98ed05db9c736d1b24e12b341e55a9c4.ppt
- Количество слайдов: 59

2016 AIPLA Patent Prosecution Boot Camp Continuation & RCE Practice October 2016 Mercedes K. Meyer, Ph. D. , J. D.

I am NOT Your Lawyer • • These materials are public information and have been prepared solely for educational and entertainment purposes to contribute to the understanding of U. S. intellectual property law and practice. These materials reflect only the personal views of the speaker and are not individualized legal advice. It is understood that each case is fact-specific, and that the appropriate solution in every case will vary. Therefore, these materials may or may not be relevant to any particular situation. Thus, Drinker Biddle & Reath LLP, and the speaker cannot be bound either philosophically or a representative of their various present and future clients to the comments expressed in these materials. The presentation of these materials does not establish any form of attorney-client relationship with the speaker and members of the firm or anyone else. While every attempt was made to insure that these materials are accurate, errors or omissions may be contained therein, for which any liability is disclaimed. And, nothing represents the views of any sentient life form on the earth or universe, or any parallel universe, alive or dead, fictitious or real! This is for entertainment purposes only. 2
Topics • Theme = Constant Change! • RCEs (Requests for Continued Examination) • Continuing Patent Applications – – Continuation (CON) Divisional (DIV) Continuation in part (CIP) Continued Prosecution Application (CPA) • Should be dying out, but you may see it – Transitional procedures for limited examination after final rejection and restriction practice. Rule 1. 129(a) practice – File Wrapper Continuation (FWC) – 1. 62 • Transitional Applications • Problems: Related to RCEs/CONs/Transitional Apps 3
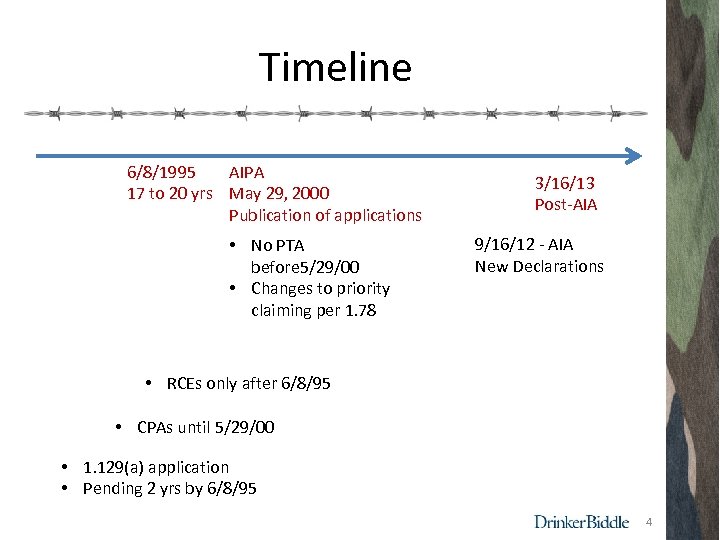
Timeline 6/8/1995 AIPA 17 to 20 yrs May 29, 2000 Publication of applications • No PTA before 5/29/00 • Changes to priority claiming per 1. 78 3/16/13 Post-AIA 9/16/12 - AIA New Declarations • RCEs only after 6/8/95 • CPAs until 5/29/00 • 1. 129(a) application • Pending 2 yrs by 6/8/95 4

RCE’s 5

RCE • Purpose – to re-open prosecution of same application – No longer expedited – pendency like a CON • Applicable situations: – Rejected claims after a final Office Action – Not ready to appeal – Submitting declarations and/or making claim amendments after final – Must withdraw from issue if Notice of Allowance / Allowability mailed • No “missing parts” practice • Cannot apply to applications filed before 6/8/1995 6
RCE Four Requirements (under 37 CFR § 1. 114): • Request • Fee • Full Response to any pending action • “Submission” – see SB/30 and SB 30 EFS-WEB – Amendment (not previously entered) – Information disclosure statement (IDS) – New argument, or – New evidence (e. g. , declaration) 7
RCE & QPIDS • QPIDS = Quick Path Information Disclosure Statement – http: //www. uspto. gov/patents/init_events/qpids. jsp – Guide: http: //www. uspto. gov/patents/init_events/qpids_qsg. pdf – See also: http: //www. uspto. gov/patent/initiatives/quick-path-information-disclosurestatement-qpids • A process to file an IDS if needed AFTER an issue fee is paid and you discover new art – Must file the IDS, transmittal form, and deposit account payment – You do not have to file an RCE • Extended through Sep. 30, 2016 • Use the SB/09 Form – http: //www. uspto. gov/forms/sb 0009. pdf 8

