7a3661752d8fed6fb20f7e1b2ceb89e2.ppt
- Количество слайдов: 59
2. Potentials, Kinetics, and d Orbitals: Featuring E and EC mechanisms Note to alanah: importance Of the fast electron transfer Effect of iron aqueous for -Ubiquitous – total iron Environment, aquated etc
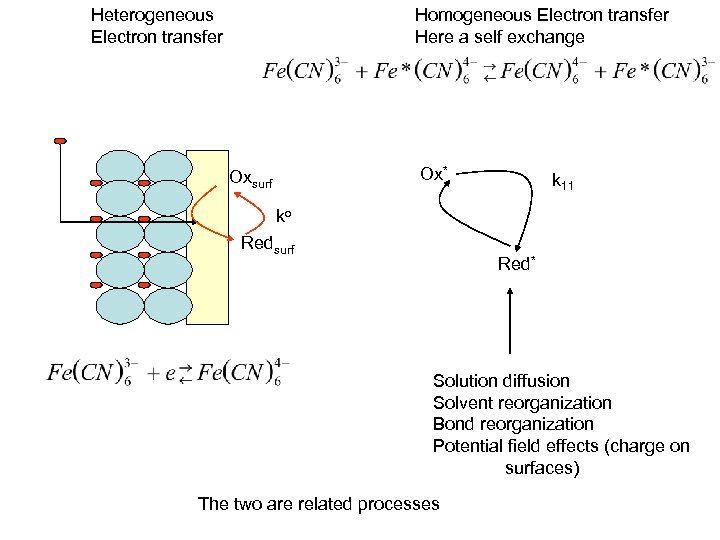
Heterogeneous Electron transfer Homogeneous Electron transfer Here a self exchange Ox* Oxsurf k 11 ko Redsurf Red* Solution diffusion Solvent reorganization Bond reorganization Potential field effects (charge on surfaces) The two are related processes
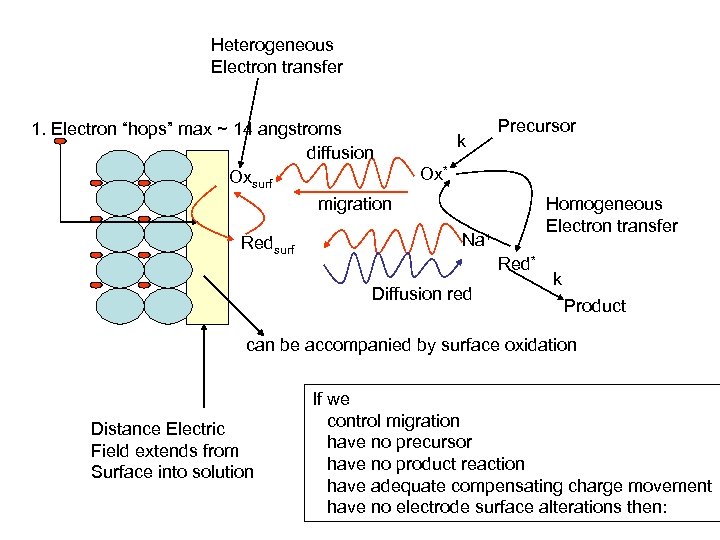
Heterogeneous Electron transfer 1. Electron “hops” max ~ 14 angstroms diffusion Oxsurf migration Redsurf k Precursor Ox* Homogeneous Electron transfer Na+ Red* Diffusion red k Product can be accompanied by surface oxidation Distance Electric Field extends from Surface into solution If we control migration have no precursor have no product reaction have adequate compensating charge movement have no electrode surface alterations then:
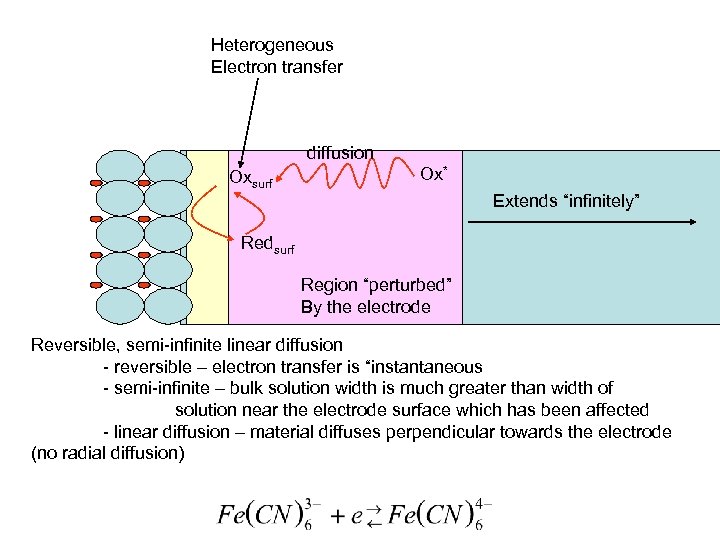
Heterogeneous Electron transfer diffusion Oxsurf Ox* Extends “infinitely” Redsurf Region “perturbed” By the electrode Reversible, semi-infinite linear diffusion - reversible – electron transfer is “instantaneous - semi-infinite – bulk solution width is much greater than width of solution near the electrode surface which has been affected - linear diffusion – material diffuses perpendicular towards the electrode (no radial diffusion)
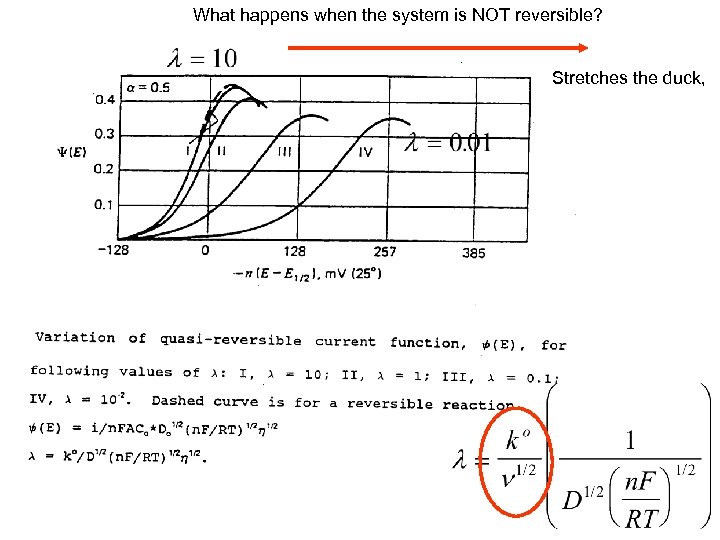
What happens when the system is NOT reversible? Stretches the duck,
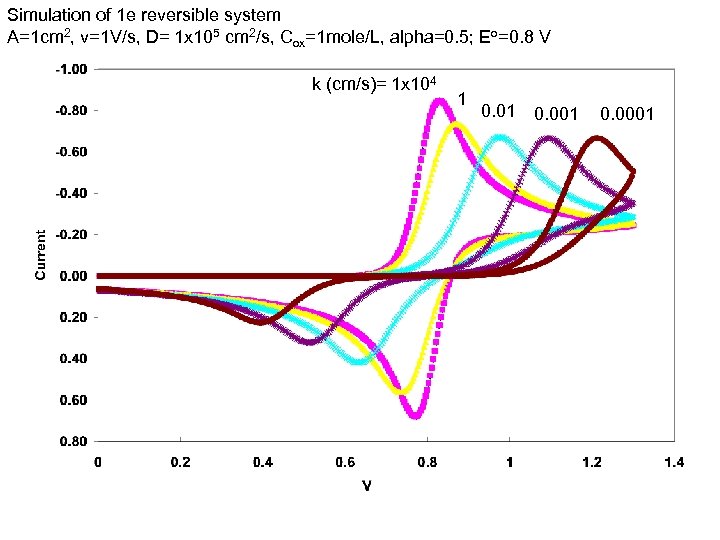
Simulation of 1 e reversible system A=1 cm 2, v=1 V/s, D= 1 x 105 cm 2/s, Cox=1 mole/L, alpha=0. 5; Eo=0. 8 V k (cm/s)= 1 x 104 1 0. 001 0. 0001

What controls whether or not the electron Transfer is “reversible”? Use Example Metal Complex Chemistry
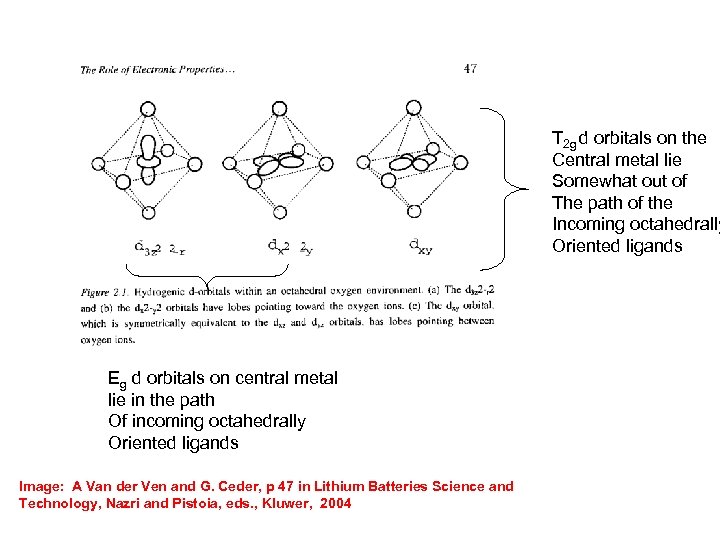
T 2 g d orbitals on the Central metal lie Somewhat out of The path of the Incoming octahedrally Oriented ligands Eg d orbitals on central metal lie in the path Of incoming octahedrally Oriented ligands Image: A Van der Ven and G. Ceder, p 47 in Lithium Batteries Science and Technology, Nazri and Pistoia, eds. , Kluwer, 2004
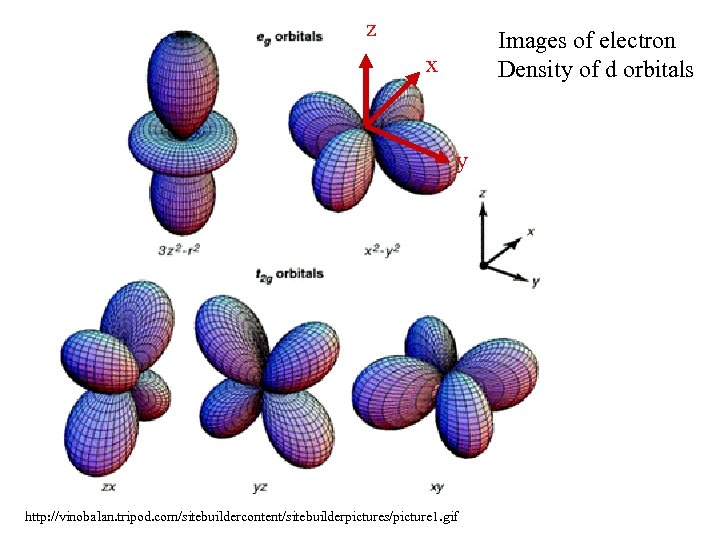
z Images of electron Density of d orbitals x y http: //vinobalan. tripod. com/sitebuildercontent/sitebuilderpictures/picture 1. gif
