1 Polymers in functionalization of nanoparticles and nanocomposites.

















paris_presentation_zaichenko_2_last-last.ppt
- Размер: 20.9 Mегабайта
- Количество слайдов: 70
Описание презентации 1 Polymers in functionalization of nanoparticles and nanocomposites. по слайдам
 1 Polymers in functionalization of nanoparticles and nanocomposites. Functional oligoperoxide-based luminescent and scintillation polymer and mineral nanocomposites. Applications in cellular research. A. Zaichenko , zaichenk@polynet. lviv. ua Lviv Polytechnic National University
1 Polymers in functionalization of nanoparticles and nanocomposites. Functional oligoperoxide-based luminescent and scintillation polymer and mineral nanocomposites. Applications in cellular research. A. Zaichenko , zaichenk@polynet. lviv. ua Lviv Polytechnic National University
 Lviv Polytechnic National University
Lviv Polytechnic National University
 The aim of the study: oligoperoxide based routes of tailored synthesis and functionalization of luminescent and scintillation polymer and mineral nanoparticles for biomedical application
The aim of the study: oligoperoxide based routes of tailored synthesis and functionalization of luminescent and scintillation polymer and mineral nanoparticles for biomedical application
 Talk outline . I. Functional surface-active oligoperoxides and derived oligoelectrolyte and nonionic surfactants of block, comb-like or branched structures. Synthesis and characterization. II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
Talk outline . I. Functional surface-active oligoperoxides and derived oligoelectrolyte and nonionic surfactants of block, comb-like or branched structures. Synthesis and characterization. II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
 5 The main approaches of synthesis of functional oligoperoxide and derived polymeric surfactants
5 The main approaches of synthesis of functional oligoperoxide and derived polymeric surfactants
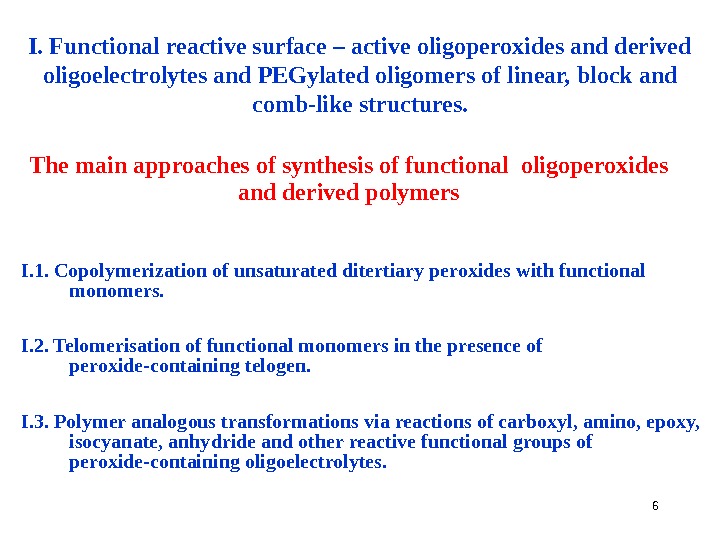
 6 I. Functional reactive surface – active oligoperoxides and derived oligoelectrolytes and PEGylated oligomers of linear, block and comb-like structures. The main approaches of synthesis of functional oligoperoxides and derived polymers I. 1. Copolymerization of unsaturated ditertiary peroxides with functional monomers. I. 2. Telomerisation of functional monomers in the presence of peroxide-containing telogen. I. 3. Polymer analogous transformations via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups of peroxide-containing oligoelectrolytes.
6 I. Functional reactive surface – active oligoperoxides and derived oligoelectrolytes and PEGylated oligomers of linear, block and comb-like structures. The main approaches of synthesis of functional oligoperoxides and derived polymers I. 1. Copolymerization of unsaturated ditertiary peroxides with functional monomers. I. 2. Telomerisation of functional monomers in the presence of peroxide-containing telogen. I. 3. Polymer analogous transformations via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups of peroxide-containing oligoelectrolytes.