Continuing Applications
Continuing Applications (CONs) • Definition: new patent application with a priority claim under 35 USC § 120 – Divisional – Continuation-in-part – more common pre-1995 • Transitional applications…. 10
Continuing Applications (CONs) Four Requirements of 35 USC § 120 Priority 1. Priority claim to earlier U. S. application 2. Priority application fully supports claim 3. At least one common inventor – 37 CFR §§ 1. 78(a)(1) or (a)(4) 4. Copendency 11
Continuing Applications The Glue: priority claims • Earlier filing date for later applications Uses/benefits: • Avoid intervening 3 rd party prior art • Avoid 35 USC § 102(b) issues, foreign self collision (pre. AIA) • Helpful for instituting interferences (for applications claiming priority before March 13, 2013) • Precautionary application • Pursuing an embodiment canceled earlier or not yet pursued 12
Continuing Applications Background: • Patent (one) vs. Family (many) • Reasons for multiple related patents: – CONs – keeps family tree alive without new matter, 37 CFR 1. 53(b), MPEP § 201. 07 – CON off a PCT – 35 USC § 111 / 1. 53(b); MPEP § 1895 – DIVs– one specification covering a separate, restricted invention from a parent specification; MPEP § 201. 06 – CIPs – combination of old and new –improvements MPEP § 201. 08 (Generally discouraged) • Also: – CPA’s – unavailable for applications filed after 5/29/00 (1. 53(d)) 13
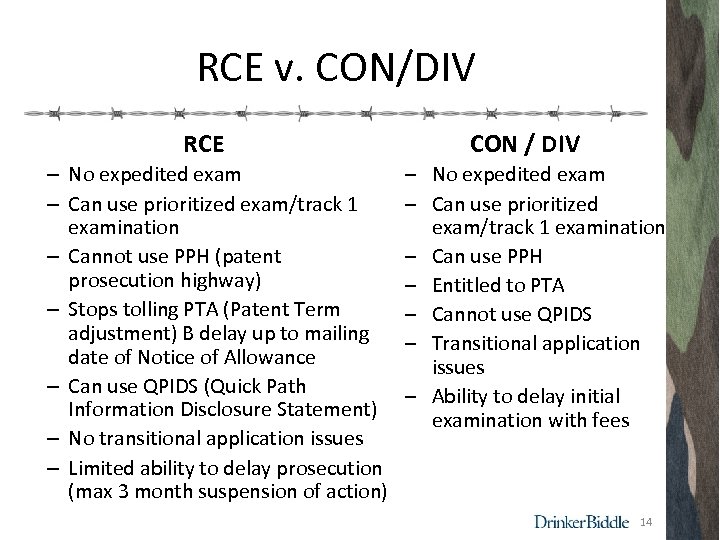
RCE v. CON/DIV RCE – No expedited exam – Can use prioritized exam/track 1 examination – Cannot use PPH (patent prosecution highway) – Stops tolling PTA (Patent Term adjustment) B delay up to mailing date of Notice of Allowance – Can use QPIDS (Quick Path Information Disclosure Statement) – No transitional application issues – Limited ability to delay prosecution (max 3 month suspension of action) CON / DIV ‒ No expedited exam ‒ Can use prioritized exam/track 1 examination ‒ Can use PPH ‒ Entitled to PTA ‒ Cannot use QPIDS ‒ Transitional application issues ‒ Ability to delay initial examination with fees 14