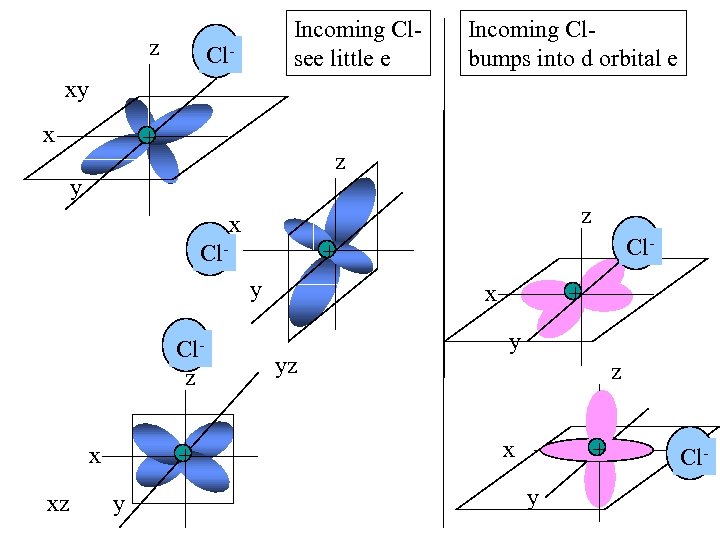
z Incoming Clsee little e Cl- Incoming Clbumps into d orbital e xy x + z y z x y Clz x xz + y Cl- + Cl- x yz + y z + x y Cl-

1. 2. 3. 4. 5. Initially all orbitals same energy Incoming anion interacts with d orbitals Energy levels change due to the interaction 3 orbitals move down in energy 2 move up z Cl- xy x + y z z Cl- x + y Cl- y + x + y z

T 2 g d orbitals on the Central metal lie Somewhat out of The path of the Incoming octahedrally Oriented ligands Eg d orbitals on central metal lie in the path Of incoming octahedrally Oriented ligands Image: A Van der Ven and G. Ceder, p 47 in Lithium Batteries Science and Technology, Nazri and Pistoia, eds. , Kluwer, 2004
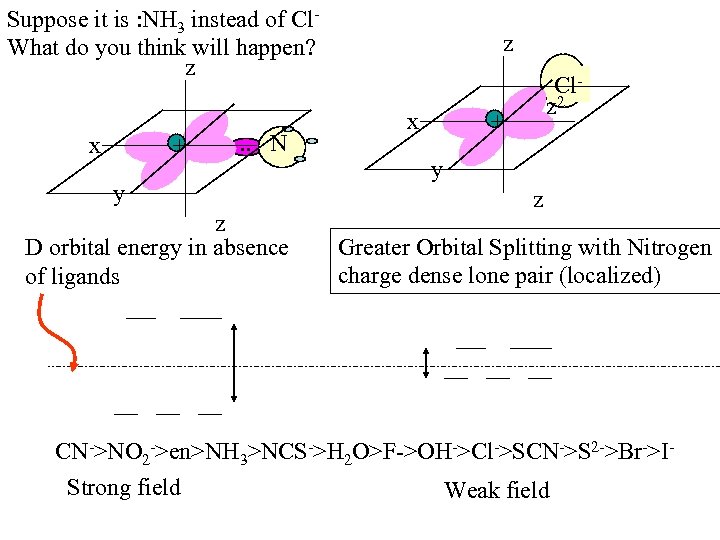
Suppose it is : NH 3 instead of Cl. What do you think will happen? z x + . . N y z D orbital energy in absence of ligands z x Clz 2 + y z Greater Orbital Splitting with Nitrogen charge dense lone pair (localized) CN->NO 2 ->en>NH 3>NCS->H 2 O>F->OH->Cl->SCN->S 2 ->Br->IStrong field Weak field
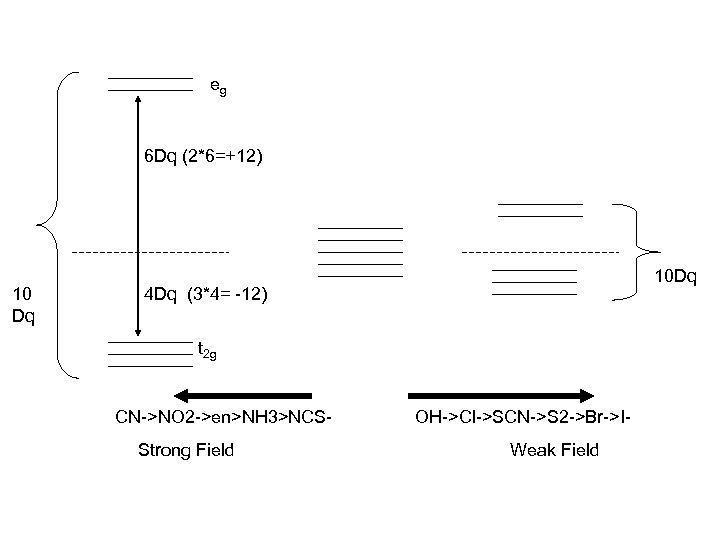
eg 6 Dq (2*6=+12) 10 Dq 10 Dq 4 Dq (3*4= -12) t 2 g CN->NO 2 ->en>NH 3>NCSStrong Field OH->Cl->SCN->S 2 ->Br->IWeak Field

Co: 3 d 74 s 2 Co 3+: 3 d 6 Co 2+: 3 d 7 Crystal Field Splitting also Affects the self exchange rate eg 6 Dq 4 Dq t 2 g CN->NO 2 ->en>NH 3>NCSStrong Field OH->Cl->SCN->S 2 ->Br->IWeak Field
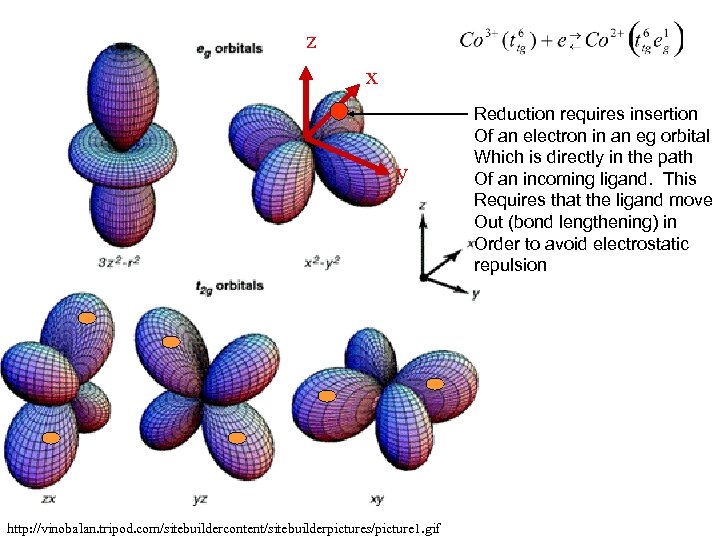
z x y http: //vinobalan. tripod. com/sitebuildercontent/sitebuilderpictures/picture 1. gif Reduction requires insertion Of an electron in an eg orbital Which is directly in the path Of an incoming ligand. This Requires that the ligand move Out (bond lengthening) in Order to avoid electrostatic repulsion
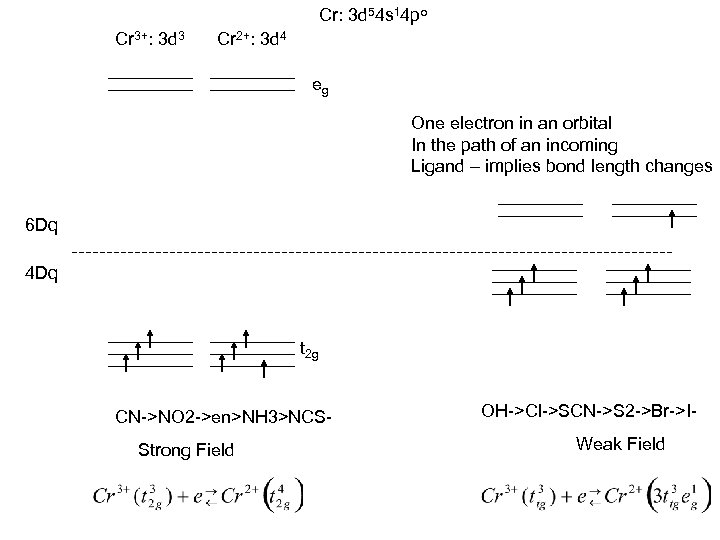
Cr: 3 d 54 s 14 po Cr 3+: 3 d 3 Cr 2+: 3 d 4 eg One electron in an orbital In the path of an incoming Ligand – implies bond length changes 6 Dq 4 Dq t 2 g CN->NO 2 ->en>NH 3>NCSStrong Field OH->Cl->SCN->S 2 ->Br->IWeak Field
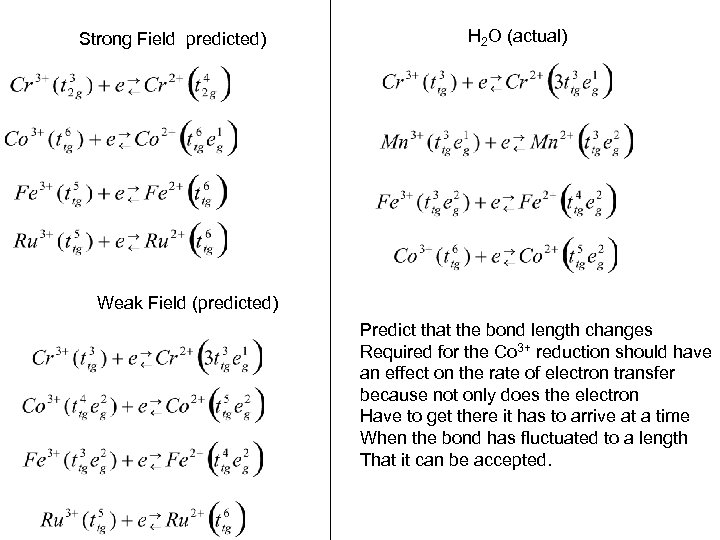
Strong Field predicted) H 2 O (actual) Weak Field (predicted) Predict that the bond length changes Required for the Co 3+ reduction should have an effect on the rate of electron transfer because not only does the electron Have to get there it has to arrive at a time When the bond has fluctuated to a length That it can be accepted.
