128 52 Te. Group 6 A 16 8
















- Размер: 3.8 Mегабайта
- Количество слайдов: 30
Описание презентации 128 52 Te. Group 6 A 16 8 по слайдам
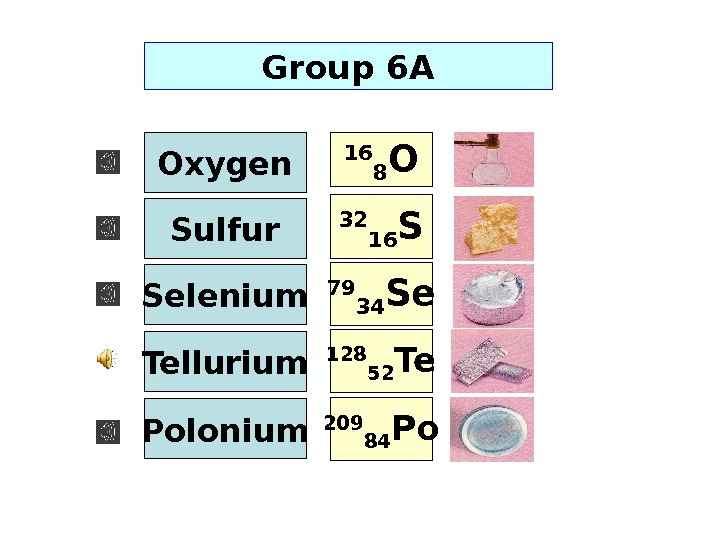
 128 52 Te. Group 6 A 16 8 O 32 16 S 79 34 Se 209 84 Po. Selenium Oxygen Sulfur Tellurium Polonium
128 52 Te. Group 6 A 16 8 O 32 16 S 79 34 Se 209 84 Po. Selenium Oxygen Sulfur Tellurium Polonium
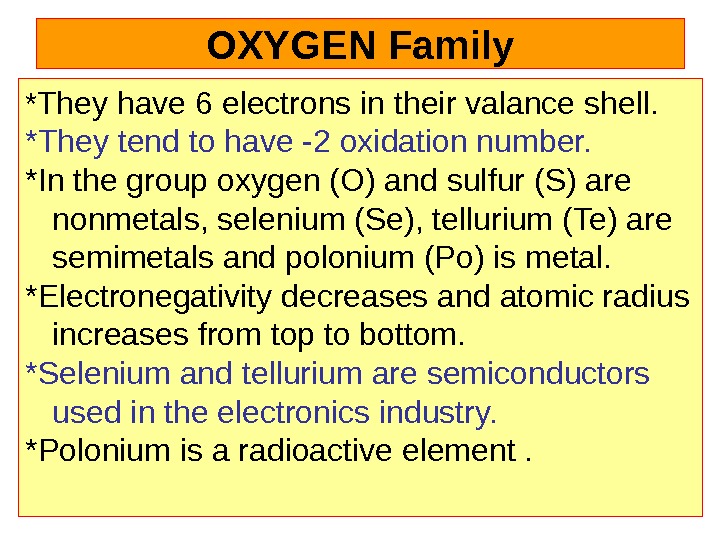
 OXYGEN Family * They have 6 electrons in their valance shell. *They tend to have -2 oxidation number. *In the group oxygen (O) and sulfur (S) are nonmetals, selenium (Se), tellurium (Te) are semimetals and polonium (Po) is metal. *Electronegativity decreases and atomic radius increases from top to bottom. *Selenium and tellurium are semiconductors used in the electronics industry. *Polonium is a radioactive element .
OXYGEN Family * They have 6 electrons in their valance shell. *They tend to have -2 oxidation number. *In the group oxygen (O) and sulfur (S) are nonmetals, selenium (Se), tellurium (Te) are semimetals and polonium (Po) is metal. *Electronegativity decreases and atomic radius increases from top to bottom. *Selenium and tellurium are semiconductors used in the electronics industry. *Polonium is a radioactive element .
