f86acae3c9bc377b2418c374f453952b.ppt
- Количество слайдов: 37
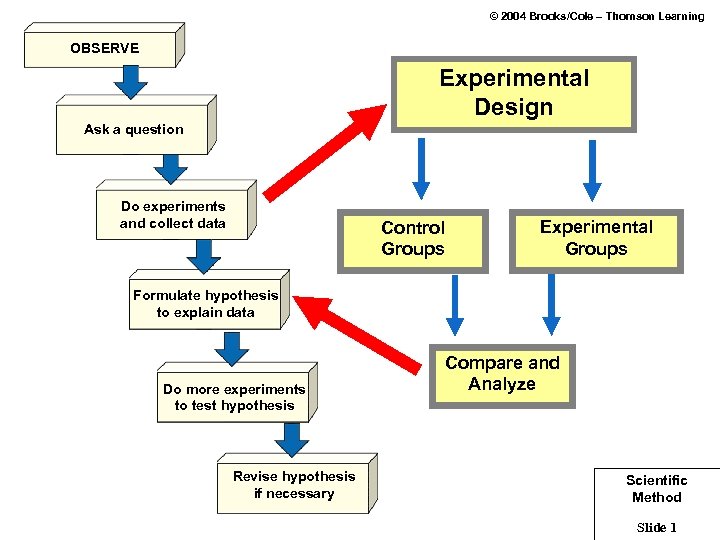
© 2004 Brooks/Cole – Thomson Learning OBSERVE Experimental Design Ask a question Do experiments and collect data Control Groups Experimental Groups Formulate hypothesis to explain data Do more experiments to test hypothesis Revise hypothesis if necessary Compare and Analyze Scientific Method Slide 1
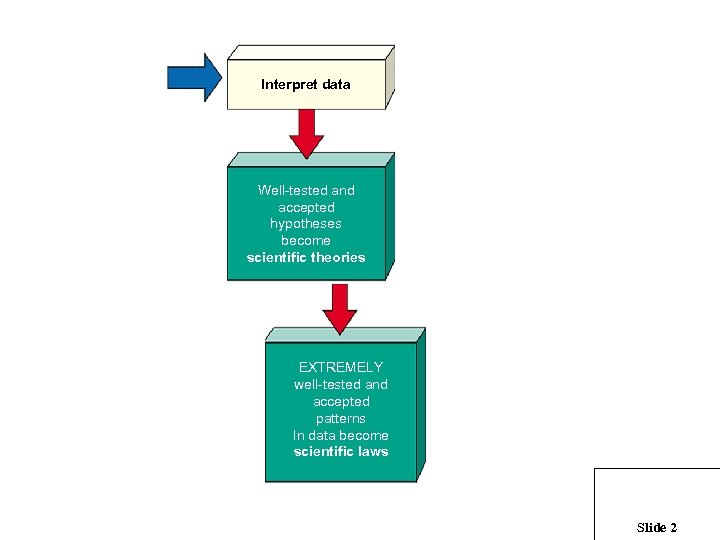
Interpret data Well-tested and accepted hypotheses become scientific theories EXTREMELY well-tested and accepted patterns In data become scientific laws Slide 2
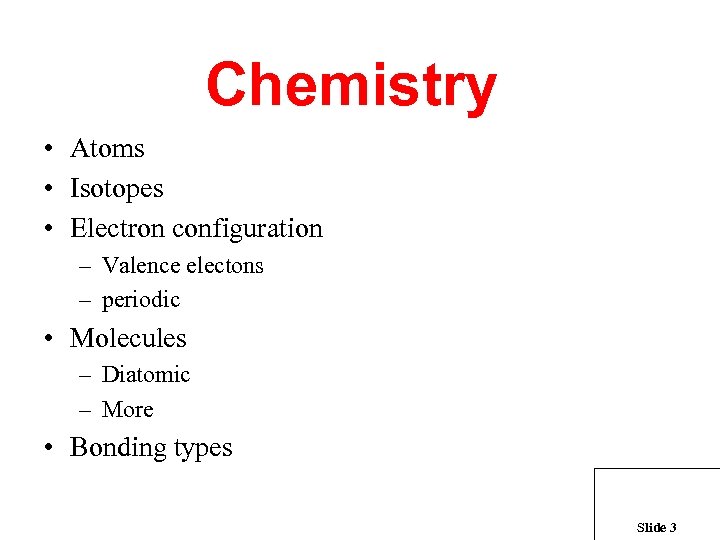
Chemistry • Atoms • Isotopes • Electron configuration – Valence electons – periodic • Molecules – Diatomic – More • Bonding types Slide 3
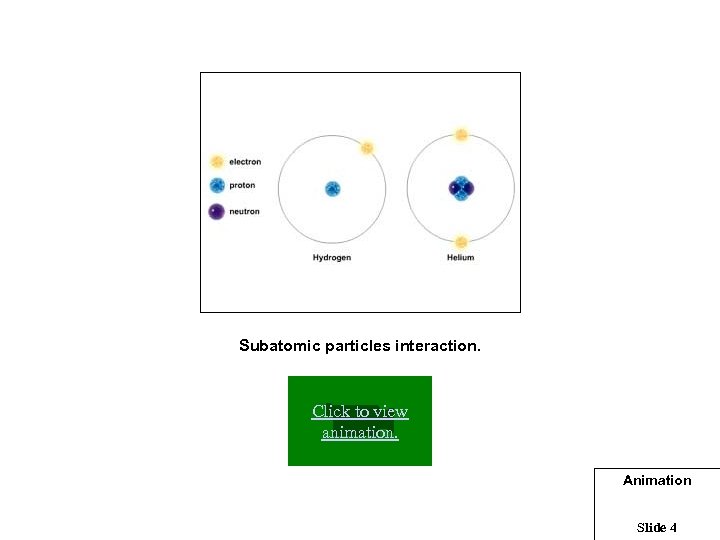
Subatomic particles interaction. Click to view animation. Animation Slide 4
Atomic number, mass number interaction. Click to view animation. Animation Slide 5
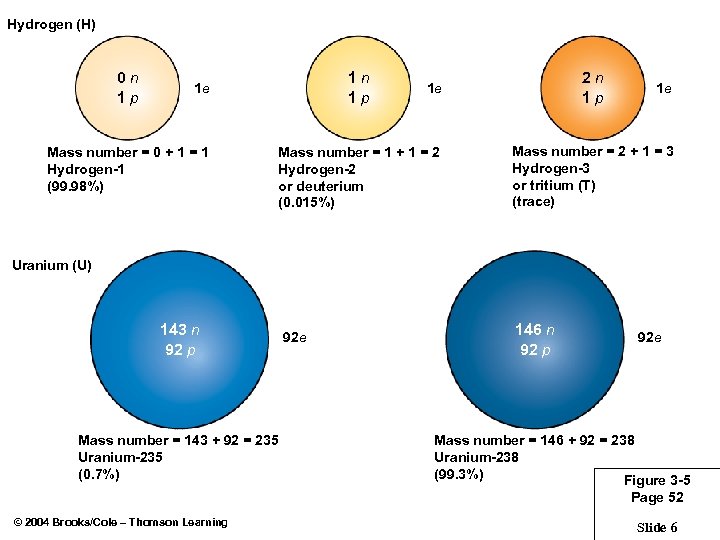
