28f7eda7989b63265b7084efa8eaf393.ppt
- Количество слайдов: 40
 1260 personnes, 90 nationalités Recherche clinique -Croissance de l’enfant : endocrinologie, nutrition Prise en charge nutritionnelle des enfants atteints de pathologies intestinales (SBS) Nutrition précoce et croissance - Risque d’obésité (B. Koletzko) - Devenir du prématuré (N. Embleton) - Allaitement et croissance (D. Turck) - « Défis, enjeux » (F. Haschke)
1260 personnes, 90 nationalités Recherche clinique -Croissance de l’enfant : endocrinologie, nutrition Prise en charge nutritionnelle des enfants atteints de pathologies intestinales (SBS) Nutrition précoce et croissance - Risque d’obésité (B. Koletzko) - Devenir du prématuré (N. Embleton) - Allaitement et croissance (D. Turck) - « Défis, enjeux » (F. Haschke)
 - B. Koltezko Allaitement risque d’obésité Prot: 1. 25 g/d. L vs 2. 05 ( IGF 1 et insuline) Effet à long-terme sur poids qui reste supérieur - N. Embleton Importance de la supplémentation en protéines chez les prématurés - développement du cerveau Est-ce la vitesse de croissance (trop rapide) ou le nutriment (aa) qui joue le plus sur l’insulinorésistance, adiposité? -D. Turck Enfants allaités (18 mois) - croissance plus rapide les 2 -3 1 ers mois - prennent moins de poids à partir de 6 -7 mois : écart de 650 g à un an - plus de différences à 2 -3 ans - Pas de diff de périmètre crânien - E intake plus faible chez les allaités - Voir taux adiponectine dans le lait? - F. Haschke -Courbes standard de croissance et BMI non adaptés aux enfants de mères obèses car poids à la naissance déjà plus élevés
- B. Koltezko Allaitement risque d’obésité Prot: 1. 25 g/d. L vs 2. 05 ( IGF 1 et insuline) Effet à long-terme sur poids qui reste supérieur - N. Embleton Importance de la supplémentation en protéines chez les prématurés - développement du cerveau Est-ce la vitesse de croissance (trop rapide) ou le nutriment (aa) qui joue le plus sur l’insulinorésistance, adiposité? -D. Turck Enfants allaités (18 mois) - croissance plus rapide les 2 -3 1 ers mois - prennent moins de poids à partir de 6 -7 mois : écart de 650 g à un an - plus de différences à 2 -3 ans - Pas de diff de périmètre crânien - E intake plus faible chez les allaités - Voir taux adiponectine dans le lait? - F. Haschke -Courbes standard de croissance et BMI non adaptés aux enfants de mères obèses car poids à la naissance déjà plus élevés
 Nutrition et croissance: Santé du squelette Nutrition et hormones de croissance - IGF (M. Savage; JM Wit) - Diabètes (T. Battelino) Prématurés : différencier suffisance et excès - optimiser la consommation de protéines pour une croissance optimale (R. van Elburg) - connaître les effets à long-terme d’une croissance « accélérée » (A. Singhal) Importance du petit déjeuner (et de sa composition) - cognition - glycémie Courbes de croissances (OMS)
Nutrition et croissance: Santé du squelette Nutrition et hormones de croissance - IGF (M. Savage; JM Wit) - Diabètes (T. Battelino) Prématurés : différencier suffisance et excès - optimiser la consommation de protéines pour une croissance optimale (R. van Elburg) - connaître les effets à long-terme d’une croissance « accélérée » (A. Singhal) Importance du petit déjeuner (et de sa composition) - cognition - glycémie Courbes de croissances (OMS)
 Accelerated growth and effects on long terms health outcomes Atul Singhal • 200 millions d’enfants naissent avec un petit poids de naissance chaque année • 96% dans les pays en voie de développement
Accelerated growth and effects on long terms health outcomes Atul Singhal • 200 millions d’enfants naissent avec un petit poids de naissance chaque année • 96% dans les pays en voie de développement
 • *ALSPAC cohort: « Catch up growth » entre la naissance et 2 ans + lourd, + grand, + gras • Soto et al. : « Catch up growth » entre la naissance et 1 an sensibilité insuline, TG • SWEDES study: gain de poids rapide entre 0 et 6 mois risque cardio vasculaire * Avon Longitudinal Study of Parents and Children (n children =13 000, measuring behavioral problem, Bristol University, UK)
• *ALSPAC cohort: « Catch up growth » entre la naissance et 2 ans + lourd, + grand, + gras • Soto et al. : « Catch up growth » entre la naissance et 1 an sensibilité insuline, TG • SWEDES study: gain de poids rapide entre 0 et 6 mois risque cardio vasculaire * Avon Longitudinal Study of Parents and Children (n children =13 000, measuring behavioral problem, Bristol University, UK)
 • *Project Viva: gain de poids rapide entre 0 et 6 mois pression systolique, IMC • Ibanez et al. : Persistance des effets à long terme • **New Delhi birth cohort: ↑ rapide de IMC jusqu’à l’âge de 1 an syndrome métabolique à l’âge adulte *Cohorte mères-enfants NIH, 1999 ** UK Southampton (n=9000)
• *Project Viva: gain de poids rapide entre 0 et 6 mois pression systolique, IMC • Ibanez et al. : Persistance des effets à long terme • **New Delhi birth cohort: ↑ rapide de IMC jusqu’à l’âge de 1 an syndrome métabolique à l’âge adulte *Cohorte mères-enfants NIH, 1999 ** UK Southampton (n=9000)
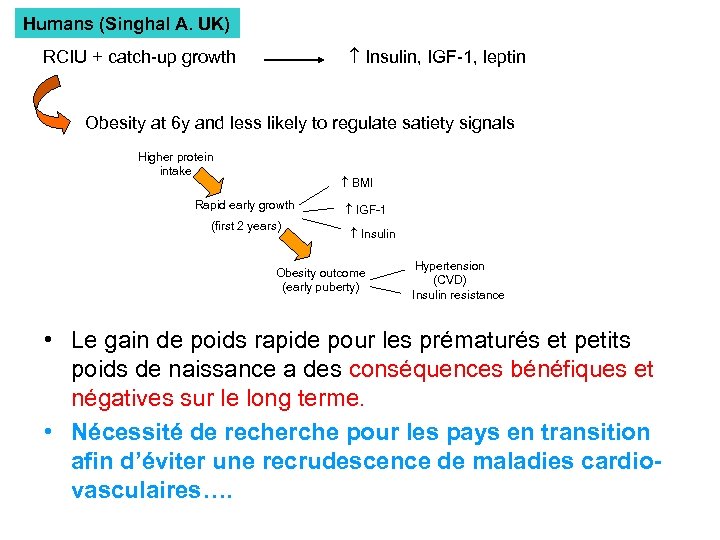 Humans (Singhal A. UK) Insulin, IGF-1, leptin RCIU + catch-up growth Obesity at 6 y and less likely to regulate satiety signals Higher protein intake BMI Rapid early growth (first 2 years) IGF-1 Insulin Obesity outcome (early puberty) Hypertension (CVD) Insulin resistance • Le gain de poids rapide pour les prématurés et petits poids de naissance a des conséquences bénéfiques et négatives sur le long terme. • Nécessité de recherche pour les pays en transition afin d’éviter une recrudescence de maladies cardiovasculaires….
Humans (Singhal A. UK) Insulin, IGF-1, leptin RCIU + catch-up growth Obesity at 6 y and less likely to regulate satiety signals Higher protein intake BMI Rapid early growth (first 2 years) IGF-1 Insulin Obesity outcome (early puberty) Hypertension (CVD) Insulin resistance • Le gain de poids rapide pour les prématurés et petits poids de naissance a des conséquences bénéfiques et négatives sur le long terme. • Nécessité de recherche pour les pays en transition afin d’éviter une recrudescence de maladies cardiovasculaires….
 Rattrapage de croissance - Nutrition et catch-up ( G. Gat-Yablonski) - Pb de croissance dans les pays en voie de développement (A. Prentice) - Compléments et croissance (V. Grote) - Mécanismes cellulaires et moléculaires du CU (J. Baron) Obésité et croissance (enfance et puberté) - Traitement des formes monogéniques (I. Aldhoon Hainerova) - Régime, IGF, croissance et obésité (K. F. Michaelsen) - Prévention de l’obésité (L. Moreno) - Rebond d’adiposité (MF Rolland Cachera) - Obésité et croissance durant l’enfance et la puberté (F. Chiarelli) Fenêtre critique pour la programmation nutritionnelle - Mécanismes (S. Ozanne) - Rôle de la nutrition de l’enfant sur risque d’obésité à long-terme (A. Singhal) NG dans les cas de maladies chroniques
Rattrapage de croissance - Nutrition et catch-up ( G. Gat-Yablonski) - Pb de croissance dans les pays en voie de développement (A. Prentice) - Compléments et croissance (V. Grote) - Mécanismes cellulaires et moléculaires du CU (J. Baron) Obésité et croissance (enfance et puberté) - Traitement des formes monogéniques (I. Aldhoon Hainerova) - Régime, IGF, croissance et obésité (K. F. Michaelsen) - Prévention de l’obésité (L. Moreno) - Rebond d’adiposité (MF Rolland Cachera) - Obésité et croissance durant l’enfance et la puberté (F. Chiarelli) Fenêtre critique pour la programmation nutritionnelle - Mécanismes (S. Ozanne) - Rôle de la nutrition de l’enfant sur risque d’obésité à long-terme (A. Singhal) NG dans les cas de maladies chroniques
 Breastfed infants Formula fed infants Lower protein content Higher protein content (9 -11 g/l) (14 -18 g/l) - Higher weight till 2 -5 month -Higher energy intake (kcal/kg/day) - Smaller lenght - Smaller weight - Smaller fat mass - Equal head circumference At 1 year - Huge amount of caseins (cows’ milk) - Earlier adiposity rebound Bioactive proteins + antibodies, excusively provided in human milk TG survive digestion + absorption in HM (oleic-palmitic-oleic acids, main TG structure in HM)
Breastfed infants Formula fed infants Lower protein content Higher protein content (9 -11 g/l) (14 -18 g/l) - Higher weight till 2 -5 month -Higher energy intake (kcal/kg/day) - Smaller lenght - Smaller weight - Smaller fat mass - Equal head circumference At 1 year - Huge amount of caseins (cows’ milk) - Earlier adiposity rebound Bioactive proteins + antibodies, excusively provided in human milk TG survive digestion + absorption in HM (oleic-palmitic-oleic acids, main TG structure in HM)
