8865b87d27fa55184c2bdad1f41e71c0.ppt
- Количество слайдов: 19
 13 th National Neonatal Screening Symposium: Poster Session March 2 - 4, 1998 A MODIFICATION OF AN IN VITRO FLUOROMETRIC MICROASSAY FOR THE DETERMINATION OF GALACTOSE-1 -PHOSPHATE FROM DRIED BLOOD SPOTS FROM NEONATES T. G. Deck, R. West, Arkansas Department of Health, J. B. Gibson, Arkansas Children’s Hospital, Little Rock, AR, USA and M. Rosenthal, Isolab, Inc. Akron, OH, USA. A D H Arkansas Department of Health Division of Public Health Laboratories 1/30/98
13 th National Neonatal Screening Symposium: Poster Session March 2 - 4, 1998 A MODIFICATION OF AN IN VITRO FLUOROMETRIC MICROASSAY FOR THE DETERMINATION OF GALACTOSE-1 -PHOSPHATE FROM DRIED BLOOD SPOTS FROM NEONATES T. G. Deck, R. West, Arkansas Department of Health, J. B. Gibson, Arkansas Children’s Hospital, Little Rock, AR, USA and M. Rosenthal, Isolab, Inc. Akron, OH, USA. A D H Arkansas Department of Health Division of Public Health Laboratories 1/30/98
 Introduction u Galactosemia is a toxicity syndrome associated with an intolerance to dietary galactose. This recessively inherited disorder is most often caused by a deficiency of the enzyme galactose-1 phosphate uridyl transferase (GALT). GALT Galactose-1 -phosphate + UDP-glucose u u u UDP-galactose + Glucose-1 -phosphate Epimerization of the hydroxyl group at the carbon-4 position on the galactose structure is the main pathway to glucose. The reaction catalyzed by galactose-1 -phosphate uridyl transferase converts galactose-1 -phosphate plus UDP-glucose to UDP-galactose plus glucose-1 -phosphate. The UDP-galactose can be metabolized to UDP plus glucose-1 -phosphate. Therefore, humans are capable of metabolizing large amounts of galactose. However, when the transferase is deficient, galactose may be reduced to galactitol or oxidized to galactonate. The accumulation of these products has direct toxic effects and results in the clinical manifestation of galactosemia. Dietary control of the disease in the first few years of life can result in normal growth and development. [1]
Introduction u Galactosemia is a toxicity syndrome associated with an intolerance to dietary galactose. This recessively inherited disorder is most often caused by a deficiency of the enzyme galactose-1 phosphate uridyl transferase (GALT). GALT Galactose-1 -phosphate + UDP-glucose u u u UDP-galactose + Glucose-1 -phosphate Epimerization of the hydroxyl group at the carbon-4 position on the galactose structure is the main pathway to glucose. The reaction catalyzed by galactose-1 -phosphate uridyl transferase converts galactose-1 -phosphate plus UDP-glucose to UDP-galactose plus glucose-1 -phosphate. The UDP-galactose can be metabolized to UDP plus glucose-1 -phosphate. Therefore, humans are capable of metabolizing large amounts of galactose. However, when the transferase is deficient, galactose may be reduced to galactitol or oxidized to galactonate. The accumulation of these products has direct toxic effects and results in the clinical manifestation of galactosemia. Dietary control of the disease in the first few years of life can result in normal growth and development. [1]
 Materials and Methods u u u Neonatal samples were assayed at the Arkansas Department of Health (ADH), Newborn Screening Laboratory, Little Rock, AR, USA. The addition of an AP-free test to the NCS software allowed measurement of total galactose and free galactose in one sample plate. Isolab’s (Akron, OH) Neonatal Chemistry System (NCS) total galactose assay, which is based on galactose oxidase methodology[2] , and a modified assay for GAL were run in the sample plate. Isolab’s galactose-1 -phosphate uridyl transferase (GALT) assay [3] was also used in these studies. The GALT determination was performed using the NCS: in our experience, the mean + SD for “normals” using this system is 7. 8 +1. 8 U/g. Hb.
Materials and Methods u u u Neonatal samples were assayed at the Arkansas Department of Health (ADH), Newborn Screening Laboratory, Little Rock, AR, USA. The addition of an AP-free test to the NCS software allowed measurement of total galactose and free galactose in one sample plate. Isolab’s (Akron, OH) Neonatal Chemistry System (NCS) total galactose assay, which is based on galactose oxidase methodology[2] , and a modified assay for GAL were run in the sample plate. Isolab’s galactose-1 -phosphate uridyl transferase (GALT) assay [3] was also used in these studies. The GALT determination was performed using the NCS: in our experience, the mean + SD for “normals” using this system is 7. 8 +1. 8 U/g. Hb.