Hydrogen (H) 0 n 1 p 1 e Mass number = 0 + 1 = 1 Hydrogen-1 (99. 98%) 2 n 1 p 1 e Mass number = 1 + 1 = 2 Hydrogen-2 or deuterium (0. 015%) 1 e Mass number = 2 + 1 = 3 Hydrogen-3 or tritium (T) (trace) Uranium (U) 143 n 92 p Mass number = 143 + 92 = 235 Uranium-235 (0. 7%) 92 e 146 n 92 p 92 e Mass number = 146 + 92 = 238 Uranium-238 (99. 3%) Figure 3 -5 Page 52 © 2004 Brooks/Cole – Thomson Learning Slide 6

Positron emission tomography (PET) animation. Click to view animation. Animation Slide 7
Ionic bonding animation. Click to view animation. Animation Slide 8
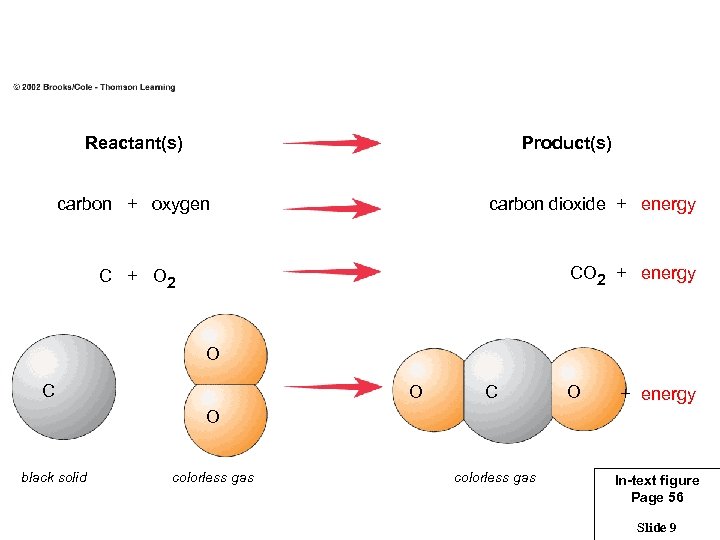
Reactant(s) Product(s) carbon + oxygen carbon dioxide + energy CO 2 + energy C + O 2 O C O + energy O black solid colorless gas In-text figure Page 56 Slide 9

High Quality Low Quality Solid Gas Salt © 2004 Brooks/Cole – Thomson Learning Solution of salt in water Coal-fired power plant emissions Gasoline Automobile emissions Figure 3 -6 Page 53 Aluminum can Aluminum ore Slide 10
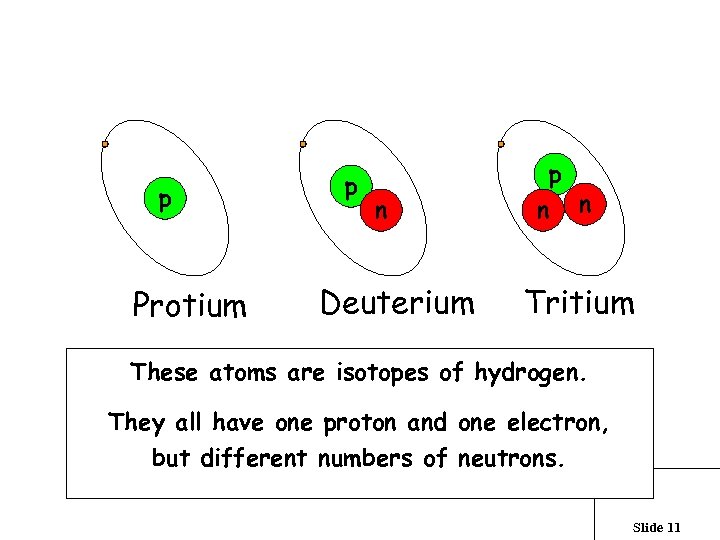