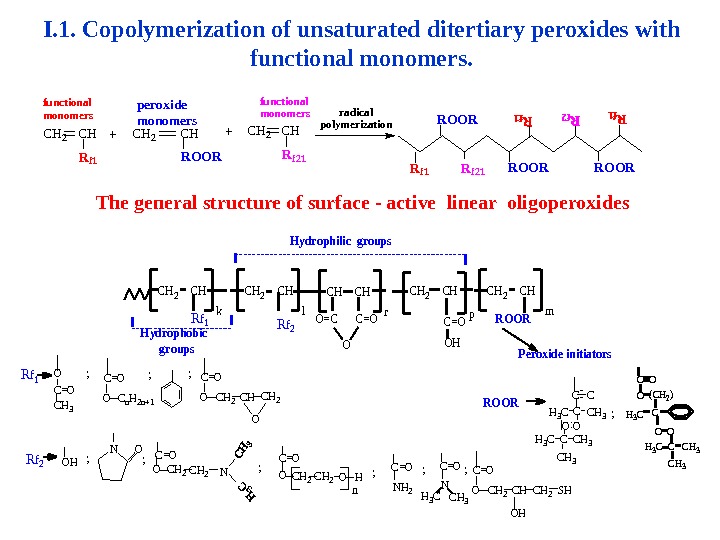
 The general structure of surface — active linear oligoperoxides. I. 1. Copolymerization of unsaturated ditertiary peroxides with functional monomers. f u n c t i o n a l m o n o m e r sp e r o x i d e m o n o m e r sf u n c t i o n a l m o n o m e r s r a d i c a l p o l y m e r i z a t i o n C H 2 C H R f 1 + + R O O RR O O R R f 2 1 C H 2 C H R f 1 R f 2 1 R O O RCH 2 CH kl CH 2 CH O=CC=Or CH O p CH 2 CH C=O OH CH 2 C=O CH 2 NOOH ON OOHCH 2 C=O CH 2 n C=O OCn. H 2 n+1 C=O O CH 3 C=O NH 2 N C=O CH 3 H 3 C Rf 1 Rf 2 CH 2 CH m CH 2 O C=O CHCH 2 O: O H 3 CC H 3 C CH 3 C C C O O(CH 2) CH 3 C O O CCH 3 H 3 C CH 3 ROOR Rf 1 Rf 2 Hydrophobic groups Hydrophilic groups Peroxide initiators ROOR CH 2 CH C=O OCH 2 SH OH ; ; ; ; ;
The general structure of surface — active linear oligoperoxides. I. 1. Copolymerization of unsaturated ditertiary peroxides with functional monomers. f u n c t i o n a l m o n o m e r sp e r o x i d e m o n o m e r sf u n c t i o n a l m o n o m e r s r a d i c a l p o l y m e r i z a t i o n C H 2 C H R f 1 + + R O O RR O O R R f 2 1 C H 2 C H R f 1 R f 2 1 R O O RCH 2 CH kl CH 2 CH O=CC=Or CH O p CH 2 CH C=O OH CH 2 C=O CH 2 NOOH ON OOHCH 2 C=O CH 2 n C=O OCn. H 2 n+1 C=O O CH 3 C=O NH 2 N C=O CH 3 H 3 C Rf 1 Rf 2 CH 2 CH m CH 2 O C=O CHCH 2 O: O H 3 CC H 3 C CH 3 C C C O O(CH 2) CH 3 C O O CCH 3 H 3 C CH 3 ROOR Rf 1 Rf 2 Hydrophobic groups Hydrophilic groups Peroxide initiators ROOR CH 2 CH C=O OCH 2 SH OH ; ; ; ; ;