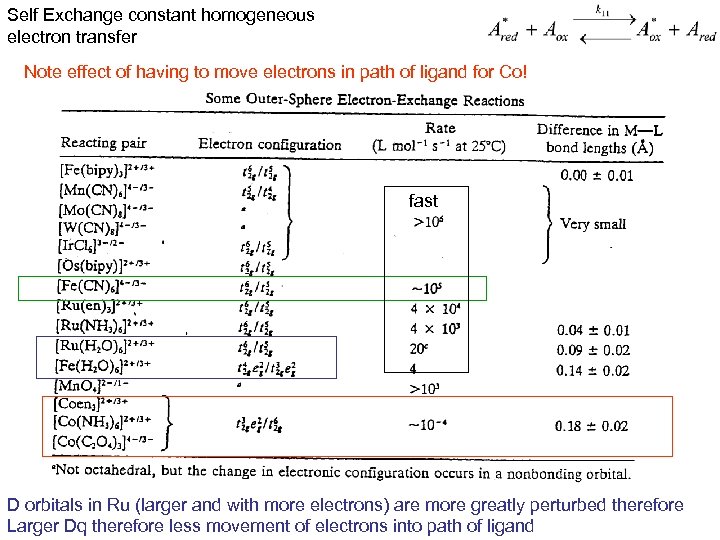
Self Exchange constant homogeneous electron transfer Note effect of having to move electrons in path of ligand for Co! fast D orbitals in Ru (larger and with more electrons) are more greatly perturbed therefore Larger Dq therefore less movement of electrons into path of ligand
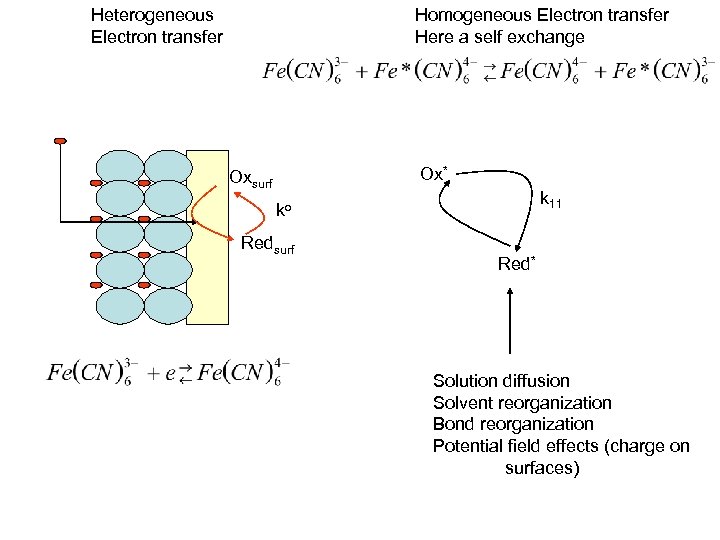
Heterogeneous Electron transfer Homogeneous Electron transfer Here a self exchange Ox* Oxsurf k 11 ko Redsurf Red* Solution diffusion Solvent reorganization Bond reorganization Potential field effects (charge on surfaces)
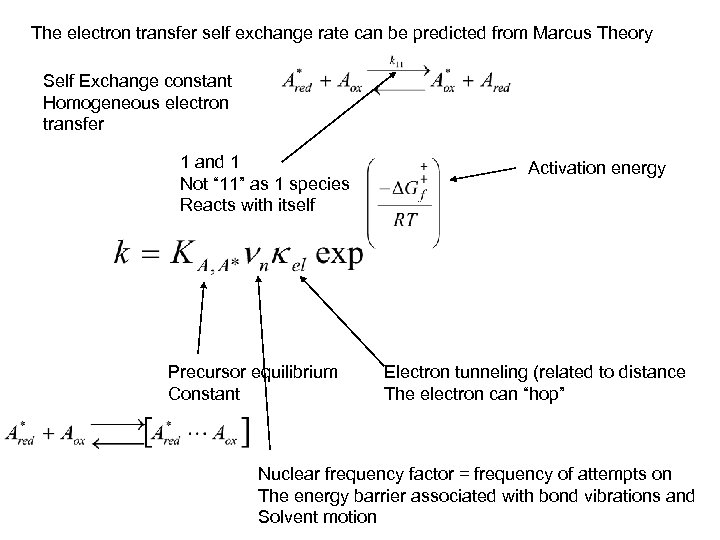
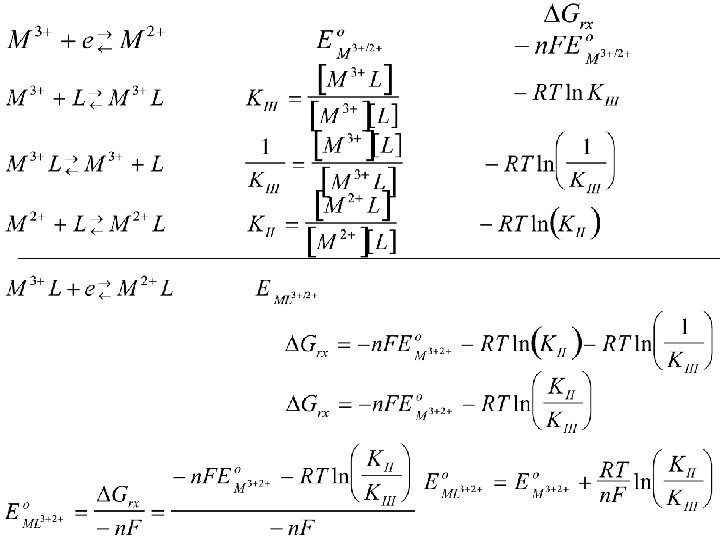
The electron transfer self exchange rate can be predicted from Marcus Theory Self Exchange constant Homogeneous electron transfer 1 and 1 Not “ 11” as 1 species Reacts with itself Precursor equilibrium Constant Activation energy Electron tunneling (related to distance The electron can “hop” Nuclear frequency factor = frequency of attempts on The energy barrier associated with bond vibrations and Solvent motion
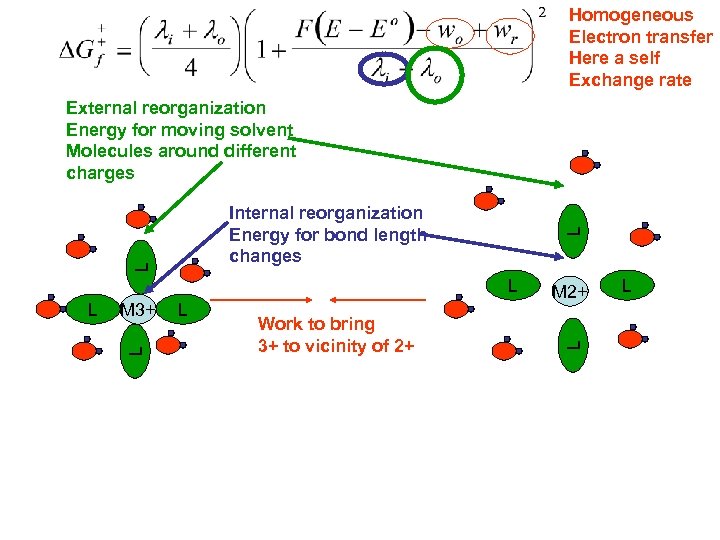
Homogeneous Electron transfer Here a self Exchange rate External reorganization Energy for moving solvent Molecules around different charges L L M 3+ L L L Work to bring 3+ to vicinity of 2+ M 2+ L L Internal reorganization Energy for bond length changes L
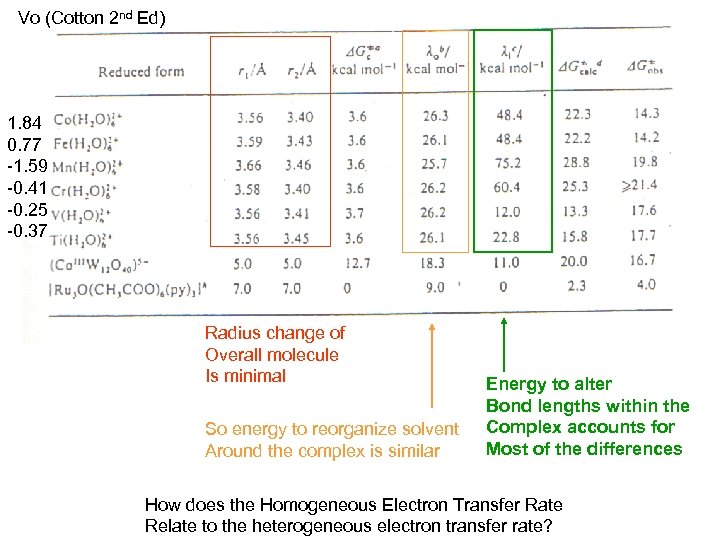
Vo (Cotton 2 nd Ed) 1. 84 0. 77 -1. 59 -0. 41 -0. 25 -0. 37 Radius change of Overall molecule Is minimal So energy to reorganize solvent Around the complex is similar Energy to alter Bond lengths within the Complex accounts for Most of the differences How does the Homogeneous Electron Transfer Rate Relate to the heterogeneous electron transfer rate?