What are isotopes? p Protium p n Deuterium p n n Tritium These atoms are isotopes of hydrogen. They all have one proton and one electron, but different numbers of neutrons. Slide 11
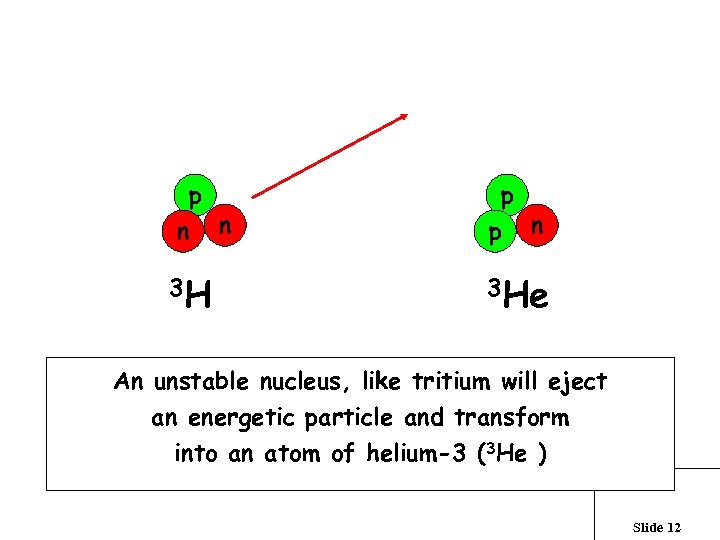
What is radioactivity? p n n 3 H p p n 3 He An unstable nucleus, like tritium will eject an energetic particle and transform into an atom of helium-3 (3 He ) Slide 12

While tritium is radioactive, the energy of the beta particle is very low. Tritium is the only radioactive isotope that you can buy in large quantities at the local mall. The tritium present in the entire Livermore Valley groundwater basin equals that found in 30 ‘tritium dial’ watches Slide 13
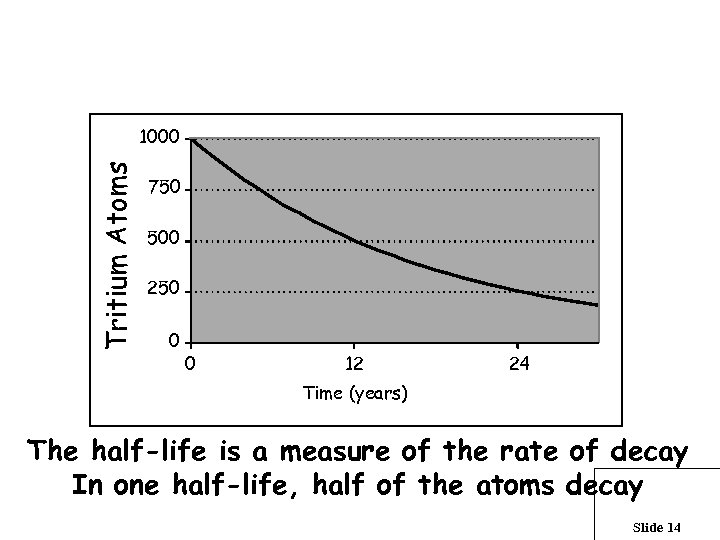
What is a half-life ? Tritium Atoms 1000 750 500 250 0 0 12 24 Time (years) The half-life is a measure of the rate of decay In one half-life, half of the atoms decay Slide 14
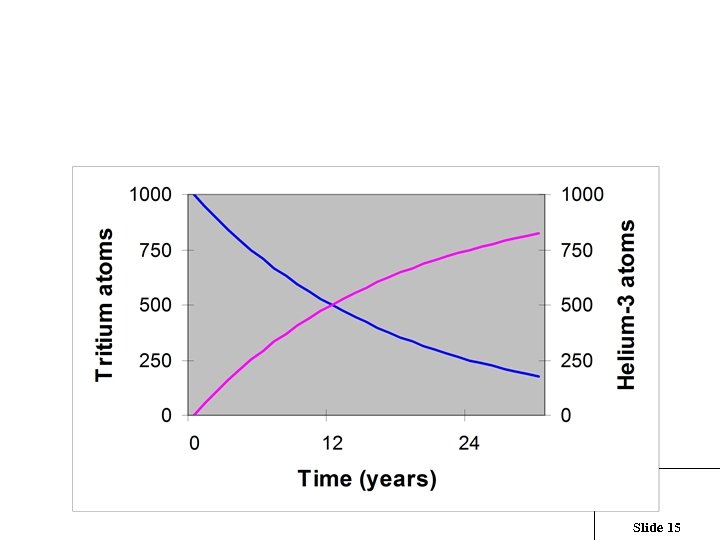
For every tritium decay, an atom of 3 He is produced Slide 15
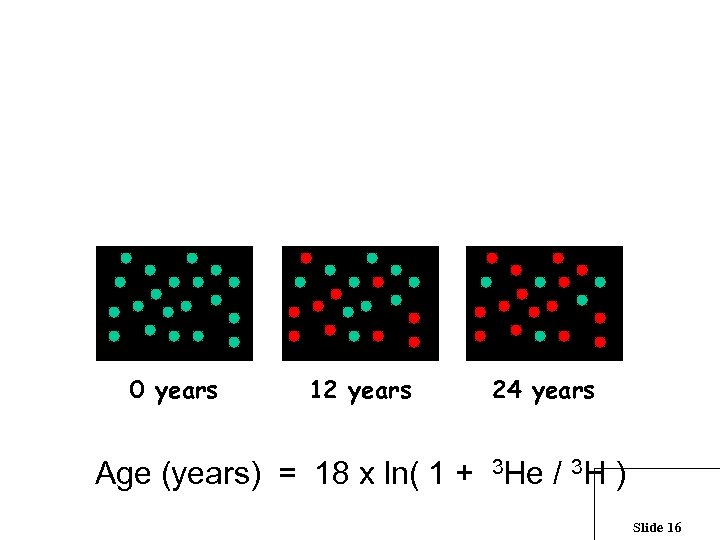