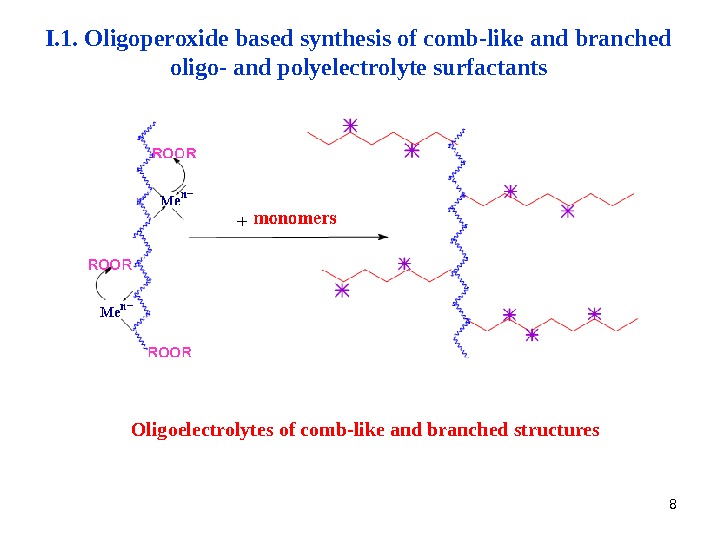
 8 Oligoelectrolytes of comb-like and branched structures. I. 1. Oligoperoxide based synthesis of comb-like and branched oligo- and polyelectrolyte surfactants
8 Oligoelectrolytes of comb-like and branched structures. I. 1. Oligoperoxide based synthesis of comb-like and branched oligo- and polyelectrolyte surfactants
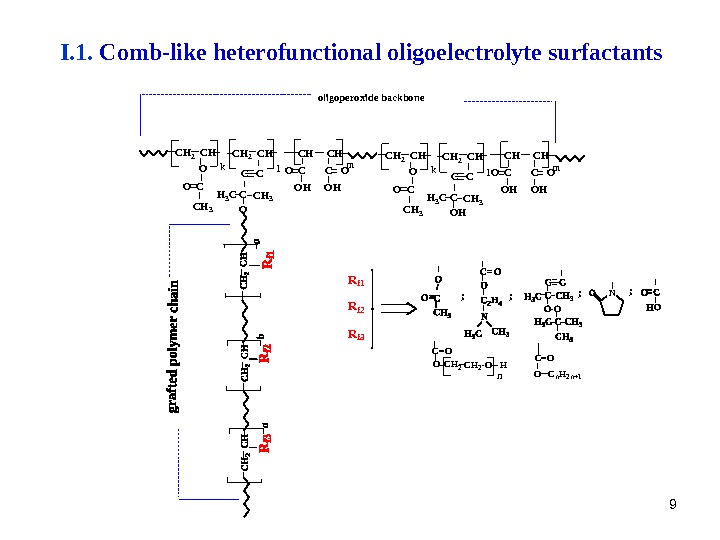
 99 o l i g o p e r o x i d e b a c k b o n e C H 2 C H O C H 3 C C CH 3 C C H 3 O HO C k lm lk O HO C C O O C H 3 H 3 C C C H 3 O C HC H 2 C H NR f 1 R f 3 R f 2 OO HC H 2 C = O C H 2 n C n H 2 n + 1 O C = O; ; C H C OO C O H m. I. 1. Comb-like heterofunctional oligoelectrolyte surfactants
99 o l i g o p e r o x i d e b a c k b o n e C H 2 C H O C H 3 C C CH 3 C C H 3 O HO C k lm lk O HO C C O O C H 3 H 3 C C C H 3 O C HC H 2 C H NR f 1 R f 3 R f 2 OO HC H 2 C = O C H 2 n C n H 2 n + 1 O C = O; ; C H C OO C O H m. I. 1. Comb-like heterofunctional oligoelectrolyte surfactants
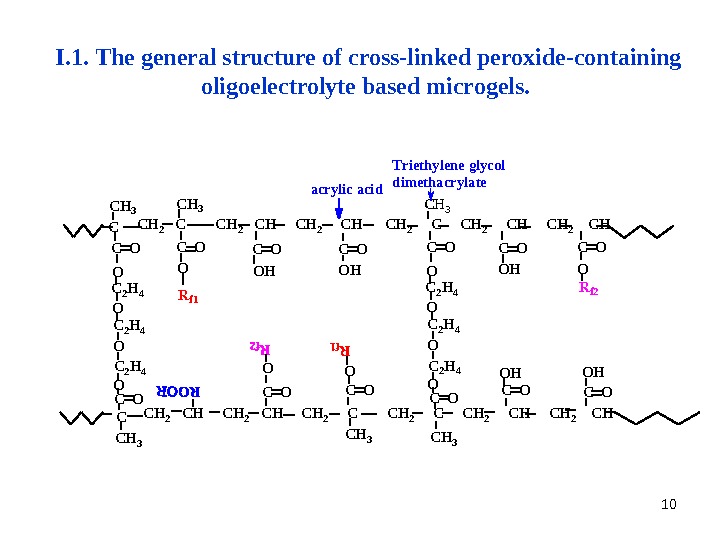
 10 O C O O HO O O H C O C O C H 3 C 2 H 4 OC 2 H 4 C H 3 C H 2 C H 2 C C H 2 C HO OO H O H C OC OC H 2 C H 2 C C H 2 C H 3 C H 3 a c r y l i c a c i d R f 1 T r i e t h y l e n e g l y c o l d i m e t h a c r y l a t e R f 2 C O OC H 3 C 2 H 4 O C 2 H 4 C H 3 C OOC CI. 1. The general structure of cross-linked peroxide-containing oligoelectrolyte based microgels.