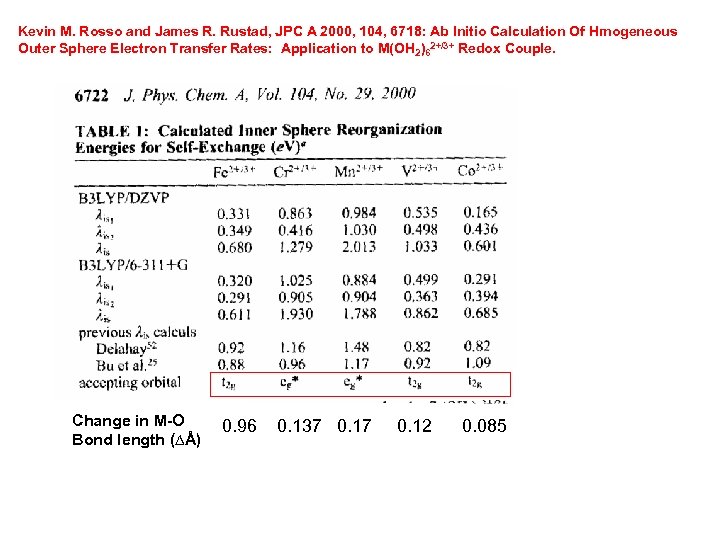
Kevin M. Rosso and James R. Rustad, JPC A 2000, 104, 6718: Ab Initio Calculation Of Hmogeneous Outer Sphere Electron Transfer Rates: Application to M(OH 2)62+/3+ Redox Couple. Change in M-O Bond length (Ɓ) 0. 96 0. 137 0. 12 0. 085
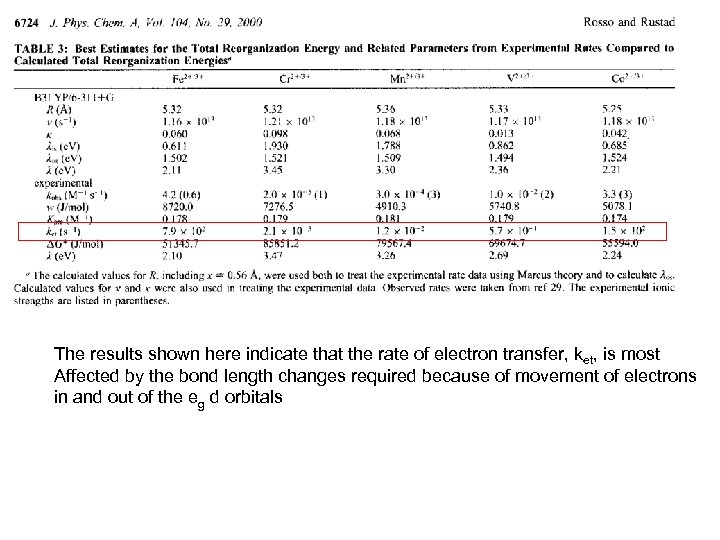
The results shown here indicate that the rate of electron transfer, ket, is most Affected by the bond length changes required because of movement of electrons in and out of the eg d orbitals

An extreme example of the inner sphere work required is to reduce an iron containing crystal Charge transport in micas: The kinetics of Fe. II/III electron transfer in the octahedral sheet, Keven M. Rosso and Eugene S. Ilton, . J of Chemical Physics, 119, 17, 9207 Hydroxyl group at “waist” (cis) Hydroxyl group at apex (trans) Calculate the self exchange Of Fe. II to Fe. III from the M 1 And M 2 sites using a cluster
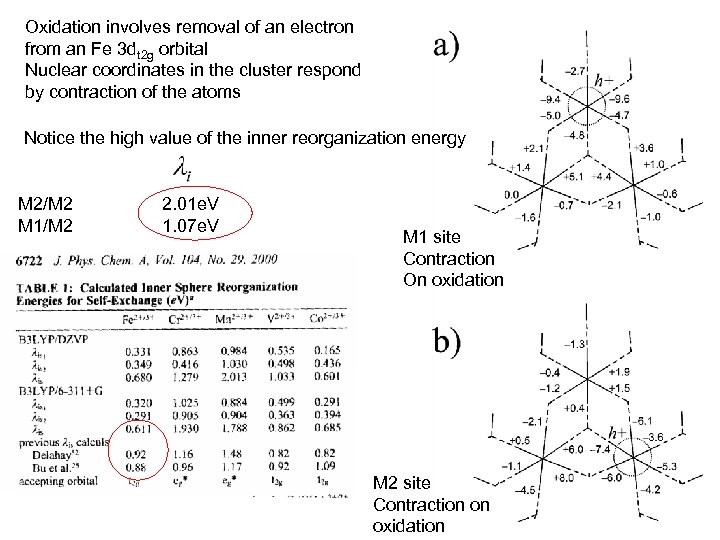
Oxidation involves removal of an electron from an Fe 3 dt 2 g orbital Nuclear coordinates in the cluster respond by contraction of the atoms Notice the high value of the inner reorganization energy M 2/M 2 M 1/M 2 2. 01 e. V 1. 07 e. V M 1 site Contraction On oxidation M 2 site Contraction on oxidation
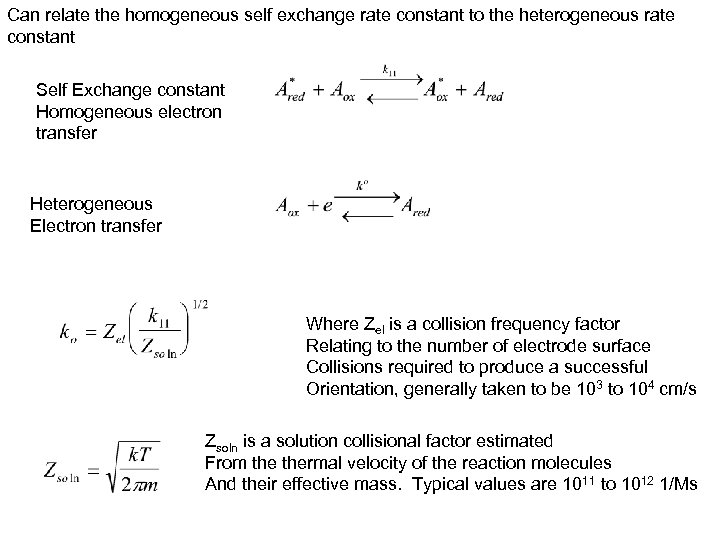
Can relate the homogeneous self exchange rate constant to the heterogeneous rate constant Self Exchange constant Homogeneous electron transfer Heterogeneous Electron transfer Where Zel is a collision frequency factor Relating to the number of electrode surface Collisions