 Infant metabolism 1. Healths benefits of Structured Lipids S. M Innis, University of British Columbia, Ca Triglycérides du lait maternel: oléate-palmitate-oléate Forme majoritairement représentée glycérol Oléate C 18: 1 Palmitate C 18: 0 Oléate C 18: 1 Digestion: lipase libère les 2 oléates et forme le sn-2 palmitate, soluble Triglycérides huiles végétales glycérol Palmitate C 18: 0 Digestion lipase : 2 palmitates sous forme d’AGNE peu solubles en milieu alcalin + point de fusion élevé: savons de calcium excrétés Oléate C 18: 1 Palmitate C 18: 0
Infant metabolism 1. Healths benefits of Structured Lipids S. M Innis, University of British Columbia, Ca Triglycérides du lait maternel: oléate-palmitate-oléate Forme majoritairement représentée glycérol Oléate C 18: 1 Palmitate C 18: 0 Oléate C 18: 1 Digestion: lipase libère les 2 oléates et forme le sn-2 palmitate, soluble Triglycérides huiles végétales glycérol Palmitate C 18: 0 Digestion lipase : 2 palmitates sous forme d’AGNE peu solubles en milieu alcalin + point de fusion élevé: savons de calcium excrétés Oléate C 18: 1 Palmitate C 18: 0
 - Typicité de structure du palmitate dans le lait maternel permet d’éviter les problèmes de dureté des selles, - Améliore l’absorption du calcium et du palmitate, par rapport à du palmitate en 1, 3 - Améliore la rétention minérale osseuse (absorption calcium) ELEVATED COUNTS OF BIFIDOBACTERIA AND LACTOBACILLI FOLLOWING CONSUMPTION OF INFANT FORMULA CONTAINING BETA PALMITATE: A DOUBLE BLIND RANDOMIZED PILOT TRIAL S. Yaron, A. Riskin, D. Bader, I. Litmanovitz, F. Bar-Yoseph, Y. Lifshitz, R. Shamir, R. Shaoul (Israël) -14 enfants à terme BF -22 enfants à terme FF: 14 reçoivent une formule enrichie en beta palmitate (43% du palmitate total sous forme beta) et 8 une formule contrôle à base d’huile végétale (13%) - Bactério sur selles à l’inclusion et à 6 semaines Lactobacilli et Bifidobacteria plus élevés (et Clostridia tend à diminuer) dans les groupes BF et beta palmitate / formule contrôle
- Typicité de structure du palmitate dans le lait maternel permet d’éviter les problèmes de dureté des selles, - Améliore l’absorption du calcium et du palmitate, par rapport à du palmitate en 1, 3 - Améliore la rétention minérale osseuse (absorption calcium) ELEVATED COUNTS OF BIFIDOBACTERIA AND LACTOBACILLI FOLLOWING CONSUMPTION OF INFANT FORMULA CONTAINING BETA PALMITATE: A DOUBLE BLIND RANDOMIZED PILOT TRIAL S. Yaron, A. Riskin, D. Bader, I. Litmanovitz, F. Bar-Yoseph, Y. Lifshitz, R. Shamir, R. Shaoul (Israël) -14 enfants à terme BF -22 enfants à terme FF: 14 reçoivent une formule enrichie en beta palmitate (43% du palmitate total sous forme beta) et 8 une formule contrôle à base d’huile végétale (13%) - Bactério sur selles à l’inclusion et à 6 semaines Lactobacilli et Bifidobacteria plus élevés (et Clostridia tend à diminuer) dans les groupes BF et beta palmitate / formule contrôle
 2. Health impact of Enriched Alpha-lactalbumin in Infant nutrition C. Dupont, Hôpital Necker, Paris -Formules plus proches du lait maternel améliorer la qualité des protéines et diminuer la quantité Lait maternel 9 -12 g protéines/L Evolue au cours du temps Court-terme Taux circulant d’AA Insuline IGF-1 Hypothèse protéique Consommation de protéines en excès/besoins G ain de poids P oids / Taille Formules (enfants à terme) 14 -18 g protéines/L Constant Long-terme * Formules plus riches en protéines pour couvrir l’ensemble des besoins en AAE Risque d’obésité?
2. Health impact of Enriched Alpha-lactalbumin in Infant nutrition C. Dupont, Hôpital Necker, Paris -Formules plus proches du lait maternel améliorer la qualité des protéines et diminuer la quantité Lait maternel 9 -12 g protéines/L Evolue au cours du temps Court-terme Taux circulant d’AA Insuline IGF-1 Hypothèse protéique Consommation de protéines en excès/besoins G ain de poids P oids / Taille Formules (enfants à terme) 14 -18 g protéines/L Constant Long-terme * Formules plus riches en protéines pour couvrir l’ensemble des besoins en AAE Risque d’obésité?
 - Lait maternel: 2. 4 g/L d’alpha-lactalbumine - Formules base caséines: 0. 5 g/L - Formules base lactosérum: 1. 3 g/L - Formules enrichies en alpha-lactalbumine: 2. 2 g/L (permet de diminuer la quantité totale de protéines car riche en AAE) FE: Gain de poids intermédiaire entre FS et LM Vitesse de croissance, 120 jours -Formule standard: 14. 1 g/L protéines 662 kcal/L -Formule enrichie en alpha-lactalbumine: 12. 8 g/L -Lait maternel FS (n=108) FE (n=103) LM (n=110) Gain de poids (g/j) 28. 1 (5. 4)a 27. 8 (5. 3) 26. 6 (5. 4) Gain de taille (cm/mois) 3. 21 (0. 33) 3. 22 (0. 35)b 3. 12 (0. 32) Périmètre crânien (cm/mois) 1. 60 (0. 20) 1. 61 (0. 22)b 1. 55 (0. 18) a, différence entre FS et LM (p<0. 05) b, différence entre FE et LM (p<0. 05) Trabulsi et al. , 2011
- Lait maternel: 2. 4 g/L d’alpha-lactalbumine - Formules base caséines: 0. 5 g/L - Formules base lactosérum: 1. 3 g/L - Formules enrichies en alpha-lactalbumine: 2. 2 g/L (permet de diminuer la quantité totale de protéines car riche en AAE) FE: Gain de poids intermédiaire entre FS et LM Vitesse de croissance, 120 jours -Formule standard: 14. 1 g/L protéines 662 kcal/L -Formule enrichie en alpha-lactalbumine: 12. 8 g/L -Lait maternel FS (n=108) FE (n=103) LM (n=110) Gain de poids (g/j) 28. 1 (5. 4)a 27. 8 (5. 3) 26. 6 (5. 4) Gain de taille (cm/mois) 3. 21 (0. 33) 3. 22 (0. 35)b 3. 12 (0. 32) Périmètre crânien (cm/mois) 1. 60 (0. 20) 1. 61 (0. 22)b 1. 55 (0. 18) a, différence entre FS et LM (p<0. 05) b, différence entre FE et LM (p<0. 05) Trabulsi et al. , 2011
 Mechanisms of early programming, S. Ozanne, UK 1. Modèles de restriction protéique Souris R=recuperated offspring (RC) PLP=postnatal low-protein (CR) Groupe R restent plus gros à l’âge adulte que C (et PLP). Growth curves of pups of control, postnatal low protein and recuperated mice during lactation. Body weights of pups were recorded at days 3, 7, 14 and 21 of age. To maximize the effects of maternal diet, recuperated pups (R) were culled to 4 and control pups (C) were culled to 8 (if litter size was greater than 8) whereas postnatal low protein pups (PLP) were unculled. Means±SEM are shown (* P<0. 05, ** P<0. 01, *** P<0. 001 compared to control; n = C: 13, PLP: 11, R: 16). Ozanne: CR, restriction après le sevrage (Caloric Restriction)
Mechanisms of early programming, S. Ozanne, UK 1. Modèles de restriction protéique Souris R=recuperated offspring (RC) PLP=postnatal low-protein (CR) Groupe R restent plus gros à l’âge adulte que C (et PLP). Growth curves of pups of control, postnatal low protein and recuperated mice during lactation. Body weights of pups were recorded at days 3, 7, 14 and 21 of age. To maximize the effects of maternal diet, recuperated pups (R) were culled to 4 and control pups (C) were culled to 8 (if litter size was greater than 8) whereas postnatal low protein pups (PLP) were unculled. Means±SEM are shown (* P<0. 05, ** P<0. 01, *** P<0. 001 compared to control; n = C: 13, PLP: 11, R: 16). Ozanne: CR, restriction après le sevrage (Caloric Restriction)
 2. Régimes, croissance et espérance de vie Régime gestation (% protein) Régime lactation (% protein) Régime au sevrage Normal chow 20 20 Chow 765 22 Normal WD 20 20 WD 715 21 Catch-up chow 8 20 Chow 568 36 Catch up WD 8 20 WD 517 35 PLP chow 20 8 Chow 814 25 PLP WD 20 8 WD 807 28 (Souris mâle) Age moyen « at death » (j) - Effet bénéfique de la croissance lente (restriction protéique) - Effet protecteur contre WD Ilôts pancréatiques: -Rats RC: raccourcissement télomérique/CC - Marqueurs de sénescence cellulaire p 21 et p 16 : expressions augmentent chez RC à 3 mois (éq au niveau d’expression à 15 mois)
2. Régimes, croissance et espérance de vie Régime gestation (% protein) Régime lactation (% protein) Régime au sevrage Normal chow 20 20 Chow 765 22 Normal WD 20 20 WD 715 21 Catch-up chow 8 20 Chow 568 36 Catch up WD 8 20 WD 517 35 PLP chow 20 8 Chow 814 25 PLP WD 20 8 WD 807 28 (Souris mâle) Age moyen « at death » (j) - Effet bénéfique de la croissance lente (restriction protéique) - Effet protecteur contre WD Ilôts pancréatiques: -Rats RC: raccourcissement télomérique/CC - Marqueurs de sénescence cellulaire p 21 et p 16 : expressions augmentent chez RC à 3 mois (éq au niveau d’expression à 15 mois)
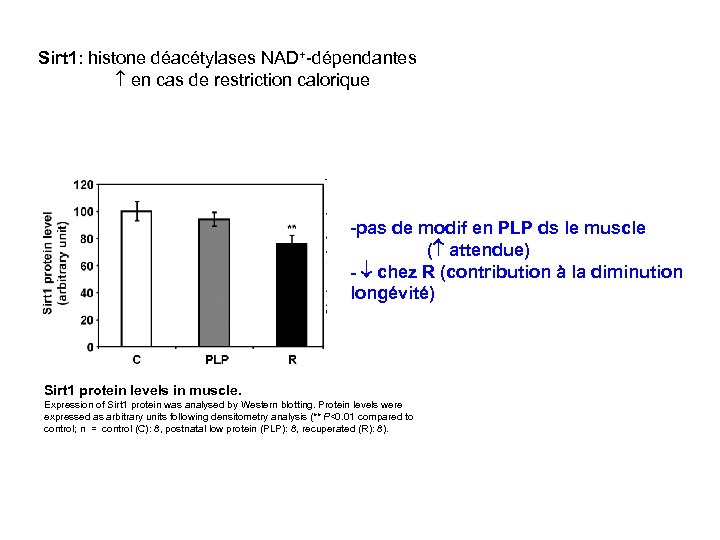 Sirt 1: histone déacétylases NAD+-dépendantes en cas de restriction calorique -pas de modif en PLP ds le muscle ( attendue) - chez R (contribution à la diminution longévité) Sirt 1 protein levels in muscle. Expression of Sirt 1 protein was analysed by Western blotting. Protein levels were expressed as arbitrary units following densitometry analysis (** P<0. 01 compared to control; n = control (C): 8, postnatal low protein (PLP): 8, recuperated (R): 8).
