b85b33ed3f0e65add0483d0f4b15e436.ppt
- Количество слайдов: 43
 1
1
 Seminar Chemical Methods for Electronic Wastes Recovery Gholamhossein Paniri Supervisor: Professor H. S. Ghaziaskar Department of Chemistry Isfahan University of Technology 2
Seminar Chemical Methods for Electronic Wastes Recovery Gholamhossein Paniri Supervisor: Professor H. S. Ghaziaskar Department of Chemistry Isfahan University of Technology 2
 Content ü What is electronic wastes ü Why E-Wastes are recycling ü E-waste recycling steps ü Methods of materials chemical recovery ü Conclusions ü References 3
Content ü What is electronic wastes ü Why E-Wastes are recycling ü E-waste recycling steps ü Methods of materials chemical recovery ü Conclusions ü References 3
 What is Electronic Waste? Electronic Waste E- Waste 4
What is Electronic Waste? Electronic Waste E- Waste 4
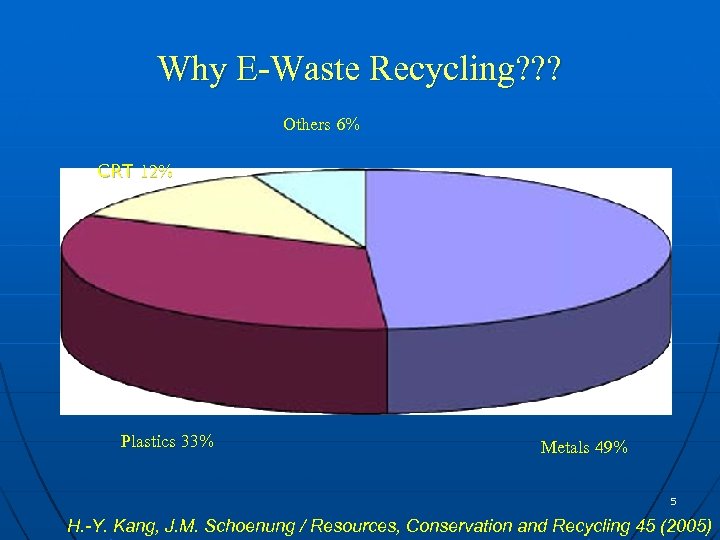 Why E-Waste Recycling? ? ? Others 6% CRT 12% Plastics 33% Metals 49% 5 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005)
Why E-Waste Recycling? ? ? Others 6% CRT 12% Plastics 33% Metals 49% 5 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005)
 Types material of E-Waste üprecious metals ühazardous material 6
Types material of E-Waste üprecious metals ühazardous material 6
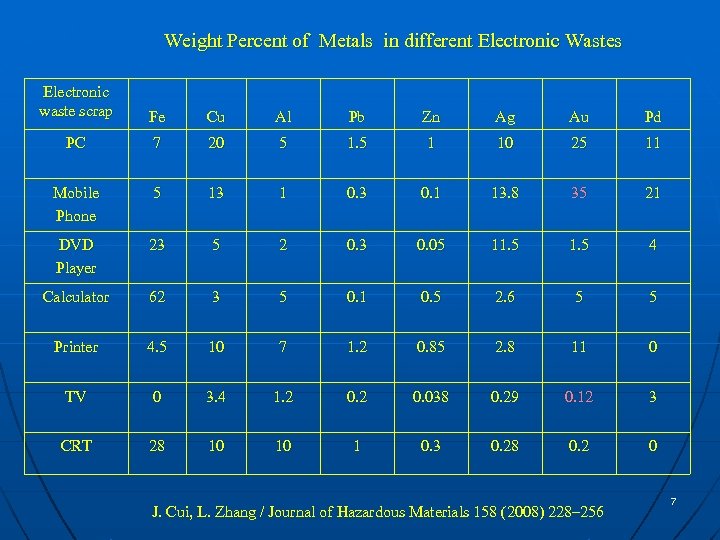 Weight Percent of Metals in different Electronic Wastes Electronic waste scrap Fe Cu Al Pb Zn Ag Au Pd PC 7 20 5 1 10 25 11 Mobile Phone 5 13 1 0. 3 0. 1 13. 8 35 21 DVD Player 23 5 2 0. 3 0. 05 11. 5 4 Calculator 62 3 5 0. 1 0. 5 2. 6 5 5 Printer 4. 5 10 7 1. 2 0. 85 2. 8 11 0 TV 0 3. 4 1. 2 0. 038 0. 29 0. 12 3 CRT 28 10 10 1 0. 3 0. 28 0. 2 0 J. Cui, L. Zhang / Journal of Hazardous Materials 158 (2008) 228– 256 7