 Oxygen is firstly discovered by English scientists, Joseph Priestley, in 1774. “Oxygen” word is deduced from the word “oxy” in Latin meaning acid producer. Oxygen has two allotropes: O 2 and O
Oxygen is firstly discovered by English scientists, Joseph Priestley, in 1774. “Oxygen” word is deduced from the word “oxy” in Latin meaning acid producer. Oxygen has two allotropes: O 2 and O
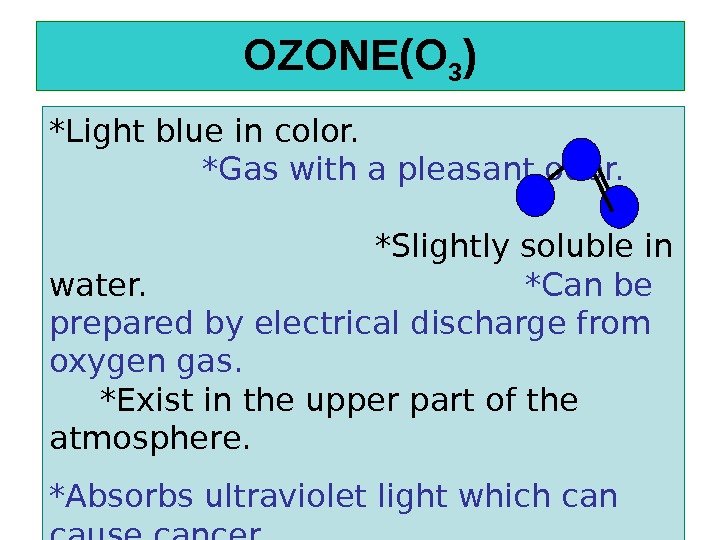
 OZONE(O 3 ) *Light blue in color. *Gas with a pleasant odor. *Slightly soluble in water. *Can be prepared by electrical discharge from oxygen gas. *Exist in the upper part of the atmosphere. *Absorbs ultraviolet light which can cause cancer.
OZONE(O 3 ) *Light blue in color. *Gas with a pleasant odor. *Slightly soluble in water. *Can be prepared by electrical discharge from oxygen gas. *Exist in the upper part of the atmosphere. *Absorbs ultraviolet light which can cause cancer.
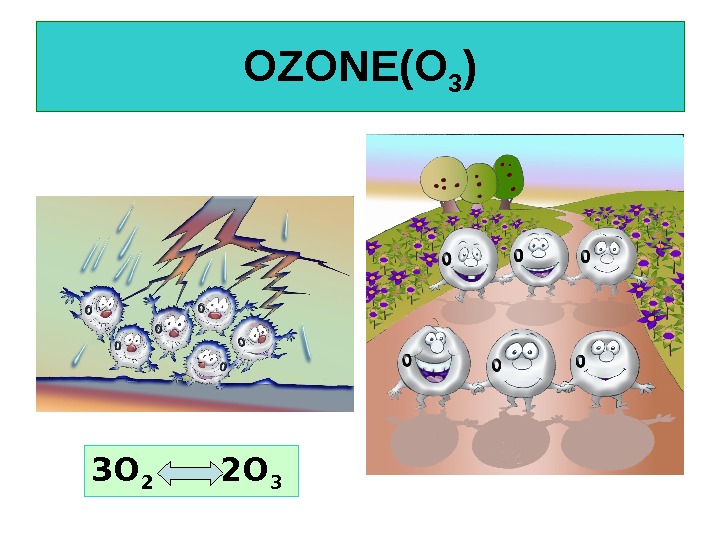
 OZONE(O 3 ) 3 O 2 2 O
OZONE(O 3 ) 3 O 2 2 O
 O zone layer of the atmosphere protects life on earth from the full force of the sun’s cancer-causing ultraviolet radiation, it is critically important.
O zone layer of the atmosphere protects life on earth from the full force of the sun’s cancer-causing ultraviolet radiation, it is critically important.
 Oxygen is the most abundant element in earth crust(46%). Oxygen composes 21 percent by volume of the atmosphere; Oxygen comprises 60 percent of the human body.
Oxygen is the most abundant element in earth crust(46%). Oxygen composes 21 percent by volume of the atmosphere; Oxygen comprises 60 percent of the human body.