The 3 He from 3 H decay starts to accumulate once the water has become groundwater 0 years 12 years 24 years Age (years) = 18 x ln( 1 + 3 He / 3 H ) Slide 16
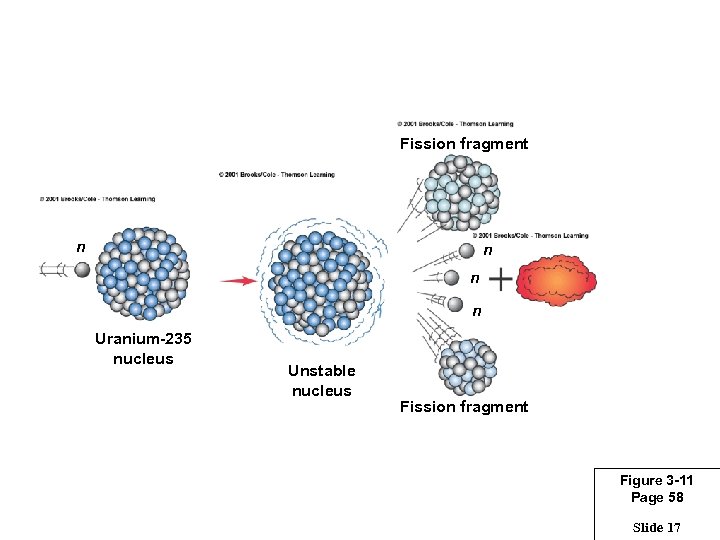
Fission fragment n n n Energy n Uranium-235 nucleus Unstable nucleus Fission fragment Figure 3 -11 Page 58 Slide 17
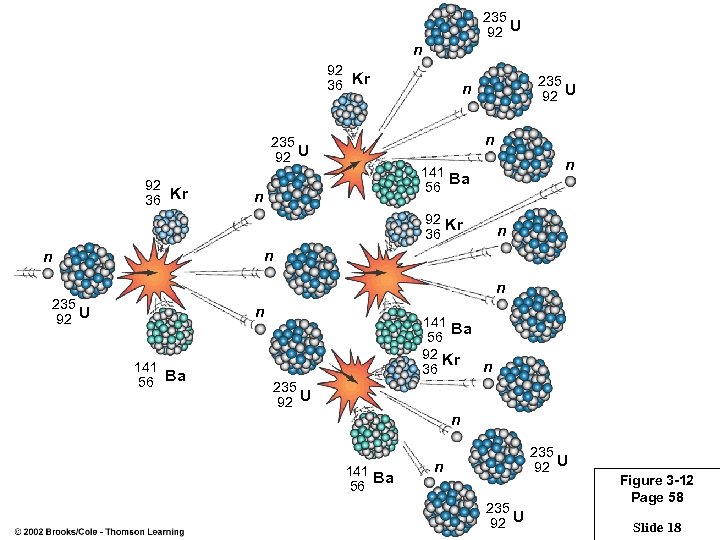
235 92 U n 92 36 Kr n 235 92 U 92 36 Kr 235 92 U n n 141 Ba 56 n 92 Kr 36 n n 235 92 U n 141 56 Ba 141 Ba 56 92 Kr n 36 235 92 U n 141 Ba 56 235 92 U n 235 92 U Figure 3 -12 Page 58 Slide 18
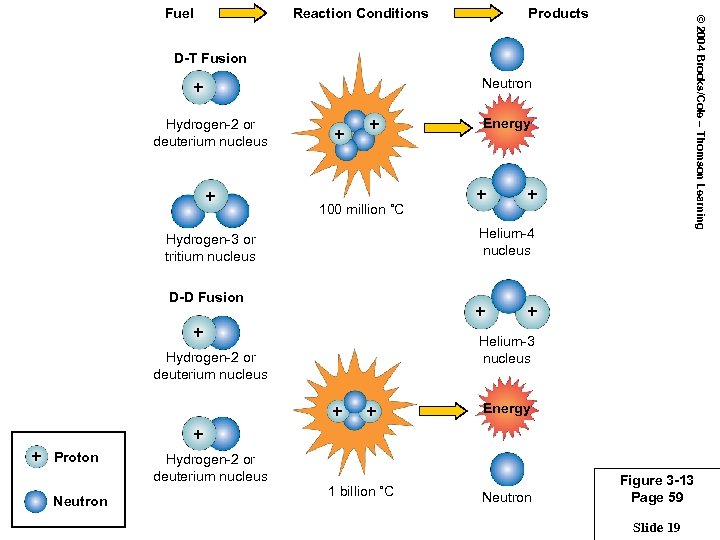
Reaction Conditions Products © 2004 Brooks/Cole – Thomson Learning Fuel D-T Fusion Neutron + Hydrogen-2 or deuterium nucleus + + + 100 million ˚C Energy + + Helium-4 nucleus Hydrogen-3 or tritium nucleus D-D Fusion + + + Helium-3 nucleus Hydrogen-2 or deuterium nucleus + + Energy + + Proton Neutron Hydrogen-2 or deuterium nucleus 1 billion ˚C Neutron Figure 3 -13 Page 59 Slide 19
Half-life interaction. Click to view animation. Animation Slide 20

Energy Slide 21