Weight Percent of Metals in different Electronic Wastes Electronic waste scrap Fe Cu Al Pb Zn Ag Au Pd PC 7 20 5 1 10 25 11 Mobile Phone 5 13 1 0. 3 0. 1 13. 8 35 21 DVD Player 23 5 2 0. 3 0. 05 11. 5 4 Calculator 62 3 5 0. 1 0. 5 2. 6 5 5 Printer 4. 5 10 7 1. 2 0. 85 2. 8 11 0 TV 0 3. 4 1. 2 0. 038 0. 29 0. 12 3 CRT 28 10 10 1 0. 3 0. 28 0. 2 0 J. Cui, L. Zhang / Journal of Hazardous Materials 158 (2008) 228– 256 7
 Vi = 100 Wti. Pri ∑ Wti. Pri Vi= Value distribution Wti= Weight precent of metal i in the electronic scrap sample Pri= the current price of metal i 8
Vi = 100 Wti. Pri ∑ Wti. Pri Vi= Value distribution Wti= Weight precent of metal i in the electronic scrap sample Pri= the current price of metal i 8
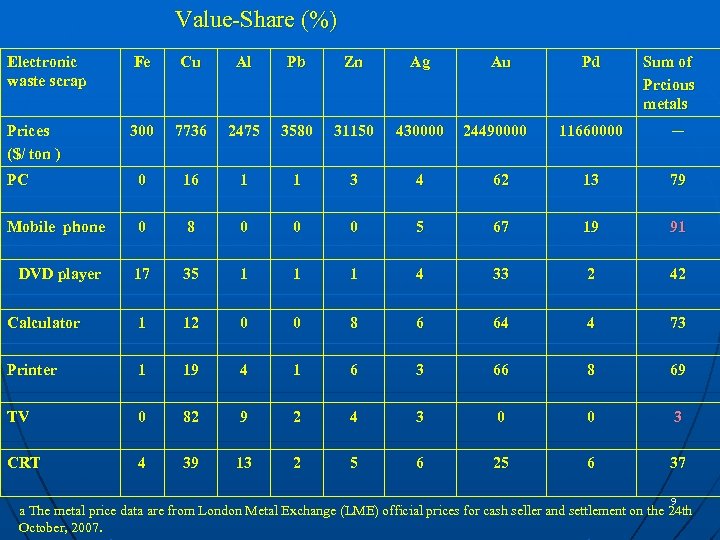 Value-Share (%) Electronic waste scrap Fe Cu Al Pb Zn Ag Prices ($/ ton ) 300 7736 2475 3580 31150 430000 PC 0 16 1 1 3 4 Mobile phone 0 8 0 0 0 DVD player 17 35 1 1 Calculator 1 12 0 Printer 1 19 TV 0 CRT 4 Au 24490000 Pd Sum of Prcious metals 11660000 ─ 62 13 79 5 67 19 91 1 4 33 2 42 0 8 6 64 4 73 4 1 6 3 66 8 69 82 9 2 4 3 0 0 3 39 13 2 5 6 25 6 37 9 a The metal price data are from London Metal Exchange (LME) official prices for cash seller and settlement on the 24 th October, 2007.
Value-Share (%) Electronic waste scrap Fe Cu Al Pb Zn Ag Prices ($/ ton ) 300 7736 2475 3580 31150 430000 PC 0 16 1 1 3 4 Mobile phone 0 8 0 0 0 DVD player 17 35 1 1 Calculator 1 12 0 Printer 1 19 TV 0 CRT 4 Au 24490000 Pd Sum of Prcious metals 11660000 ─ 62 13 79 5 67 19 91 1 4 33 2 42 0 8 6 64 4 73 4 1 6 3 66 8 69 82 9 2 4 3 0 0 3 39 13 2 5 6 25 6 37 9 a The metal price data are from London Metal Exchange (LME) official prices for cash seller and settlement on the 24 th October, 2007.