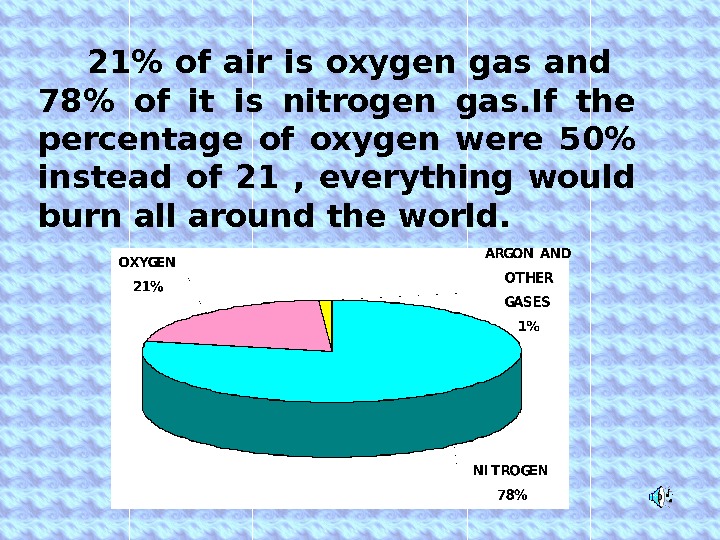
 21% of air is oxygen gas and 78% of it is nitrogen gas. If the percentage of oxygen were 50% instead of 21 , everything would burn all around the world.
21% of air is oxygen gas and 78% of it is nitrogen gas. If the percentage of oxygen were 50% instead of 21 , everything would burn all around the world.
 Preparation in the laboratory a) Heating of some metal oxides. Ag 2 O(s) 2 Ag (s) + ½ O 2(g) b) Heating of peroxides. 2 H 2 O 2(l) 2 H 2 O (l) + O 2(g) c) Heating of NO 3 — and KCl. O 3 — compounds. 2 KCl. O 3(s) 2 KCl (s) + 3 O 2(g) d) Heating of Mn. O 4 — compounds. 2 KMn. O 4(s) K 2 Mn. O 4(s) +Mn. O 2 +O 2(g) e) Electrolysis of water. 2 H 2 O (l) O 2(g) + 2 H 2(g)
Preparation in the laboratory a) Heating of some metal oxides. Ag 2 O(s) 2 Ag (s) + ½ O 2(g) b) Heating of peroxides. 2 H 2 O 2(l) 2 H 2 O (l) + O 2(g) c) Heating of NO 3 — and KCl. O 3 — compounds. 2 KCl. O 3(s) 2 KCl (s) + 3 O 2(g) d) Heating of Mn. O 4 — compounds. 2 KMn. O 4(s) K 2 Mn. O 4(s) +Mn. O 2 +O 2(g) e) Electrolysis of water. 2 H 2 O (l) O 2(g) + 2 H 2(g)
 Preparation in the industry In industry oxygen is prepared from air. There are two steps for that process. 2) Fractional distillation of liquid air. When we heat the liquid air, after nitrogen oxygen is evaporated at -183 0 C. 1) Liquefying of air. After removing of CO 2 pressure is applied about 200 atm. After that at -200 0 C liquid air is formed.
Preparation in the industry In industry oxygen is prepared from air. There are two steps for that process. 2) Fractional distillation of liquid air. When we heat the liquid air, after nitrogen oxygen is evaporated at -183 0 C. 1) Liquefying of air. After removing of CO 2 pressure is applied about 200 atm. After that at -200 0 C liquid air is formed.