Sirt 1: histone déacétylases NAD+-dépendantes en cas de restriction calorique -pas de modif en PLP ds le muscle ( attendue) - chez R (contribution à la diminution longévité) Sirt 1 protein levels in muscle. Expression of Sirt 1 protein was analysed by Western blotting. Protein levels were expressed as arbitrary units following densitometry analysis (** P<0. 01 compared to control; n = control (C): 8, postnatal low protein (PLP): 8, recuperated (R): 8).
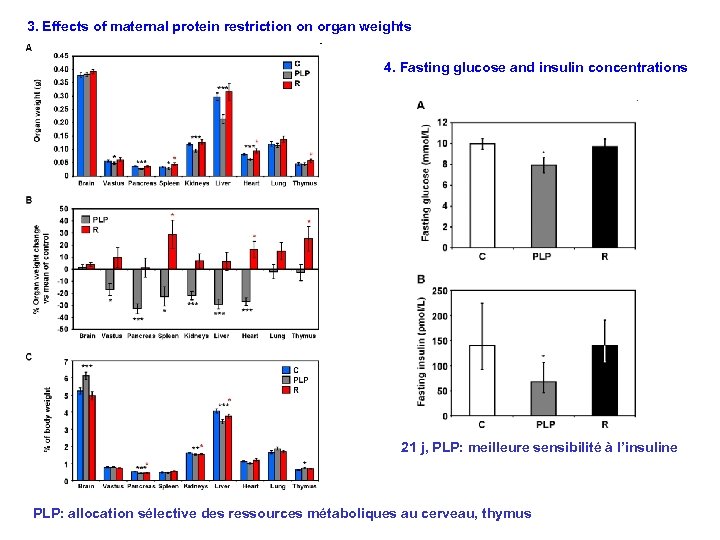 3. Effects of maternal protein restriction on organ weights 4. Fasting glucose and insulin concentrations 21 j, PLP: meilleure sensibilité à l’insuline PLP: allocation sélective des ressources métaboliques au cerveau, thymus
3. Effects of maternal protein restriction on organ weights 4. Fasting glucose and insulin concentrations 21 j, PLP: meilleure sensibilité à l’insuline PLP: allocation sélective des ressources métaboliques au cerveau, thymus
 Meilleure sensibilité à l’insuline contribue à une longévité augmentée Chez PLP - IRS 1 (insulin signalling) et PKC zeta /C (IS, active T glucose) - phosphorylation de IRS 1 (Ser 307) (régule négativement IS) Muscle 21 j Chez RC - IGF 1 R - PKC zeta et SU p 85 et p 110ß de la PI 3 kinase - phosphorylation de IRS 1 (Tyr 612) malgré taux d’insuline à jeun identique au C ( capacité régulatrice) - phosphorylation de Akt (Ser 473) - Data chez le rat - Cohorte danoise (hommes 19 ans, LBW/NBW) - PKC zeta et SU p 85 et p 110ß de la PI 3 kinase, fasting GLUT 4 Perspectives Placenta: p 110ß corrélation avec BW
Meilleure sensibilité à l’insuline contribue à une longévité augmentée Chez PLP - IRS 1 (insulin signalling) et PKC zeta /C (IS, active T glucose) - phosphorylation de IRS 1 (Ser 307) (régule négativement IS) Muscle 21 j Chez RC - IGF 1 R - PKC zeta et SU p 85 et p 110ß de la PI 3 kinase - phosphorylation de IRS 1 (Tyr 612) malgré taux d’insuline à jeun identique au C ( capacité régulatrice) - phosphorylation de Akt (Ser 473) - Data chez le rat - Cohorte danoise (hommes 19 ans, LBW/NBW) - PKC zeta et SU p 85 et p 110ß de la PI 3 kinase, fasting GLUT 4 Perspectives Placenta: p 110ß corrélation avec BW
 PNAS 2011 Contrôle épigénétique des interactions promoteur-enhancer Gène *HFN 4 a (ilôts pancréatiques) - Expression diminuée de 50% dans le diabète de type 2 - chez **RR (PCR et WB) à 3 mois et à 15 mois/ CC (+ effet âge) *hepatocyte nuclear factor 4 alpha, facteur de transcription, différentiation des cellules beta et homéostasie glucidique ** Éq. à nos RR Contrôle épigénétique de HFN 4 a Petite augmentation (5%) de la méthylation de l’ADN : région promotrice P 2 (développement précoce des ilôts et âge adulte) chez RR/CC qq soit l’âge Insuffisant pour expliquer la diminution de 60% d’ HFN 4 a chez RR
PNAS 2011 Contrôle épigénétique des interactions promoteur-enhancer Gène *HFN 4 a (ilôts pancréatiques) - Expression diminuée de 50% dans le diabète de type 2 - chez **RR (PCR et WB) à 3 mois et à 15 mois/ CC (+ effet âge) *hepatocyte nuclear factor 4 alpha, facteur de transcription, différentiation des cellules beta et homéostasie glucidique ** Éq. à nos RR Contrôle épigénétique de HFN 4 a Petite augmentation (5%) de la méthylation de l’ADN : région promotrice P 2 (développement précoce des ilôts et âge adulte) chez RR/CC qq soit l’âge Insuffisant pour expliquer la diminution de 60% d’ HFN 4 a chez RR
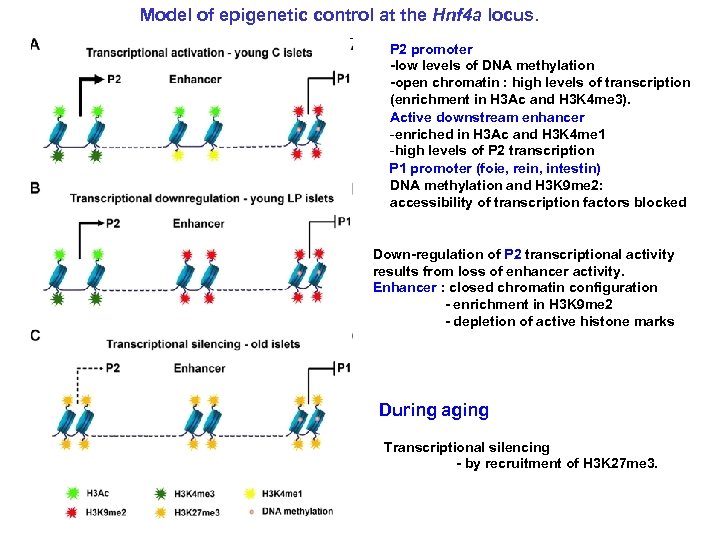 Model of epigenetic control at the Hnf 4 a locus. P 2 promoter -low levels of DNA methylation -open chromatin : high levels of transcription (enrichment in H 3 Ac and H 3 K 4 me 3). Active downstream enhancer -enriched in H 3 Ac and H 3 K 4 me 1 -high levels of P 2 transcription P 1 promoter (foie, rein, intestin) DNA methylation and H 3 K 9 me 2: accessibility of transcription factors blocked Down-regulation of P 2 transcriptional activity results from loss of enhancer activity. Enhancer : closed chromatin configuration - enrichment in H 3 K 9 me 2 - depletion of active histone marks During aging Transcriptional silencing - by recruitment of H 3 K 27 me 3.
Model of epigenetic control at the Hnf 4 a locus. P 2 promoter -low levels of DNA methylation -open chromatin : high levels of transcription (enrichment in H 3 Ac and H 3 K 4 me 3). Active downstream enhancer -enriched in H 3 Ac and H 3 K 4 me 1 -high levels of P 2 transcription P 1 promoter (foie, rein, intestin) DNA methylation and H 3 K 9 me 2: accessibility of transcription factors blocked Down-regulation of P 2 transcriptional activity results from loss of enhancer activity. Enhancer : closed chromatin configuration - enrichment in H 3 K 9 me 2 - depletion of active histone marks During aging Transcriptional silencing - by recruitment of H 3 K 27 me 3.
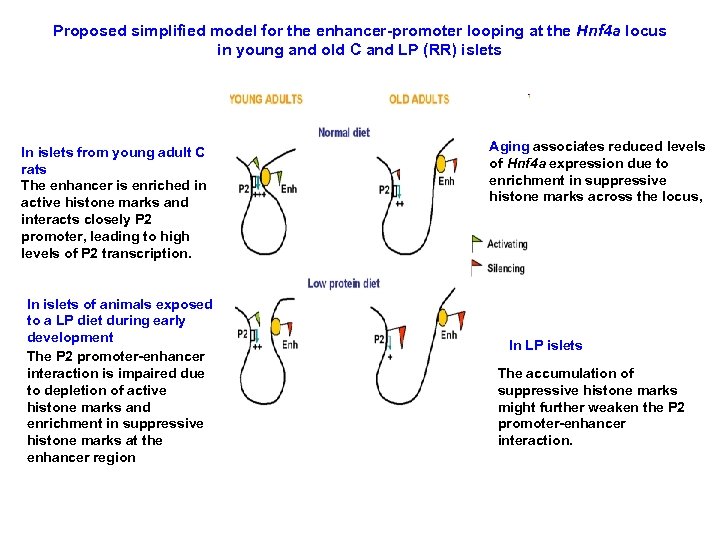 Proposed simplified model for the enhancer-promoter looping at the Hnf 4 a locus in young and old C and LP (RR) islets In islets from young adult C rats The enhancer is enriched in active histone marks and interacts closely P 2 promoter, leading to high levels of P 2 transcription. In islets of animals exposed to a LP diet during early development The P 2 promoter-enhancer interaction is impaired due to depletion of active histone marks and enrichment in suppressive histone marks at the enhancer region Aging associates reduced levels of Hnf 4 a expression due to enrichment in suppressive histone marks across the locus, In LP islets The accumulation of suppressive histone marks might further weaken the P 2 promoter-enhancer interaction.
Proposed simplified model for the enhancer-promoter looping at the Hnf 4 a locus in young and old C and LP (RR) islets In islets from young adult C rats The enhancer is enriched in active histone marks and interacts closely P 2 promoter, leading to high levels of P 2 transcription. In islets of animals exposed to a LP diet during early development The P 2 promoter-enhancer interaction is impaired due to depletion of active histone marks and enrichment in suppressive histone marks at the enhancer region Aging associates reduced levels of Hnf 4 a expression due to enrichment in suppressive histone marks across the locus, In LP islets The accumulation of suppressive histone marks might further weaken the P 2 promoter-enhancer interaction.
 DNA methylation A. Prentice, UK Supplémentation en micronutriments « autour de la conception » au Gambie - supplémentation (UNIMMAP) en folates (400µg/j), Zn et vitamines A, B, C, D. vs placebo - arrêt de la supplémentation à en moy 9, 5 semaines de grossesse - supplémentation anténatale en fer et folates (250µg/j) DNA extrait du sang de cordon Quantification de méthylation par spectrométrie de masse sur 13 « imprinted genes » , soumis à l’empreinte parentale Expression monoallélique initiée par méthylation différentielle entre gamètes - DMR: differentially methylated regions Effet sur 2 loci: diminution significative de la méthylation sur IGF 2 R chez filles, GTL 2 -DMR 2 chez les garçons Pas retrouvé chez les enfants (9 mois) Conclusions? ? ?