 Materials and Methods (Cont’d) u u One 1/8”(3 mm) disk was punched from an initial DBS sample collected on Schleicher & Schuell 903 filter paper. Blood spot controls and calibrators were obtained from Isolab, Inc. (Akron, Ohio, USA). External blood spot controls were obtained from CDC (Atlanta, Georgia, USA). The procedure for Isolab’s total galactose assay[4] was used substituting Isolab’s APfree GAL reagent or in-house AP-free GAL reagent[5] in the place of the normal reagent in appropriate wells. GAL Assay
Materials and Methods (Cont’d) u u One 1/8”(3 mm) disk was punched from an initial DBS sample collected on Schleicher & Schuell 903 filter paper. Blood spot controls and calibrators were obtained from Isolab, Inc. (Akron, Ohio, USA). External blood spot controls were obtained from CDC (Atlanta, Georgia, USA). The procedure for Isolab’s total galactose assay[4] was used substituting Isolab’s APfree GAL reagent or in-house AP-free GAL reagent[5] in the place of the normal reagent in appropriate wells. GAL Assay
 Materials and Methods (Cont’d) u u u This study used 27, 101 initial screening samples collected from January 2, 1996 to September 30, 1996, and 34, 672 initial samples collected from November 1, 1996 to September 30, 1997. Samples were initially tested on Isolab’s Neonatal Chemistry System within two weeks of collection. Samples were stored at -20°C with desiccant in a air-tight bag. The plates were read in a Fluorscan II fluorescent plate reader (excitation 320 nm and emission 405 nm) controlled by Isolab’s Neonatal Chemistry Software. GAL-1 -P was calculated as the difference between total GAL and free GAL.
Materials and Methods (Cont’d) u u u This study used 27, 101 initial screening samples collected from January 2, 1996 to September 30, 1996, and 34, 672 initial samples collected from November 1, 1996 to September 30, 1997. Samples were initially tested on Isolab’s Neonatal Chemistry System within two weeks of collection. Samples were stored at -20°C with desiccant in a air-tight bag. The plates were read in a Fluorscan II fluorescent plate reader (excitation 320 nm and emission 405 nm) controlled by Isolab’s Neonatal Chemistry Software. GAL-1 -P was calculated as the difference between total GAL and free GAL.
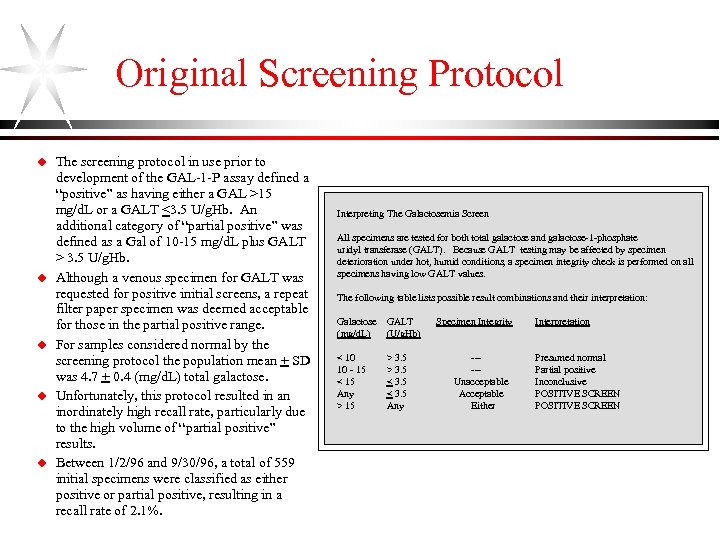 Original Screening Protocol u u u The screening protocol in use prior to development of the GAL-1 -P assay defined a “positive” as having either a GAL >15 mg/d. L or a GALT <3. 5 U/g. Hb. An additional category of “partial positive” was defined as a Gal of 10 -15 mg/d. L plus GALT > 3. 5 U/g. Hb. Although a venous specimen for GALT was requested for positive initial screens, a repeat filter paper specimen was deemed acceptable for those in the partial positive range. For samples considered normal by the screening protocol the population mean + SD was 4. 7 + 0. 4 (mg/d. L) total galactose. Unfortunately, this protocol resulted in an inordinately high recall rate, particularly due to the high volume of “partial positive” results. Between 1/2/96 and 9/30/96, a total of 559 initial specimens were classified as either positive or partial positive, resulting in a recall rate of 2. 1%. Interpreting The Galactosemia Screen All specimens are tested for both total galactose and galactose-1 -phosphate uridyl transferase (GALT). Because GALT testing may be affected by specimen deterioration under hot, humid conditions, a specimen integrity check is performed on all specimens having low GALT values. The following table lists possible result combinations and their interpretation: Galactose (mg/d. L) GALT (U/g. Hb) < 10 10 - 15 < 15 Any > 15 > 3. 5 < 3. 5 Any Specimen Integrity ----Unacceptable Acceptable Either Interpretation Presumed normal Partial positive Inconclusive POSITIVE SCREEN