 COMPOUNDS: 1 -OXIDES Oxygen is a component of many organic and inorganic compounds. It forms compounds called oxides with almost all the elements, including some of the noble gases. A chemical reaction in which an oxide forms is called oxidation. EXAMPLE: S + O 2 SO 2 (Sulfur dioxide) C + O 2 CO 2 (Carbon dioxide)
COMPOUNDS: 1 -OXIDES Oxygen is a component of many organic and inorganic compounds. It forms compounds called oxides with almost all the elements, including some of the noble gases. A chemical reaction in which an oxide forms is called oxidation. EXAMPLE: S + O 2 SO 2 (Sulfur dioxide) C + O 2 CO 2 (Carbon dioxide)
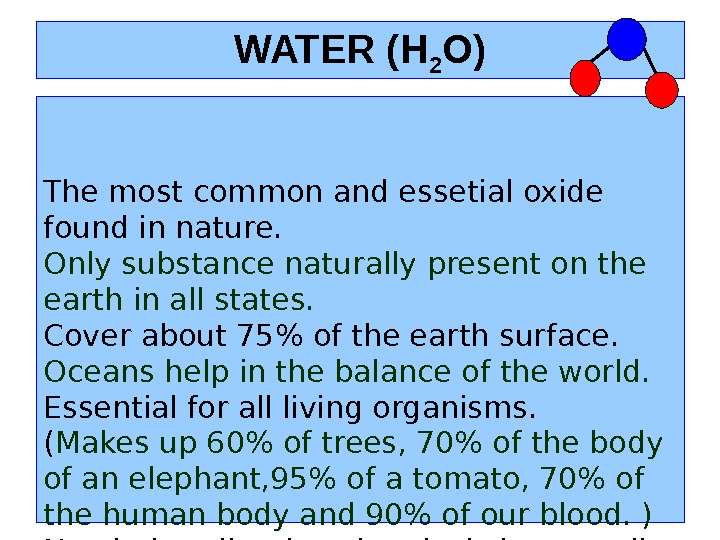
 WATER (H 2 O) The most common and essetial oxide found in nature. Only substance naturally present on the earth in all states. Cover about 75% of the earth surface. Oceans help i n the balance of the world. Essential for all living organisms. ( Makes up 60% of trees, 70% of the body of an elephant, 95% of a tomato, 70% of the human body and 90% of our blood. ) Needed to dissolve chemicals in our cells and to carry chemicals in our body.
WATER (H 2 O) The most common and essetial oxide found in nature. Only substance naturally present on the earth in all states. Cover about 75% of the earth surface. Oceans help i n the balance of the world. Essential for all living organisms. ( Makes up 60% of trees, 70% of the body of an elephant, 95% of a tomato, 70% of the human body and 90% of our blood. ) Needed to dissolve chemicals in our cells and to carry chemicals in our body.
 Uses of water In industry solvent and cooler. In automobile radiators and nuclear power plants. Production of steel and paper. In the home for cooking, drinking and washing. Water steam is used to generate electricity. To extract minerals and to manufacture of several chemicals.
Uses of water In industry solvent and cooler. In automobile radiators and nuclear power plants. Production of steel and paper. In the home for cooking, drinking and washing. Water steam is used to generate electricity. To extract minerals and to manufacture of several chemicals.
 Uses of O 2 *In respiration tubes for divers, patients and astronauts. *Used in oxygen welding to cut very hard metals. *Rocket fuel with hydrogen. *Production of some chemicals.
Uses of O 2 *In respiration tubes for divers, patients and astronauts. *Used in oxygen welding to cut very hard metals. *Rocket fuel with hydrogen. *Production of some chemicals.
 Oxygen O
Oxygen O
 Tasteless, odorless, light yellow nonmetallic element. All forms of sulfur are insoluble in water, but the crystalline forms are soluble in carbon disulfide.
Tasteless, odorless, light yellow nonmetallic element. All forms of sulfur are insoluble in water, but the crystalline forms are soluble in carbon disulfide.
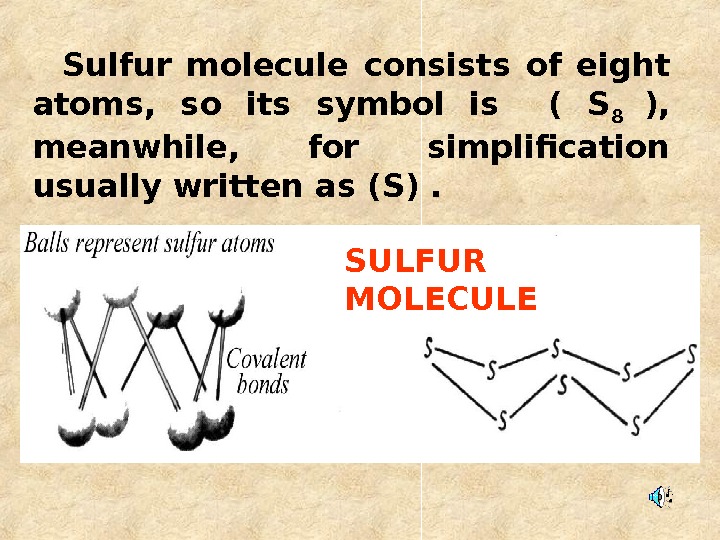
 Sulfur molecule consists of eight atoms, so its symbol is ( S 8 ), meanwhile, for simplification usually written as (S). SULFUR MOLECUL
Sulfur molecule consists of eight atoms, so its symbol is ( S 8 ), meanwhile, for simplification usually written as (S). SULFUR MOLECUL
 Naturally, sulfur occurs, in the free form, in underground deposits, also occurs in combined form such as sulfide and sulfites. Sulfur is one of the elements, which is present in the composition of the living bodies.