DNA methylation A. Prentice, UK Supplémentation en micronutriments « autour de la conception » au Gambie - supplémentation (UNIMMAP) en folates (400µg/j), Zn et vitamines A, B, C, D. vs placebo - arrêt de la supplémentation à en moy 9, 5 semaines de grossesse - supplémentation anténatale en fer et folates (250µg/j) DNA extrait du sang de cordon Quantification de méthylation par spectrométrie de masse sur 13 « imprinted genes » , soumis à l’empreinte parentale Expression monoallélique initiée par méthylation différentielle entre gamètes - DMR: differentially methylated regions Effet sur 2 loci: diminution significative de la méthylation sur IGF 2 R chez filles, GTL 2 -DMR 2 chez les garçons Pas retrouvé chez les enfants (9 mois) Conclusions? ? ?
 Modèle animal d’IUGR (mouton) 239 INFLUENCE OF BIRTH WEIGHT AND GENDER ON EARLY POSTNATAL GROWTH, METABOLISM AND BODY COMPOSITION. J. Wallace, J. Milne, R. Aitken, C. Adam, Aberdeen, UK Modèle IUGR: Repro par transfert d’embryon, gestation et lactation par des jeunes agnelles « overnourished » (altération de la répartition des nutriments vers l’utérus gravide et donc pb de développement du placenta et fœtus IUGR) -Growth rates measured at 5 -day intervals -FGR, fractional growth rate: absloute weight realtive to birth weight -Glucose tolerance test (GTT) at 50 d. - At 77 d: carcass composition analysis. At birth: IUGR lambs had - decreased weight, girth and height (P< 0. 001) - absolute growth rate for weight and girth lower (P<0. 05) and for height higher (P< 0. 02) - FGR for all measures of body size were 29 -35% higher (P< 0. 001) At GTT: - fasting glucose (IUGR>N, males>females; P< 0. 03) - first phase insulin response (IUGR< N, males
Modèle animal d’IUGR (mouton) 239 INFLUENCE OF BIRTH WEIGHT AND GENDER ON EARLY POSTNATAL GROWTH, METABOLISM AND BODY COMPOSITION. J. Wallace, J. Milne, R. Aitken, C. Adam, Aberdeen, UK Modèle IUGR: Repro par transfert d’embryon, gestation et lactation par des jeunes agnelles « overnourished » (altération de la répartition des nutriments vers l’utérus gravide et donc pb de développement du placenta et fœtus IUGR) -Growth rates measured at 5 -day intervals -FGR, fractional growth rate: absloute weight realtive to birth weight -Glucose tolerance test (GTT) at 50 d. - At 77 d: carcass composition analysis. At birth: IUGR lambs had - decreased weight, girth and height (P< 0. 001) - absolute growth rate for weight and girth lower (P<0. 05) and for height higher (P< 0. 02) - FGR for all measures of body size were 29 -35% higher (P< 0. 001) At GTT: - fasting glucose (IUGR>N, males>females; P< 0. 03) - first phase insulin response (IUGR< N, males
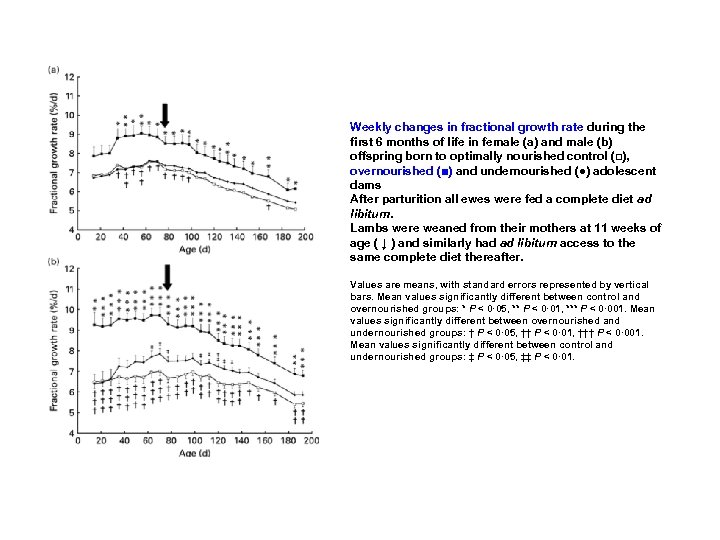 Weekly changes in fractional growth rate during the first 6 months of life in female (a) and male (b) offspring born to optimally nourished control (□), overnourished (■) and undernourished (●) adolescent dams After parturition all ewes were fed a complete diet ad libitum. Lambs were weaned from their mothers at 11 weeks of age ( ↓ ) and similarly had ad libitum access to the same complete diet thereafter. Values are means, with standard errors represented by vertical bars. Mean values significantly different between control and overnourished groups: * P < 0· 05, ** P < 0· 01, *** P < 0· 001. Mean values significantly different between overnourished and undernourished groups: † P < 0· 05, †† P < 0· 01, ††† P < 0· 001. Mean values significantly different between control and undernourished groups: ‡ P < 0· 05, ‡‡ P < 0· 01.
Weekly changes in fractional growth rate during the first 6 months of life in female (a) and male (b) offspring born to optimally nourished control (□), overnourished (■) and undernourished (●) adolescent dams After parturition all ewes were fed a complete diet ad libitum. Lambs were weaned from their mothers at 11 weeks of age ( ↓ ) and similarly had ad libitum access to the same complete diet thereafter. Values are means, with standard errors represented by vertical bars. Mean values significantly different between control and overnourished groups: * P < 0· 05, ** P < 0· 01, *** P < 0· 001. Mean values significantly different between overnourished and undernourished groups: † P < 0· 05, †† P < 0· 01, ††† P < 0· 001. Mean values significantly different between control and undernourished groups: ‡ P < 0· 05, ‡‡ P < 0· 01.
 Modèle animal d’IUGR (mouton) 240 INFLUENCE OF BIRTHWEIGHT AND GENDER ON EARLY POSTNATAL HYPOTHALAMIC ENERGY BALANCE REGULATORY GENE EXPRESSION C. Adam, T. Bake, P. Findlay, J. Milne, R. Aitken, J. Wallace Même modèle At 77 d, no difference in body weight and adiposity between N and IUGR but males were heavier than females, with lower adiposity and leptinaemia and higher insulinaemia. In situ hybridisation in the hypothalamic arcuate nucleus - greater gene expression for anorexigenic cocaine- and amphetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC) in females than males (P< 0. 01 -0. 05), - greater gene expression for leptin receptor (OB-Rb) and orexigenic neuropeptide Y (NPY) and agouti-related peptide (AGRP) in males than females (P< 0. 001 -0. 01), with no effect of birthweight. OB-Rb gene expression correlated negatively with body fat, plasma leptin, CART and POMC (P< 0. 01 -0. 05) and positively with NPY and AGRP gene expression (P< 0. 001). No effect of birthweight or gender on insulin receptor gene expression, which showed no correlation with insulinaemia or neuropeptide gene expression. This study revealed no effect of IUGR on early postnatal hypothalamic energy balance gene expression but a major effect of gender linked to sex differences in postnatal growth, adiposity and leptinaemia.
Modèle animal d’IUGR (mouton) 240 INFLUENCE OF BIRTHWEIGHT AND GENDER ON EARLY POSTNATAL HYPOTHALAMIC ENERGY BALANCE REGULATORY GENE EXPRESSION C. Adam, T. Bake, P. Findlay, J. Milne, R. Aitken, J. Wallace Même modèle At 77 d, no difference in body weight and adiposity between N and IUGR but males were heavier than females, with lower adiposity and leptinaemia and higher insulinaemia. In situ hybridisation in the hypothalamic arcuate nucleus - greater gene expression for anorexigenic cocaine- and amphetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC) in females than males (P< 0. 01 -0. 05), - greater gene expression for leptin receptor (OB-Rb) and orexigenic neuropeptide Y (NPY) and agouti-related peptide (AGRP) in males than females (P< 0. 001 -0. 01), with no effect of birthweight. OB-Rb gene expression correlated negatively with body fat, plasma leptin, CART and POMC (P< 0. 01 -0. 05) and positively with NPY and AGRP gene expression (P< 0. 001). No effect of birthweight or gender on insulin receptor gene expression, which showed no correlation with insulinaemia or neuropeptide gene expression. This study revealed no effect of IUGR on early postnatal hypothalamic energy balance gene expression but a major effect of gender linked to sex differences in postnatal growth, adiposity and leptinaemia.
 Composition lipidique et régulation hypothalamique du métabolisme énergétique 69 DEVELOPMENT OF HYPOTHALAMIC PATHWAYS REGULATING METABOLISM IS AFFECTED BY FATTY ACID COMPOSITION OF POSTNATAL NUTRITION IN MICE E. M. van der Beek 1, L. Schipper 2, K. Bouyer 3, A. Oosting 2, R. B. Simerly 3, 4 1 Danone Research - Singapore, 2 Danone Research, Wageningen, The Netherlands, 3 Saban Research Institute, Los Angeles, 4 Department of Pediatrics, Los Angeles, CA, USA Postnatal diets / fat quality : moins d’ω6 ou plus d’ω3 protection contre une accumulation excessive de masse grasse à l’âge adulte (C 57 Bl/6 j mice), lors d’une exposition à un régime « mild obesogenic » . Est-ce la conséquence d’une modification de la régulation centrale de la prise alimentaire et de la balance énergétique? Study: effects of modest differences in dietary fatty acid composition on hypothalamic fibre network development. Male mouse, three diets: - 50% reduced ω6 (LOWn 6, specifically linoleic acid) - increased ω3 (HIGHn 3, 5% DHA) - control (CTRL) from postnatal day 2 to 28. (? ) Density of fibers (Ag. RP and αMSH) in the arcuate nucleus in mice fed LOWn 6 or HIGHn 3 was significantly reduced compared to CTRL mice. Fat quality in postnatal diets affect hypothalamic pathways regulating energy intake and expenditure. Programmation à long-terme de la balance E : pourrait expliquer la moindre accumulation de masse grasse à l’âge adulte
Composition lipidique et régulation hypothalamique du métabolisme énergétique 69 DEVELOPMENT OF HYPOTHALAMIC PATHWAYS REGULATING METABOLISM IS AFFECTED BY FATTY ACID COMPOSITION OF POSTNATAL NUTRITION IN MICE E. M. van der Beek 1, L. Schipper 2, K. Bouyer 3, A. Oosting 2, R. B. Simerly 3, 4 1 Danone Research - Singapore, 2 Danone Research, Wageningen, The Netherlands, 3 Saban Research Institute, Los Angeles, 4 Department of Pediatrics, Los Angeles, CA, USA Postnatal diets / fat quality : moins d’ω6 ou plus d’ω3 protection contre une accumulation excessive de masse grasse à l’âge adulte (C 57 Bl/6 j mice), lors d’une exposition à un régime « mild obesogenic » . Est-ce la conséquence d’une modification de la régulation centrale de la prise alimentaire et de la balance énergétique? Study: effects of modest differences in dietary fatty acid composition on hypothalamic fibre network development. Male mouse, three diets: - 50% reduced ω6 (LOWn 6, specifically linoleic acid) - increased ω3 (HIGHn 3, 5% DHA) - control (CTRL) from postnatal day 2 to 28. (? ) Density of fibers (Ag. RP and αMSH) in the arcuate nucleus in mice fed LOWn 6 or HIGHn 3 was significantly reduced compared to CTRL mice. Fat quality in postnatal diets affect hypothalamic pathways regulating energy intake and expenditure. Programmation à long-terme de la balance E : pourrait expliquer la moindre accumulation de masse grasse à l’âge adulte
 Propriétés physique des gouttelettes lipidiques du lait 12 A LIPID MATRIX WITH PROPERTIES CLOSER TO THOSE IN HUMAN MILK REDUCES EXCESSIVE BODY FAT ACCUMULATION IN ADULT MICE A. Oosting 1, E. Engels 1, D. Kegler 1, M. Abrahamse-Berkeveld 1, I. C. Teller 1, E. M. van der Beek 2 1 Danone, Wageningen, The Netherlands, 2 Danone, Singapore - Sustained effect of fatty acid composition of postnatal diet on fat mass accumulation in adult mice. - Compared to infant formula (IF), lipid globules in human milk are up to ten times larger and coated with a phospholipid membrane. - Developement of an IF concept with a complex lipid matrix containing larger lipid droplets coated with phospholipids (Nuturis®). Study: to evaluate long-term effects of Nuturis® as part of early postnatal diet on body composition development in adult C 57 BI/6 j mice. From postnatal day (PN) 16 to 42; male mice; fed with a diet containing either Nuturis® or standard IF (CTRL). Subsequently, mice were challenged with a moderate Western style diet (20% fat) until PN 98. Mice raised on CTRL and switched to standard chow served as non-challenged reference. Analyses: Body composition (DEXA) and fat depots at PN 98. - Adult body weight and total, visceral and subcutaneous fat weights of the Nuturis® group were lower than CTRL (éq. reference). No differences were observed for food intake and lean body mass between groups. - Adipocyte number was comparable in all groups but adipocytes of Nuturis® fed animals were smaller than CTRL (éq. reference). Conclusion: besides fatty acid composition other aspects of lipid quality in the early diet can reduce susceptibility for excessive fat accumulation of adult mice in a mildly obesogenic environment.