 Toxic Content of E-waste 10
Toxic Content of E-waste 10
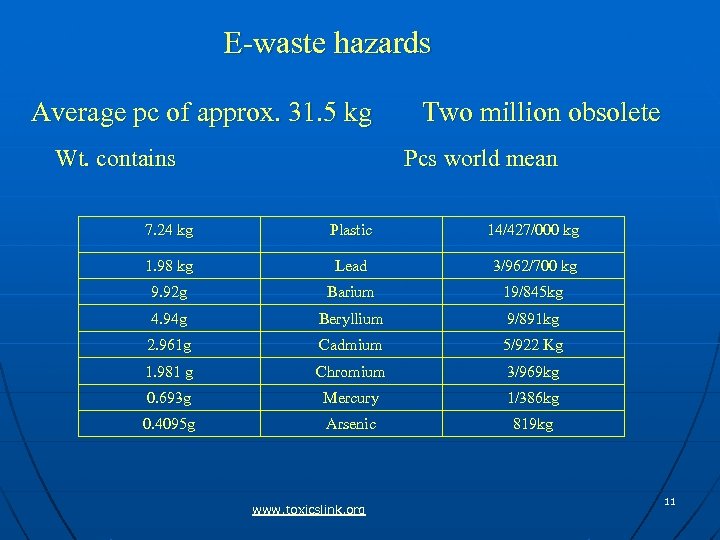 E-waste hazards Average pc of approx. 31. 5 kg Wt. contains Two million obsolete Pcs world mean 7. 24 kg Plastic 14/427/000 kg 1. 98 kg Lead 3/962/700 kg 9. 92 g Barium 19/845 kg 4. 94 g Beryllium 9/891 kg 2. 961 g Cadmium 5/922 Kg 1. 981 g Chromium 3/969 kg 0. 693 g Mercury 1/386 kg 0. 4095 g Arsenic 819 kg www. toxicslink. org 11
E-waste hazards Average pc of approx. 31. 5 kg Wt. contains Two million obsolete Pcs world mean 7. 24 kg Plastic 14/427/000 kg 1. 98 kg Lead 3/962/700 kg 9. 92 g Barium 19/845 kg 4. 94 g Beryllium 9/891 kg 2. 961 g Cadmium 5/922 Kg 1. 981 g Chromium 3/969 kg 0. 693 g Mercury 1/386 kg 0. 4095 g Arsenic 819 kg www. toxicslink. org 11
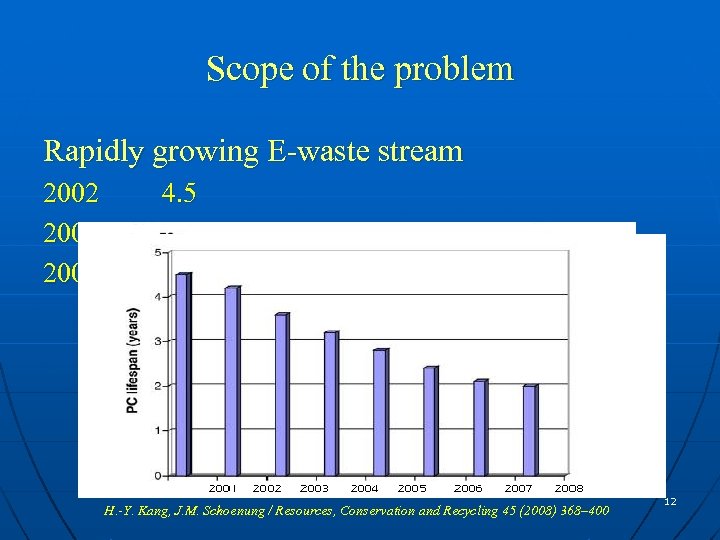 Scope of the problem Rapidly growing E-waste stream 2002 2005 2009 4. 5 3 2 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2008) 368– 400 12
Scope of the problem Rapidly growing E-waste stream 2002 2005 2009 4. 5 3 2 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2008) 368– 400 12
 Why recycling E-waste? 1. E-Waste large amount of precious metals 2. E-Waste contain hazardous material 3. E-Waste is a huge secondary resource above (mine ground) should not wasted 13
Why recycling E-waste? 1. E-Waste large amount of precious metals 2. E-Waste contain hazardous material 3. E-Waste is a huge secondary resource above (mine ground) should not wasted 13
 E-waste recycling content 1. Ban export and disposal as well as incineration landfilling 2. Advertisement for recycling 3. Collection and transportation 4. Market for reuse 5. Materials recovery facility (MRF) Resources, Conservation and Recycling 45 (2005) 368– 400 14
E-waste recycling content 1. Ban export and disposal as well as incineration landfilling 2. Advertisement for recycling 3. Collection and transportation 4. Market for reuse 5. Materials recovery facility (MRF) Resources, Conservation and Recycling 45 (2005) 368– 400 14
 Ban export and disposal The Basel convention Disposal = reuse + recycling The amount of secondhand personal computer exported from Japan in 2005 Hong Kong Vietnam 1354963 197815 Thailand Malaysia Chaina Cambodia Korea Others 65323 27264 14440 13011 8598 18015 The amount of seconhand television exported from Japan in 2005 Hong Kong Vietnam Cambodia Malaysia Myenmar Chaina 28356 658293 92779 281124 167723 166010 Thailand Others 35463 15 45259
