ba8eeef481be6c692864edbe3c6127c0.ppt
- Количество слайдов: 25
 11 Heat • Homework: • 1, 3, 4, 5, 6, 9, 11, 23, 54, 63, 64. 1
11 Heat • Homework: • 1, 3, 4, 5, 6, 9, 11, 23, 54, 63, 64. 1
![Heat • Heat is energy transferred due to temperature difference. • Symbol, Q [J] Heat • Heat is energy transferred due to temperature difference. • Symbol, Q [J]](https://present5.com/presentation/ba8eeef481be6c692864edbe3c6127c0/image-2.jpg) Heat • Heat is energy transferred due to temperature difference. • Symbol, Q [J] • Ex. 4186 J heat needed to raise 1 kg of water one degree C. 2
Heat • Heat is energy transferred due to temperature difference. • Symbol, Q [J] • Ex. 4186 J heat needed to raise 1 kg of water one degree C. 2
![specific heat • c = Q/m. DT [J/(kg·K)] • heat needed per kg to specific heat • c = Q/m. DT [J/(kg·K)] • heat needed per kg to](https://present5.com/presentation/ba8eeef481be6c692864edbe3c6127c0/image-3.jpg) specific heat • c = Q/m. DT [J/(kg·K)] • heat needed per kg to raise temperature by 1 degree C or K. • slope warming water = DT/Q = 1/(mc) 3
specific heat • c = Q/m. DT [J/(kg·K)] • heat needed per kg to raise temperature by 1 degree C or K. • slope warming water = DT/Q = 1/(mc) 3
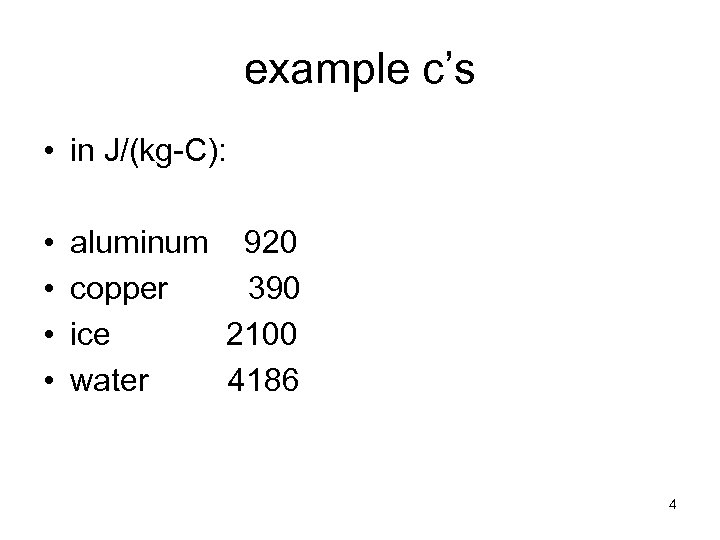 example c’s • in J/(kg-C): • • aluminum 920 copper 390 ice 2100 water 4186 4
example c’s • in J/(kg-C): • • aluminum 920 copper 390 ice 2100 water 4186 4
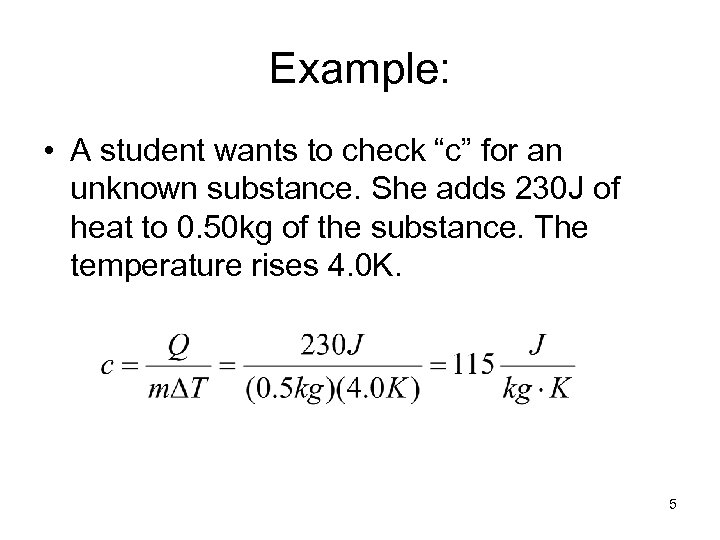 Example: • A student wants to check “c” for an unknown substance. She adds 230 J of heat to 0. 50 kg of the substance. The temperature rises 4. 0 K. 5
Example: • A student wants to check “c” for an unknown substance. She adds 230 J of heat to 0. 50 kg of the substance. The temperature rises 4. 0 K. 5
 Calorimetry • literally: ‘meter’ the calories emitted by a substance as it cools. • Ex. Heated object is added to water. change in temperature of water determines specific heat of object. 6
Calorimetry • literally: ‘meter’ the calories emitted by a substance as it cools. • Ex. Heated object is added to water. change in temperature of water determines specific heat of object. 6
 Example Calorimetry • 2 kg of “substance-A” heated to 100 C. Placed in 5 kg of water at 20 C. After five minutes the water temp. is 25 C. • heat lost by substance = heat gained water. 7