Naturally, sulfur occurs, in the free form, in underground deposits, also occurs in combined form such as sulfide and sulfites. Sulfur is one of the elements, which is present in the composition of the living bodies.
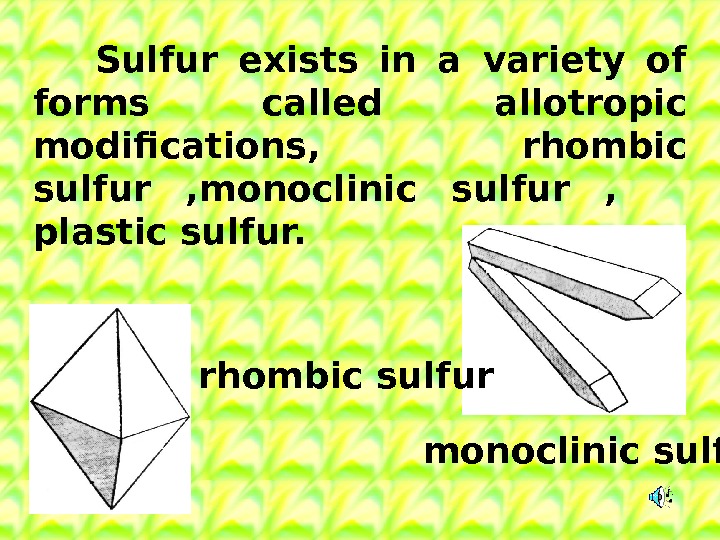
 Sulfur exists in a variety of forms called allotropic modifications, rhombic sulfur , monoclinic sulfur , plastic sulfur. rhombic sulfur monoclinic sulfur
Sulfur exists in a variety of forms called allotropic modifications, rhombic sulfur , monoclinic sulfur , plastic sulfur. rhombic sulfur monoclinic sulfur
 Allotropes RHOMBIC; A crystal which is stable below 96 0 C. Bright yellow in color and octahedral (rhombic) in shape. Odorless and have S 8 molecules. MONOCLINIC; A crystal which is stable between 96 -119 0 C. Crystals are long, thin and needle shaped. Reverts to rhombic one at room temperature. PLASTIC; Obtained by heating sulfur to its boiling point. When it is poured into cold water , it will roll up into yellow ribbons called plastic sulfur.
Allotropes RHOMBIC; A crystal which is stable below 96 0 C. Bright yellow in color and octahedral (rhombic) in shape. Odorless and have S 8 molecules. MONOCLINIC; A crystal which is stable between 96 -119 0 C. Crystals are long, thin and needle shaped. Reverts to rhombic one at room temperature. PLASTIC; Obtained by heating sulfur to its boiling point. When it is poured into cold water , it will roll up into yellow ribbons called plastic sulfur.