Propriétés physique des gouttelettes lipidiques du lait 12 A LIPID MATRIX WITH PROPERTIES CLOSER TO THOSE IN HUMAN MILK REDUCES EXCESSIVE BODY FAT ACCUMULATION IN ADULT MICE A. Oosting 1, E. Engels 1, D. Kegler 1, M. Abrahamse-Berkeveld 1, I. C. Teller 1, E. M. van der Beek 2 1 Danone, Wageningen, The Netherlands, 2 Danone, Singapore - Sustained effect of fatty acid composition of postnatal diet on fat mass accumulation in adult mice. - Compared to infant formula (IF), lipid globules in human milk are up to ten times larger and coated with a phospholipid membrane. - Developement of an IF concept with a complex lipid matrix containing larger lipid droplets coated with phospholipids (Nuturis®). Study: to evaluate long-term effects of Nuturis® as part of early postnatal diet on body composition development in adult C 57 BI/6 j mice. From postnatal day (PN) 16 to 42; male mice; fed with a diet containing either Nuturis® or standard IF (CTRL). Subsequently, mice were challenged with a moderate Western style diet (20% fat) until PN 98. Mice raised on CTRL and switched to standard chow served as non-challenged reference. Analyses: Body composition (DEXA) and fat depots at PN 98. - Adult body weight and total, visceral and subcutaneous fat weights of the Nuturis® group were lower than CTRL (éq. reference). No differences were observed for food intake and lean body mass between groups. - Adipocyte number was comparable in all groups but adipocytes of Nuturis® fed animals were smaller than CTRL (éq. reference). Conclusion: besides fatty acid composition other aspects of lipid quality in the early diet can reduce susceptibility for excessive fat accumulation of adult mice in a mildly obesogenic environment.
 Supplémentation maternelle Qualité des lipides 29 MATERNAL DOCOSAHEXAENOIC ACID (DHA) SUPPLEMENTATION IN OVERWEIGHT/OBESE WOMEN LESSENS INFANT ADIPOSITY AT BIRTH D. A. Krummel 1, A. Baker 2, A. Cassin 2, S. Davis 2, A. Gundamaraju 2, J. Khoury 3, T. Powell 4; USA Obesity and pregnancy : - low-grade inflammatory states - increase the risk of fetal adiposity in the short term - and metabolic syndrome with related diseases in the long term. DHA decreases inflammatory markers (improve insulin action in nonpregnant women) DHA and risk for fetal overgrowth? Aim: to investigate the effectiveness of DHA supplementation in overweight/obese pregnant women on optimizing fetal growth. Methods: Healthy, pregnant women (N=78; pre-pregnant BMI 25 -58 were randomized to the placebo (corn/soy oil blend - 0 mg DHA) or DHA (algal oil - 800 mg DHA) group at 26 weeks of pregnancy until study end (36 -37 weeks). Adherence was assessed by erythrocyte (RBC) DHA. Infant physical growth (recumbent length, weight) and adiposity ( {BMI}, ponderal index {PI}, weight for length) was assessed at birth and plotted on WHO growth curves. Results: The DHA group had higher RBC, DHA at study end (p<. 0001). The WHO length-forage percentile was higher (p<. 05) and PI lower (p<. 02) in infants born to mothers in the DHA group versus the placebo group. Having an infant with a BMI > than the 85 th percentile was 9. 3 times greater for mothers in the placebo group (OR: 9. 33, 95%CI 1. 10 -79. 9) (P=. 04). Conclusions: DHA supplementation in women with pregravid overweight/obesity resulted in infants with lower adiposity at birth.
Supplémentation maternelle Qualité des lipides 29 MATERNAL DOCOSAHEXAENOIC ACID (DHA) SUPPLEMENTATION IN OVERWEIGHT/OBESE WOMEN LESSENS INFANT ADIPOSITY AT BIRTH D. A. Krummel 1, A. Baker 2, A. Cassin 2, S. Davis 2, A. Gundamaraju 2, J. Khoury 3, T. Powell 4; USA Obesity and pregnancy : - low-grade inflammatory states - increase the risk of fetal adiposity in the short term - and metabolic syndrome with related diseases in the long term. DHA decreases inflammatory markers (improve insulin action in nonpregnant women) DHA and risk for fetal overgrowth? Aim: to investigate the effectiveness of DHA supplementation in overweight/obese pregnant women on optimizing fetal growth. Methods: Healthy, pregnant women (N=78; pre-pregnant BMI 25 -58 were randomized to the placebo (corn/soy oil blend - 0 mg DHA) or DHA (algal oil - 800 mg DHA) group at 26 weeks of pregnancy until study end (36 -37 weeks). Adherence was assessed by erythrocyte (RBC) DHA. Infant physical growth (recumbent length, weight) and adiposity ( {BMI}, ponderal index {PI}, weight for length) was assessed at birth and plotted on WHO growth curves. Results: The DHA group had higher RBC, DHA at study end (p<. 0001). The WHO length-forage percentile was higher (p<. 05) and PI lower (p<. 02) in infants born to mothers in the DHA group versus the placebo group. Having an infant with a BMI > than the 85 th percentile was 9. 3 times greater for mothers in the placebo group (OR: 9. 33, 95%CI 1. 10 -79. 9) (P=. 04). Conclusions: DHA supplementation in women with pregravid overweight/obesity resulted in infants with lower adiposity at birth.
 Obésité et supplémentation maternelle en taurine 325 MATERNAL TAURINE SUPPLEMENTATION MODIFIES GROWTH AND METABOLISM IN MALE RAT OFFSPRING OF MOTHERS FED AN OBESOGENIC DIET DURING PREGNANCY AND LACTATION M. Li, D. Sloboda, K. Hepple, M. Vickers (New Zealand) - Maternal obesity results in obese offspring with metabolic dysfunction. - Taurine ameliorates adverse metabolic outcomes in offspring of undernourished mothers. Maternal obesogenic diet? - Control (CONT): dams fed control diet during pregnancy and lactation - CONT-Taurine : dams supplemented with 1. 5% taurine - Maternal obesity (MO): dams fed an obesogenic diet during pregnancy and lactation - MO dams supplemented with taurine (MO+Tau) Offspring were fed a control diet postweaning. Pubertal onset was assessed and a glucose tolerance test and obesogenic diet challenge performed in offspring. Results: Puberty was advanced in MO males compared to CONT (pas d’effet taurine) As adults, MO offspring : increased body fat / CONT. MO+Tau offspring : reduced absolute body weight gain / MO offspring and similar to CONT offspring. MO offspring : - impaired glucose tolerance/ CONT, but no differences between MO+Tau /CONT. - increased caloric intake and weight gain / CONT when offered an obesogenic diet but no differences between MO+Tau and CONT. Conclusions: A maternal obesogenic diet led to increased fat mass, body weight gain, appetite and impaired glucose tolerance in offspring. Taurine modified metabolic dysfunction in offspring of obese mothers and may act as a conditionally essential amino acid during early life development.
Obésité et supplémentation maternelle en taurine 325 MATERNAL TAURINE SUPPLEMENTATION MODIFIES GROWTH AND METABOLISM IN MALE RAT OFFSPRING OF MOTHERS FED AN OBESOGENIC DIET DURING PREGNANCY AND LACTATION M. Li, D. Sloboda, K. Hepple, M. Vickers (New Zealand) - Maternal obesity results in obese offspring with metabolic dysfunction. - Taurine ameliorates adverse metabolic outcomes in offspring of undernourished mothers. Maternal obesogenic diet? - Control (CONT): dams fed control diet during pregnancy and lactation - CONT-Taurine : dams supplemented with 1. 5% taurine - Maternal obesity (MO): dams fed an obesogenic diet during pregnancy and lactation - MO dams supplemented with taurine (MO+Tau) Offspring were fed a control diet postweaning. Pubertal onset was assessed and a glucose tolerance test and obesogenic diet challenge performed in offspring. Results: Puberty was advanced in MO males compared to CONT (pas d’effet taurine) As adults, MO offspring : increased body fat / CONT. MO+Tau offspring : reduced absolute body weight gain / MO offspring and similar to CONT offspring. MO offspring : - impaired glucose tolerance/ CONT, but no differences between MO+Tau /CONT. - increased caloric intake and weight gain / CONT when offered an obesogenic diet but no differences between MO+Tau and CONT. Conclusions: A maternal obesogenic diet led to increased fat mass, body weight gain, appetite and impaired glucose tolerance in offspring. Taurine modified metabolic dysfunction in offspring of obese mothers and may act as a conditionally essential amino acid during early life development.