Types of Energy • Potential – Stored chemical – Physical position • Kinetic – Motion – Temperature / Heat Slide 22
Metabolic Use of Energy • Homeostasis • Feedback Loops • Heat Production Slide 23
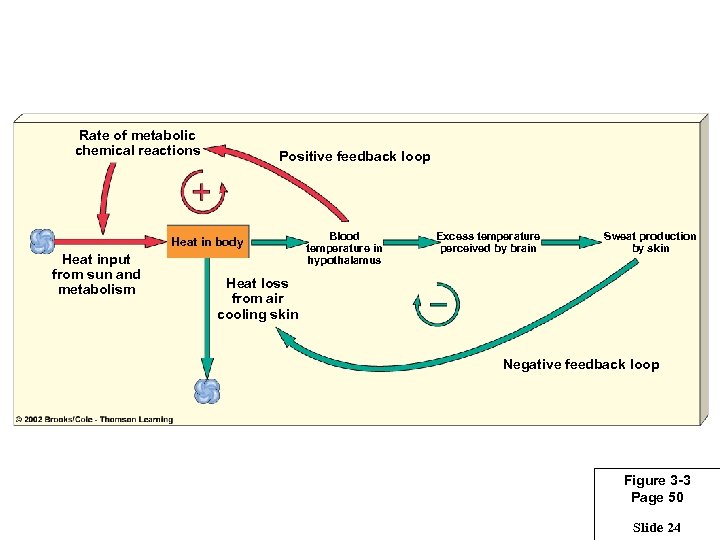
Rate of metabolic chemical reactions Positive feedback loop Heat in body Heat input from sun and metabolism Blood temperature in hypothalamus Excess temperature perceived by brain Sweat production by skin Heat loss from air cooling skin Negative feedback loop Figure 3 -3 Page 50 Slide 24
Homeostatic control of temperature animation. Click to view animation. Animation Slide 25
Total energy remains constant animation. Click to view animation. Animation Slide 26
st 1 Law of Thermodynamics Slide 27

Example of mechanical work animation. Click to view animation. Animation Slide 28
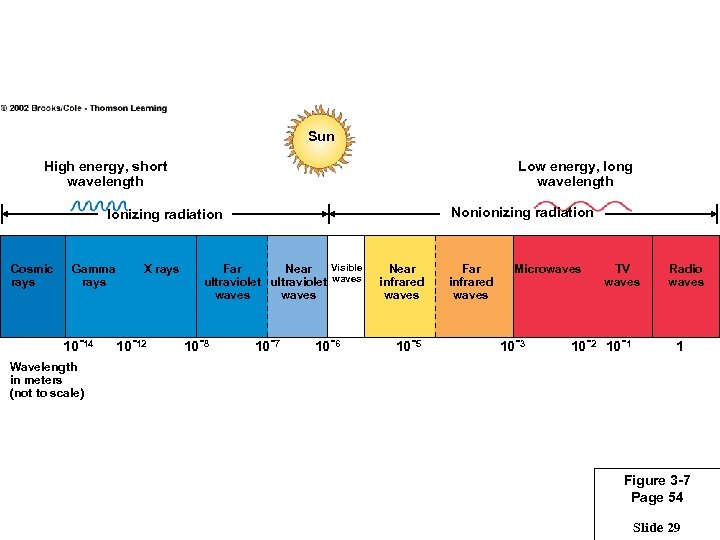
Sun High energy, short wavelength Low energy, long wavelength Nonionizing radiation Ionizing radiation Cosmic rays Gamma rays 10 -14 X rays 10 -12 Visible Far Near ultraviolet waves 10 -8 10 -7 10 -6 Near infrared waves 10 -5 Far infrared waves Microwaves 10 -3 TV waves 10 -2 10 -1 Radio waves 1 Wavelength in meters (not to scale) Figure 3 -7 Page 54 Slide 29