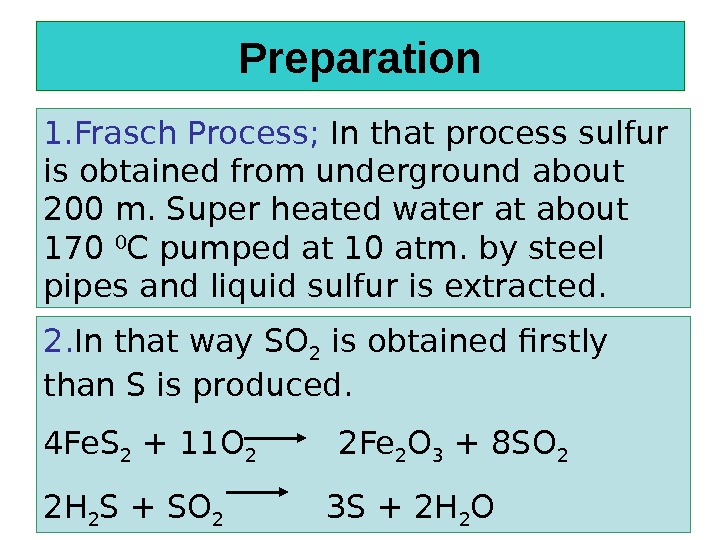
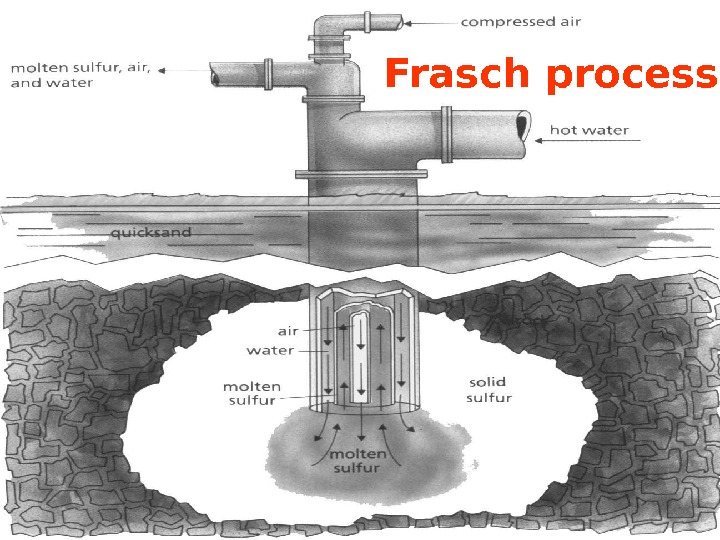
 Preparation 1. Frasch Process; In that process sulfur is obtained from underground about 200 m. Super heated water at about 170 0 C pumped at 10 atm. by steel pipes and liquid sulfur is extracted. 2. In that way SO 2 is obtained firstly than S is produced. 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2 2 H 2 S + SO 2 3 S + 2 H 2 O
Preparation 1. Frasch Process; In that process sulfur is obtained from underground about 200 m. Super heated water at about 170 0 C pumped at 10 atm. by steel pipes and liquid sulfur is extracted. 2. In that way SO 2 is obtained firstly than S is produced. 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2 2 H 2 S + SO 2 3 S + 2 H 2 O
 Frasch process
Frasch process
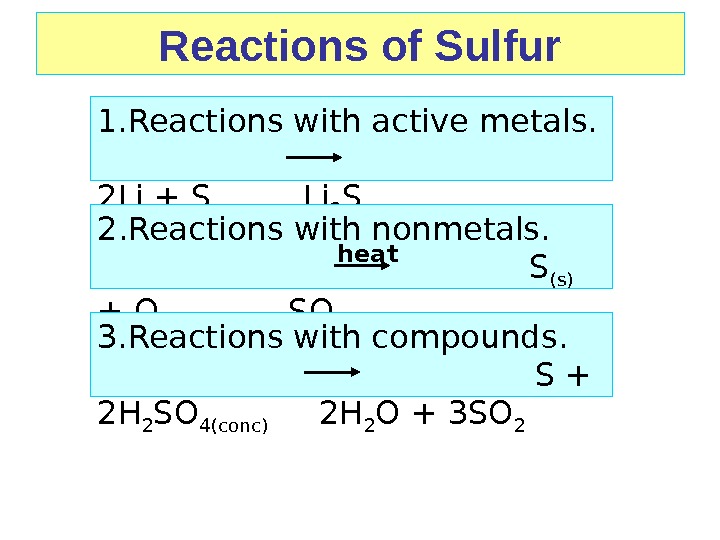
 Reactions of Sulfur 1. Reactions with active metals. 2 Li + S Li 2 S 2. Reactions with nonmetals. S (s) + O 2(g) SO 2(s) 3. Reactions with compounds. S + 2 H 2 SO 4(conc) 2 H 2 O + 3 SO 2 heat