Example Calorimetry • 2 kg of “substance-A” heated to 100 C. Placed in 5 kg of water at 20 C. After five minutes the water temp. is 25 C. • heat lost by substance = heat gained water. 7
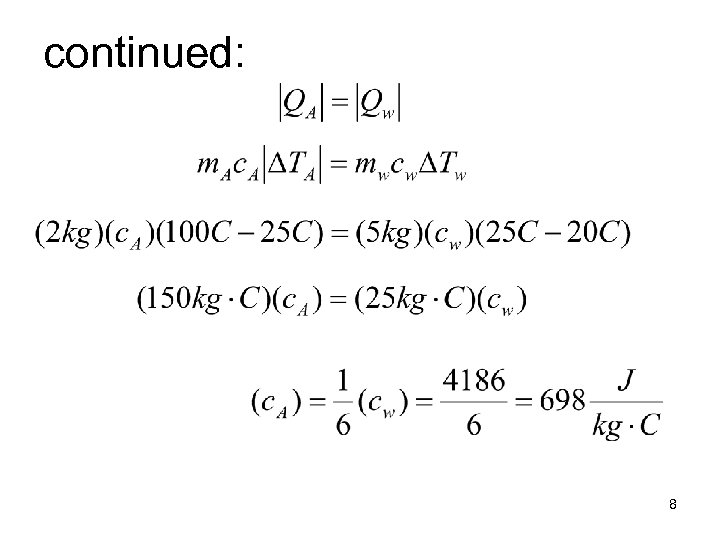 continued: 8
continued: 8
![latent heat • L = Q/m [J/(kg)] • heat needed per kg to melt latent heat • L = Q/m [J/(kg)] • heat needed per kg to melt](https://present5.com/presentation/ba8eeef481be6c692864edbe3c6127c0/image-9.jpg) latent heat • L = Q/m [J/(kg)] • heat needed per kg to melt (f) or vaporize (v) a substance 9
latent heat • L = Q/m [J/(kg)] • heat needed per kg to melt (f) or vaporize (v) a substance 9
 example L’s • in J/kg: • melting (f) • alcohol 100, 000 • water 333, 000 vaporization (v) 850, 000 2, 226, 000 10
example L’s • in J/kg: • melting (f) • alcohol 100, 000 • water 333, 000 vaporization (v) 850, 000 2, 226, 000 10
 Example: • How much heat must be added to 0. 5 kg of ice at 0 C to melt it? • Q = m. L = (0. 5 kg)(333, 000 J/kg) • = 167, 000 J • same amount of heat must be removed from 0. 5 kg water at 0 C to freeze it. 11
Example: • How much heat must be added to 0. 5 kg of ice at 0 C to melt it? • Q = m. L = (0. 5 kg)(333, 000 J/kg) • = 167, 000 J • same amount of heat must be removed from 0. 5 kg water at 0 C to freeze it. 11
 Heat Transfer • Conduction • Convection • Radiation 12
Heat Transfer • Conduction • Convection • Radiation 12
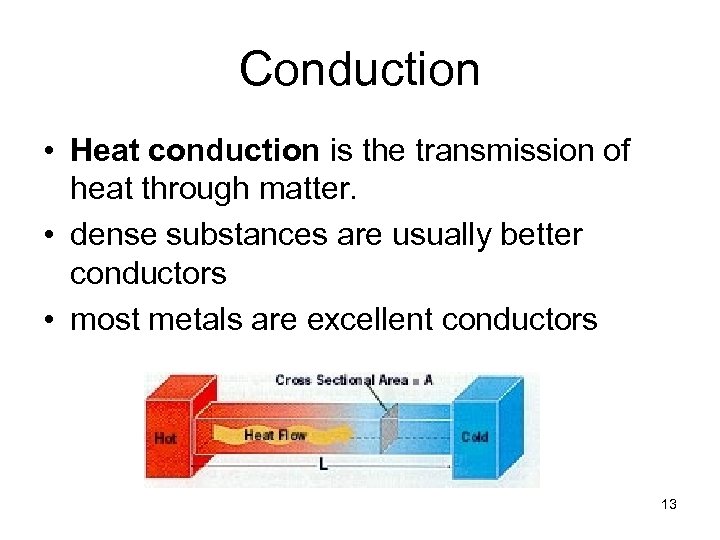 Conduction • Heat conduction is the transmission of heat through matter. • dense substances are usually better conductors • most metals are excellent conductors 13
Conduction • Heat conduction is the transmission of heat through matter. • dense substances are usually better conductors • most metals are excellent conductors 13
![conduction equation • • heat current = energy/time [watts] heat current = k. ADT/L conduction equation • • heat current = energy/time [watts] heat current = k. ADT/L](https://present5.com/presentation/ba8eeef481be6c692864edbe3c6127c0/image-14.jpg) conduction equation • • heat current = energy/time [watts] heat current = k. ADT/L k = thermal conductivity & DT = temperature difference, L below 14
conduction equation • • heat current = energy/time [watts] heat current = k. ADT/L k = thermal conductivity & DT = temperature difference, L below 14