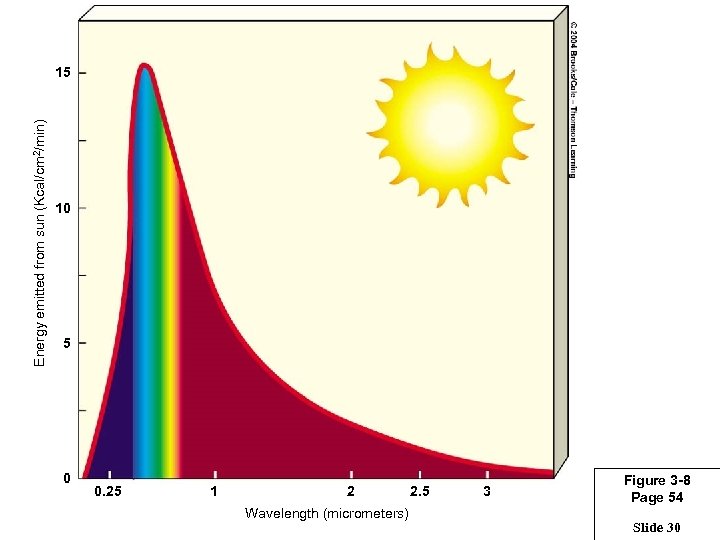
Energy emitted from sun (Kcal/cm 2/min) 15 10 5 0 0. 25 1 2 Wavelength (micrometers) 2. 5 3 Figure 3 -8 Page 54 Slide 30
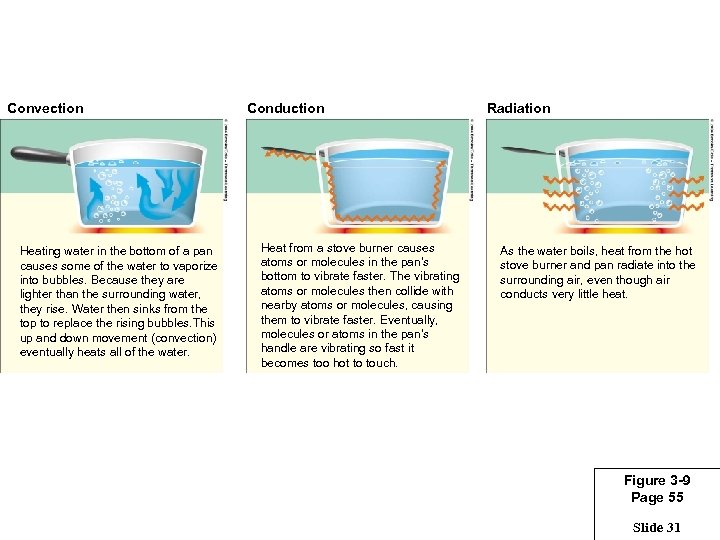
Convection Heating water in the bottom of a pan causes some of the water to vaporize into bubbles. Because they are lighter than the surrounding water, they rise. Water then sinks from the top to replace the rising bubbles. This up and down movement (convection) eventually heats all of the water. Conduction Heat from a stove burner causes atoms or molecules in the pan’s bottom to vibrate faster. The vibrating atoms or molecules then collide with nearby atoms or molecules, causing them to vibrate faster. Eventually, molecules or atoms in the pan’s handle are vibrating so fast it becomes too hot to touch. Radiation As the water boils, heat from the hot stove burner and pan radiate into the surrounding air, even though air conducts very little heat. Figure 3 -9 Page 55 Slide 31