Ban export and disposal The Basel convention Disposal = reuse + recycling The amount of secondhand personal computer exported from Japan in 2005 Hong Kong Vietnam 1354963 197815 Thailand Malaysia Chaina Cambodia Korea Others 65323 27264 14440 13011 8598 18015 The amount of seconhand television exported from Japan in 2005 Hong Kong Vietnam Cambodia Malaysia Myenmar Chaina 28356 658293 92779 281124 167723 166010 Thailand Others 35463 15 45259
 Advertisement and sorting Advertisement Sorting 16
Advertisement and sorting Advertisement Sorting 16
 Collection and transportation 17
Collection and transportation 17
 MRF Market for reuse Materials Recovery Facility 18
MRF Market for reuse Materials Recovery Facility 18
 Electronic Waste in IRAN 19
Electronic Waste in IRAN 19
 20
20
 Materials recovery of chemical methods 1. percious metals Recovery 2. hazardous materials Recovery 21
Materials recovery of chemical methods 1. percious metals Recovery 2. hazardous materials Recovery 21
 Precious metals recovery 1. Pyrometallurgical 2. Hydrometallurgical 22
Precious metals recovery 1. Pyrometallurgical 2. Hydrometallurgical 22
 Recovery of precios metals from E- waste by pyrometallurgical processing 1. Incineration 2. Smelting in a blast furnace 3. Drossing 4. Sintering 5. Reaction in a gas phase at high tempertures 23
Recovery of precios metals from E- waste by pyrometallurgical processing 1. Incineration 2. Smelting in a blast furnace 3. Drossing 4. Sintering 5. Reaction in a gas phase at high tempertures 23
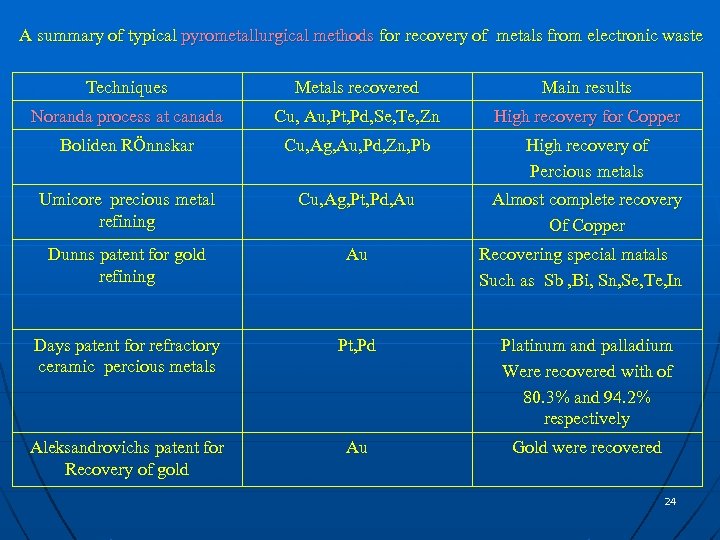 A summary of typical pyrometallurgical methods for recovery of metals from electronic waste Techniques Metals recovered Main results Noranda process at canada Cu, Au, Pt, Pd, Se, Te, Zn High recovery for Copper Boliden RÖnnskar Cu, Ag, Au, Pd, Zn, Pb High recovery of Percious metals Umicore precious metal refining Cu, Ag, Pt, Pd, Au Almost complete recovery Of Copper Dunns patent for gold refining Au Days patent for refractory ceramic percious metals Pt, Pd Platinum and palladium Were recovered with of 80. 3% and 94. 2% respectively Aleksandrovichs patent for Recovery of gold Au Gold were recovered Recovering special matals Such as Sb , Bi, Sn, Se, Te, In 24