 Peu importe le poids de naissance, c’est la vitesse de croissance qui crée le risque de syndrome métabolique 327 EFFECTS OF INTRAUTERINE GROWTH ON INSULIN RESISTANCE AND METABOLIC DERANGEMENTS IN CHILDHOOD AND ADOLESCENCE V. P. Wickramasinghe 1, C. Arambepola 2, P. Bandara 1, M. Abeysekera 1, S. Kuruppu 1, P. Dilshan 1, B. S. Dissanayake 1 Sri Lanka Objective: To identify effects of intrauterine and later growth on metabolic derangements among 5 -15 year old children in Colombo, Sri Lanka. Materials and methods: 833 (boys 494) children - Fasting blood glucose/insulin, lipid profile, 2 hour OGTT/insulin, height, weight, fat mass (FM) and blood pressure (BP) - Sample was stratified by age (5 -10, >10 -15 yrs). Each group was categorized to tertiles of birth weight and current BMI. Results: LBW et highest BMI tertile = poor metabolic profile. Résultats significatifs dans la catégorie 10 -15 ans. Pas d’effet sexe. In the 10 -15 year group, LBW high BMI LBW low BMI % fat mass 32. 6 16. 1 Blood pressure (cm Hg) 10. 9 9. 8 Cholesterol (mg/d. L) 169. 7 162. 8 TG (mg/d. L) 98. 5 72. 7 Fasting insulin (U/L) 69. 9 28. 5 Postprandial insulin 577 157 HOMA (IR) 2. 1 0. 81 Conclusion: Birth weight independently is not a risk factor for abnormal metabolic profile in childhood. Those who gain higher BMI are at a higher risk than those who remain small. This favours the accelerated post natal growth hypothesis.
Peu importe le poids de naissance, c’est la vitesse de croissance qui crée le risque de syndrome métabolique 327 EFFECTS OF INTRAUTERINE GROWTH ON INSULIN RESISTANCE AND METABOLIC DERANGEMENTS IN CHILDHOOD AND ADOLESCENCE V. P. Wickramasinghe 1, C. Arambepola 2, P. Bandara 1, M. Abeysekera 1, S. Kuruppu 1, P. Dilshan 1, B. S. Dissanayake 1 Sri Lanka Objective: To identify effects of intrauterine and later growth on metabolic derangements among 5 -15 year old children in Colombo, Sri Lanka. Materials and methods: 833 (boys 494) children - Fasting blood glucose/insulin, lipid profile, 2 hour OGTT/insulin, height, weight, fat mass (FM) and blood pressure (BP) - Sample was stratified by age (5 -10, >10 -15 yrs). Each group was categorized to tertiles of birth weight and current BMI. Results: LBW et highest BMI tertile = poor metabolic profile. Résultats significatifs dans la catégorie 10 -15 ans. Pas d’effet sexe. In the 10 -15 year group, LBW high BMI LBW low BMI % fat mass 32. 6 16. 1 Blood pressure (cm Hg) 10. 9 9. 8 Cholesterol (mg/d. L) 169. 7 162. 8 TG (mg/d. L) 98. 5 72. 7 Fasting insulin (U/L) 69. 9 28. 5 Postprandial insulin 577 157 HOMA (IR) 2. 1 0. 81 Conclusion: Birth weight independently is not a risk factor for abnormal metabolic profile in childhood. Those who gain higher BMI are at a higher risk than those who remain small. This favours the accelerated post natal growth hypothesis.
 39 ASSOCIATION OF WEIGHT GAIN IN EARLY LIFE WITH BODY COMPOSITION AND HORMONAL STATUS AT 20 YEARS S. Péneau, M. -F. Rolland-Cachera CRNH Id. F, UMR U 557 Inserm, U 1125 Inra, Cnam, Paris 13, Bobigny, France Long term follow-up : early growth velocity with body composition and hormonal status at 20 years. 73 healthy French children recruited in Health Centres. At 20 years, anthropometric measurements, body composition (BIA) and serum leptin Birth measurements were recorded in health booklets. Results: Association between weight gain at 0 -2 years and measurements at 20 years using linear regression analysis. (Adjusted for gender) Body Mass Index (kg/m 2 ) ; Waist circumference (cm) ; Subscapular skinfold (mm); Triceps skinfold (mm) ; Fat Mass BIA (kg) (Adjusted for height); Lean Mass BIA (kg) (Adjustedfor height); Fat Mass BIA (%) ; Leptin Conclusions: -Association between rapid growth in early life, high body fat, particularly at the trunk site, and leptin concentration at adult age reflects a deleterious influence of rapid growth. -Rapid growth in early life may not be considered optimal. Topo MF Rolland-Cachera: Rebond d’adiposité (normal à 6 ans) RA svt associé à un low BMI avant → 3 -4 ans Low BMI High BMI Dvpmt fat mass (pb cardiovasc. , diabète…) High BMI Dvpmt fat mass et fat free mass (obésité mais moins de problèmes métaboliques)
39 ASSOCIATION OF WEIGHT GAIN IN EARLY LIFE WITH BODY COMPOSITION AND HORMONAL STATUS AT 20 YEARS S. Péneau, M. -F. Rolland-Cachera CRNH Id. F, UMR U 557 Inserm, U 1125 Inra, Cnam, Paris 13, Bobigny, France Long term follow-up : early growth velocity with body composition and hormonal status at 20 years. 73 healthy French children recruited in Health Centres. At 20 years, anthropometric measurements, body composition (BIA) and serum leptin Birth measurements were recorded in health booklets. Results: Association between weight gain at 0 -2 years and measurements at 20 years using linear regression analysis. (Adjusted for gender) Body Mass Index (kg/m 2 ) ; Waist circumference (cm) ; Subscapular skinfold (mm); Triceps skinfold (mm) ; Fat Mass BIA (kg) (Adjusted for height); Lean Mass BIA (kg) (Adjustedfor height); Fat Mass BIA (%) ; Leptin Conclusions: -Association between rapid growth in early life, high body fat, particularly at the trunk site, and leptin concentration at adult age reflects a deleterious influence of rapid growth. -Rapid growth in early life may not be considered optimal. Topo MF Rolland-Cachera: Rebond d’adiposité (normal à 6 ans) RA svt associé à un low BMI avant → 3 -4 ans Low BMI High BMI Dvpmt fat mass (pb cardiovasc. , diabète…) High BMI Dvpmt fat mass et fat free mass (obésité mais moins de problèmes métaboliques)
 Sensibilité à l’insuline 376 ADIPONECTIN LEVELS AND INFANT WEIGHT GAIN OVER THE FIRST TWO YEARS OF LIFE P. Prentice 1, P. Barker 2, K. Burling 2, K. Ong 1, D. Dunger 1 Cambridge, UK Background: - Adiponectin affects insulin sensitivity, glucose and fatty acid metabolism, and the immune system - Early life adiponectin has been associated with childhood adiposity in small-for gestational age (SGA) infants showing 'catch-up' growth. In non-SGA infants? Study: Potential association between adiponectin and weight gain between 0 -2 years in a cohort of non-SGA infants (≥ 36 weeks gestation). Method: The Cambridge Baby Growth Study (CBGS) has detailed infant anthropometry and dried blood spot (DBS) collection from birth to 2 years. A subset of DBS collected from 63 children (36 female) at 12 months were processed using an adiponectin immunoassay. Samples were selected for infants showing 'catch-up' (n=21, ≥ 0. 67 sds weight gain), 'catch-down' (n=19, ≤ 0. 67 sds) or 'normal' growth (n=23) over 0 -2 years. Results: -Adiponectin normally distributed, without differences between breast and formulated infants -lower adiponectin levels in female infants with 'catch-up' (6. 75+/-2. 81μg/ml), compared with 'catch-down' (8. 86+/2. 52μg/ml) and 'normal' weight-gain (9. 56+/-3. 35μg/ml). -lower adiponectin in females showing 'catch-up' between 12 -24 months (p=0. 037), but not between 0 -12 months -Female adiponectin levels were significantly inversely correlated with 12 -24 month weight-gain (r=-0. 348; p=0. 038). Conclusion: results suggest an association between adiponectin levels at 12 months and weight gain over the first 2 years of life.
Sensibilité à l’insuline 376 ADIPONECTIN LEVELS AND INFANT WEIGHT GAIN OVER THE FIRST TWO YEARS OF LIFE P. Prentice 1, P. Barker 2, K. Burling 2, K. Ong 1, D. Dunger 1 Cambridge, UK Background: - Adiponectin affects insulin sensitivity, glucose and fatty acid metabolism, and the immune system - Early life adiponectin has been associated with childhood adiposity in small-for gestational age (SGA) infants showing 'catch-up' growth. In non-SGA infants? Study: Potential association between adiponectin and weight gain between 0 -2 years in a cohort of non-SGA infants (≥ 36 weeks gestation). Method: The Cambridge Baby Growth Study (CBGS) has detailed infant anthropometry and dried blood spot (DBS) collection from birth to 2 years. A subset of DBS collected from 63 children (36 female) at 12 months were processed using an adiponectin immunoassay. Samples were selected for infants showing 'catch-up' (n=21, ≥ 0. 67 sds weight gain), 'catch-down' (n=19, ≤ 0. 67 sds) or 'normal' growth (n=23) over 0 -2 years. Results: -Adiponectin normally distributed, without differences between breast and formulated infants -lower adiponectin levels in female infants with 'catch-up' (6. 75+/-2. 81μg/ml), compared with 'catch-down' (8. 86+/2. 52μg/ml) and 'normal' weight-gain (9. 56+/-3. 35μg/ml). -lower adiponectin in females showing 'catch-up' between 12 -24 months (p=0. 037), but not between 0 -12 months -Female adiponectin levels were significantly inversely correlated with 12 -24 month weight-gain (r=-0. 348; p=0. 038). Conclusion: results suggest an association between adiponectin levels at 12 months and weight gain over the first 2 years of life.
 Biomarqueurs 348 PLASMA CYSTEINE: AN UNRECOGNIZED PREDICTOR OF CHILDHOOD ADIPOSITY AND INSULIN RESISTANCE A. Elshorbagy 1, 2, M. Valdivia-Garcia 1, A. D. Smith 1, H. Refsum 1, 3, N. Butte 4, UK, Egypt, Norway, USA Background aims: -The sulfur amino acid cysteine promotes adiposity and decreases glucose tolerance in rodents. -Plasma total cysteine (t. Cys) is strongly associated with fat mass in adults. -Is t. Cys associated with inflammatory obesity and insulin resistance in children? Methods: relations of fasting plasma t. Cys and related metabolites with body composition (DEXA) in 984 Hispanic children/adolescents aged 4 -19 y from the Viva La Familia Study. Linear and logistic regression and dose-response curves were used to evaluate relations of t. Cys with obesity, insulin resistance and inflammatory biomarkers. Results: - t. Cys, methionine and total homocysteine (t. Hcy) increased with age, while folate and vitamin B 12 decreased. -t. GSH and vitamin B 12 were lower in overweight/obese children and correlated inversely with body fat%. - Upper t. Cys quartile was independently associated with a 5 -fold increased risk of obesity (95% CI: 3. 5 -8. 0, P< 0. 001), and nearly 3 -fold risk for insulin resistance independent of body fat% (95% CI: 1. 65. 0, P< 0. 001). - Within the overweight/obese subgroup, but not in lean children, t. Cys accounted for 9% of the variability in body fat% (partial r= 0. 30, P< 0. 001, after multiple adjustments), and also correlated with insulin resistance (partial r=0. 20, P< 0. 001; age and gender adjusted) and serum CRP, leptin and adiponectin. Conclusion: t. Cys is associated with obesity, systemic inflammation and insulin resistance in children and adolescents substantiating the novel link between the sulfur amino acid pathway and obesity and cardiometabolic risk.