Original Screening Protocol u u u The screening protocol in use prior to development of the GAL-1 -P assay defined a “positive” as having either a GAL >15 mg/d. L or a GALT <3. 5 U/g. Hb. An additional category of “partial positive” was defined as a Gal of 10 -15 mg/d. L plus GALT > 3. 5 U/g. Hb. Although a venous specimen for GALT was requested for positive initial screens, a repeat filter paper specimen was deemed acceptable for those in the partial positive range. For samples considered normal by the screening protocol the population mean + SD was 4. 7 + 0. 4 (mg/d. L) total galactose. Unfortunately, this protocol resulted in an inordinately high recall rate, particularly due to the high volume of “partial positive” results. Between 1/2/96 and 9/30/96, a total of 559 initial specimens were classified as either positive or partial positive, resulting in a recall rate of 2. 1%. Interpreting The Galactosemia Screen All specimens are tested for both total galactose and galactose-1 -phosphate uridyl transferase (GALT). Because GALT testing may be affected by specimen deterioration under hot, humid conditions, a specimen integrity check is performed on all specimens having low GALT values. The following table lists possible result combinations and their interpretation: Galactose (mg/d. L) GALT (U/g. Hb) < 10 10 - 15 < 15 Any > 15 > 3. 5 < 3. 5 Any Specimen Integrity ----Unacceptable Acceptable Either Interpretation Presumed normal Partial positive Inconclusive POSITIVE SCREEN
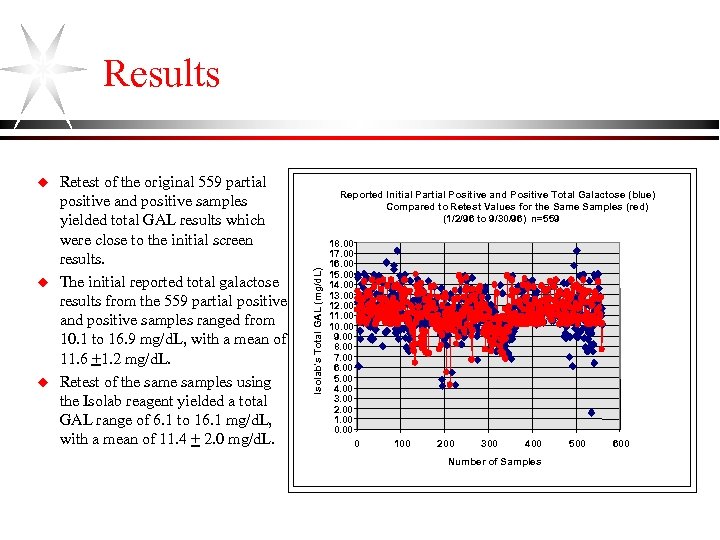 Results u u Retest of the original 559 partial positive and positive samples yielded total GAL results which were close to the initial screen results. The initial reported total galactose results from the 559 partial positive and positive samples ranged from 10. 1 to 16. 9 mg/d. L, with a mean of 11. 6 +1. 2 mg/d. L. Retest of the samples using the Isolab reagent yielded a total GAL range of 6. 1 to 16. 1 mg/d. L, with a mean of 11. 4 + 2. 0 mg/d. L. Reported Initial Partial Positive and Positive Total Galactose (blue) Compared to Retest Values for the Samples (red) (1/2/96 to 9/30/96) n=559 Isolab's Total GAL (mg/d. L) u 18. 00 17. 00 16. 00 15. 00 14. 00 13. 00 12. 00 11. 00 10. 00 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. 00 Series 1 0 100 200 300 400 Number of Samples 500 600
Results u u Retest of the original 559 partial positive and positive samples yielded total GAL results which were close to the initial screen results. The initial reported total galactose results from the 559 partial positive and positive samples ranged from 10. 1 to 16. 9 mg/d. L, with a mean of 11. 6 +1. 2 mg/d. L. Retest of the samples using the Isolab reagent yielded a total GAL range of 6. 1 to 16. 1 mg/d. L, with a mean of 11. 4 + 2. 0 mg/d. L. Reported Initial Partial Positive and Positive Total Galactose (blue) Compared to Retest Values for the Samples (red) (1/2/96 to 9/30/96) n=559 Isolab's Total GAL (mg/d. L) u 18. 00 17. 00 16. 00 15. 00 14. 00 13. 00 12. 00 11. 00 10. 00 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. 00 Series 1 0 100 200 300 400 Number of Samples 500 600
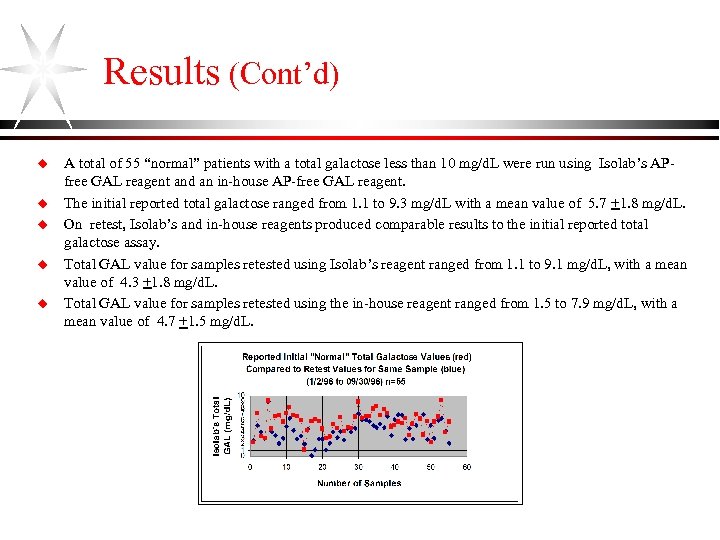 Results (Cont’d) u u u A total of 55 “normal” patients with a total galactose less than 10 mg/d. L were run using Isolab’s APfree GAL reagent and an in-house AP-free GAL reagent. The initial reported total galactose ranged from 1. 1 to 9. 3 mg/d. L with a mean value of 5. 7 +1. 8 mg/d. L. On retest, Isolab’s and in-house reagents produced comparable results to the initial reported total galactose assay. Total GAL value for samples retested using Isolab’s reagent ranged from 1. 1 to 9. 1 mg/d. L, with a mean value of 4. 3 +1. 8 mg/d. L. Total GAL value for samples retested using the in-house reagent ranged from 1. 5 to 7. 9 mg/d. L, with a mean value of 4. 7 +1. 5 mg/d. L.