A summary of typical pyrometallurgical methods for recovery of metals from electronic waste Techniques Metals recovered Main results Noranda process at canada Cu, Au, Pt, Pd, Se, Te, Zn High recovery for Copper Boliden RÖnnskar Cu, Ag, Au, Pd, Zn, Pb High recovery of Percious metals Umicore precious metal refining Cu, Ag, Pt, Pd, Au Almost complete recovery Of Copper Dunns patent for gold refining Au Days patent for refractory ceramic percious metals Pt, Pd Platinum and palladium Were recovered with of 80. 3% and 94. 2% respectively Aleksandrovichs patent for Recovery of gold Au Gold were recovered Recovering special matals Such as Sb , Bi, Sn, Se, Te, In 24
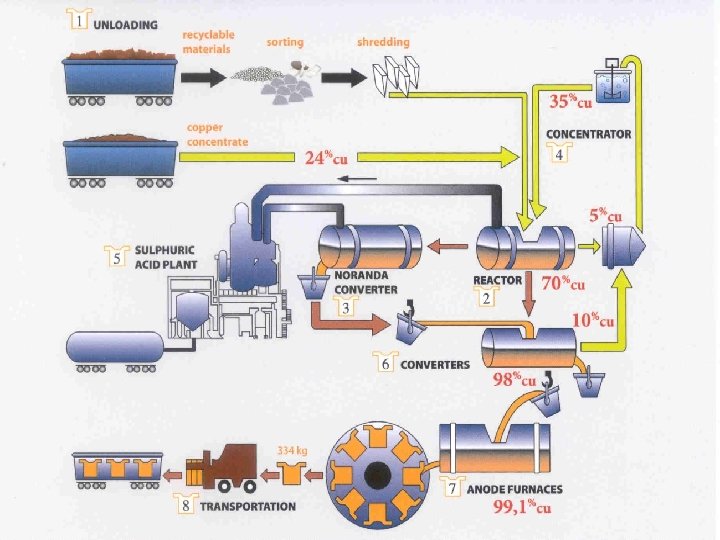 25
25
 Advantages pyrometallurgical processing 1. High efficiency recovery precious metals from E-waste 2. Recovery of energy from PC waste gives an example for using of plastic in E-waste 26
Advantages pyrometallurgical processing 1. High efficiency recovery precious metals from E-waste 2. Recovery of energy from PC waste gives an example for using of plastic in E-waste 26
 Disavantages pyrometallurgical processing 1. Integrated smelters cannot recover aluminum and iron as metals 2. Ceramic componets and glass in the E-waste increase the amount of slag from Blast furnaces 3. Precious metals stay for a long time in the pyrometallurgical processing 27
Disavantages pyrometallurgical processing 1. Integrated smelters cannot recover aluminum and iron as metals 2. Ceramic componets and glass in the E-waste increase the amount of slag from Blast furnaces 3. Precious metals stay for a long time in the pyrometallurgical processing 27
 Disavantages pyrometallurgical processing 4. Energy recovery and utilizing of organic constituents as a reducing agent are only on its beginning 5. Thermal processing of e-waste provides a feasible approach for recovery of energy from e-waste if a comprehensive emission control system is installed 28
Disavantages pyrometallurgical processing 4. Energy recovery and utilizing of organic constituents as a reducing agent are only on its beginning 5. Thermal processing of e-waste provides a feasible approach for recovery of energy from e-waste if a comprehensive emission control system is installed 28
 Recovery of precious metals from E-Waste by hydrometallurgical processing The main steps in hydrometallurgical Separation and purification Processing consist of a series of acid Precipitation of impurities Or caustic leaches of solid material Solvent extraction Adsorption Cementation…. . 29
Recovery of precious metals from E-Waste by hydrometallurgical processing The main steps in hydrometallurgical Separation and purification Processing consist of a series of acid Precipitation of impurities Or caustic leaches of solid material Solvent extraction Adsorption Cementation…. . 29