10 O C O O HO O O H C O C O C H 3 C 2 H 4 OC 2 H 4 C H 3 C H 2 C H 2 C C H 2 C HO OO H O H C OC OC H 2 C H 2 C C H 2 C H 3 C H 3 a c r y l i c a c i d R f 1 T r i e t h y l e n e g l y c o l d i m e t h a c r y l a t e R f 2 C O OC H 3 C 2 H 4 O C 2 H 4 C H 3 C OOC CI. 1. The general structure of cross-linked peroxide-containing oligoelectrolyte based microgels.
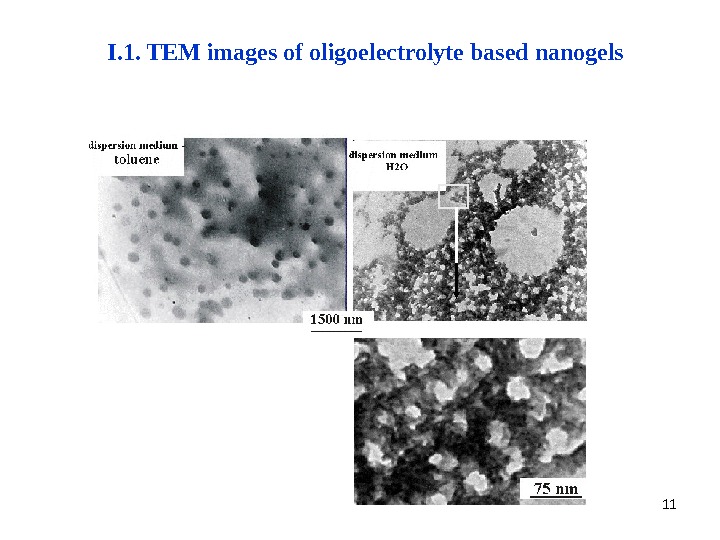
 11 I. 1. TEM images of oligoelectrolyte based nanogels
11 I. 1. TEM images of oligoelectrolyte based nanogels
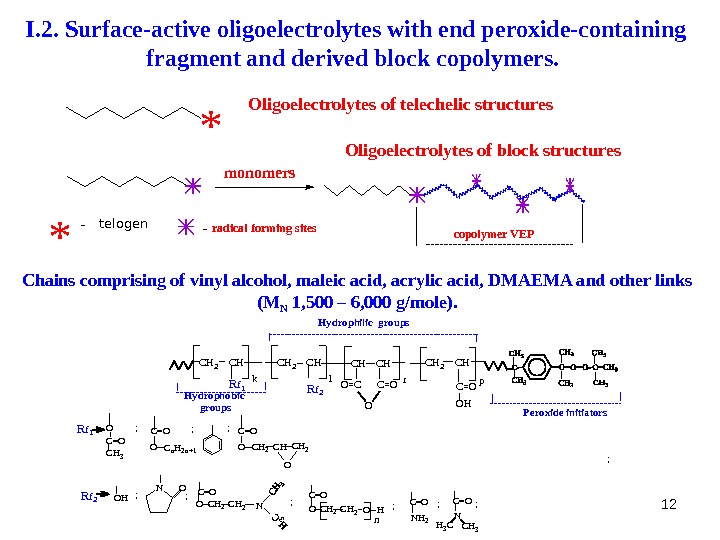
 12 I. 2. Surface-active oligoelectrolytes with end peroxide-containing fragment and derived block copolymers. Chains comprising of vinyl alcohol, maleic acid, acrylic acid, DMAEMA and other links (M N 1 , 500 – 6 , 000 g/mole). * * — telogen — radical forming sites copolymer VEPOligoelectrolytes of block structures. Oligoelectrolytes of telechelic structures monomers C H 2 C H k l. C H 2 C H O = C C = O r. C H O p. C H 2 C H C = O O H C H 2 C = O C H 2 NOO H ON OO HC H 2 C = O C H 2 n. C = O O C n H 2 n + 1 C = OO C H 3 C = O N H 2 NC = O C H 3 H 3 CR f 1 R f 2 C H 2 O C = O C H 2 OR f 1 R f 2 H y d r o p h o b i c g r o u p s H y d r o p h i l i c g r o u p s P e r o x i d e i n i t i a t o r s ; ; ; ; ;