Results (Cont’d) u u u A total of 55 “normal” patients with a total galactose less than 10 mg/d. L were run using Isolab’s APfree GAL reagent and an in-house AP-free GAL reagent. The initial reported total galactose ranged from 1. 1 to 9. 3 mg/d. L with a mean value of 5. 7 +1. 8 mg/d. L. On retest, Isolab’s and in-house reagents produced comparable results to the initial reported total galactose assay. Total GAL value for samples retested using Isolab’s reagent ranged from 1. 1 to 9. 1 mg/d. L, with a mean value of 4. 3 +1. 8 mg/d. L. Total GAL value for samples retested using the in-house reagent ranged from 1. 5 to 7. 9 mg/d. L, with a mean value of 4. 7 +1. 5 mg/d. L.
 Results (Cont’d) u Retest of the original 559 partial positive and positive samples using Isolab’s AP-free galactose oxidase reagent and an in-house galactose reagent yielded comparable results for both total galactose and free galactose. The sample retest values are shown below: Isolab Rgt. In-House Rgt.
Results (Cont’d) u Retest of the original 559 partial positive and positive samples using Isolab’s AP-free galactose oxidase reagent and an in-house galactose reagent yielded comparable results for both total galactose and free galactose. The sample retest values are shown below: Isolab Rgt. In-House Rgt.
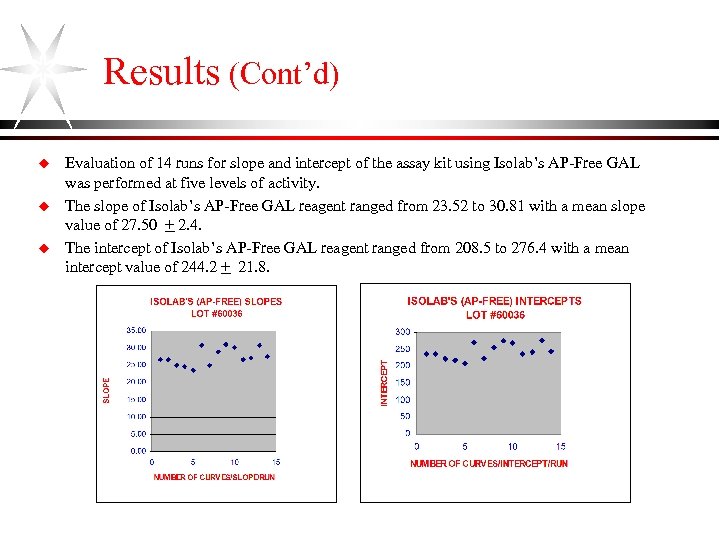 Results (Cont’d) u u u Evaluation of 14 runs for slope and intercept of the assay kit using Isolab’s AP-Free GAL was performed at five levels of activity. The slope of Isolab’s AP-Free GAL reagent ranged from 23. 52 to 30. 81 with a mean slope value of 27. 50 + 2. 4. The intercept of Isolab’s AP-Free GAL reagent ranged from 208. 5 to 276. 4 with a mean intercept value of 244. 2 + 21. 8.
Results (Cont’d) u u u Evaluation of 14 runs for slope and intercept of the assay kit using Isolab’s AP-Free GAL was performed at five levels of activity. The slope of Isolab’s AP-Free GAL reagent ranged from 23. 52 to 30. 81 with a mean slope value of 27. 50 + 2. 4. The intercept of Isolab’s AP-Free GAL reagent ranged from 208. 5 to 276. 4 with a mean intercept value of 244. 2 + 21. 8.