Costs of Continuing Applications • Costs and government fees for filing and prosecuting each divisional are comparable to the parent application – $6, 000 – $15, 000, depending on the technology and complexity • Applicants currently must ensure proper inventorship • Reporting requirements (IDSs) between applications with “substantially similar” claims – Mc. Kesson & Larson 15
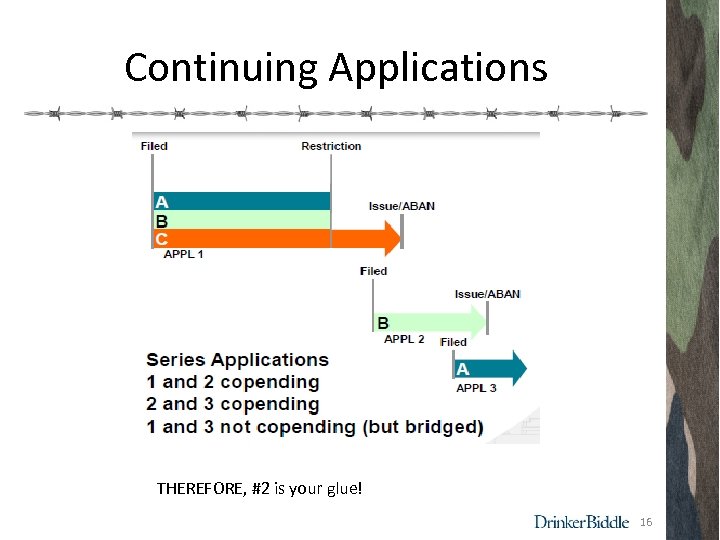
Continuing Applications THEREFORE, #2 is your glue! 16
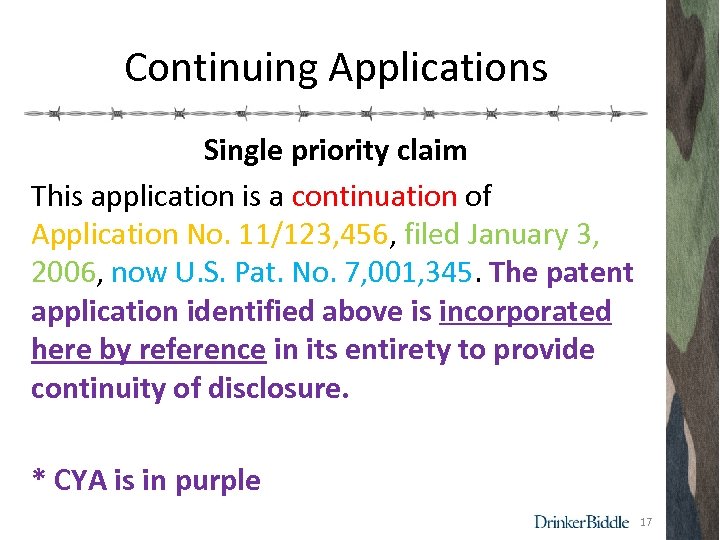
Continuing Applications Single priority claim This application is a continuation of Application No. 11/123, 456, filed January 3, 2006, now U. S. Pat. No. 7, 001, 345. The patent application identified above is incorporated here by reference in its entirety to provide continuity of disclosure. * CYA is in purple 17
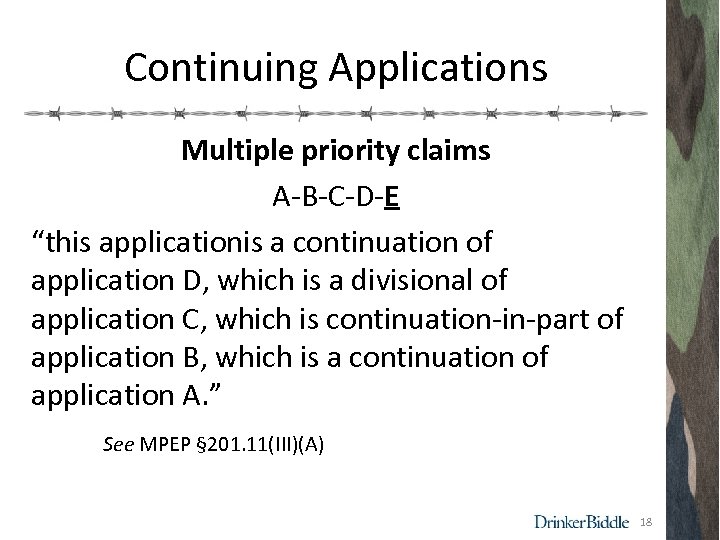
Continuing Applications Multiple priority claims A-B-C-D-E “this applicationis a continuation of application D, which is a divisional of application C, which is continuation-in-part of application B, which is a continuation of application A. ” See MPEP § 201. 11(III)(A) 18
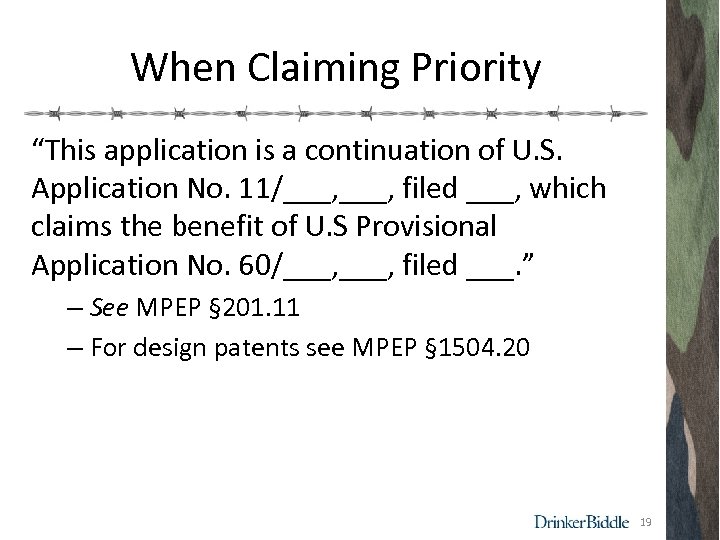
When Claiming Priority “This application is a continuation of U. S. Application No. 11/___, filed ___, which claims the benefit of U. S Provisional Application No. 60/___, filed ___. ” – See MPEP § 201. 11 – For design patents see MPEP § 1504. 20 19
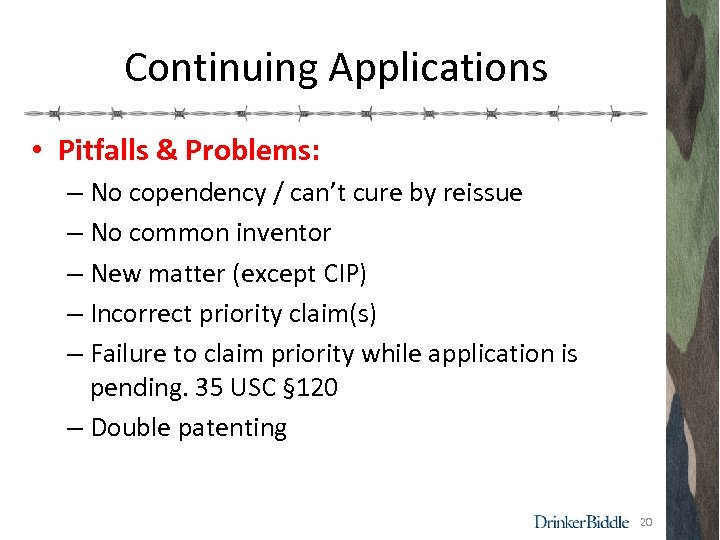