Reactions of Sulfur 1. Reactions with active metals. 2 Li + S Li 2 S 2. Reactions with nonmetals. S (s) + O 2(g) SO 2(s) 3. Reactions with compounds. S + 2 H 2 SO 4(conc) 2 H 2 O + 3 SO 2 heat
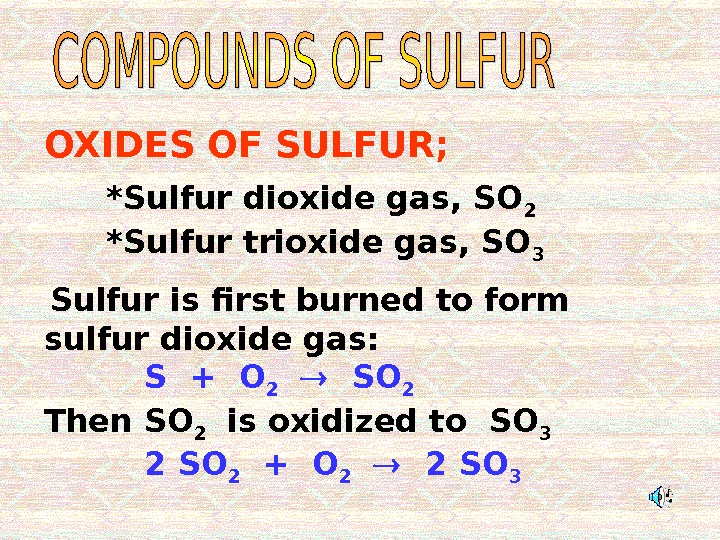
 Sulfur is first burned to form sulfur dioxide gas: S + O 2 SO 2 Then SO 2 is oxidized to SO 3 2 SO 2 + O 2 2 SO 3*Sulfur dioxide gas, SO 2 *Sulfur trioxide gas, SO 3 OXIDES OF SULFUR;
Sulfur is first burned to form sulfur dioxide gas: S + O 2 SO 2 Then SO 2 is oxidized to SO 3 2 SO 2 + O 2 2 SO 3*Sulfur dioxide gas, SO 2 *Sulfur trioxide gas, SO 3 OXIDES OF SULFUR;
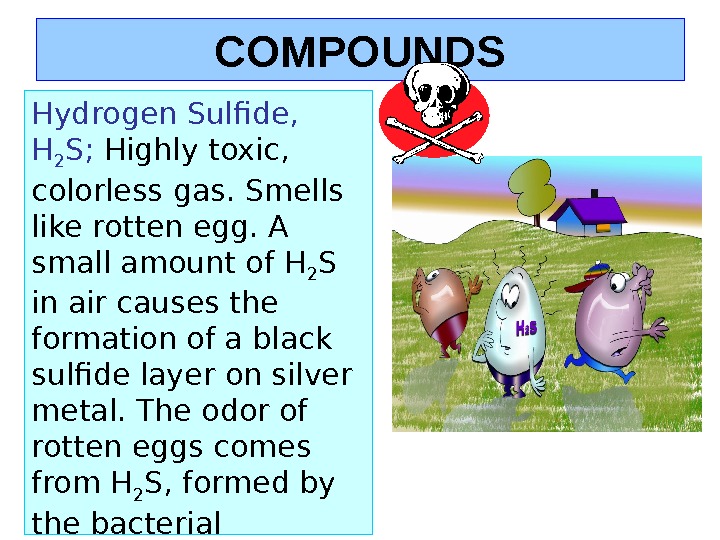
 COMPOUNDS Hydrogen Sulfide, H 2 S; Highly toxic, colorless gas. Smells like rotten egg. A small amount of H 2 S in air causes the formation of a black sulfide layer on silver metal. The odor of rotten eggs comes from H 2 S, formed by the bacterial decomposition of sulfur compounds.
COMPOUNDS Hydrogen Sulfide, H 2 S; Highly toxic, colorless gas. Smells like rotten egg. A small amount of H 2 S in air causes the formation of a black sulfide layer on silver metal. The odor of rotten eggs comes from H 2 S, formed by the bacterial decomposition of sulfur compounds.