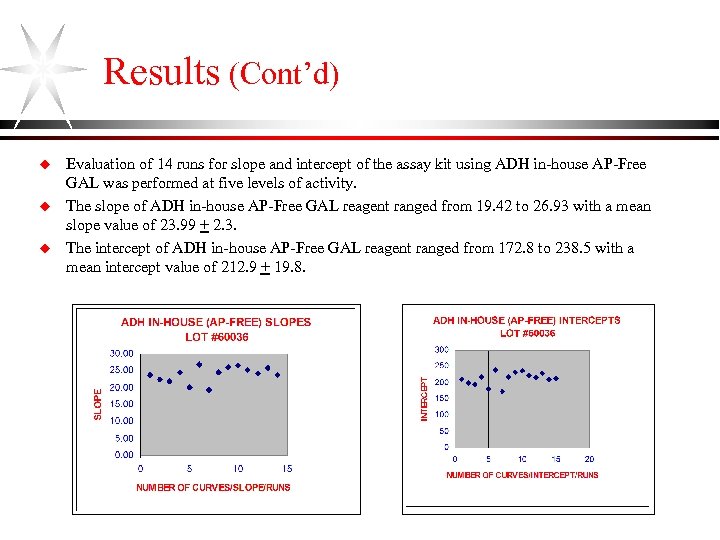 Results (Cont’d) u u u Evaluation of 14 runs for slope and intercept of the assay kit using ADH in-house AP-Free GAL was performed at five levels of activity. The slope of ADH in-house AP-Free GAL reagent ranged from 19. 42 to 26. 93 with a mean slope value of 23. 99 + 2. 3. The intercept of ADH in-house AP-Free GAL reagent ranged from 172. 8 to 238. 5 with a mean intercept value of 212. 9 + 19. 8.
Results (Cont’d) u u u Evaluation of 14 runs for slope and intercept of the assay kit using ADH in-house AP-Free GAL was performed at five levels of activity. The slope of ADH in-house AP-Free GAL reagent ranged from 19. 42 to 26. 93 with a mean slope value of 23. 99 + 2. 3. The intercept of ADH in-house AP-Free GAL reagent ranged from 172. 8 to 238. 5 with a mean intercept value of 212. 9 + 19. 8.
 Results (Cont’d) u A reproducibility study was performed on dried blood calibrators spotted on S&S 903 filter paper with concentrations of 2. 0 and 25. 8 mg/d. L. These two calibrators were run for eight days, twice a day in duplicate on the Isolab’s AP-Free GAL reagent and the in-house AP-Free GAL reagent. Results are summarized below. u The mean of CAL A were 1. 92 mg/d. L and CAL D were 25. 2 mg/d. L with the Isolab’s AP-Free GAL reagent. The mean CAL A were 1. 9 mg/d. L and CAL D were 25. 3 mg/d. L with the ADH in-house AP-Free GAL reagent. The analytical recoveries for CAL A (2. 0 mg/d. L) and CAL D (25. 8 mg/d. L) for the AP-Free GAL assays were 96% and 98% respectively. u u
Results (Cont’d) u A reproducibility study was performed on dried blood calibrators spotted on S&S 903 filter paper with concentrations of 2. 0 and 25. 8 mg/d. L. These two calibrators were run for eight days, twice a day in duplicate on the Isolab’s AP-Free GAL reagent and the in-house AP-Free GAL reagent. Results are summarized below. u The mean of CAL A were 1. 92 mg/d. L and CAL D were 25. 2 mg/d. L with the Isolab’s AP-Free GAL reagent. The mean CAL A were 1. 9 mg/d. L and CAL D were 25. 3 mg/d. L with the ADH in-house AP-Free GAL reagent. The analytical recoveries for CAL A (2. 0 mg/d. L) and CAL D (25. 8 mg/d. L) for the AP-Free GAL assays were 96% and 98% respectively. u u
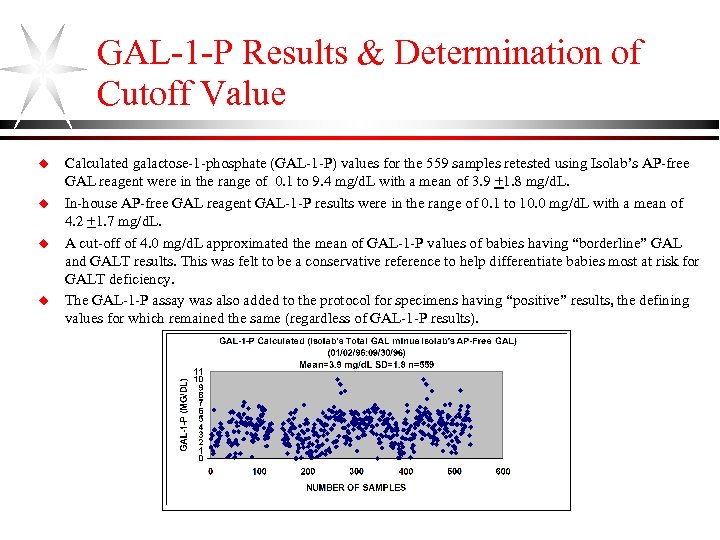 GAL-1 -P Results & Determination of Cutoff Value u u Calculated galactose-1 -phosphate (GAL-1 -P) values for the 559 samples retested using Isolab’s AP-free GAL reagent were in the range of 0. 1 to 9. 4 mg/d. L with a mean of 3. 9 +1. 8 mg/d. L. In-house AP-free GAL reagent GAL-1 -P results were in the range of 0. 1 to 10. 0 mg/d. L with a mean of 4. 2 +1. 7 mg/d. L. A cut-off of 4. 0 mg/d. L approximated the mean of GAL-1 -P values of babies having “borderline” GAL and GALT results. This was felt to be a conservative reference to help differentiate babies most at risk for GALT deficiency. The GAL-1 -P assay was also added to the protocol for specimens having “positive” results, the defining values for which remained the same (regardless of GAL-1 -P results).