 Leaching of precious metals Cyanide leaching Halide leaching Thiourea leaching Thiosulfate leaching 30
Leaching of precious metals Cyanide leaching Halide leaching Thiourea leaching Thiosulfate leaching 30
 Halide leaching Exceptions of flurine and astatine Gold forms both Au(I) and Au(III) complexes with all halogens Low p. H High halogen level Icreased temperature High surface area 31
Halide leaching Exceptions of flurine and astatine Gold forms both Au(I) and Au(III) complexes with all halogens Low p. H High halogen level Icreased temperature High surface area 31
 Aqua regia Advantage 2 HNO 3 + 6 HCl 2 NO 4 H 2 O +3 Cl Halide+leaching 2 The process generally fast 2 Au + 11 HCl + 3 HNO 3 2 HAu. Cl 4 + 3 NOCl + 6 H 2 O Low reagent consumption disavantage Highly corrosive acid Highly poisonous 32
Aqua regia Advantage 2 HNO 3 + 6 HCl 2 NO 4 H 2 O +3 Cl Halide+leaching 2 The process generally fast 2 Au + 11 HCl + 3 HNO 3 2 HAu. Cl 4 + 3 NOCl + 6 H 2 O Low reagent consumption disavantage Highly corrosive acid Highly poisonous 32
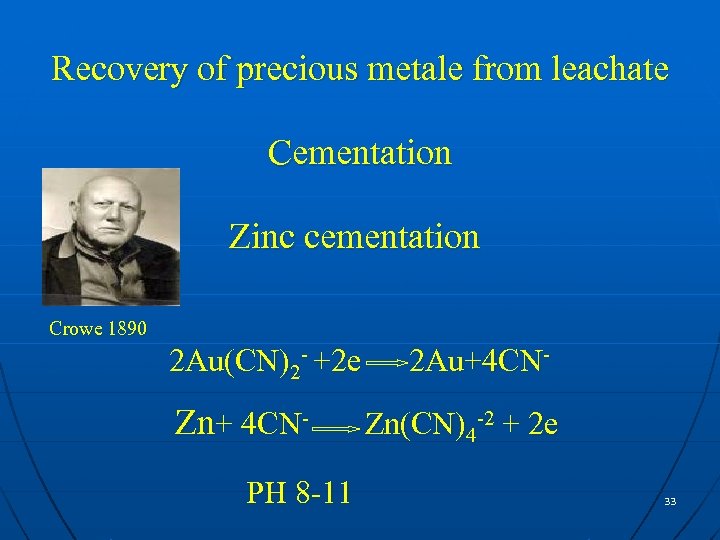 Recovery of precious metale from leachate Cementation Zinc cementation Crowe 1890 2 Au(CN)2 - +2 e Zn+ 4 CNPH 8 -11 2 Au+4 CNZn(CN)4 -2 + 2 e 33
Recovery of precious metale from leachate Cementation Zinc cementation Crowe 1890 2 Au(CN)2 - +2 e Zn+ 4 CNPH 8 -11 2 Au+4 CNZn(CN)4 -2 + 2 e 33
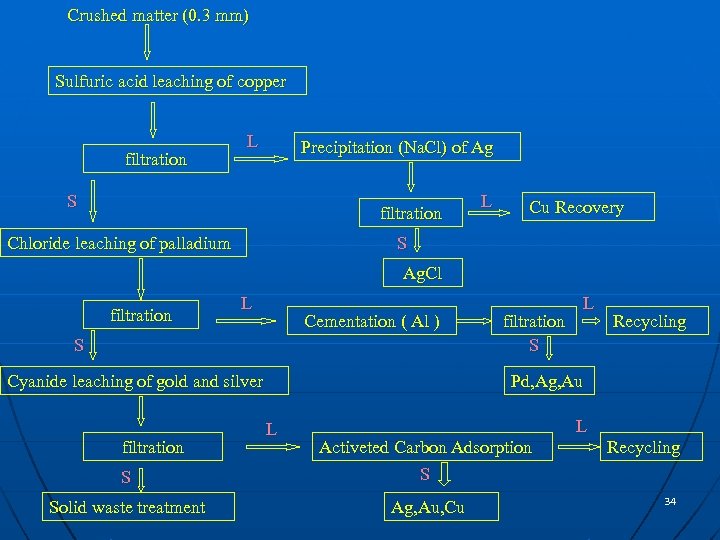 Crushed matter (0. 3 mm) Sulfuric acid leaching of copper filtration L Precipitation (Na. Cl) of Ag S filtration Chloride leaching of palladium L Cu Recovery S Ag. Cl filtration L Cementation ( Al ) S Cyanide leaching of gold and silver filtration S L Recycling Pd, Ag, Au L L S Activeted Carbon Adsorption S Solid waste treatment Ag, Au, Cu Recycling 34
Crushed matter (0. 3 mm) Sulfuric acid leaching of copper filtration L Precipitation (Na. Cl) of Ag S filtration Chloride leaching of palladium L Cu Recovery S Ag. Cl filtration L Cementation ( Al ) S Cyanide leaching of gold and silver filtration S L Recycling Pd, Ag, Au L L S Activeted Carbon Adsorption S Solid waste treatment Ag, Au, Cu Recycling 34
 Comparing with the pyrometallurgical processing hydrometallurgical methode is More exact More predictable More easily controlled 35
Comparing with the pyrometallurgical processing hydrometallurgical methode is More exact More predictable More easily controlled 35
 Recovery of hazardious metals from E-Waste Cathod Ray Tube C RT 0. 5 – 5 kg pb 36
