8678368ded44eb7090924ed347414fce.ppt
- Количество слайдов: 22

2004 November 16, 2004 Pharmaceutical Regulatory and Compliance Congress Government Price Reporting – Operational Challenges and Solutions 4. 02 Pharmaceutical Price Reporting Issues, Challenges, and Solutions Jody Ann Noon RN, JD National Practice Leader, Life Sciences & Health Care Regulatory Deloitte & Touche LLP Ben Barrameda CPA, JD Senior Manager, Life Sciences & Health Care Regulatory Deloitte & Touche LLP © 2004 Deloitte Development LLC. All rights reserved.
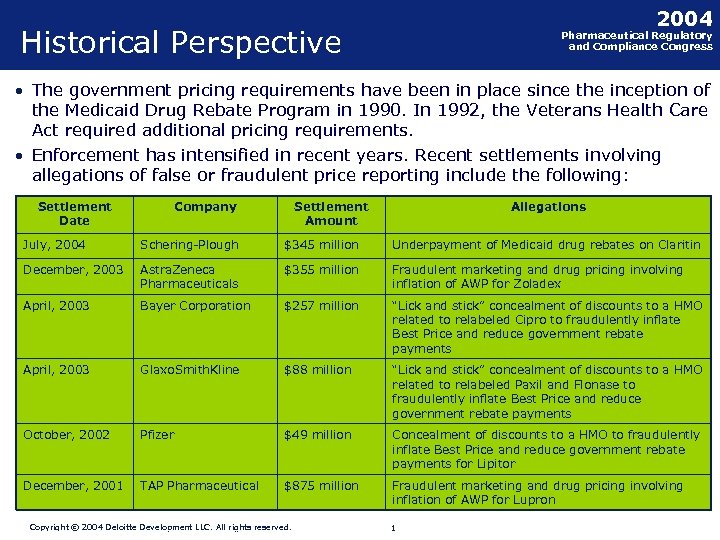
2004 Historical Perspective Pharmaceutical Regulatory and Compliance Congress • The government pricing requirements have been in place since the inception of the Medicaid Drug Rebate Program in 1990. In 1992, the Veterans Health Care Act required additional pricing requirements. • Enforcement has intensified in recent years. Recent settlements involving allegations of false or fraudulent price reporting include the following: Settlement Date Company Settlement Amount Allegations July, 2004 Schering-Plough $345 million Underpayment of Medicaid drug rebates on Claritin December, 2003 Astra. Zeneca Pharmaceuticals $355 million Fraudulent marketing and drug pricing involving inflation of AWP for Zoladex April, 2003 Bayer Corporation $257 million “Lick and stick” concealment of discounts to a HMO related to relabeled Cipro to fraudulently inflate Best Price and reduce government rebate payments April, 2003 Glaxo. Smith. Kline $88 million “Lick and stick” concealment of discounts to a HMO related to relabeled Paxil and Flonase to fraudulently inflate Best Price and reduce government rebate payments October, 2002 Pfizer $49 million Concealment of discounts to a HMO to fraudulently inflate Best Price and reduce government rebate payments for Lipitor December, 2001 TAP Pharmaceutical $875 million Fraudulent marketing and drug pricing involving inflation of AWP for Lupron Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 11

2004 Today – Pharmaceutical Regulatory and Compliance Congress • Governmental Price Reporting is a monumental challenge due to – – Numerous legal & contractual requirements that have varied price calculations – Complex business processes with many types of customers & transactions – A variety of business IT systems that do not integrate – Information that is required but unobtainable Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 22

2004 Pricing Requirements Medicaid Rebate Program • Best Price (BP) • Average Manufacturers Price (AMP) • Unit Rebate Amount (URA) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved Pharmaceutical Regulatory and Compliance Congress • Manufacturers must calculate and report BP and AMP to CMS within 30 days of the end of each quarter for all outpatient drugs covered under the manufacturer’s rebate agreement with CMS. • Manufacturers must pay rebates to each State Medicaid agency within 30 days of receiving Medicaid Utilization information for the covered drugs paid for by the Agency in the quarter. 33