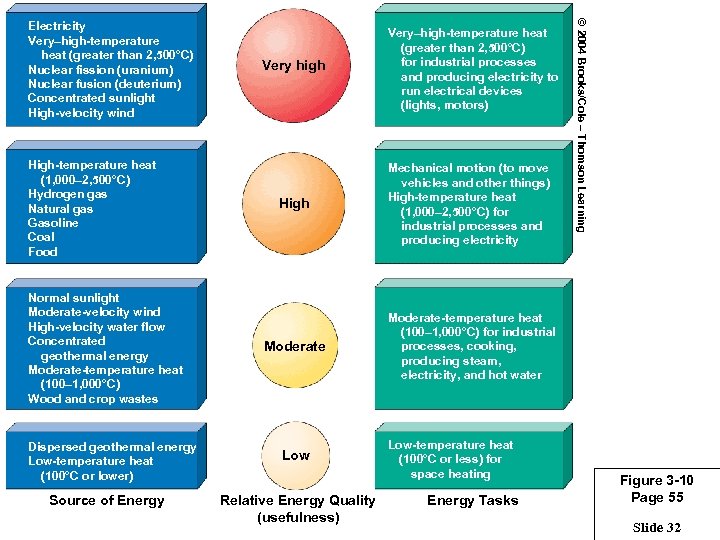
High-temperature heat (1, 000– 2, 500°C) Hydrogen gas Natural gas Gasoline Coal Food Normal sunlight Moderate-velocity wind High-velocity water flow Concentrated geothermal energy Moderate-temperature heat (100– 1, 000°C) Wood and crop wastes Dispersed geothermal energy Low-temperature heat (100°C or lower) Source of Energy Very high Very–high-temperature heat (greater than 2, 500°C) for industrial processes and producing electricity to run electrical devices (lights, motors) High Mechanical motion (to move vehicles and other things) High-temperature heat (1, 000– 2, 500°C) for industrial processes and producing electricity Moderate © 2004 Brooks/Cole – Thomson Learning Electricity Very–high-temperature heat (greater than 2, 500°C) Nuclear fission (uranium) Nuclear fusion (deuterium) Concentrated sunlight High-velocity wind Moderate-temperature heat (100– 1, 000°C) for industrial processes, cooking, producing steam, electricity, and hot water Low Relative Energy Quality (usefulness) Low-temperature heat (100°C or less) for space heating Energy Tasks Figure 3 -10 Page 55 Slide 32