GAL-1 -P Results & Determination of Cutoff Value u u Calculated galactose-1 -phosphate (GAL-1 -P) values for the 559 samples retested using Isolab’s AP-free GAL reagent were in the range of 0. 1 to 9. 4 mg/d. L with a mean of 3. 9 +1. 8 mg/d. L. In-house AP-free GAL reagent GAL-1 -P results were in the range of 0. 1 to 10. 0 mg/d. L with a mean of 4. 2 +1. 7 mg/d. L. A cut-off of 4. 0 mg/d. L approximated the mean of GAL-1 -P values of babies having “borderline” GAL and GALT results. This was felt to be a conservative reference to help differentiate babies most at risk for GALT deficiency. The GAL-1 -P assay was also added to the protocol for specimens having “positive” results, the defining values for which remained the same (regardless of GAL-1 -P results).
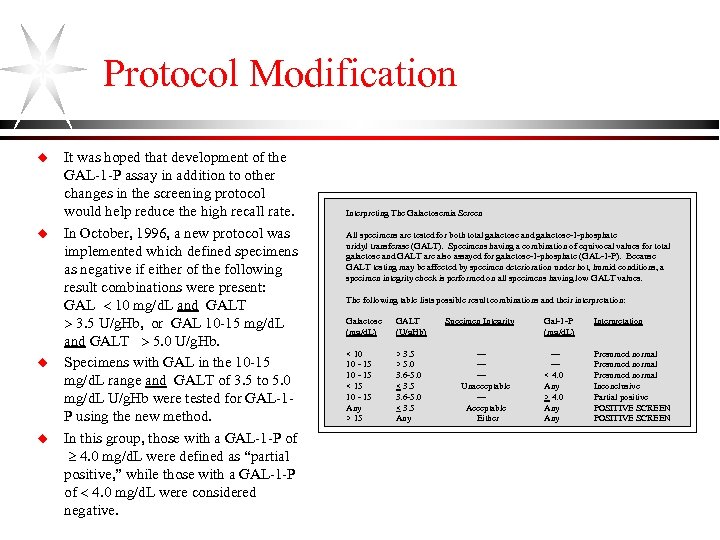 Protocol Modification u u It was hoped that development of the GAL-1 -P assay in addition to other changes in the screening protocol would help reduce the high recall rate. In October, 1996, a new protocol was implemented which defined specimens as negative if either of the following result combinations were present: GAL 10 mg/d. L and GALT 3. 5 U/g. Hb, or GAL 10 -15 mg/d. L and GALT 5. 0 U/g. Hb. Specimens with GAL in the 10 -15 mg/d. L range and GALT of 3. 5 to 5. 0 mg/d. L U/g. Hb were tested for GAL-1 P using the new method. In this group, those with a GAL-1 -P of 4. 0 mg/d. L were defined as “partial positive, ” while those with a GAL-1 -P of 4. 0 mg/d. L were considered negative. Interpreting The Galactosemia Screen All specimens are tested for both total galactose and galactose-1 -phosphate uridyl transferase (GALT). Specimens having a combination of equivocal values for total galactose and GALT are also assayed for galactose-1 -phosphate (GAL-1 -P). Because GALT testing may be affected by specimen deterioration under hot, humid conditions, a specimen integrity check is performed on all specimens having low GALT values. The following table lists possible result combinations and their interpretation: Galactose (mg/d. L) GALT (U/g. Hb) < 10 10 - 15 < 15 10 - 15 Any > 15 > 3. 5 > 5. 0 3. 6 -5. 0 < 3. 5 Any Specimen Integrity ------Unacceptable --Acceptable Either Gal-1 -P (mg/d. L) Interpretation ----< 4. 0 Any > 4. 0 Any Presumed normal Inconclusive Partial positive POSITIVE SCREEN
Protocol Modification u u It was hoped that development of the GAL-1 -P assay in addition to other changes in the screening protocol would help reduce the high recall rate. In October, 1996, a new protocol was implemented which defined specimens as negative if either of the following result combinations were present: GAL 10 mg/d. L and GALT 3. 5 U/g. Hb, or GAL 10 -15 mg/d. L and GALT 5. 0 U/g. Hb. Specimens with GAL in the 10 -15 mg/d. L range and GALT of 3. 5 to 5. 0 mg/d. L U/g. Hb were tested for GAL-1 P using the new method. In this group, those with a GAL-1 -P of 4. 0 mg/d. L were defined as “partial positive, ” while those with a GAL-1 -P of 4. 0 mg/d. L were considered negative. Interpreting The Galactosemia Screen All specimens are tested for both total galactose and galactose-1 -phosphate uridyl transferase (GALT). Specimens having a combination of equivocal values for total galactose and GALT are also assayed for galactose-1 -phosphate (GAL-1 -P). Because GALT testing may be affected by specimen deterioration under hot, humid conditions, a specimen integrity check is performed on all specimens having low GALT values. The following table lists possible result combinations and their interpretation: Galactose (mg/d. L) GALT (U/g. Hb) < 10 10 - 15 < 15 10 - 15 Any > 15 > 3. 5 > 5. 0 3. 6 -5. 0 < 3. 5 Any Specimen Integrity ------Unacceptable --Acceptable Either Gal-1 -P (mg/d. L) Interpretation ----< 4. 0 Any > 4. 0 Any Presumed normal Inconclusive Partial positive POSITIVE SCREEN