2004 Pricing Requirements Veterans Affairs • Non-Federal AMP (non -FAMP) • Federal Ceiling Price (FCP) • Federal Supply Schedule (FSS) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved Pharmaceutical Regulatory and Compliance Congress • Manufacturers must calculate and report non-FAMP to the Dept of Veterans Affairs (VA) within 30 days of the end of each quarter for all drug products covered under the manufacturer’s Pharmaceutical Pricing Agreement with the VA. • Manufacturers must calculate and report non-FAMP for all covered drug products by November 15 of each year for the one-year period ended September 30. • Manufacturers may not charge more than the FCP for covered drug products purchased by Federal Agencies and State homes represented by the VA. 44

2004 Pricing Requirements Medicare Modernization Act (MMA) • Average Sales Price (ASP) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved Pharmaceutical Regulatory and Compliance Congress • Manufacturers must calculate and report ASP to CMS within 30 days of the end of each quarter for all Medicare and Part B drugs and biologics covered under the Medicare Prescription Drug, Improvement, and Modernization Act and the Social Security Act. • ASP will be used to calculate payment allowances to manufacturers for covered drugs and biologics effective January 1, 2005. 55

2004 Pricing Requirements Public Health Service Act (PHSA) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved Pharmaceutical Regulatory and Compliance Congress • Manufacturers may not charge Federally-qualified health centers and other entities enrolled in the 340 B Program more than AMP reduced by a rebate percentage calculated for the preceding calendar quarter for outpatient drugs covered under State plans for medical assistance. 66
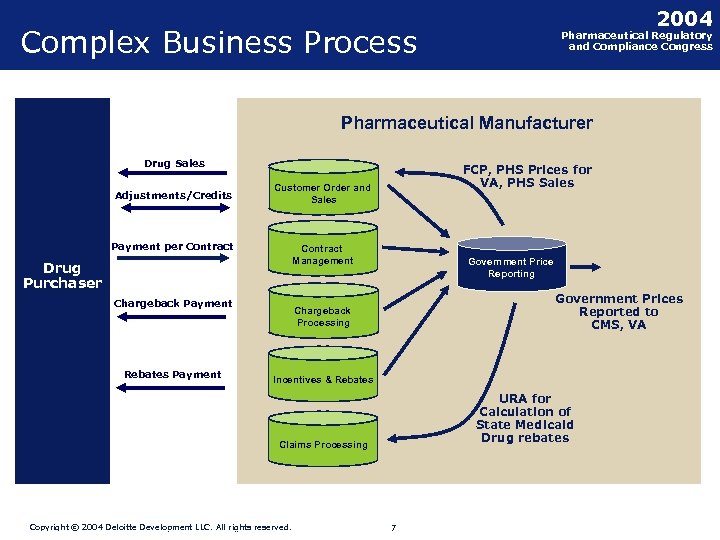
2004 Complex Business Process Pharmaceutical Regulatory and Compliance Congress Pharmaceutical Manufacturer Drug Sales Adjustments/Credits Customer Order and Sales Payment per Contract Management Drug Purchaser Chargeback Payment Rebates Payment FCP, PHS Prices for VA, PHS Sales Government Price Reporting Government Prices Reported to CMS, VA Chargeback Processing Incentives & Rebates URA for Calculation of State Medicaid Drug rebates Claims Processing Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 77

Complex Business Process - Sales 2004 Pharmaceutical Regulatory and Compliance Congress • All U. S. sales (and Puerto Rico for non-FAMP) • All products participating (practically all) • All distribution channels • All rebates and discounts given • All adjustments to transactions (e. g. shipment in error, picking error, damaged shipments, etc. ) • All other marketing incentives given to induce sale (e. g. non-bona fide grants) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 88