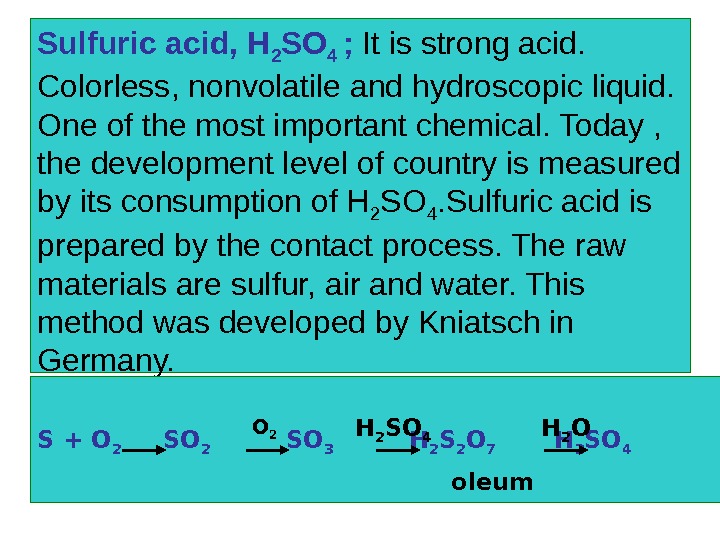
 Sulfuric acid, H 2 SO 4 ; It is strong acid. Colorless, nonvolatile and hydroscopic liquid. One of the most important chemical. Today , the development level of country is measured by its consumption of H 2 SO 4. Sulfuric acid is prepared by the contact process. The raw materials are sulfur, air and water. This method was developed by Kniatsch in Germany. S + O 2 SO 2 SO 3 H 2 S 2 O 7 H 2 SO 4 O 2 H 2 SO 4 H 2 O oleum
Sulfuric acid, H 2 SO 4 ; It is strong acid. Colorless, nonvolatile and hydroscopic liquid. One of the most important chemical. Today , the development level of country is measured by its consumption of H 2 SO 4. Sulfuric acid is prepared by the contact process. The raw materials are sulfur, air and water. This method was developed by Kniatsch in Germany. S + O 2 SO 2 SO 3 H 2 S 2 O 7 H 2 SO 4 O 2 H 2 SO 4 H 2 O oleum
 Carbon disulfide, CS 2 ; Flammable and colorless liquid. Toxic and have pleasant smelling. Good solvent for chemicals. Sulfur dioxide, SO 2 ; Formed by the combustion reaction of S or Fe. S 2. Toxic, colorless gas with a sharp, bad odor. Plays a major role in producing acid rain. Sulfur trioxide, SO 3 ; Formed by the reaction of SO 2 with O 2. Very active and volatile substance. Plays a major role in producing acid rain.
Carbon disulfide, CS 2 ; Flammable and colorless liquid. Toxic and have pleasant smelling. Good solvent for chemicals. Sulfur dioxide, SO 2 ; Formed by the combustion reaction of S or Fe. S 2. Toxic, colorless gas with a sharp, bad odor. Plays a major role in producing acid rain. Sulfur trioxide, SO 3 ; Formed by the reaction of SO 2 with O 2. Very active and volatile substance. Plays a major role in producing acid rain.
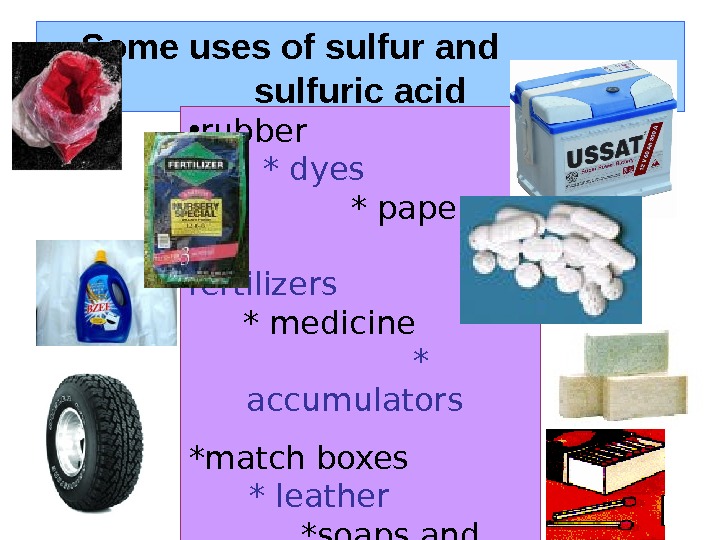
 Some uses of sulfur and sulfuric acid • rubber * dyes * paper * fertilizers * medicine * accumulators *match boxes * leather *soaps and detergents • Gun powder
Some uses of sulfur and sulfuric acid • rubber * dyes * paper * fertilizers * medicine * accumulators *match boxes * leather *soaps and detergents • Gun powder
 Sulfur S
Sulfur S
 THE END!
THE END!