Continuing Applications • Pitfalls & Problems: – No copendency / can’t cure by reissue – No common inventor – New matter (except CIP) – Incorrect priority claim(s) – Failure to claim priority while application is pending. 35 USC § 120 – Double patenting 20
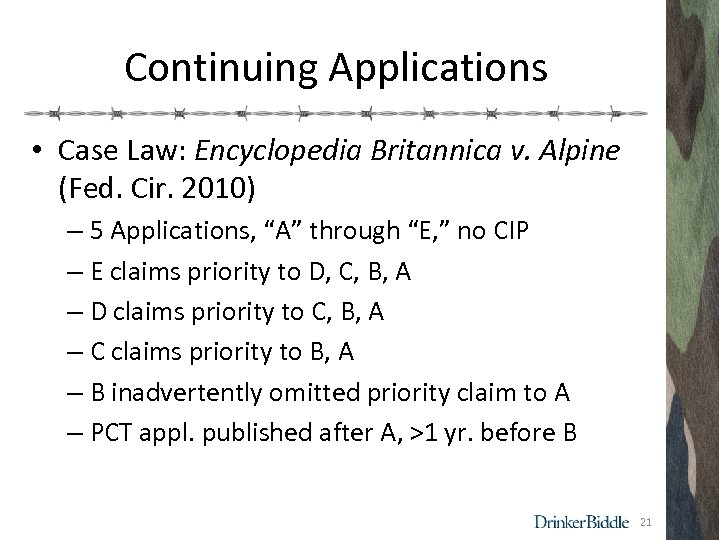
Continuing Applications • Case Law: Encyclopedia Britannica v. Alpine (Fed. Cir. 2010) – 5 Applications, “A” through “E, ” no CIP – E claims priority to D, C, B, A – D claims priority to C, B, A – C claims priority to B, A – B inadvertently omitted priority claim to A – PCT appl. published after A, >1 yr. before B 21
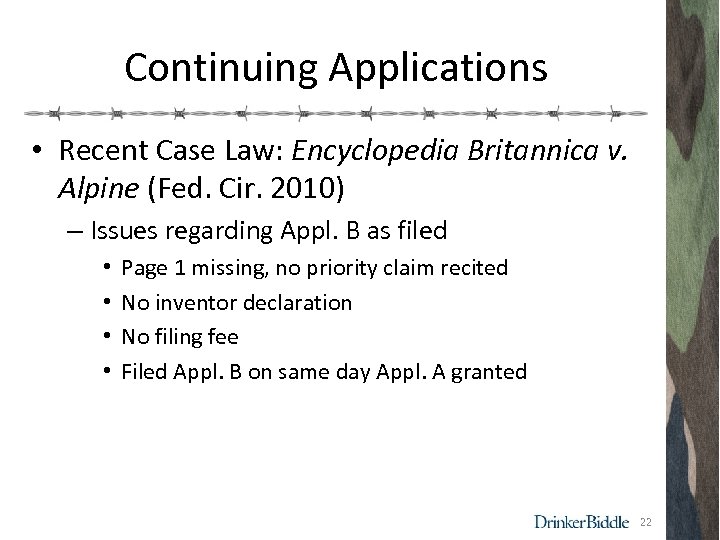
Continuing Applications • Recent Case Law: Encyclopedia Britannica v. Alpine (Fed. Cir. 2010) – Issues regarding Appl. B as filed • • Page 1 missing, no priority claim recited No inventor declaration No filing fee Filed Appl. B on same day Appl. A granted 22
Continuing Applications • Recent Case Law: Encyclopedia Britannica v. Alpine (Fed. Cir. 2010) – B abandoned without correction – C, D, E all claim priority to B, A • RESULTS: – E only gets priority of D and C, not B and A – PCT Appl. is § 102(b) prior art to E – Patent owner loses 23
Continuing Applications • Take-aways from Encyclopedia Britannica v. Alpine: – Check appl. priority claim at the point of filing – Late priority claim would win; no priority claim loses • Dicta foreshadow other issues – Incomplete application (B) not a proper link in priority chain – Is filing B on issue date of A copendency? 24