Complex Business Process – Accounts Receivable & Accounts Payable 2004 Pharmaceutical Regulatory and Compliance Congress • Sales are recorded in period that they occur • Chargebacks are recorded when they are received and may have 15– 45 days of lag time. Chargeback adjustments are received up to 6 -8 months later • Rebates are recorded when they are paid – usually with 2 -3 quarters of lag time • Returns are recorded when received – as much as 1 year of lag time • Other adjustments may come with several quarters of lag time (e. g. volume discount adjustments, disputed items, etc. ) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 99

Business Process – Medicaid Rebates 2004 Pharmaceutical Regulatory and Compliance Congress Pharmaceutical Manufacturer Government Price Reporting Prescriptions Doctors Medicaid Patients Bill for Medicaid Rebate State Medicaid Agency Payments Per Contract Drug Sales C Pa laim ym s en t Price Information C Ut lai ili ms za / tio n What Can Be Prescribed (Formulary) Federal Government (CMS) Pay Medicaid Rebate Pharmacies Wholesalers Rx Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 10 10

Business Process – Managed Care Rebates 2004 Pharmaceutical Regulatory and Compliance Congress Calculations for Government Price Reporting Generate Summary of Claims Filed/Paid HMOs PPOs PBM Rebates Contract Insurers -Process/Administer Data Remit Rebates Maintain Contract Networks Transmit Enrollment Data - Program Changes Transmit Data Pharma Company - Formulary Employers Payment Transmit Claim Summary Formulary; Claims Payment What Can Be Prescribed (Formulary) Pharmacies ent aym P Prescription Doctors Copyright © 2004 Deloitte Development LLC. All rights reserved. ion at dic pt cri es Pr Patients Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 11 11 Me

2004 Business Process – Chargebacks Calculations for Government Price Reporting Chargebacks Pharmaceutical Manufacturer Chargeback (CB) = $2 ($20 -$18) Pharmaceutical Regulatory and Compliance Congress Contract Price = $18 Customer/ Patient Wholesale Price = $20 Customer Pays Contract Price = $18 Wholesaler Delivers Drugs Wholesaler Rx Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 12 12

A Variety of Non-integrated IT Systems • Many of the systems are legacy and/or customized systems that rely heavily on manual inputs – Contracting system – Invoicing System – Chargeback System – Rebate System – Sales ERP System – Financial Reporting System (general ledger) – Accounts Payable System – Government Price Reporting System – Medicaid Rebate System – Sales ERP system – Various Excel spreadsheets – Access database – Medicaid rebate system – Hardcopy (e. g. customer contracts) Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 13 13 2004 Pharmaceutical Regulatory and Compliance Congress