 conduction example • some conductivities in J/(m-s-C): • silver 429 copper 401 aluminum 240 • Ex: Water in aluminum pot. bottom = 101 C, inside = 100 C, thickness = 3 mm, area = 280 sq. cm. • Q/t = k. A(Th-Tc)/L • = (240)(0. 028)(101 -100)/(0. 003) • = 2, 240 watts heat current 15
conduction example • some conductivities in J/(m-s-C): • silver 429 copper 401 aluminum 240 • Ex: Water in aluminum pot. bottom = 101 C, inside = 100 C, thickness = 3 mm, area = 280 sq. cm. • Q/t = k. A(Th-Tc)/L • = (240)(0. 028)(101 -100)/(0. 003) • = 2, 240 watts heat current 15
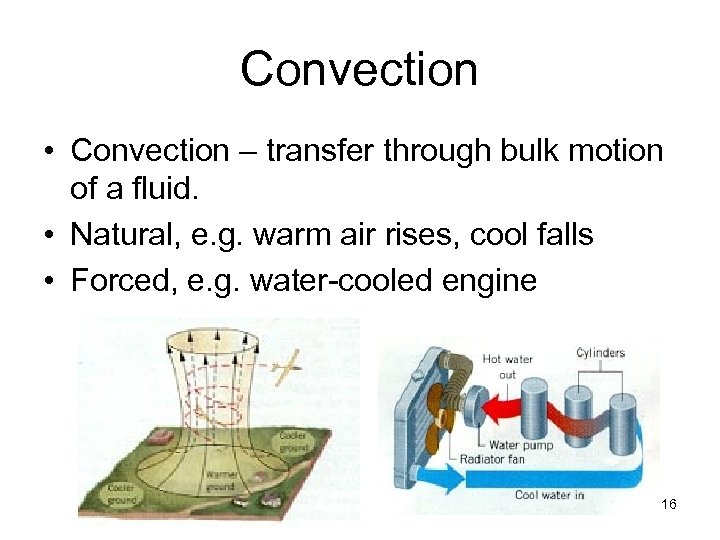 Convection • Convection – transfer through bulk motion of a fluid. • Natural, e. g. warm air rises, cool falls • Forced, e. g. water-cooled engine 16
Convection • Convection – transfer through bulk motion of a fluid. • Natural, e. g. warm air rises, cool falls • Forced, e. g. water-cooled engine 16
 Radiation • Heat transfer by electromagnetic radiation, e. g. infrared. • Examples: • space heaters with the shiny reflector use radiation to heat. • If they add a fan, they use both radiation and convection 17
Radiation • Heat transfer by electromagnetic radiation, e. g. infrared. • Examples: • space heaters with the shiny reflector use radiation to heat. • If they add a fan, they use both radiation and convection 17
 Greenhouse Effect • ‘dirtier’ air must be at higher temperature to radiate out as much as Earth receives • higher temperature air is associated with higher surface temperatures, thus the term ‘global warming’ • very complicated model! 18
Greenhouse Effect • ‘dirtier’ air must be at higher temperature to radiate out as much as Earth receives • higher temperature air is associated with higher surface temperatures, thus the term ‘global warming’ • very complicated model! 18
 Summary • T measured in C, K, F. Use K for gas laws. • thermometry uses thermometric properties • change in length is proportional to change in temperature for many solids • c: heat needed to raise 1 kg by 1 C. • L: heat needed to melt or vaporize 1 kg. • Heat transfer 19
Summary • T measured in C, K, F. Use K for gas laws. • thermometry uses thermometric properties • change in length is proportional to change in temperature for many solids • c: heat needed to raise 1 kg by 1 C. • L: heat needed to melt or vaporize 1 kg. • Heat transfer 19
 Phase Change • • • freeze (liquid to solid) melt (solid to liquid) evaporate (liquid to gas) sublime (solid to gas) phase changes occur at constant temperature 20