Divisional Applications Aka DIVs
Divisional • Purpose – prosecution of a separately patentable invention, restricted by the PTO from parent • 37 C. F. R. § 1. 53(b); previously under § 1. 60 • Why file? Safe Harbor benefit of 35 USC § 121 from a restriction requirement – One invention prosecuted, other(s) withdrawn, canceled – Divisional application(s) filed to prosecute non-elected invention(s) 26

Restriction Practice and Divisionals • Pitfalls & Problems: – Invalidity if the Divisional Does not have Consonance with the Restriction in the Parent – Loss of Embodiments of the Invention for failure to file an application • Cannot seek revival through reissue (35 USC 251); any subject matter not sought becomes dedicated to the public – See Watkinson 27

Problems - Invalidity • The 35 U. S. C. § 121 safe harbor against obviousness-type double patenting is very narrowly applied: – The claims must be pending before the restriction requirement is made. Geneva Pharm – Line of demarcation between inventions must be clear and maintained. Symbol Techs – Double patenting cannot always be cured with a Terminal Disclaimer. Boehringer – A method of using a composition may not be patentably distinct from an earlier claim to the identical composition in a patent disclosing the identical use. Pfizer – Safe harbor applies only to “divisionals, ” not continuations or CIPs. Pfizer, Amgen 28
Problems – DP Jeopardy • Double patenting rejections may be made under the following circumstances identified in MPEP § 804. 01: A. Two or more applications filed without a restriction (Cons, CIPs, Div of U. S. National Stage App. Under 35 U. S. C. 371, unrelated applications - Pfizer v. Teva); B. Claims in a “divisional” are not consonant with a restriction; MPEP §§ 804. 01, 822 C. Same invention – double patent (DP) D. Withdrawal of restriction because a linking or generic claim is rejoined; E. “Lack of unity” made only in an International Application; F. Restriction withdrawn 29
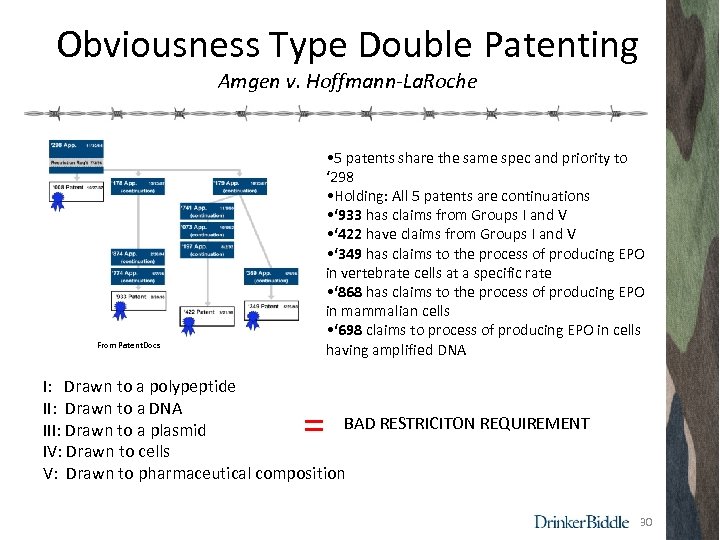
Obviousness Type Double Patenting Amgen v. Hoffmann-La. Roche • 5 patents share the same spec and priority to ‘ 298 • Holding: All 5 patents are continuations • ‘ 933 has claims from Groups I and V • ‘ 422 have claims from Groups I and V • ‘ 349 has claims to the process of producing EPO in vertebrate cells at a specific rate • ‘ 868 has claims to the process of producing EPO in mammalian cells • ‘ 698 claims to process of producing EPO in cells having amplified DNA From Patent. Docs I: Drawn to a polypeptide II: Drawn to a DNA BAD RESTRICITON REQUIREMENT III: Drawn to a plasmid IV: Drawn to cells V: Drawn to pharmaceutical composition = 30
Obviousness Type Double Patenting (OTDP) • OTDP Appearing in Litigation – Amgen v. Hoffmann. La. Roche – Takeda v. Doll – sent back for review – Cabilly (Genentech) Reexams – OTDP was asserted in Cabilly II (USPN 6, 331, 415) over Cabilly I (USPN 4, 816, 567) 31

Correct Restriction Requirements • For 121 Safe Harbor – make sure your restriction is correct – Petition is necessary – not appealable. – This is strictly a procedural issue outside appellate review by the Board or Fed. Cir. – Hengehold – USPTO procedural requirements are complex, and improper application occurs 32

Elections of Species • The PTO cannot necessarily examine all species or subcombinations of a generic claim – E. g. : A DNA array comprising one or more oligonucleotide probes selected from the group consisting of SEQ ID NOS: 1 – 100, 000 • Other species will be considered when the elected species is found to be allowable. – History: To promote “quality” examination, the PTO required Applicants to limit the claims to the elected species – This is no longer the case. See 76 Fed. Reg. 7166 (Feb. 9, 2011), at http: //edocket. access. gpo. gov/2011/pdf/2011 -2841. pdf 33

Review: Substantive vs. Procedural Issues • Review: The Petitions Process 1. Traverse / request reconsideration. • Many improper requirements can be withdrawn or modified before petition 2. File a petition, if reconsideration is denied. • Reviewed by various PTO personnel, usually a Technology Center Director 3. Request reconsideration, if petition denied • • Office of the Deputy Commissioner for Patent Examination Policy reviews A denial is a “final agency action” 4. Appeal to district court, if request for reconsideration of petition denied 34