2004 Unavailable Information Pharmaceutical Regulatory and Compliance Congress • Obtaining class of trade of members of GPOs • Tracing back chargebacks to the original invoice • Tracing back expired products to the original sales invoice • Obtaining units of returned damaged products Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 14 14
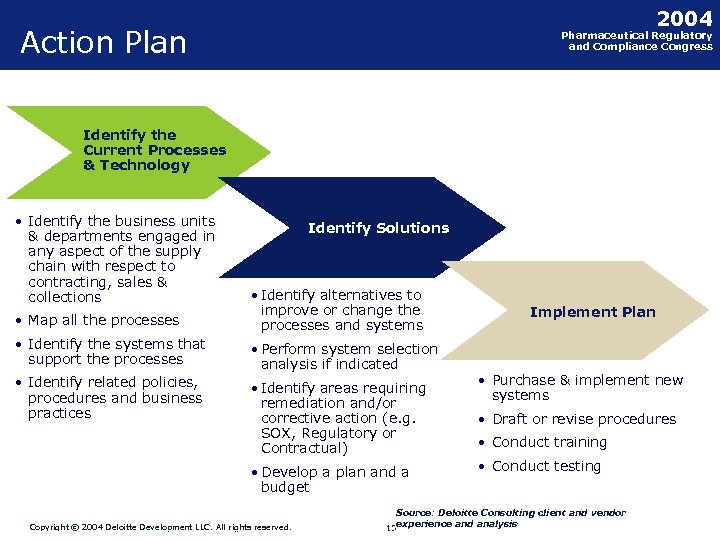
2004 Action Plan Pharmaceutical Regulatory and Compliance Congress Identify the Current Processes & Technology • Identify the business units & departments engaged in any aspect of the supply chain with respect to contracting, sales & collections Identify Solutions • Map all the processes • Identify alternatives to improve or change the processes and systems • Identify the systems that support the processes • Perform system selection analysis if indicated • Identify related policies, procedures and business practices • Identify areas requiring remediation and/or corrective action (e. g. SOX, Regulatory or Contractual) • Develop a plan and a budget Implement Plan • Purchase & implement new systems • Draft or revise procedures • Conduct training • Conduct testing Source: Deloitte Consulting client and vendor Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 15 experience and analysis 15
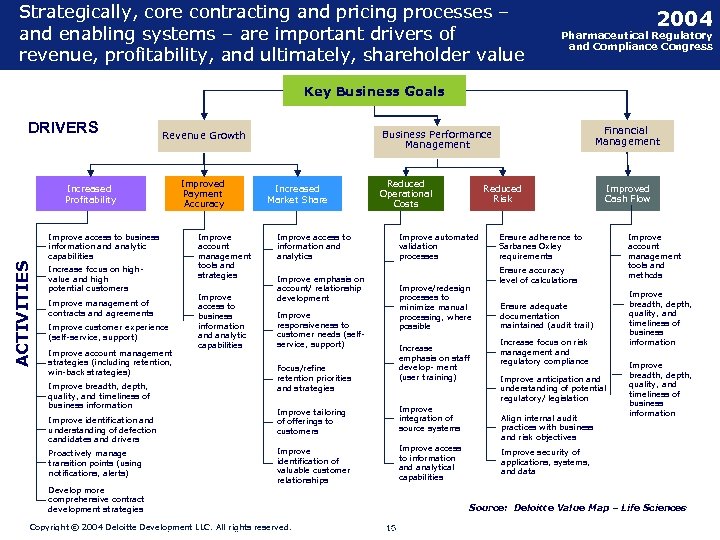
Strategically, core contracting and pricing processes – and enabling systems – are important drivers of revenue, profitability, and ultimately, shareholder value 2004 Pharmaceutical Regulatory and Compliance Congress Key Business Goals DRIVERS Increased Profitability ACTIVITIES Improve access to business information and analytic capabilities Increase focus on highvalue and high potential customers Improve management of contracts and agreements Improve customer experience (self-service, support) Improve account management strategies (including retention, win-back strategies) Improve breadth, depth, quality, and timeliness of business information Improve identification and understanding of defection candidates and drivers Proactively manage transition points (using notifications, alerts) Improved Payment Accuracy Improve account management tools and strategies Improve access to business information and analytic capabilities Financial Management Business Performance Management Revenue Growth Increased Market Share Reduced Operational Costs Improve access to information and analytics Improve automated validation processes Improve emphasis on account/ relationship development Improve/redesign processes to minimize manual processing, where possible Improve responsiveness to customer needs (selfservice, support) Increase emphasis on staff develop- ment (user training) Focus/refine retention priorities and strategies Improve tailoring of offerings to customers Improve integration of source systems Improve identification of valuable customer relationships Improve access to information and analytical capabilities Develop more comprehensive contract development strategies Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved Reduced Risk Improved Cash Flow Ensure adherence to Sarbanes Oxley requirements Ensure accuracy level of calculations Ensure adequate documentation maintained (audit trail) Increase focus on risk management and regulatory compliance Improve anticipation and understanding of potential regulatory/ legislation Align internal audit practices with business and risk objectives Improve account management tools and methods Improve breadth, depth, quality, and timeliness of business information Improve security of applications, systems, and data Source: Deloitte Value Map – Life Sciences 16 16
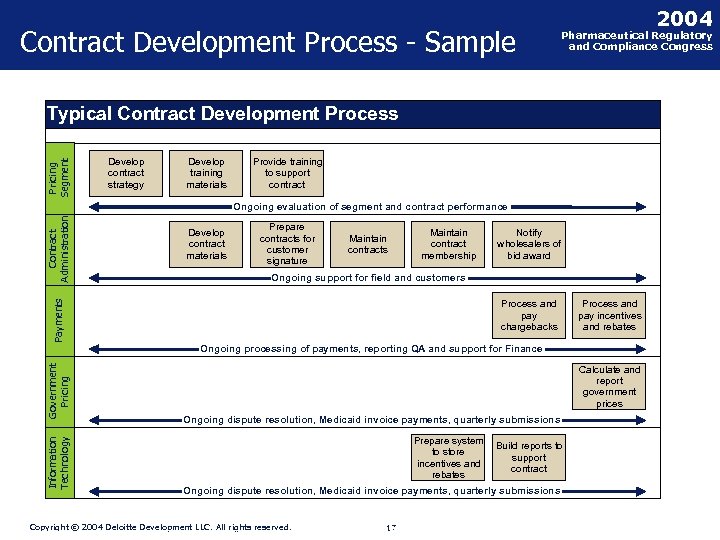
Contract Development Process - Sample 2004 Pharmaceutical Regulatory and Compliance Congress Pricing Segment Typical Contract Development Process Develop contract strategy Develop training materials Provide training to support contract Information Technology Government Pricing Payments Contract Administration Ongoing evaluation of segment and contract performance Develop contract materials Prepare contracts for customer signature Maintain contracts Maintain contract membership Notify wholesalers of bid award Ongoing support for field and customers Process and pay chargebacks Process and pay incentives and rebates Ongoing processing of payments, reporting QA and support for Finance Calculate and report government prices Ongoing dispute resolution, Medicaid invoice payments, quarterly submissions Prepare system to store incentives and rebates Build reports to support contract Ongoing dispute resolution, Medicaid invoice payments, quarterly submissions Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 17 17
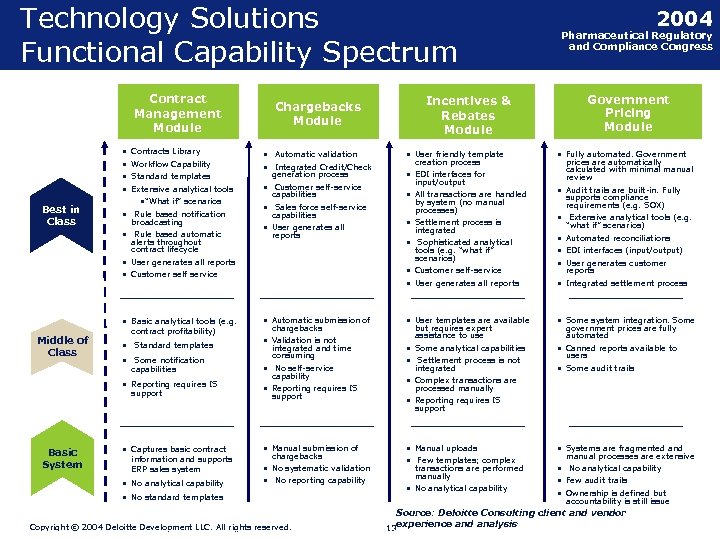
Technology Solutions Functional Capability Spectrum Contract Management Module • • Best in Class Contracts Library Workflow Capability Standard templates Extensive analytical tools • “What if” scenarios • Rule based notification broadcasting • Rule based automatic alerts throughout contract lifecycle Chargebacks Module • Automatic validation • Integrated Credit/Check generation process • Customer self-service capabilities • Sales force self-service capabilities • User generates all reports Middle of Class contract profitability) • Standard templates • Some notification capabilities • Reporting requires IS support • User friendly template creation process • EDI interfaces for input/output • All transactions are handled by system (no manual processes) • Settlement process is integrated • Sophisticated analytical tools (e. g. “what if” scenarios) • User generates all reports • Customer self service • Basic analytical tools (e. g. Incentives & Rebates Module • Customer self-service • User generates all reports • Automatic submission of chargebacks • Validation is not integrated and time consuming • No self-service capability • Reporting requires IS support 2004 Pharmaceutical Regulatory and Compliance Congress Government Pricing Module • Fully automated. Government prices are automatically calculated with minimal manual review • Audit trails are built-in. Fully supports compliance requirements (e. g. SOX) • Extensive analytical tools (e. g. “what if” scenarios) • Automated reconciliations • EDI interfaces (input/output) • User generates customer reports • Integrated settlement process • User templates are available • Some system integration. Some • Some analytical capabilities • Settlement process is not • Canned reports available to but requires expert assistance to use integrated government prices are fully automated users • Some audit trails • Complex transactions are processed manually • Reporting requires IS support • Captures basic contract • Manual submission of • No analytical capability Basic System • No systematic validation • No reporting capability information and supports ERP sales system chargebacks • No standard templates • Manual uploads • Few templates; complex transactions are performed manually • No analytical capability • Systems are fragmented and manual processes are extensive • No analytical capability • Few audit trails • Ownership is defined but accountability is still issue Source: Deloitte Consulting client and vendor Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 18 experience and analysis 18