12 I. 2. Surface-active oligoelectrolytes with end peroxide-containing fragment and derived block copolymers. Chains comprising of vinyl alcohol, maleic acid, acrylic acid, DMAEMA and other links (M N 1 , 500 – 6 , 000 g/mole). * * — telogen — radical forming sites copolymer VEPOligoelectrolytes of block structures. Oligoelectrolytes of telechelic structures monomers C H 2 C H k l. C H 2 C H O = C C = O r. C H O p. C H 2 C H C = O O H C H 2 C = O C H 2 NOO H ON OO HC H 2 C = O C H 2 n. C = O O C n H 2 n + 1 C = OO C H 3 C = O N H 2 NC = O C H 3 H 3 CR f 1 R f 2 C H 2 O C = O C H 2 OR f 1 R f 2 H y d r o p h o b i c g r o u p s H y d r o p h i l i c g r o u p s P e r o x i d e i n i t i a t o r s ; ; ; ; ;
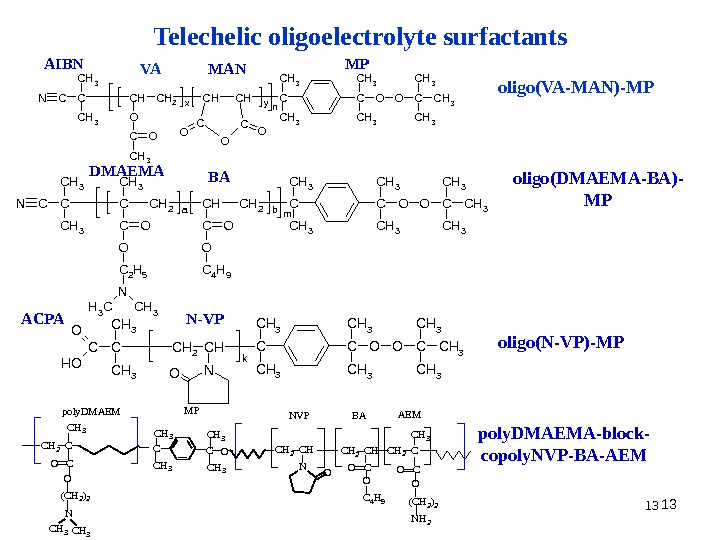
 13 13 13 Telechelic oligoelectrolyte surfactants VA MAN MP oligo ( VA — MAN )- MPNCC CH 3 CHCH 2 O CO CH 3 x CHCH CC OOO y n C CH 3 OOC CH 3 AIBN NCC CH 3 CCH 2 a CHCH 2 b m C CH 3 OOC CH 3 CO O C 2 H 5 N CH 3 CO O C 4 H 9 DMAEMA BA oligo ( DMAEMA — BA )- MP C CC H 3 C H 2 C Hk C C H 3 CC H 3 O O CC H 3 N OOH O N-VP oligo ( N-VP )- MP C H 2 C C H 3 CO O ( C H 2 ) 2 N C H 3 C C H 3 O C H 2 C H N O C H 2 C H CO O C 4 H 9 C H 2 C C H 3 OO C ( C H 2 ) 2 N H 2 p o l y D M A E M M P N V P B A A E M poly. DMAEMA-block- copoly. NVP-BA-AEMACP
13 13 13 Telechelic oligoelectrolyte surfactants VA MAN MP oligo ( VA — MAN )- MPNCC CH 3 CHCH 2 O CO CH 3 x CHCH CC OOO y n C CH 3 OOC CH 3 AIBN NCC CH 3 CCH 2 a CHCH 2 b m C CH 3 OOC CH 3 CO O C 2 H 5 N CH 3 CO O C 4 H 9 DMAEMA BA oligo ( DMAEMA — BA )- MP