Continuation-in-Part Rare & Endangered Species
Continuation-in-Part (CIP) • Purpose – Combine old and new matter in a single application • Applicable situations: – Capture new developments respecting same basic invention – Fix omissions – Consolidation of patents: saves fees • MPEP 201. 08; 37 CFR § 1. 53(b) 36
Continuation-in-Part (CIP) • Timing: – Co-pendency - 35 USC 120 – Before § 102(b) bars if pre-AIA – Before publication post-AIA • How: – This application is a continuation of Application No. D, filed ---, which is a continuation-in-part of Application No. C, filed ---, Application No. D claims the benefit of provisional Application No. B, filed ---, and Application No. C claims the benefit of provisional Application No. A, filed ---. ” MPEP 201. 11 37
Continuation-in-Part (CIP) – Priority claim doesn’t protect any claim not fully supported in parent(s) – If priority claim fails, parent(s) may be 35 USC § 102 prior art – Length of priority chain limited by pre-AIA 102(b) bars – CIP needs a common inventor with each priority application – Self-collision between CIP and an antecedent application by same inventor (In re Ruscetta) 38
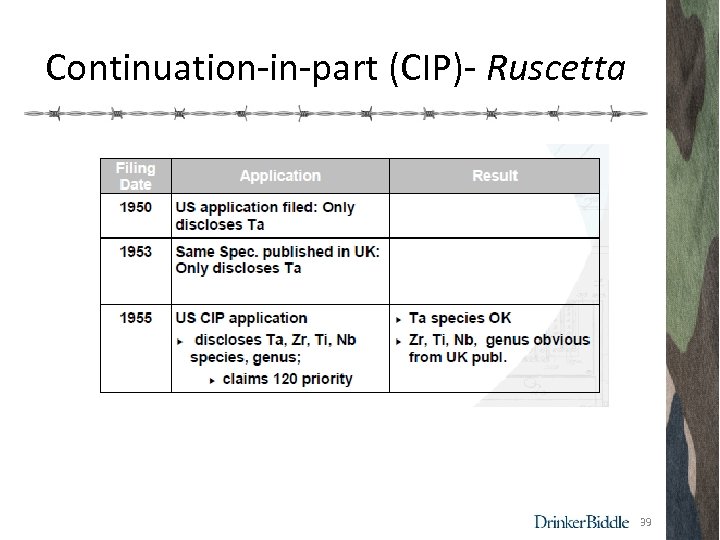
Continuation-in-part (CIP)- Ruscetta 39
Continuation-in-part (CIP) • Pitfalls & Problems: – CANNOT combine priority applications to support a single claim – Term loss for later applications • Even if priority claim useless – Becoming an endangered species post-AIA because of effective filing date issues (to be discussed) 40

Continuation-in-part (CIP) • Many problems not caught by PTO – usually found too late – i. e. – No safety net • Paris Convention Problem – if you file on A, then more than 12 months later file on A + A’, you will only get A’ not A in that application – See Article 87 EPC, Patent Act Art. 43(1) and Paris Convention Art. 4 41

Continuation-in-part (CIP) • CIP Practice Tips: – WATCH FOR: Lack of benefit in provisionals – BEST: File CIP and PCT within one year after first application and before any disclosure of the invention (i. e. , before publication) • Preserves foreign rights in new matter • Paris Convention, 35 USC § 112 priority for claims supported by original disclosure • Consider AIA-transitional application issues (to be discussed) • No 35 USC § 102 problem even if priority claim fails 42

TRANSITIONAL APPLICATIONS A POST AIA PHENOMENON 43
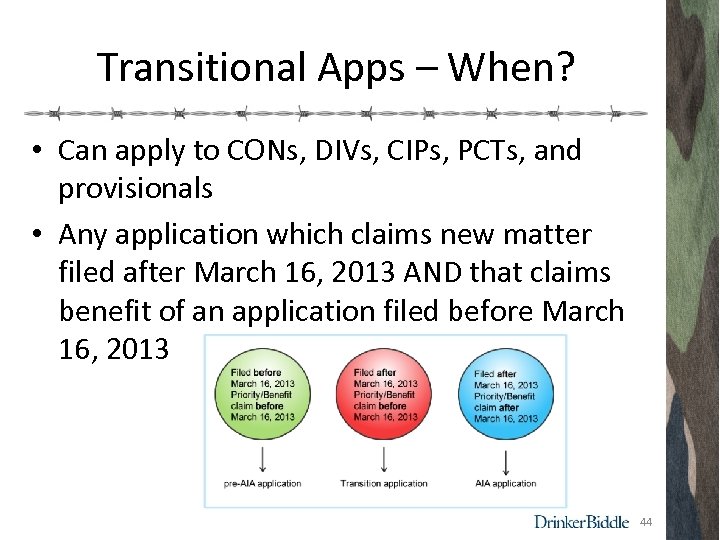
Transitional Apps – When? • Can apply to CONs, DIVs, CIPs, PCTs, and provisionals • Any application which claims new matter filed after March 16, 2013 AND that claims benefit of an application filed before March 16, 2013 44
Transitional Applications • 35 USC § 100(i)(1) “Effective filing date” (EFD) of a claim is: – Filing date of earliest application from which the application containing the claim is entitled to claim priority or filing date benefit, or, if none exists, – Filing date of the (nonprovisional) patent/application containing the claim • Under AIA, EFD informs your strategy and directs the fate of your claim • Best practice: • • • File claims with later EFDs in a separate applications Create running EFD charts for every claim in every application examined under the AIA and keep updating them Preserve the complete history of a claim in application family • Other Best: File original claims with application, then file new claims • Actual Best: Will be determined by a court in the future… 45