required to produce a successful Orientation, generally taken to be 103 to 104 cm/s Zsoln is a solution collisional factor estimated From thermal velocity of the reaction molecules And their effective mass. Typical values are 1011 to 1012 1/Ms
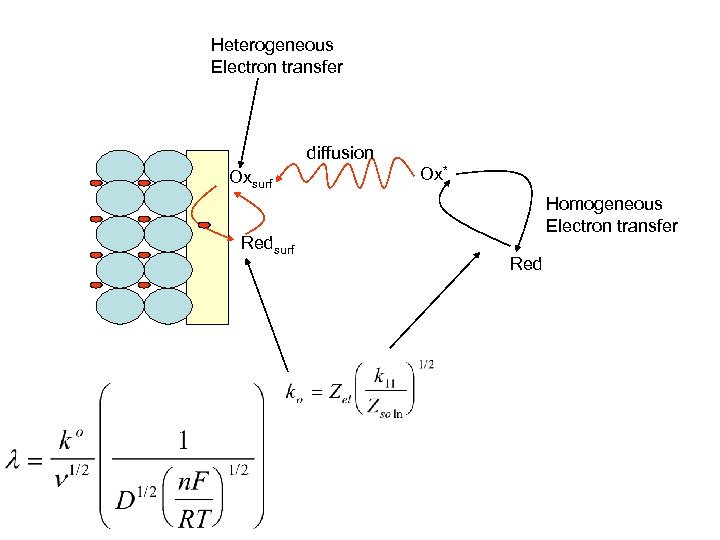
Heterogeneous Electron transfer diffusion Oxsurf Redsurf Ox* Homogeneous Electron transfer Red
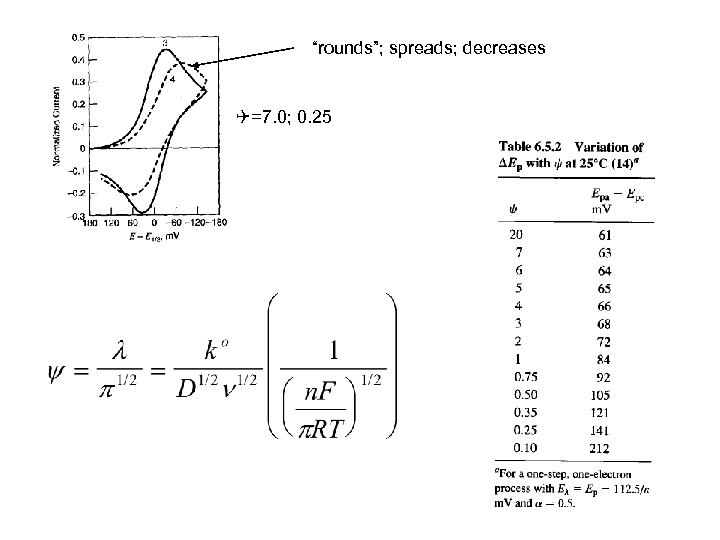
“rounds”; spreads; decreases =7. 0; 0. 25
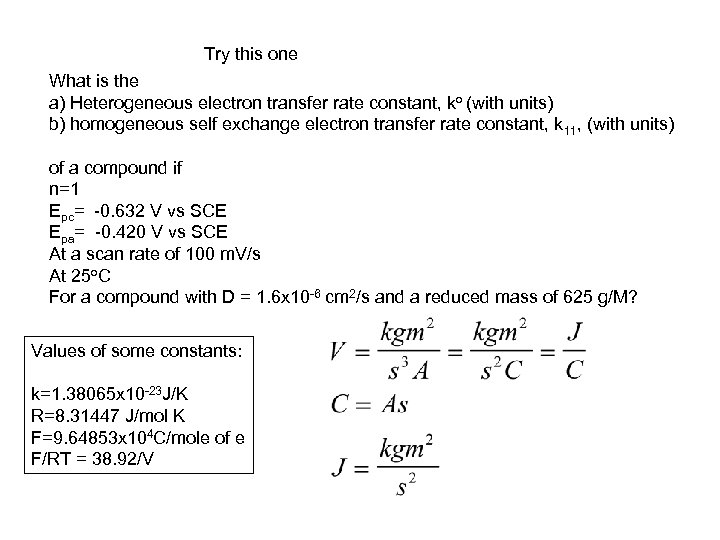
Try this one What is the a) Heterogeneous electron transfer rate constant, ko (with units) b) homogeneous self exchange electron transfer rate constant, k 11, (with units) of a compound if n=1 Epc= -0. 632 V vs SCE Epa= -0. 420 V vs SCE At a scan rate of 100 m. V/s At 25 o. C For a compound with D = 1. 6 x 10 -6 cm 2/s and a reduced mass of 625 g/M? Values of some constants: k=1. 38065 x 10 -23 J/K R=8. 31447 J/mol K F=9. 64853 x 104 C/mole of e F/RT = 38. 92/V
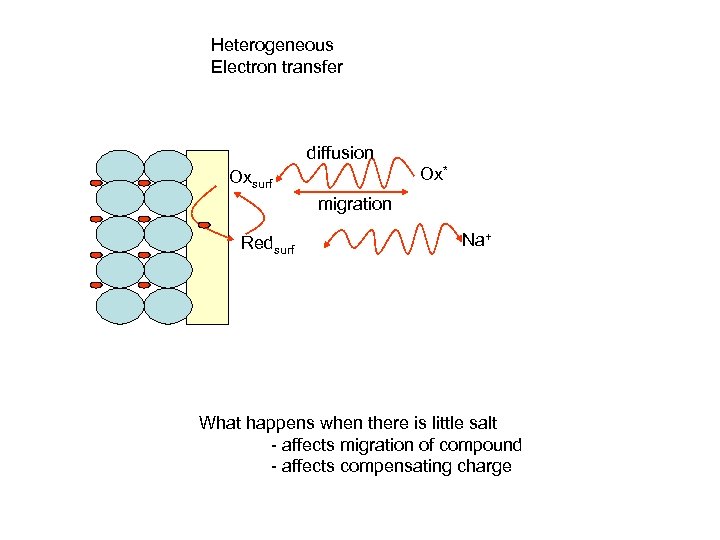
Heterogeneous Electron transfer diffusion Ox* Oxsurf migration Redsurf Na+ What happens when there is little salt - affects migration of compound - affects compensating charge
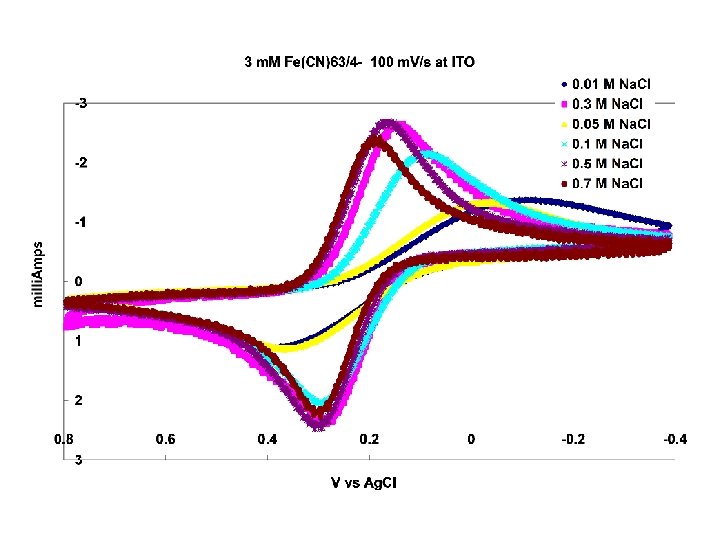
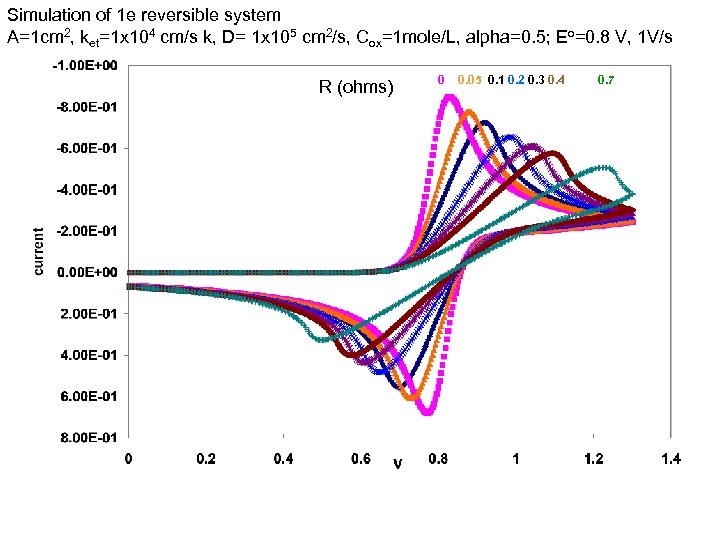
Simulation of 1 e reversible system A=1 cm 2, ket=1 x 104 cm/s k, D= 1 x 105 cm 2/s, Cox=1 mole/L, alpha=0. 5; Eo=0. 8 V, 1 V/s R (ohms) 0 0. 05 0. 1 0. 2 0. 3 0. 4 0. 7
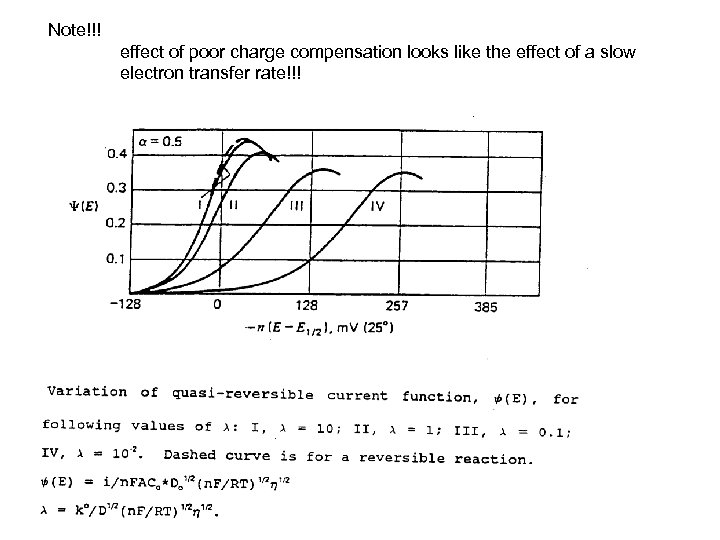
Note!!! effect of poor charge compensation looks like the effect of a slow electron transfer rate!!!