Phase Change • • • freeze (liquid to solid) melt (solid to liquid) evaporate (liquid to gas) sublime (solid to gas) phase changes occur at constant temperature 20
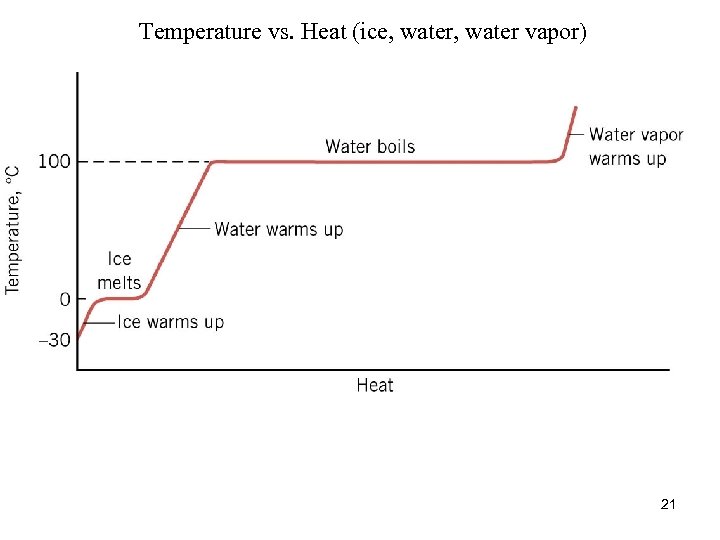 Temperature vs. Heat (ice, water vapor) 21
Temperature vs. Heat (ice, water vapor) 21
 Heat and Phase Change • Latent Heat of Fusion – heat supplied to melt or the heat removed to freeze • Latent Heat of Vaporization – heat supplied to vaporize or heat removed to liquify. 22
Heat and Phase Change • Latent Heat of Fusion – heat supplied to melt or the heat removed to freeze • Latent Heat of Vaporization – heat supplied to vaporize or heat removed to liquify. 22
 Newton’s Law of Cooling • For a body cooling in a draft (i. e. , by forced convection), the rate of heat loss is proportional to the difference in temperatures between the body and its surroundings • rate of heat-loss ~ DT 23
Newton’s Law of Cooling • For a body cooling in a draft (i. e. , by forced convection), the rate of heat loss is proportional to the difference in temperatures between the body and its surroundings • rate of heat-loss ~ DT 23
 Real Greenhouse • covering allows sunlight to enter, which warms the ground air inside the greenhouse. • the ‘house’ is mostly enclosed so the warm air cannot leave, thus keeping the greenhouse warm (a car in the sun does this very effectively!) 24
Real Greenhouse • covering allows sunlight to enter, which warms the ground air inside the greenhouse. • the ‘house’ is mostly enclosed so the warm air cannot leave, thus keeping the greenhouse warm (a car in the sun does this very effectively!) 24
 Solar Power Solar Constant • Describes the Solar Radiation that falls on an area above the atmosphere = 1. 37 k. W / m². In space, solar radiation is practically constant; on earth it varies with the time of day and year as well as with the latitude and weather. The maximum value on earth is between 0. 8 and 1. 0 k. W / m². • see: solarserver. de 25
Solar Power Solar Constant • Describes the Solar Radiation that falls on an area above the atmosphere = 1. 37 k. W / m². In space, solar radiation is practically constant; on earth it varies with the time of day and year as well as with the latitude and weather. The maximum value on earth is between 0. 8 and 1. 0 k. W / m². • see: solarserver. de 25