Transitional Applications • First Application (e. g. , provisional) filed before March 16, 2013 – All claims drawn to subject matter in the provisional eligible to be examined under pre-AIA law • Nonprovisional/PCT (or maybe CIP) filed after March 16, 2013 adding new information – If any claims drawn to new subject matter they will be examined under AIA (but may be also subject to interference) • →MUST TELL USPTO if you claim information added post-AIA on filing • This is a CERTIFICATION = RISK 46

1. 55 / 1. 78 Certification • An applicant simply needs to state that the application contains at least one claims that does not find support in one of the applications to which benefit or priority is sought. Here are example statements that an applicant could file: – This application filed on or after March 16, 2013 which claims benefit or priority to an application filed before March 16, 2013, contains one or more claims NOT entitled to a filing date before March 16, 2013. – This application claims benefit or priority to an application filed before March 16, 2013, and contains one or more claims NOT entitled to a filing date before March 16, 2013. – This application contains a claim having an effective filing date on or after March 16, 2013. – http: //www. uspto. gov/blog/aia/entry/message_from_janet_gongola_patent 2 47

Transitional Applications • One legal regime (pre v. post AIA) may be preferred over another, depending on: – The claimed subject matter – The existence of pre-AIA prior art that AIA eliminated the existence of “new, ” post-AIA prior art • Preference is easy to forfeit: – If a transition application ever contains a claim with EFD post-AIA (even if later canceled) will be considered under the post-AIA regime – Any CONs or DIVs of a transition application that ever contained such a claim will be considered under the post-AIA regime 48

Transitional Applns: How Accidents Happen? • Accidents resulting in loss of regime choice arise when: – Drafting post-AIA specification • Broaden definition of claim term—most insidious – Amending claim • Novelty-conferring feature disclosed only post-AIA – Accidentally adding a claim with post-AIA content – Filing CON or DIV intended to be pre-AIA off an AIAtainted parent 49

O T I S H E S U R E S
Race to the USPTO • Pre-AIA: file early (and “completely”/ § 112 compliant) and often • Post-AIA: file as early as possible, as often as developments warrant and in every case comply with § 112(a) (post AIA) – If not early, another applicant can scoop you – If not often, another can scoop your later developments – If not § 112 compliant: • Claims will not get the early filing date as their effective filing date • Application will not be effective upon filing as prior art against others’ subsequent filings • See this especially with CIPs and provisional practice 51
Pendency – Priority Claims • Pendency must be claimed during pendency in an ADS or in the specification as required by 37 CFR 1. 78(a)(2) or (a)(5) in the designated time frame or a Petition and surcharge under 1. 78(a) and 1. 17(t) respectively must be filed • 1. 78(a) only applies to applications that were filed on or after Nov. 29, 2000 – 4 months from filing or 16 months from priority • CERTIFICATION = RISK 52
Correcting Benefit of Priority • A petition under 1. 78(a)(3) is not required to correct (just update the application data sheet and priority claim in the specification): – Changing the relationship (Con Div) – Changing the filing date of a prior application – Changing a benefit claim – See MPEP § 201. 11 (V) 53

New Declarations • For applications filed after Sept. 16, 2012 claiming benefit of a prior filed application, then a new declaration is required – In re Chan (D 2011 -21) • Missing/ Irritated / Dead / Senile Inventors – see 37 CFR 1. 46 – BUT, not required if application is US application based on a PCT application pending before Sept. 16, 2013 – Generally separated from the Power of Attorney – See PTO Reference Guide http: //www. uspto. gov/aia_implementation/inventors-oath-ordeclaration-quick-reference-guide. pdf 54
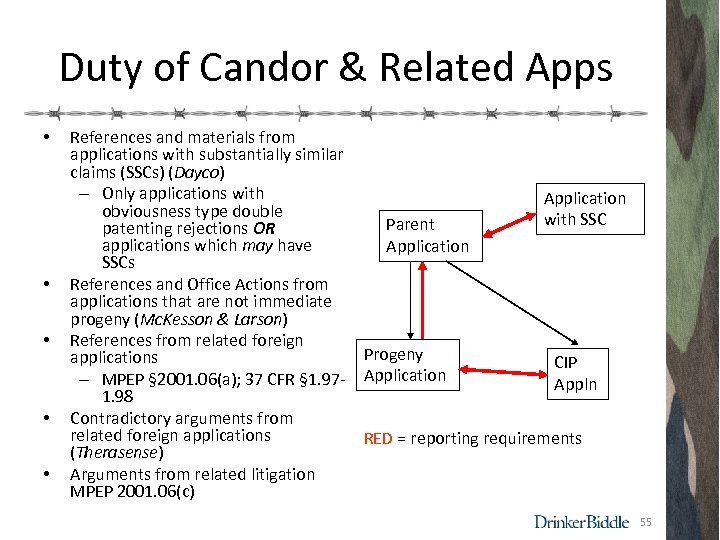
Duty of Candor & Related Apps • • • References and materials from applications with substantially similar claims (SSCs) (Dayco) – Only applications with Application obviousness type double with SSC Parent patenting rejections OR applications which may have Application SSCs References and Office Actions from applications that are not immediate progeny (Mc. Kesson & Larson) References from related foreign Progeny applications CIP Application – MPEP § 2001. 06(a); 37 CFR § 1. 97 Appln 1. 98 Contradictory arguments from related foreign applications RED = reporting requirements (Therasense) Arguments from related litigation MPEP 2001. 06(c) 55