Recovery of hazardious metals from E-Waste Cathod Ray Tube C RT 0. 5 – 5 kg pb 36
 Cathode Ray Tube Recycling 1. Glass CRT components Funel glass, panel glass, solder glass, neck Sio 2, Nao, Cao for coloring and Zno, Bao, Pbo for proctecting from X-Rays 2. Non glass Plastic, steel, copper, electron gun , phosphor coating 37
Cathode Ray Tube Recycling 1. Glass CRT components Funel glass, panel glass, solder glass, neck Sio 2, Nao, Cao for coloring and Zno, Bao, Pbo for proctecting from X-Rays 2. Non glass Plastic, steel, copper, electron gun , phosphor coating 37
 Cathode Ray Tube Recycling 1. Glass-to-glass recycling 2. Glass-to-lead recycling 38
Cathode Ray Tube Recycling 1. Glass-to-glass recycling 2. Glass-to-lead recycling 38
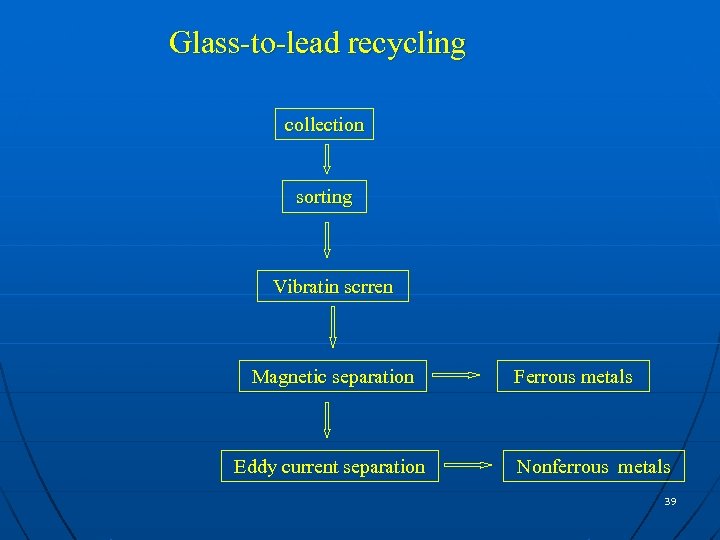 Glass-to-lead recycling collection sorting Vibratin scrren Magnetic separation Eddy current separation Ferrous metals Nonferrous metals 39
Glass-to-lead recycling collection sorting Vibratin scrren Magnetic separation Eddy current separation Ferrous metals Nonferrous metals 39
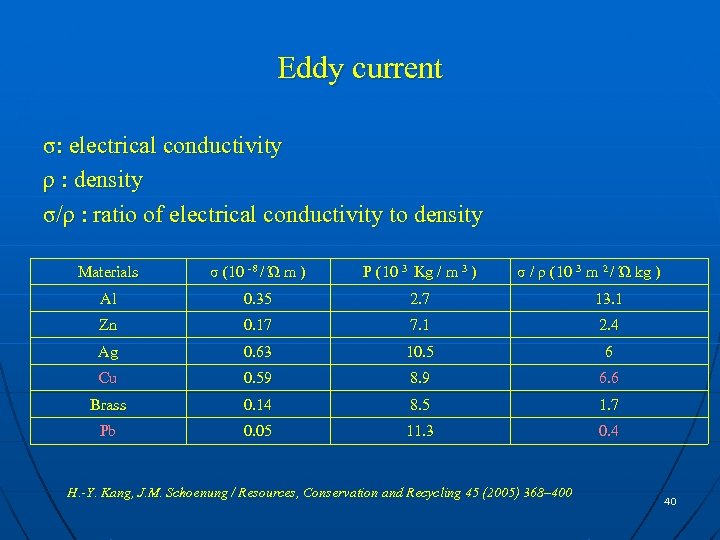 Eddy current σ: electrical conductivity ρ : density σ/ρ : ratio of electrical conductivity to density Materials σ (10 -8 / Ώ m ) Ρ (10 3 Kg / m 3 ) σ / ρ (10 3 m 2 / Ώ kg ) Al 0. 35 2. 7 13. 1 Zn 0. 17 7. 1 2. 4 Ag 0. 63 10. 5 6 Cu 0. 59 8. 9 6. 6 Brass 0. 14 8. 5 1. 7 Pb 0. 05 11. 3 0. 4 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005) 368– 400 40
Eddy current σ: electrical conductivity ρ : density σ/ρ : ratio of electrical conductivity to density Materials σ (10 -8 / Ώ m ) Ρ (10 3 Kg / m 3 ) σ / ρ (10 3 m 2 / Ώ kg ) Al 0. 35 2. 7 13. 1 Zn 0. 17 7. 1 2. 4 Ag 0. 63 10. 5 6 Cu 0. 59 8. 9 6. 6 Brass 0. 14 8. 5 1. 7 Pb 0. 05 11. 3 0. 4 H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005) 368– 400 40
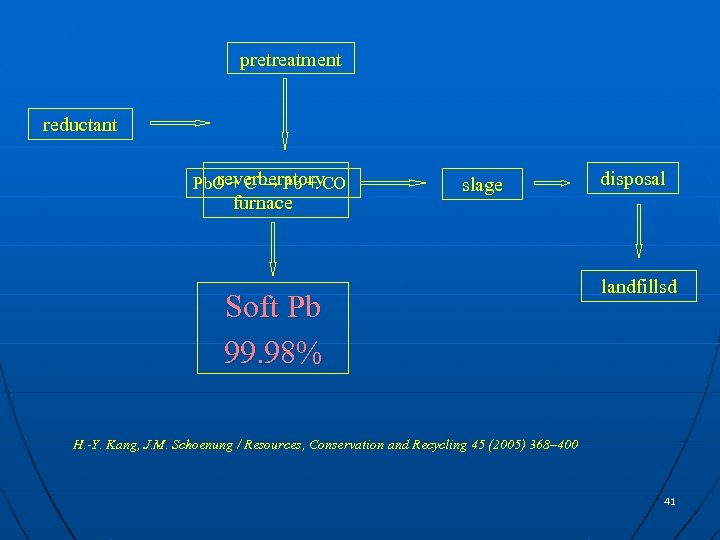 pretreatment reductant reverberatory Pb. O + C → Pb + CO furnace slage Soft Pb 99. 98% disposal landfillsd H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005) 368– 400 41
pretreatment reductant reverberatory Pb. O + C → Pb + CO furnace slage Soft Pb 99. 98% disposal landfillsd H. -Y. Kang, J. M. Schoenung / Resources, Conservation and Recycling 45 (2005) 368– 400 41
 Conclusion 1. Recycling of electronic waste is an important subject 2. E-Waste is a huge secondary resource (maine above ground) Shouh not wasted 3. The major economic driver for recycling of electronic waste is from the recovery of precious metals 42