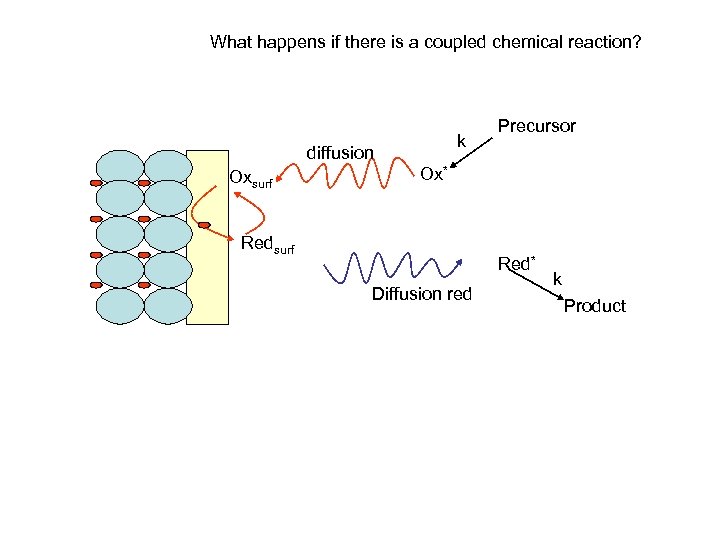
What happens if there is a coupled chemical reaction? k diffusion Oxsurf Precursor Ox* Redsurf Red* Diffusion red k Product
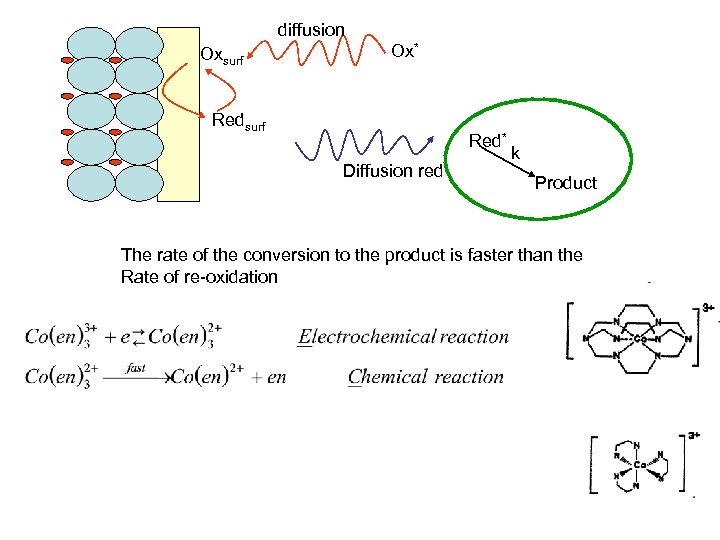
diffusion Oxsurf Ox* Redsurf Red* Diffusion red k Product The rate of the conversion to the product is faster than the Rate of re-oxidation
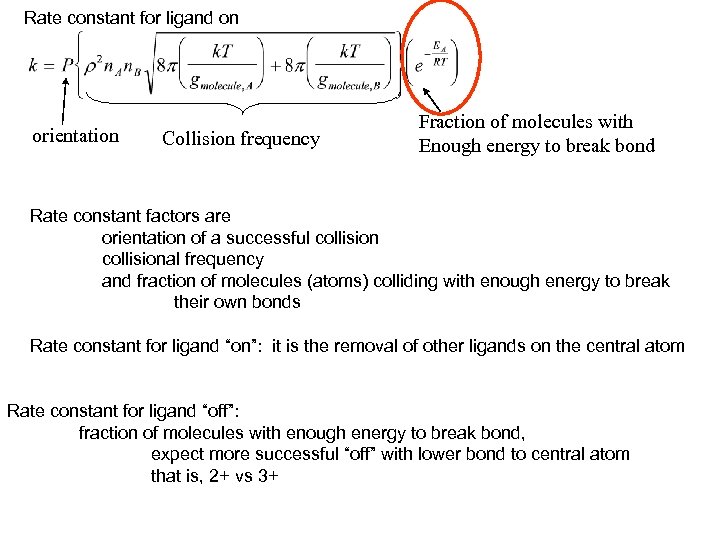
Rate constant for ligand on orientation Collision frequency Fraction of molecules with Enough energy to break bond Rate constant factors are orientation of a successful collisional frequency and fraction of molecules (atoms) colliding with enough energy to break their own bonds Rate constant for ligand “on”: it is the removal of other ligands on the central atom Rate constant for ligand “off”: fraction of molecules with enough energy to break bond, expect more successful “off” with lower bond to central atom that is, 2+ vs 3+
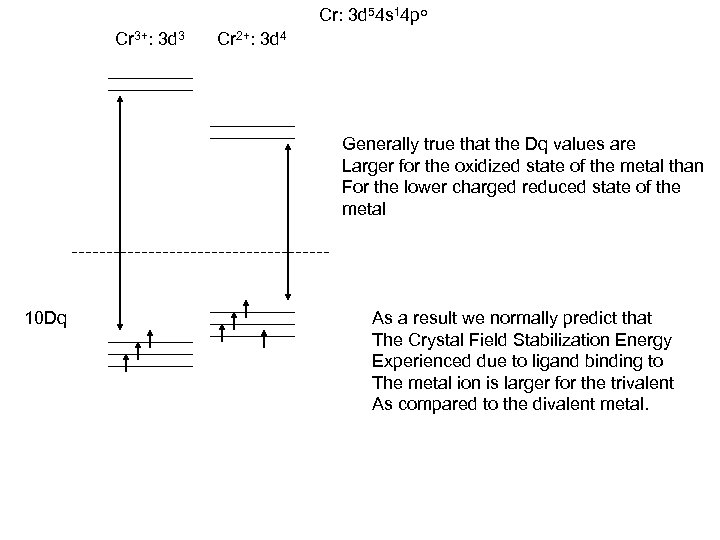
Cr: 3 d 54 s 14 po Cr 3+: 3 d 3 Cr 2+: 3 d 4 Generally true that the Dq values are Larger for the oxidized state of the metal than For the lower charged reduced state of the metal 10 Dq As a result we normally predict that The Crystal Field Stabilization Energy Experienced due to ligand binding to The metal ion is larger for the trivalent As compared to the divalent metal.
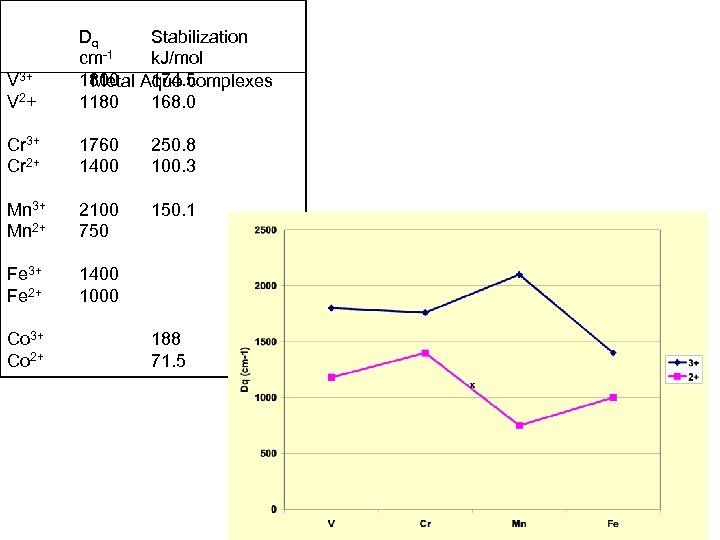
V 3+ V 2+ Dq Stabilization cm-1 k. J/mol 1800 Aquo complexes Metal 174. 5 1180 168. 0 Cr 3+ Cr 2+ 1760 1400 250. 8 100. 3 Mn 3+ Mn 2+ 2100 750 150. 1 Fe 3+ Fe 2+ 1400 1000 Co 3+ Co 2+ 188 71. 5
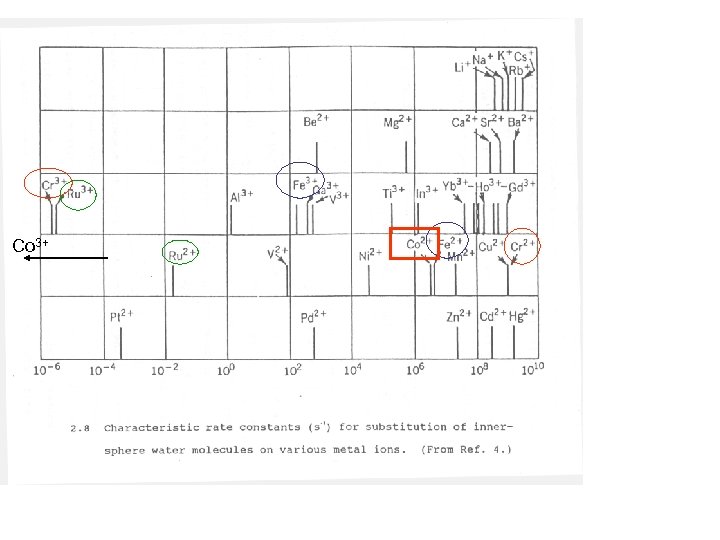
Co 3+
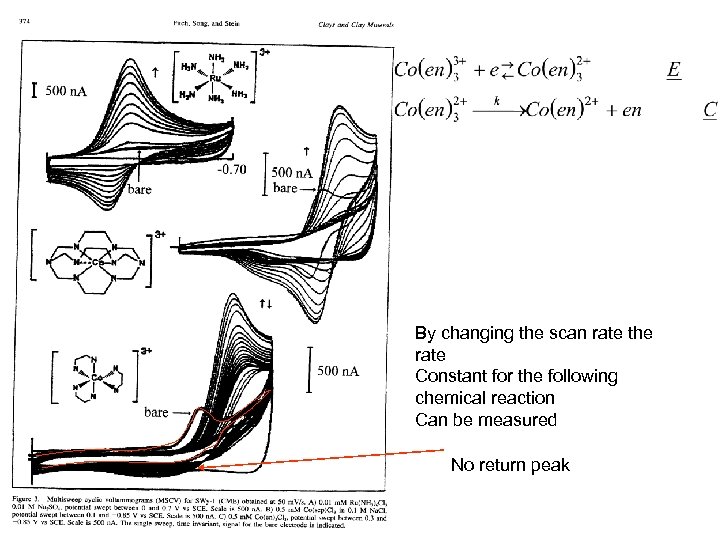