Ideas • Have filing checklists for new applications – To address formalities – Make sure you have all necessary documentation for that filing – And make sure you have your papers correct and you have all the papers (lots of new transmittals) • Use claim charts to track your changes • Opinions – Know what application animal you have (e. g. , pre- or post-AIA) and which rules apply – Use your claim charts – Have a checklist of pitfalls to look for 56

Thank You! Mercedes K. Meyer, Ph. D. , J. D. Drinker Biddle & Reath LLP 1500 K Street, N. W. Washington, DC 20005 Mercedes. Meyer@dbr. com 202 -842 -8821 Linked. In: https: //www. linkedin. com/in/mercedesmeyer Bio: urldefense. proofpoint. com/v 2/url? u=http 3 A__www. drinkerbiddle. com_people_attorneys_meyer-2 Dmercedes 2 Dk&d=BQMF-g&c=jq. QLr 8 Vjrh 9 v. QZQBMH 8 t 0 g&r=o. UUer 6 Ob 9 k. Plyimhvx. Dak. MKMC-Yqv-R 0 jkpw. JEWw. SM&m=SDhu. ZMac. Cs 7 TLf. Qcfd 49 JGg. SBk. Qbs 9 dpjti 8 xg 1 jo 8&s=dj 0 u. Sx 0 fu. Aj. Dacw. Ceb. STo. O 1 hw. Epn. CMJp 1 bt 8 Buc 2 r. Y&e= PTAB Blog: https: //urldefense. proofpoint. com/v 2/url? u=http-

EXTRA GEAR 58

Materials Adda Gogoris, “Straddling the first-to-invent/First-to-file Gap” from ACI 3 rd Comprehensive Guide to Patent Reform Amgen Inc. v. Hoffmann-La Roche Ltd. , 580 F. 3 d 1340, 92 USPQ 2 d 1289 (Fed. Cir. 2009) Boehringer Ingelheim Int’l Gmb. H v. Barr Labs. Inc. , 592 F 3 d 1340, 93 USPQ 2 d 1417 (Fed. Cir. 2010) In re Chan (D 2011 -21) – can be found on the USPTO OED website CPA v. RCE Chart: http: //www. uspto. gov/web/offices/dcom/olia/aipa/RCECPA. pdf Dayco Products, Inc. v. Total Containments, Inc. , 329 F. 3 d 1358 (Fed. Cir. 2003) Encyclopedia Britannica v. Alpine (Fed. Cir. 2010) George Wheeler “Continuation and RCE Practice” Bootcamp 2011 Geneva Pharm. , Inc. v. Glaxo. Smith. Kline PLC, 349 F. 3 d 1373, 68 USPQ 2 d 1865 (Fed. Cir. 2003) In re Haas, 580 F. 2 d 461, 198 USPQ 334 (CCPA 1978) In re Harnisch, 631 F. 2 d 716, 722, 206 USPQ 300 (CCPA 1980) In re Hengehold, 440 F. 2 d 1395, 169 USPQ 473 (CCPA 1971) Julie Burke: When not to restrict? : http: //www. google. com/url? sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=6&ved=0 CDw. QFj. AF&url=http%3 A%2 F%2 Fwww. c abic. com%2 Fbcp%2 F 090209%2 FJBurke_WNAR. ppt&ei=9 Cer. U 5 q 2 HM 2 sy. AShh. IDw. Dw&usg=AFQj. CNE 6_l_4 i. EXuu. R 9 WKMJ 8 w. B_PMt. WHQ&bvm=bv. 69620078, d. a. Ww Inventor Oath or Dec FAQs: http: //www. uspto. gov/aia_implementation/faqs_inventors_oath. jsp Inventor Dec Quick Reference: http: //www. uspto. gov/aia_implementation/inventors-oath-or-declaration-quick-referenceguide. pdf Mc. Kesson Info. Solutions, Inc. v. Bridge Med. , 487 F. 3 d 897 (2007) Naomi Abe Voegtli and Jameson Q. Ma “Prosecution in the New Frontier: Continuation Application Practice under AIA” Pfizer Inc. v. Teva Pharm. USA Inc. , 86 USPQ 2 d 1001 (Fed. Cir. 2008) In re Ruscetta, 118 USPQ 101 (CCPA 1958) Symbol Techs. , Inc. v. Opticon, Inc. , 935 F. 2 d 1569, 19 USPQ 2 d 1241 (Fed. Cir. 1991) Takeda Pharmaceutical Co. v. Doll, 90 USP 2 d 1496 (Fed. Cir. 2009) In re Watkinson, 63 USP 2 d 1161 (Fed. Cir. 2002) 59
98ed05db9c736d1b24e12b341e55a9c4.ppt