Conclusion 1. Recycling of electronic waste is an important subject 2. E-Waste is a huge secondary resource (maine above ground) Shouh not wasted 3. The major economic driver for recycling of electronic waste is from the recovery of precious metals 42
 References J. Cui, E. Fotssberg, Mechanical recycling of waste electronic and electric equipment : a reveiew , j. Hazard. mater. 99 (3) (2003) 243 -263 EPCEU, : Directive 2002 / 96/EC of the European parliament and of the council of 27 January 2003 on waste electronic and electrical equipment (WEEE) , off. j. Eur. Union (2003) 24 -38 T. Maruyama, H. Matsushita, Y. Shimada, et al. , Proteins and protein-rich biomass as environmentally friendly adsorbents selective for precious metal ions, Environ. Sci. Technol. 41 (4) (2007) 1359– 1364, Feb 15 A. N. Mabbett, D. Sanyahumbi, P. Yong, et al. , Biorecovered precious metals from industrial wastes: Single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application, Environ. Sci. Technol. 40 (3) (2006) 1015– 1021, Feb 1 J. Shibata, S. Matsumoto, Development of Environmentally Friendly Leaching and Recovery Process of Gold and Silver from Wasted Electronic Parts, 2007 -10 -29, 2007 D. Morin, A. Lips, T. Pinches, et al. , Bio. Min. E – Integrated project for the development of biotechnology for metal-bearing materials in Europe, Hydrometallurgy 83 (1– 4) (2006) 69– 76. 43
References J. Cui, E. Fotssberg, Mechanical recycling of waste electronic and electric equipment : a reveiew , j. Hazard. mater. 99 (3) (2003) 243 -263 EPCEU, : Directive 2002 / 96/EC of the European parliament and of the council of 27 January 2003 on waste electronic and electrical equipment (WEEE) , off. j. Eur. Union (2003) 24 -38 T. Maruyama, H. Matsushita, Y. Shimada, et al. , Proteins and protein-rich biomass as environmentally friendly adsorbents selective for precious metal ions, Environ. Sci. Technol. 41 (4) (2007) 1359– 1364, Feb 15 A. N. Mabbett, D. Sanyahumbi, P. Yong, et al. , Biorecovered precious metals from industrial wastes: Single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application, Environ. Sci. Technol. 40 (3) (2006) 1015– 1021, Feb 1 J. Shibata, S. Matsumoto, Development of Environmentally Friendly Leaching and Recovery Process of Gold and Silver from Wasted Electronic Parts, 2007 -10 -29, 2007 D. Morin, A. Lips, T. Pinches, et al. , Bio. Min. E – Integrated project for the development of biotechnology for metal-bearing materials in Europe, Hydrometallurgy 83 (1– 4) (2006) 69– 76. 43


