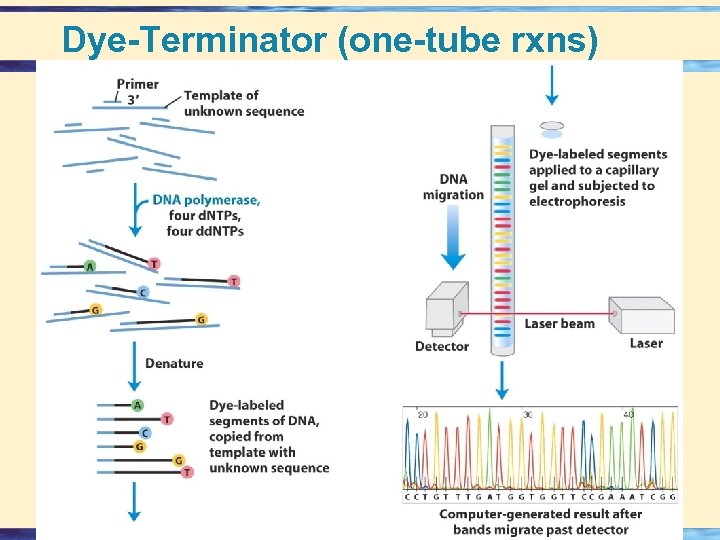 Dye-Terminator (one-tube rxns) 26 -
Dye-Terminator (one-tube rxns) 26 -
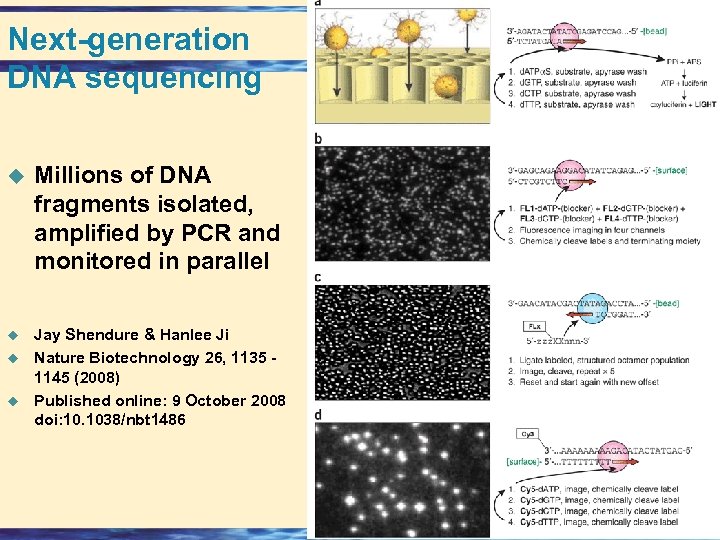 Next-generation DNA sequencing u Millions of DNA fragments isolated, amplified by PCR and monitored in parallel u Jay Shendure & Hanlee Ji Nature Biotechnology 26, 1135 - 1145 (2008) Published online: 9 October 2008 doi: 10. 1038/nbt 1486 u u 26 -
Next-generation DNA sequencing u Millions of DNA fragments isolated, amplified by PCR and monitored in parallel u Jay Shendure & Hanlee Ji Nature Biotechnology 26, 1135 - 1145 (2008) Published online: 9 October 2008 doi: 10. 1038/nbt 1486 u u 26 -