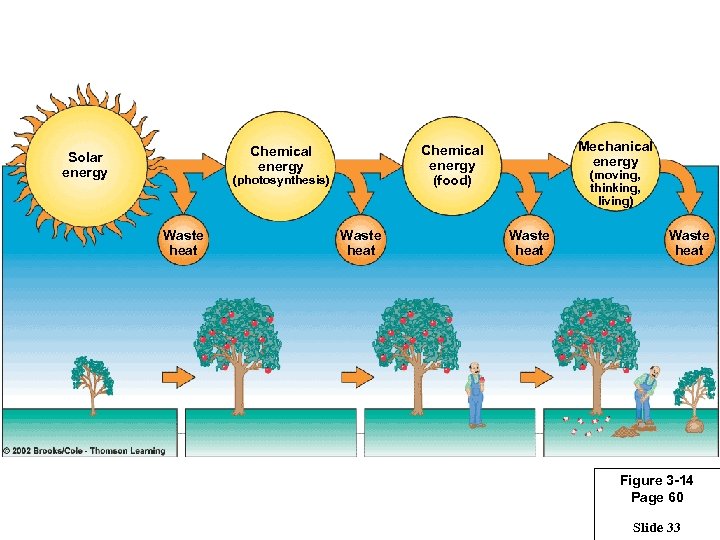
(photosynthesis) Waste heat Mechanical energy Chemical energy (food) Chemical energy Solar energy Waste heat (moving, thinking, living) Waste heat Figure 3 -14 Page 60 Slide 33
2 nd Law of Thermodynamics Slide 34
Energy flow animation. Click to view animation. Animation Slide 35
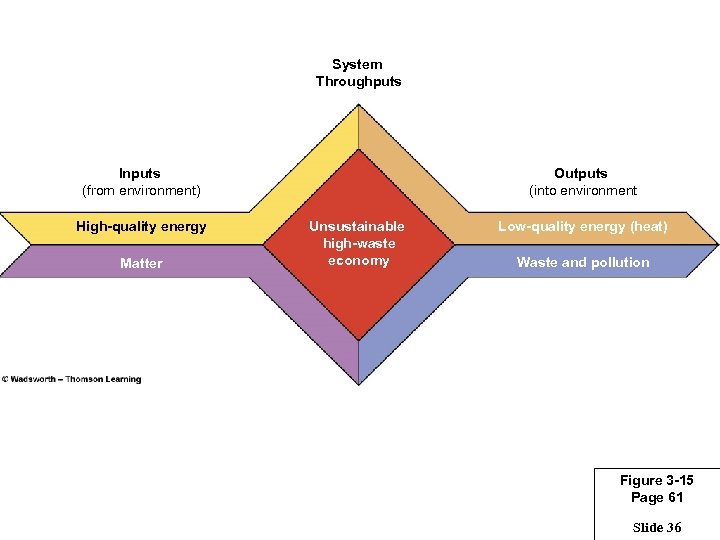
System Throughputs Inputs (from environment) High-quality energy Matter Outputs (into environment Unsustainable high-waste economy Low-quality energy (heat) Waste and pollution Figure 3 -15 Page 61 Slide 36
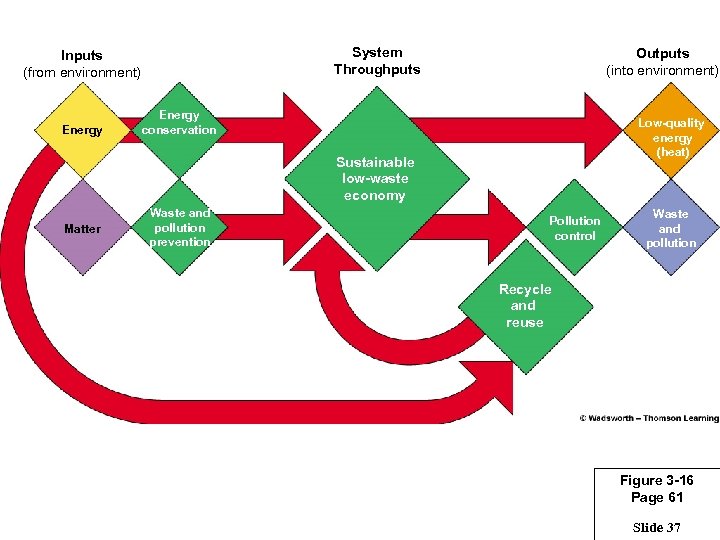
System Throughputs Inputs (from environment) Energy Outputs (into environment) Energy conservation Low-quality energy (heat) Sustainable low-waste economy Matter Waste and pollution prevention Pollution control Waste and pollution Recycle and reuse Figure 3 -16 Page 61 Slide 37
f86acae3c9bc377b2418c374f453952b.ppt