Biomarqueurs 348 PLASMA CYSTEINE: AN UNRECOGNIZED PREDICTOR OF CHILDHOOD ADIPOSITY AND INSULIN RESISTANCE A. Elshorbagy 1, 2, M. Valdivia-Garcia 1, A. D. Smith 1, H. Refsum 1, 3, N. Butte 4, UK, Egypt, Norway, USA Background aims: -The sulfur amino acid cysteine promotes adiposity and decreases glucose tolerance in rodents. -Plasma total cysteine (t. Cys) is strongly associated with fat mass in adults. -Is t. Cys associated with inflammatory obesity and insulin resistance in children? Methods: relations of fasting plasma t. Cys and related metabolites with body composition (DEXA) in 984 Hispanic children/adolescents aged 4 -19 y from the Viva La Familia Study. Linear and logistic regression and dose-response curves were used to evaluate relations of t. Cys with obesity, insulin resistance and inflammatory biomarkers. Results: - t. Cys, methionine and total homocysteine (t. Hcy) increased with age, while folate and vitamin B 12 decreased. -t. GSH and vitamin B 12 were lower in overweight/obese children and correlated inversely with body fat%. - Upper t. Cys quartile was independently associated with a 5 -fold increased risk of obesity (95% CI: 3. 5 -8. 0, P< 0. 001), and nearly 3 -fold risk for insulin resistance independent of body fat% (95% CI: 1. 65. 0, P< 0. 001). - Within the overweight/obese subgroup, but not in lean children, t. Cys accounted for 9% of the variability in body fat% (partial r= 0. 30, P< 0. 001, after multiple adjustments), and also correlated with insulin resistance (partial r=0. 20, P< 0. 001; age and gender adjusted) and serum CRP, leptin and adiponectin. Conclusion: t. Cys is associated with obesity, systemic inflammation and insulin resistance in children and adolescents substantiating the novel link between the sulfur amino acid pathway and obesity and cardiometabolic risk.
 Nutrition et composition corporelle 36 THE EFFECT OF BREAST VERSUS FORMULA FEEDING ON INFANT BODY COMPOSITION: A SYSTEMATIC REVIEW AND META-ANALYSIS C. Gale, K. M. Logan, S. Santhakumaran, J. R. C. Parkinson, H. J. Matthew, N. Modi UK Objective: To conduct a systematic review and meta-analysis of studies examining body composition in healthy, term infants in relation to breast or formula feeding. Design: Pub. Med was searched for human studies reporting the outcomes: fat-free mass, fatmass or percentage fat-mass, in breast and formula-fed infants. Differences in outcomes between feeding groups were compared at pre-specified ages, Results: We identified 15 studies for inclusion in the systematic review and 11 studies for inclusion in the meta-analysis. In formula compared to breast-fed infants, fat-free mass increased at 3 -4 months (mean difference [95%CI]) (0. 12 kg [0. 02, 0. 22]), 8 -9 months (0. 29 kg [0. 09, 0. 49]) and 12 months (0. 30 kg [0. 13, 0. 48]); fat-mass reduced at 3 -4 months (-0. 09 kg [-0. 18, -0. 01]) and 6 months (-0. 17 [-0. 33, -0. 01]). Conversely at 12 months : increase in fat-mass in formula-fed infants (0. 29 kg [-0. 03, 0. 61]). Conclusions: Formula feeding, when compared to breast feeding, is associated with altered body composition in infancy. - early increase in fat-mass associated with breast feeding may represent an evolutionary response promoting infant survival. -increase in fat-free mass, accompanied by a trend to greater adiposity in formula-fed infants by one year of age is indicative of nutritional programming
Nutrition et composition corporelle 36 THE EFFECT OF BREAST VERSUS FORMULA FEEDING ON INFANT BODY COMPOSITION: A SYSTEMATIC REVIEW AND META-ANALYSIS C. Gale, K. M. Logan, S. Santhakumaran, J. R. C. Parkinson, H. J. Matthew, N. Modi UK Objective: To conduct a systematic review and meta-analysis of studies examining body composition in healthy, term infants in relation to breast or formula feeding. Design: Pub. Med was searched for human studies reporting the outcomes: fat-free mass, fatmass or percentage fat-mass, in breast and formula-fed infants. Differences in outcomes between feeding groups were compared at pre-specified ages, Results: We identified 15 studies for inclusion in the systematic review and 11 studies for inclusion in the meta-analysis. In formula compared to breast-fed infants, fat-free mass increased at 3 -4 months (mean difference [95%CI]) (0. 12 kg [0. 02, 0. 22]), 8 -9 months (0. 29 kg [0. 09, 0. 49]) and 12 months (0. 30 kg [0. 13, 0. 48]); fat-mass reduced at 3 -4 months (-0. 09 kg [-0. 18, -0. 01]) and 6 months (-0. 17 [-0. 33, -0. 01]). Conversely at 12 months : increase in fat-mass in formula-fed infants (0. 29 kg [-0. 03, 0. 61]). Conclusions: Formula feeding, when compared to breast feeding, is associated with altered body composition in infancy. - early increase in fat-mass associated with breast feeding may represent an evolutionary response promoting infant survival. -increase in fat-free mass, accompanied by a trend to greater adiposity in formula-fed infants by one year of age is indicative of nutritional programming
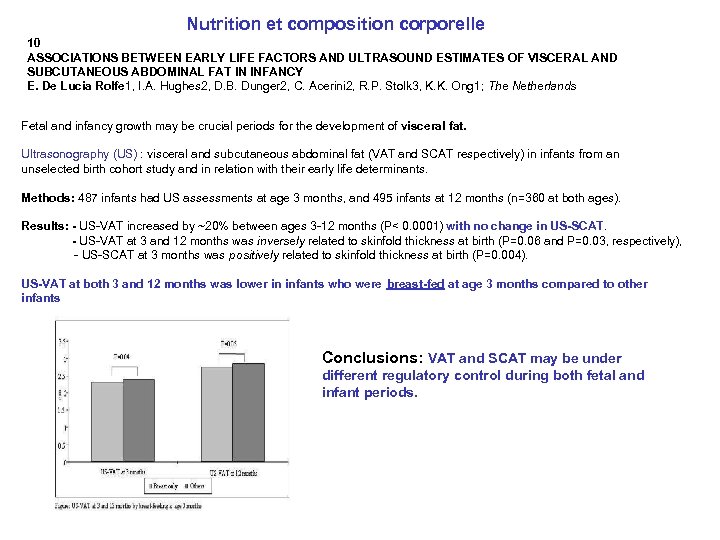 Nutrition et composition corporelle 10 ASSOCIATIONS BETWEEN EARLY LIFE FACTORS AND ULTRASOUND ESTIMATES OF VISCERAL AND SUBCUTANEOUS ABDOMINAL FAT IN INFANCY E. De Lucia Rolfe 1, I. A. Hughes 2, D. B. Dunger 2, C. Acerini 2, R. P. Stolk 3, K. K. Ong 1; The Netherlands Fetal and infancy growth may be crucial periods for the development of visceral fat. Ultrasonography (US) : visceral and subcutaneous abdominal fat (VAT and SCAT respectively) in infants from an unselected birth cohort study and in relation with their early life determinants. Methods: 487 infants had US assessments at age 3 months, and 495 infants at 12 months (n=360 at both ages). Results: - US-VAT increased by ~20% between ages 3 -12 months (P< 0. 0001) with no change in US-SCAT. - US-VAT at 3 and 12 months was inversely related to skinfold thickness at birth (P=0. 06 and P=0. 03, respectively), - US-SCAT at 3 months was positively related to skinfold thickness at birth (P=0. 004). US-VAT at both 3 and 12 months was lower in infants who were breast-fed at age 3 months compared to other infants Conclusions: VAT and SCAT may be under different regulatory control during both fetal and infant periods.
Nutrition et composition corporelle 10 ASSOCIATIONS BETWEEN EARLY LIFE FACTORS AND ULTRASOUND ESTIMATES OF VISCERAL AND SUBCUTANEOUS ABDOMINAL FAT IN INFANCY E. De Lucia Rolfe 1, I. A. Hughes 2, D. B. Dunger 2, C. Acerini 2, R. P. Stolk 3, K. K. Ong 1; The Netherlands Fetal and infancy growth may be crucial periods for the development of visceral fat. Ultrasonography (US) : visceral and subcutaneous abdominal fat (VAT and SCAT respectively) in infants from an unselected birth cohort study and in relation with their early life determinants. Methods: 487 infants had US assessments at age 3 months, and 495 infants at 12 months (n=360 at both ages). Results: - US-VAT increased by ~20% between ages 3 -12 months (P< 0. 0001) with no change in US-SCAT. - US-VAT at 3 and 12 months was inversely related to skinfold thickness at birth (P=0. 06 and P=0. 03, respectively), - US-SCAT at 3 months was positively related to skinfold thickness at birth (P=0. 004). US-VAT at both 3 and 12 months was lower in infants who were breast-fed at age 3 months compared to other infants Conclusions: VAT and SCAT may be under different regulatory control during both fetal and infant periods.
 Composition du lait maternel 28 THE POLYUNSATURATED FATTY ACID CONTENT OF MOTHERS MILK IS ACCOCIATED WITH CHILDHOOD BODY COMPOSITION L. Pedersen 1, 2, L. Lauritzen 3, M. Brasholt 2, B. Schaadt 4, H. Bisgaard 2 Denmark Objective: Relationship between the docosahexaenoic acid (DHA)-content of breast-milk and timing of adiposity rebound and body composition in children. Patients and methods: In the Copenhagen Study on Asthma in Childhood (COPSAC) birth cohort, breast-milk fatty acids were determined 1 mo after delivery and growth prospectively followed to the age of 7 y in 228 children. Dual energy X-ray scan was performed at 7 y in 207 of the children. Age of adiposity rebound was identified from 2 -7 y BMI-curves. Results: There was a bivariate association between breast-milk DHA and BMI from 2 to 7 y (P=0. 02) and fat mass at 7 y (P=0. 01) persisting after confounder adjustment. Age at adiposity rebound tended to be higher in the upper milk-DHA quartile relative to the lower (P=0. 06), but the difference did not persist in the adjusted model. Conclusion: DHA-content of breast-milk may have an impact on body composition in the offspring.
Composition du lait maternel 28 THE POLYUNSATURATED FATTY ACID CONTENT OF MOTHERS MILK IS ACCOCIATED WITH CHILDHOOD BODY COMPOSITION L. Pedersen 1, 2, L. Lauritzen 3, M. Brasholt 2, B. Schaadt 4, H. Bisgaard 2 Denmark Objective: Relationship between the docosahexaenoic acid (DHA)-content of breast-milk and timing of adiposity rebound and body composition in children. Patients and methods: In the Copenhagen Study on Asthma in Childhood (COPSAC) birth cohort, breast-milk fatty acids were determined 1 mo after delivery and growth prospectively followed to the age of 7 y in 228 children. Dual energy X-ray scan was performed at 7 y in 207 of the children. Age of adiposity rebound was identified from 2 -7 y BMI-curves. Results: There was a bivariate association between breast-milk DHA and BMI from 2 to 7 y (P=0. 02) and fat mass at 7 y (P=0. 01) persisting after confounder adjustment. Age at adiposity rebound tended to be higher in the upper milk-DHA quartile relative to the lower (P=0. 06), but the difference did not persist in the adjusted model. Conclusion: DHA-content of breast-milk may have an impact on body composition in the offspring.