Technology Solutions Functional Capability Spectrum Architecture • Business Process • Service oriented • BPM capability • Standards based – Architecture architecture • Object oriented Best in Class Change Management Interfaces • Web based MVC programming language • Rules based and configuration driven changes • Compliance with standards 2004 Pharmaceutical Regulatory and Compliance Congress General • Available Knowledge base • Workflow driven process • Well defined SLA with the • Load balancing and • Audit controls and driven integration • Search engine users BPEL, WSDL, XML • Plug and play with documentation canonical messages format • Historical analysis and H/A • Session management and Transaction recovery capability rollbacks • Standards based • Planned releases based on message format release numbers • Process driven EDI capability • Web based architecture with thin client Middle of Class • N Tired architecture • Modular division of functionality • Use of standard programming languages • Enterprise Application • Synchronous and Async capability • Publish/subscribe • • Database to log problems • • • Integration technology Manual change management architecture Proper analysis for changes Planned releases • H/A • More Configuration based changes Defined process for assignments • Application specific message formats • Basic EDI capabilities • Automated error handling • Monolithic application architecture Basic System • Mainframe driven • Highly customized with proprietary language • Non modular/tired architecture • Point to Point interfaces • Proprietary message formats • Batch/File based interfaces • Proprietary communication • Manual error handling Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 19 19 Ad hoc process for changes Ad hoc release management No prioritization No audit control and history tracking • No High availability • More than 85% change requests need changes to code • Need of specialized resources