By changing the scan rate the rate Constant for the following chemical reaction Can be measured No return peak
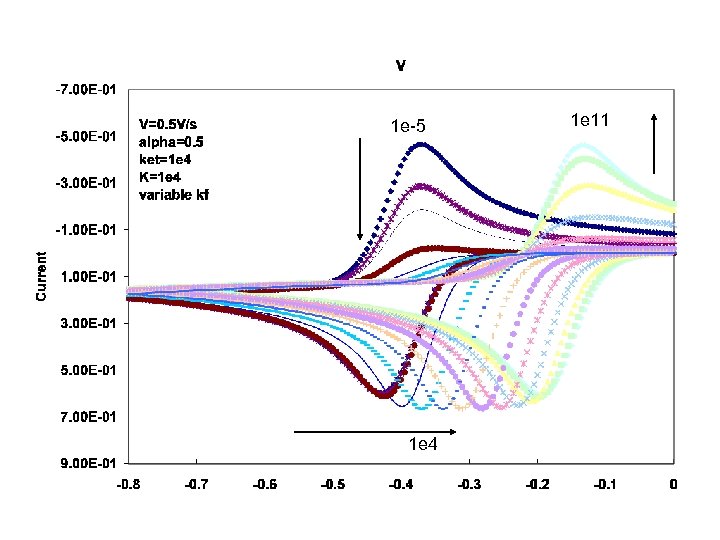
1 e-5 1 e 4 1 e 11
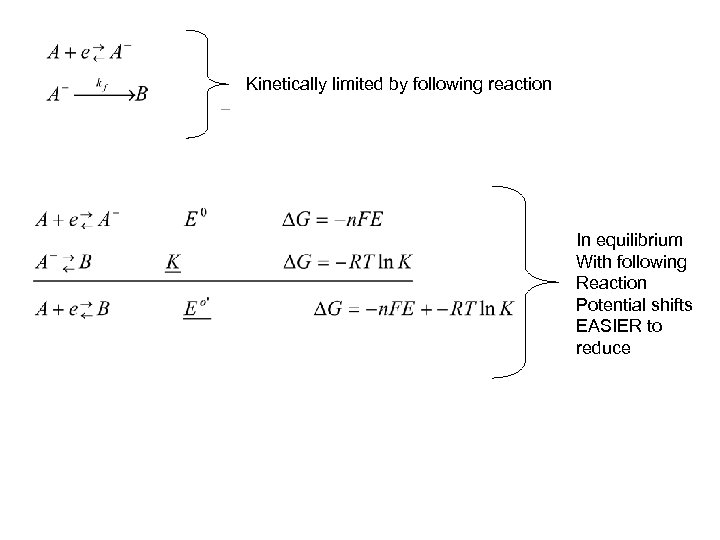
Kinetically limited by following reaction In equilibrium With following Reaction Potential shifts EASIER to reduce
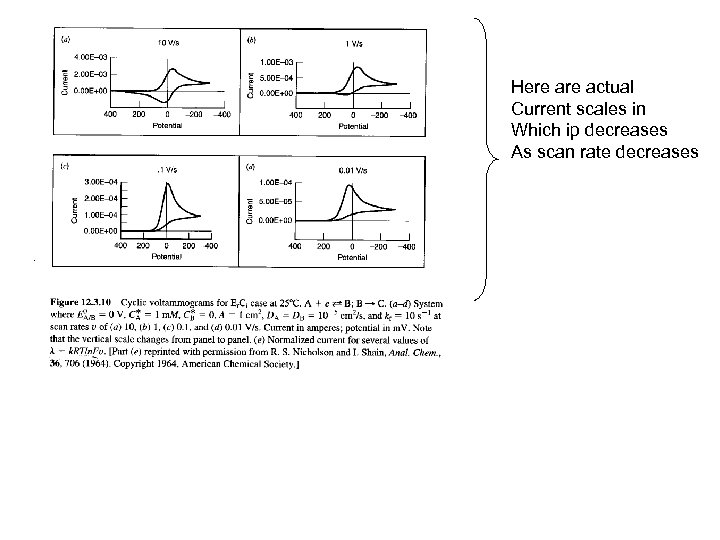
Here actual Current scales in Which ip decreases As scan rate decreases
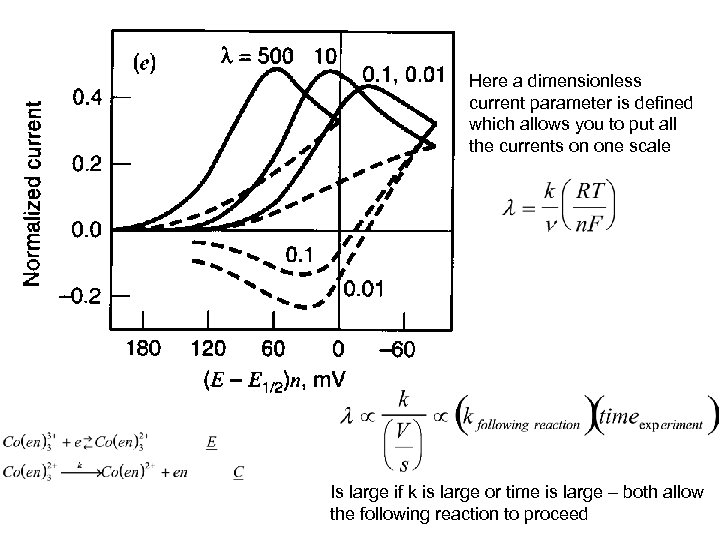
Here a dimensionless current parameter is defined which allows you to put all the currents on one scale Is large if k is large or time is large – both allow the following reaction to proceed
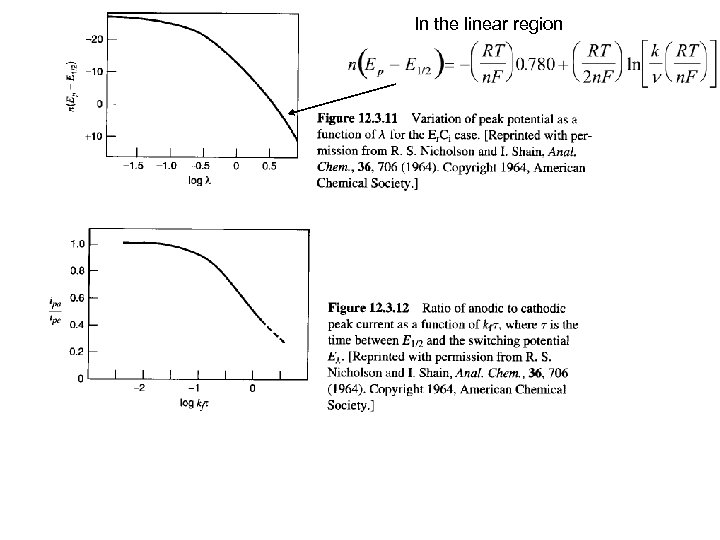
In the linear region
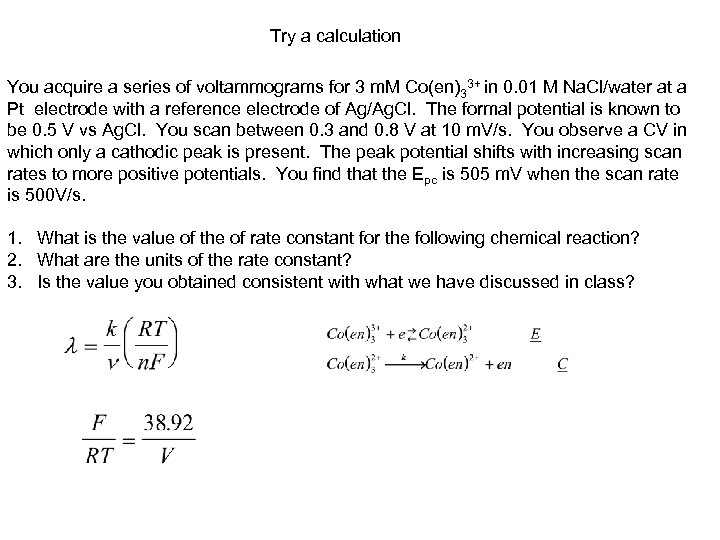
Try a calculation You acquire a series of voltammograms for 3 m. M Co(en)33+ in 0. 01 M Na. Cl/water at a Pt electrode with a reference electrode of Ag/Ag. Cl. The formal potential is known to be 0. 5 V vs Ag. Cl. You scan between 0. 3 and 0. 8 V at 10 m. V/s. You observe a CV in which only a cathodic peak is present. The peak potential shifts with increasing scan rates to more positive potentials. You find that the Epc is 505 m. V when the scan rate is 500 V/s. 1. What is the value of the of rate constant for the following chemical reaction? 2. What are the units of the rate constant? 3. Is the value you obtained consistent with what we have discussed in class?
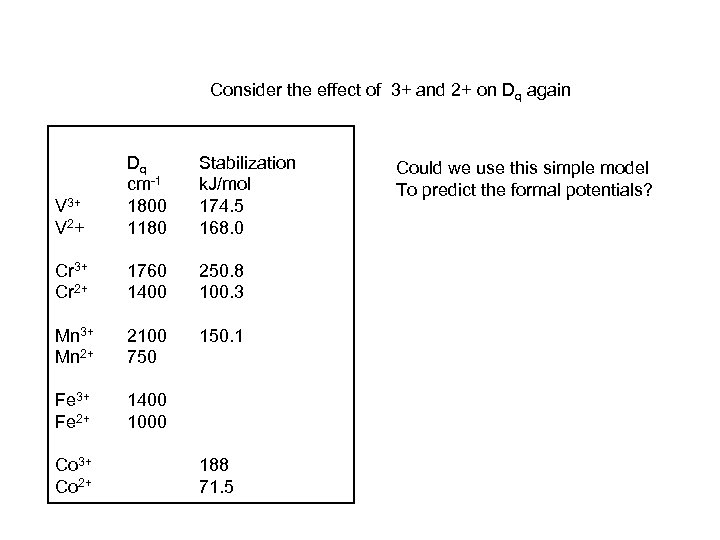
Consider the effect of 3+ and 2+ on Dq again V 3+ V 2+ Dq cm-1 1800 1180 Stabilization k. J/mol 174. 5 168. 0 Cr 3+ Cr 2+ 1760 1400 250. 8 100. 3 Mn 3+ Mn 2+ 2100 750 150. 1 Fe 3+ Fe 2+ 1400 1000 Co 3+ Co 2+ 188 71. 5 Could we use this simple model To predict the formal potentials?