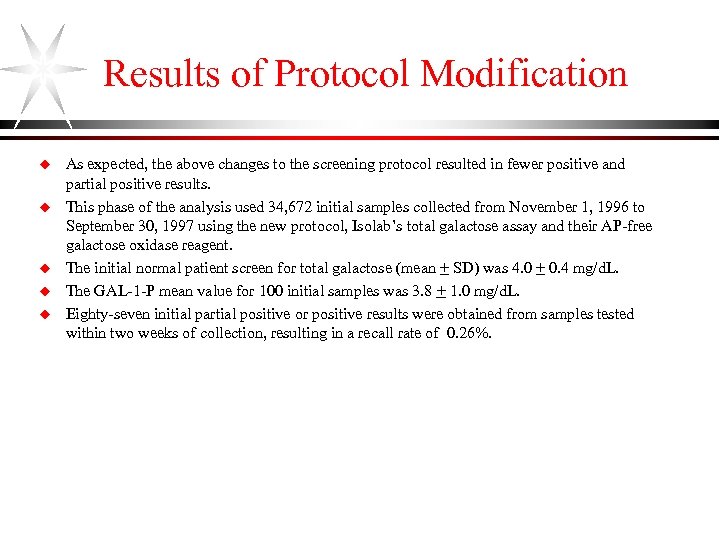 Results of Protocol Modification u u u As expected, the above changes to the screening protocol resulted in fewer positive and partial positive results. This phase of the analysis used 34, 672 initial samples collected from November 1, 1996 to September 30, 1997 using the new protocol, Isolab’s total galactose assay and their AP-free galactose oxidase reagent. The initial normal patient screen for total galactose (mean + SD) was 4. 0 + 0. 4 mg/d. L. The GAL-1 -P mean value for 100 initial samples was 3. 8 + 1. 0 mg/d. L. Eighty-seven initial partial positive or positive results were obtained from samples tested within two weeks of collection, resulting in a recall rate of 0. 26%.
Results of Protocol Modification u u u As expected, the above changes to the screening protocol resulted in fewer positive and partial positive results. This phase of the analysis used 34, 672 initial samples collected from November 1, 1996 to September 30, 1997 using the new protocol, Isolab’s total galactose assay and their AP-free galactose oxidase reagent. The initial normal patient screen for total galactose (mean + SD) was 4. 0 + 0. 4 mg/d. L. The GAL-1 -P mean value for 100 initial samples was 3. 8 + 1. 0 mg/d. L. Eighty-seven initial partial positive or positive results were obtained from samples tested within two weeks of collection, resulting in a recall rate of 0. 26%.
 Overall Program Outcomes u u u Between November 1, 1996 and September 30, 1997, a total of nine infants were detected with clinically significant transferase deficiencies (e. g. Duarte-galactosemia compound heterozygotes), for an estimated incidence of 1: 3, 850. Since this falls within the range of variant detection by other state programs, it appears that the changes in protocol have not hampered overall sensitivity of the screening process. A second major benefit of incorporation of GAL-1 -P measurement into the screening protocol has been to facilitate detection of other enzyme deficiencies associated with galactosemia, i. e. epimerase and galactokinase. In the setting of a high total GAL ( 15 mg/d. L using the current protocol) with a “normal” GALT ( 5. 0 U/g. Hb) result, a high GAL-1 -P suggests epimerase deficiency, while a low GAL-1 -P ( 1 mg/d. L or undetectable) suggests kinase deficiency. Since introduction of the GAL-1 -P assay, one child with peripheral epimerase deficiency has been detected because of screening results in the former range.
Overall Program Outcomes u u u Between November 1, 1996 and September 30, 1997, a total of nine infants were detected with clinically significant transferase deficiencies (e. g. Duarte-galactosemia compound heterozygotes), for an estimated incidence of 1: 3, 850. Since this falls within the range of variant detection by other state programs, it appears that the changes in protocol have not hampered overall sensitivity of the screening process. A second major benefit of incorporation of GAL-1 -P measurement into the screening protocol has been to facilitate detection of other enzyme deficiencies associated with galactosemia, i. e. epimerase and galactokinase. In the setting of a high total GAL ( 15 mg/d. L using the current protocol) with a “normal” GALT ( 5. 0 U/g. Hb) result, a high GAL-1 -P suggests epimerase deficiency, while a low GAL-1 -P ( 1 mg/d. L or undetectable) suggests kinase deficiency. Since introduction of the GAL-1 -P assay, one child with peripheral epimerase deficiency has been detected because of screening results in the former range.