2004 Pharmaceutical Regulatory and Compliance Congress Jody Ann Noon RN, JD Principal National Practice Leader Life Science and Health Care Regulatory Practice Deloitte & Touche LLP jodynoon@deloitte. com (212) 436 -2558 As an RN, JD, Jody utilizes her past experience practicing law to assist life science companies to develop systems to comply with complex legal and regulatory requirements. Jody has experience working with the OIG Compliance Guidance, the federal False Claims Act, Anti-kickback Statute, Medicare & Medicaid requirements, Physician Self-Referral Law (Stark) and Privacy requirements. Ben Barrameda, Esq. , CPA, MBA Senior Manager Life Science and Health Care Regulatory Practice Deloitte & Touche LLP bbarrameda@deloitte. com (212) 436 -3555 Ben is a senior manager in Deloitte’s Life Science and Health Care Regulatory practice, and he is one of Deloitte and Touche’s leading pharmaceutical pricing practitioners. Ben has over 15 years of experience in providing dispute consulting and litigation support, due diligence, internal investigations, developing, implementing and monitoring corporate compliance programs, audit, control, financial modeling, and business processes. Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 20 20

2004 Pharmaceutical Regulatory and Compliance Congress Copyright © 2004 Deloitte Development LLC. All rights reserved. Confidential and Proprietary Material of Deloitte Consulting. Copyright © 2002 Deloitte Consulting (US) LLC. All Rights Reserved 21 21
8678368ded44eb7090924ed347414fce.ppt