 Composition du lait maternel 117 INVESTIGATION OF FOOD INTAKE FOR FREE AMINO ACID CONTENT IN JAPANESE MOTHER'S BREASTMILK AT 4 -5 DAYS AFTER GIVING BIRTH F. Endo 1, K. Nakamura 1, S. Matsumoto 2, M. Iwai 2, A. Yokoyama 2, A. Sato 2, H. Tsuchiya 3, S. Fukuda 3, H. Matsumoto 4 Background aim: Breast milk contains numerous nutrients such as carbohydrate dominated by lactose, lipids, proteins and free amino acids (FAAs). - To determine the concentrations of FFAs and influence of food intake on FAAs in breast milk of Japanese women. Methods: Subjects, 12 mothers, living in Kumamoto were recruited to supply breast milk samples. Approximately 15 m. L of breast milk was obtained at a time by assistance of maternity nurses, collecting in propylene tube, immediate placed in ice and stored in deep freezer until analysis. The amino acids in respective samples were carried out by deproteinization with 0. 3% (w/v) sulfosalicylic acid solution, filtration with 0. 45 um pore size filter, ultrafiltration with 10 K filter, and amino acid contents analysis using Hitachi model L-8900 amino acid analyzer. Results and conclusions: Breast milk contained high concentrations of glutamic acid (Glu), which was over 20% of total FFAs. Glu concentrations increased at 1 and 2. 5 hours after the meal, but other FAAs are not changed by eating a meal. The free amino acids concentration in breast milk seems well maintained with the exception of glutamic acid.
Composition du lait maternel 117 INVESTIGATION OF FOOD INTAKE FOR FREE AMINO ACID CONTENT IN JAPANESE MOTHER'S BREASTMILK AT 4 -5 DAYS AFTER GIVING BIRTH F. Endo 1, K. Nakamura 1, S. Matsumoto 2, M. Iwai 2, A. Yokoyama 2, A. Sato 2, H. Tsuchiya 3, S. Fukuda 3, H. Matsumoto 4 Background aim: Breast milk contains numerous nutrients such as carbohydrate dominated by lactose, lipids, proteins and free amino acids (FAAs). - To determine the concentrations of FFAs and influence of food intake on FAAs in breast milk of Japanese women. Methods: Subjects, 12 mothers, living in Kumamoto were recruited to supply breast milk samples. Approximately 15 m. L of breast milk was obtained at a time by assistance of maternity nurses, collecting in propylene tube, immediate placed in ice and stored in deep freezer until analysis. The amino acids in respective samples were carried out by deproteinization with 0. 3% (w/v) sulfosalicylic acid solution, filtration with 0. 45 um pore size filter, ultrafiltration with 10 K filter, and amino acid contents analysis using Hitachi model L-8900 amino acid analyzer. Results and conclusions: Breast milk contained high concentrations of glutamic acid (Glu), which was over 20% of total FFAs. Glu concentrations increased at 1 and 2. 5 hours after the meal, but other FAAs are not changed by eating a meal. The free amino acids concentration in breast milk seems well maintained with the exception of glutamic acid.
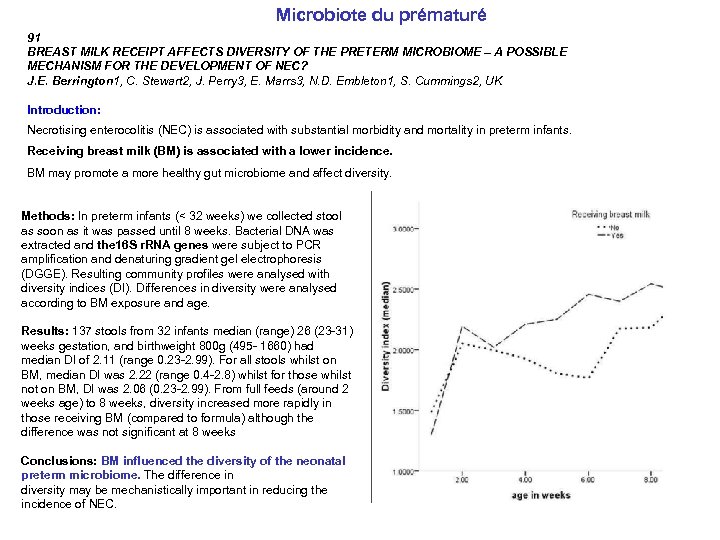 Microbiote du prématuré 91 BREAST MILK RECEIPT AFFECTS DIVERSITY OF THE PRETERM MICROBIOME – A POSSIBLE MECHANISM FOR THE DEVELOPMENT OF NEC? J. E. Berrington 1, C. Stewart 2, J. Perry 3, E. Marrs 3, N. D. Embleton 1, S. Cummings 2, UK Introduction: Necrotising enterocolitis (NEC) is associated with substantial morbidity and mortality in preterm infants. Receiving breast milk (BM) is associated with a lower incidence. BM may promote a more healthy gut microbiome and affect diversity. Methods: In preterm infants (< 32 weeks) we collected stool as soon as it was passed until 8 weeks. Bacterial DNA was extracted and the 16 S r. RNA genes were subject to PCR amplification and denaturing gradient gel electrophoresis (DGGE). Resulting community profiles were analysed with diversity indices (DI). Differences in diversity were analysed according to BM exposure and age. Results: 137 stools from 32 infants median (range) 26 (23 -31) weeks gestation, and birthweight 800 g (495 - 1660) had median DI of 2. 11 (range 0. 23 -2. 99). For all stools whilst on BM, median DI was 2. 22 (range 0. 4 -2. 8) whilst for those whilst not on BM, DI was 2. 06 (0. 23 -2. 99). From full feeds (around 2 weeks age) to 8 weeks, diversity increased more rapidly in those receiving BM (compared to formula) although the difference was not significant at 8 weeks Conclusions: BM influenced the diversity of the neonatal preterm microbiome. The difference in diversity may be mechanistically important in reducing the incidence of NEC.
Microbiote du prématuré 91 BREAST MILK RECEIPT AFFECTS DIVERSITY OF THE PRETERM MICROBIOME – A POSSIBLE MECHANISM FOR THE DEVELOPMENT OF NEC? J. E. Berrington 1, C. Stewart 2, J. Perry 3, E. Marrs 3, N. D. Embleton 1, S. Cummings 2, UK Introduction: Necrotising enterocolitis (NEC) is associated with substantial morbidity and mortality in preterm infants. Receiving breast milk (BM) is associated with a lower incidence. BM may promote a more healthy gut microbiome and affect diversity. Methods: In preterm infants (< 32 weeks) we collected stool as soon as it was passed until 8 weeks. Bacterial DNA was extracted and the 16 S r. RNA genes were subject to PCR amplification and denaturing gradient gel electrophoresis (DGGE). Resulting community profiles were analysed with diversity indices (DI). Differences in diversity were analysed according to BM exposure and age. Results: 137 stools from 32 infants median (range) 26 (23 -31) weeks gestation, and birthweight 800 g (495 - 1660) had median DI of 2. 11 (range 0. 23 -2. 99). For all stools whilst on BM, median DI was 2. 22 (range 0. 4 -2. 8) whilst for those whilst not on BM, DI was 2. 06 (0. 23 -2. 99). From full feeds (around 2 weeks age) to 8 weeks, diversity increased more rapidly in those receiving BM (compared to formula) although the difference was not significant at 8 weeks Conclusions: BM influenced the diversity of the neonatal preterm microbiome. The difference in diversity may be mechanistically important in reducing the incidence of NEC.
 139 AN ENERGY BALANCE APP FOR THE IPHONE: A TOOL TO MONITOR FOOD INTAKE AND ENERGY BALANCE A. Hills 1, F. Loewnich 2, R. Wood 2, N. Byrne 2, N. King 2, Australia. Study: to develop an improved method of monitoring food intake and energy balance. We previously developed a hand-held Electronic Appetite Rating System (EARS) - to measure subjective appetite sensations in clinical trials (Gibbons et al. , 2011) - have upgraded the EARS concept and developed a food monitoring application (app) for the Apple i. Phone. The app provides continuous feedback about food intake quantifying and expressing energy intake relative to energy requirements. Food and drink items are identified via a barcode using the i. Phone's built-in camera with portion size (g or ml) entered manually. Energy and macronutrient information is automatically retrieved from a nutrition database (AUSNUT 2007). This app provides a novel platform for improving the measurement of food intake in children, adolescents and adults, including the self-management of health behaviours.
139 AN ENERGY BALANCE APP FOR THE IPHONE: A TOOL TO MONITOR FOOD INTAKE AND ENERGY BALANCE A. Hills 1, F. Loewnich 2, R. Wood 2, N. Byrne 2, N. King 2, Australia. Study: to develop an improved method of monitoring food intake and energy balance. We previously developed a hand-held Electronic Appetite Rating System (EARS) - to measure subjective appetite sensations in clinical trials (Gibbons et al. , 2011) - have upgraded the EARS concept and developed a food monitoring application (app) for the Apple i. Phone. The app provides continuous feedback about food intake quantifying and expressing energy intake relative to energy requirements. Food and drink items are identified via a barcode using the i. Phone's built-in camera with portion size (g or ml) entered manually. Energy and macronutrient information is automatically retrieved from a nutrition database (AUSNUT 2007). This app provides a novel platform for improving the measurement of food intake in children, adolescents and adults, including the self-management of health behaviours.
 Take-home messages? 1. Le critère de prédisposition au syndrome métabolique : la vitesse de croissance (qq soit le BW) 2. Hypothèse protéique acceptée : Il faut revoir la composition des laits infantiles ou allaiter…longtemps 3. Lipides du lait maternel : composition…mais aussi structure et organisation micellaire 4. La Molécule miracle contre l’obésité: la taurine 5. The Biomarqueur de l’obésité : la cystéine plasmatique 6. Ce qu’on savait déjà: les oméga 3: c’est mieux que les oméga 6 Le DHA, c’est pas mal non plus 7. Modèles animaux RCIU : peut mieux faire? 8. Peu de Mécanistique…lien alimentation-épigénétique reste descriptif 9. Parent pauvre : le métagénome 10. Etudes randomisées « placebo vs supplémentation » chez l’homme. . . . Ethique? ? ?
Take-home messages? 1. Le critère de prédisposition au syndrome métabolique : la vitesse de croissance (qq soit le BW) 2. Hypothèse protéique acceptée : Il faut revoir la composition des laits infantiles ou allaiter…longtemps 3. Lipides du lait maternel : composition…mais aussi structure et organisation micellaire 4. La Molécule miracle contre l’obésité: la taurine 5. The Biomarqueur de l’obésité : la cystéine plasmatique 6. Ce qu’on savait déjà: les oméga 3: c’est mieux que les oméga 6 Le DHA, c’est pas mal non plus 7. Modèles animaux RCIU : peut mieux faire? 8. Peu de Mécanistique…lien alimentation-épigénétique reste descriptif 9. Parent pauvre : le métagénome 10. Etudes randomisées « placebo vs supplémentation » chez l’homme. . . . Ethique? ? ?