 Conclusions u u u Our study clearly showed the modification of the Isolab’s NCS methodology using APFree reagents is a feasible means of estimating GAL-1 -P from dried blood spots obtained from neonates. Isolab’s total galactose and their AP-Free galactose reagent assayed within one sample plate proved to be reliable, simple and convenient screening method for the calculation of GAL-1 -P in DBS. Determination of GAL-1 -P among infants having equivocal GAL and GALT results has proven particularly useful in identifying those most likely to have a significant variant for which continuation of a lactose-free diet may be beneficial. Stated differently, recall rates, and their attendant costs, have been reduced by retesting only babies with evidence of significant GAL-1 -P accumulation. Furthermore, measurement of GAL-1 -P has provided the potential to identify less common galactosemia states, including peripheral epimerase and galactokinase deficiency.
Conclusions u u u Our study clearly showed the modification of the Isolab’s NCS methodology using APFree reagents is a feasible means of estimating GAL-1 -P from dried blood spots obtained from neonates. Isolab’s total galactose and their AP-Free galactose reagent assayed within one sample plate proved to be reliable, simple and convenient screening method for the calculation of GAL-1 -P in DBS. Determination of GAL-1 -P among infants having equivocal GAL and GALT results has proven particularly useful in identifying those most likely to have a significant variant for which continuation of a lactose-free diet may be beneficial. Stated differently, recall rates, and their attendant costs, have been reduced by retesting only babies with evidence of significant GAL-1 -P accumulation. Furthermore, measurement of GAL-1 -P has provided the potential to identify less common galactosemia states, including peripheral epimerase and galactokinase deficiency.
![References u u u [1] Kaplan, Lawerence A. , Pesce, Amadeo J. , Clinical References u u u [1] Kaplan, Lawerence A. , Pesce, Amadeo J. , Clinical](https://present5.com/presentation/8865b87d27fa55184c2bdad1f41e71c0/image-18.jpg) References u u u [1] Kaplan, Lawerence A. , Pesce, Amadeo J. , Clinical Chemistry, “Theory, analysis and correlation”, p. 702, C. V. Mosby Co. , St. Louis, MO. , (1989). [2] Inoue N, Hata M, Ichiba Y, Wada H, Misami H, Mori T. Results of newborn screening for galactose metabolic disorder. J Inher Metabol Dis 1990: 13: 93 -101. [3] Insert, Isolab’s GALT Test Kit, “For Measuring Galactose-1 -Phosphate Uridyl Transferase Collected onto Filter Paper”, Revised November 1, 1995. Isolab, Inc, Akron, OH. [4] Insert, Isolab’s Galactose Test Kit, “For Measuring Galactose and Galactose-1 -Phosphate in Blood Collected onto Filter Paper”, Revised December 20, 1995. Isolab, Inc, Akron, OH. [5] Yamaguchi, Akihiro, Fukushi, Masaru, et. al. , Microassay for Screening Newborns for Galactosemia with Use of a Fluorometric Microplate Reader, Clinical Chemistry, (1989); 35: 1962 -1964.
References u u u [1] Kaplan, Lawerence A. , Pesce, Amadeo J. , Clinical Chemistry, “Theory, analysis and correlation”, p. 702, C. V. Mosby Co. , St. Louis, MO. , (1989). [2] Inoue N, Hata M, Ichiba Y, Wada H, Misami H, Mori T. Results of newborn screening for galactose metabolic disorder. J Inher Metabol Dis 1990: 13: 93 -101. [3] Insert, Isolab’s GALT Test Kit, “For Measuring Galactose-1 -Phosphate Uridyl Transferase Collected onto Filter Paper”, Revised November 1, 1995. Isolab, Inc, Akron, OH. [4] Insert, Isolab’s Galactose Test Kit, “For Measuring Galactose and Galactose-1 -Phosphate in Blood Collected onto Filter Paper”, Revised December 20, 1995. Isolab, Inc, Akron, OH. [5] Yamaguchi, Akihiro, Fukushi, Masaru, et. al. , Microassay for Screening Newborns for Galactosemia with Use of a Fluorometric Microplate Reader, Clinical Chemistry, (1989); 35: 1962 -1964.
 Acknowledgement u u Isolab, Inc. (Akron, Ohio, USA), for furnishing their AP-Free GAL reagent for these studies, and Melissa Foust for assisting in this poster presentation. We would also like to thank our colleagues in the newborn screening program and genetic services for their skillful technical assistance and their constant dedication to newborn genetic screening. A H D Arkansas Department of Health Division of Public Health Laboratories 1/30/98
Acknowledgement u u Isolab, Inc. (Akron, Ohio, USA), for furnishing their AP-Free GAL reagent for these studies, and Melissa Foust for assisting in this poster presentation. We would also like to thank our colleagues in the newborn screening program and genetic services for their skillful technical assistance and their constant dedication to newborn genetic screening. A H D Arkansas Department of Health Division of Public Health Laboratories 1/30/98