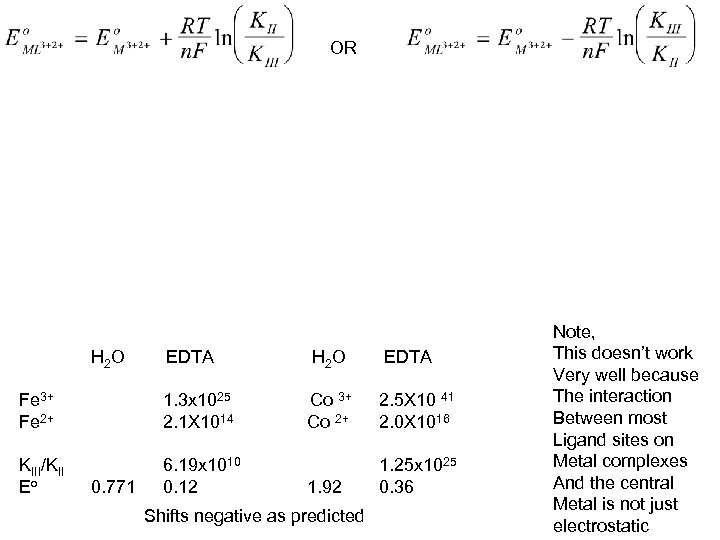
OR H 2 O EDTA H 2 O EDTA Fe 3+ Fe 2+ 1. 3 x 1025 2. 1 X 1014 Co 3+ Co 2+ 2. 5 X 10 41 2. 0 X 1016 KIII/KII Eo 6. 19 x 1010 0. 12 1. 92 1. 25 x 1025 0. 36 0. 771 Shifts negative as predicted Note, This doesn’t work Very well because The interaction Between most Ligand sites on Metal complexes And the central Metal is not just electrostatic

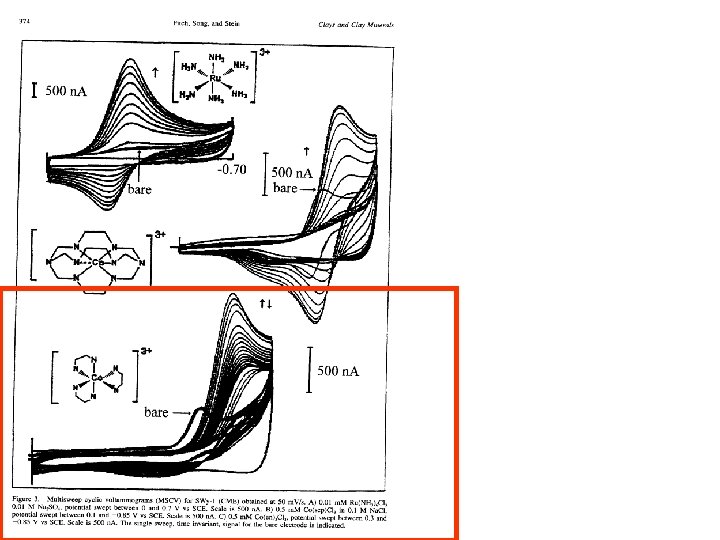
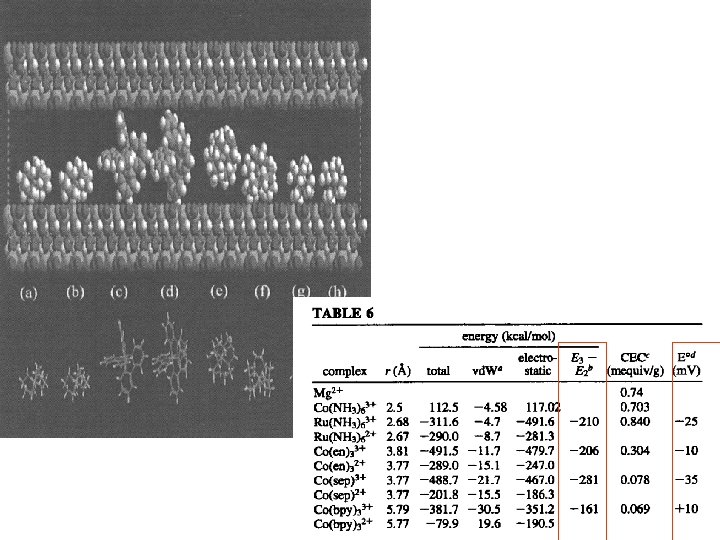
Electrostatic Charge Effects Show up In other places Example is the potential shift of a cobalt sepulchrate complex When ion exchanged into an anionic film
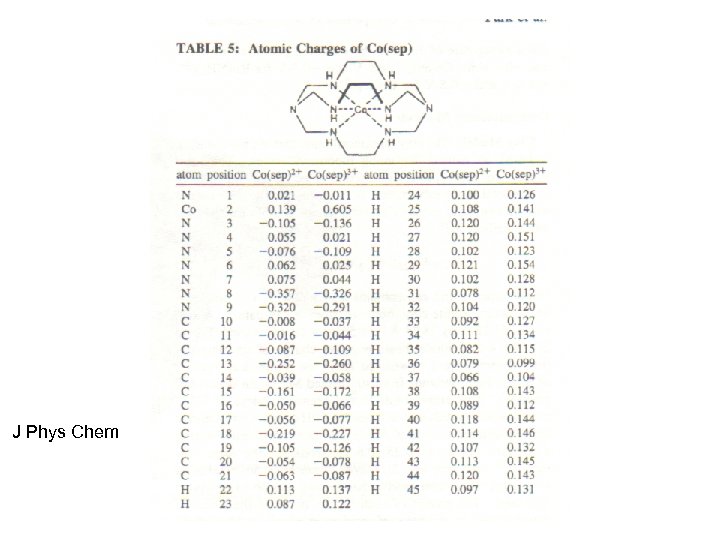
J Phys Chem
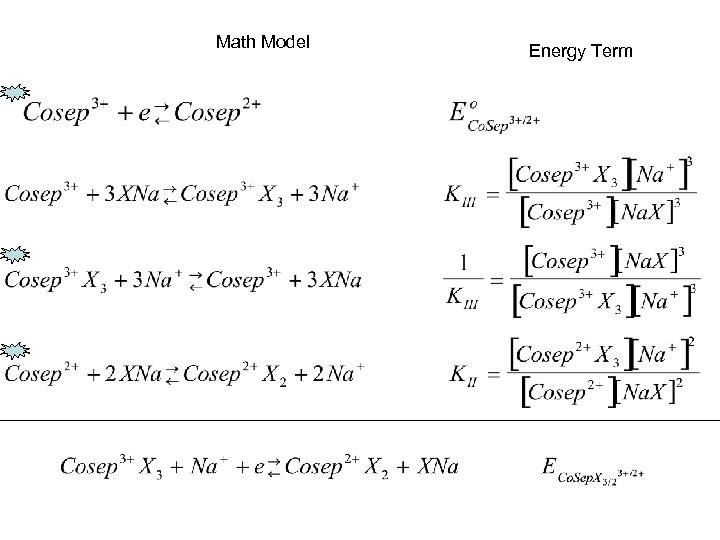
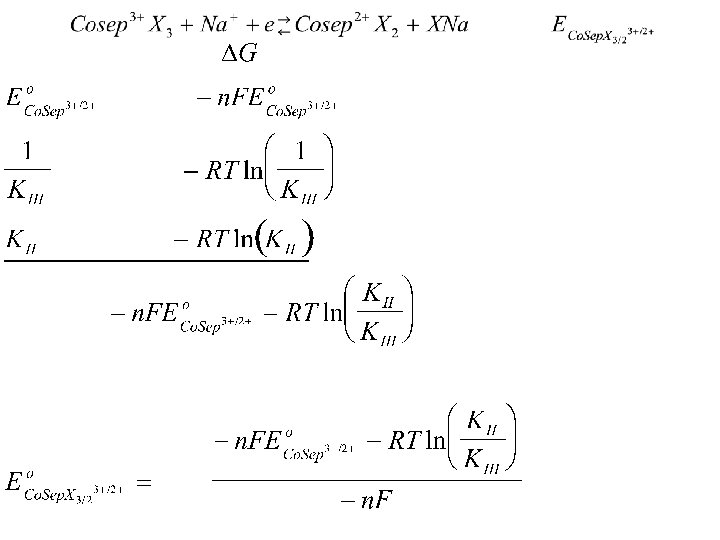
Math Model Energy Term
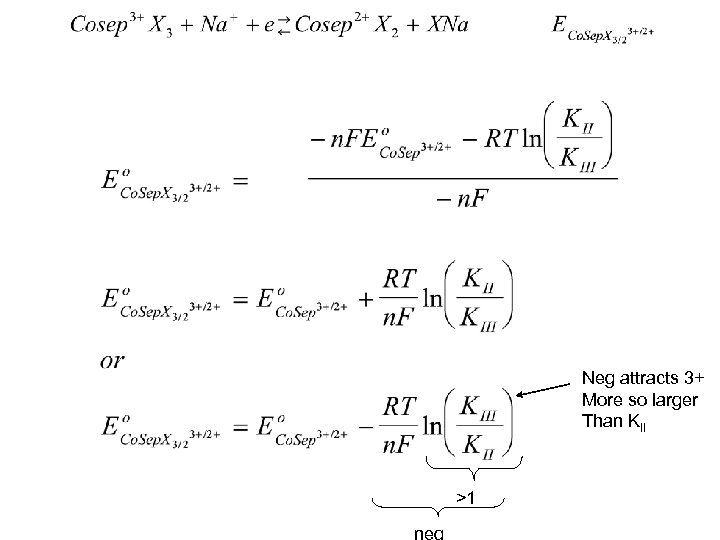
Neg attracts 3+ More so larger Than KII >1 neg
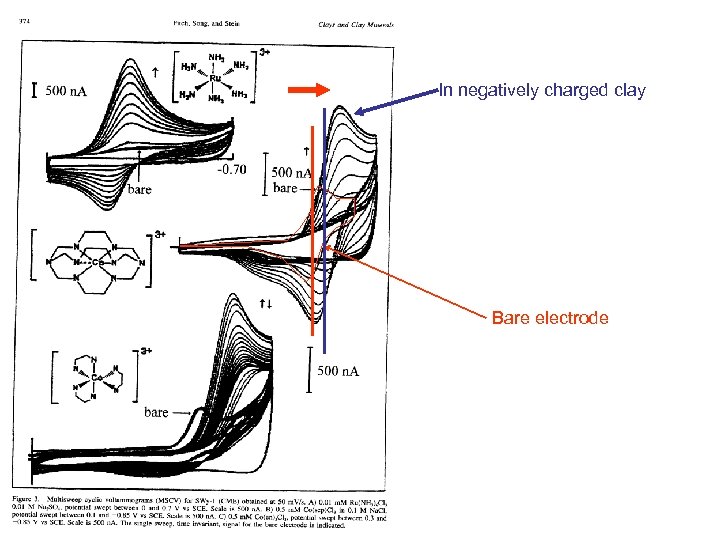
In negatively charged clay Bare electrode
7a3661752d8fed6fb20f7e1b2ceb89e2.ppt