C CC H 3 C H 2 C Hk C C H 3 CC H 3 O O CC H 3 N OOH O N-VP oligo ( N-VP )- MP C H 2 C C H 3 CO O ( C H 2 ) 2 N C H 3 C C H 3 O C H 2 C H N O C H 2 C H CO O C 4 H 9 C H 2 C C H 3 OO C ( C H 2 ) 2 N H 2 p o l y D M A E M M P N V P B A A E M poly. DMAEMA-block- copoly. NVP-BA-AEMACP
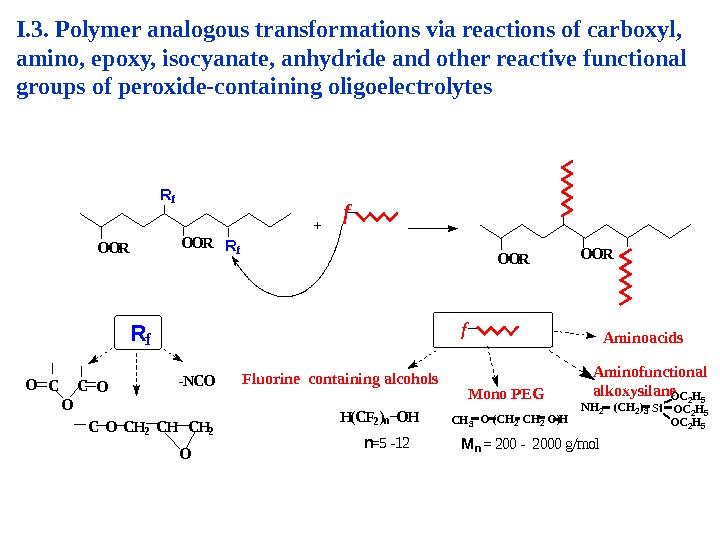
 O O R R f O O R + f O O R f R f C O C H 2 OC C OO О — N C O H ( C F 2 ) n O H n = 5 — 1 2 Fluorine containing alcohols Mono PEG M n = 2 0 0 — 2 0 0 0 g / m o l Aminoacids N H 2 ( C H 2 )3 S i O C 2 H 5 C H 3 O ( C H 2 O ) H Aminofunctional alkoxysilane. I. 3. Polymer analogous transformations via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups of peroxide-containing oligoelectrolytes
O O R R f O O R + f O O R f R f C O C H 2 OC C OO О — N C O H ( C F 2 ) n O H n = 5 — 1 2 Fluorine containing alcohols Mono PEG M n = 2 0 0 — 2 0 0 0 g / m o l Aminoacids N H 2 ( C H 2 )3 S i O C 2 H 5 C H 3 O ( C H 2 O ) H Aminofunctional alkoxysilane. I. 3. Polymer analogous transformations via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups of peroxide-containing oligoelectrolytes
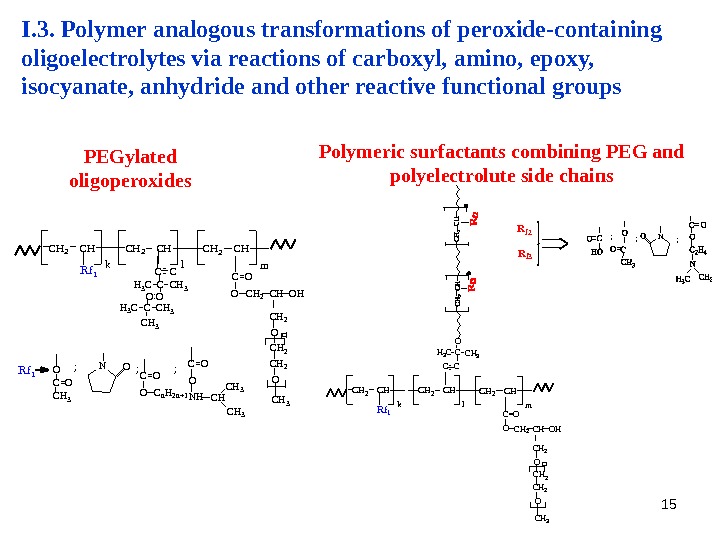
 15 I. 3. Polymer analogous transformations of peroxide-containing oligoelectrolytes via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups C H 3 O C H 2 O HC H 2 C H k l. C H 2 C H m R f 1 C H 2 O C = O C H 2 C H 2 C H : O H 3 C C H 3 CC C O C H 3 C HN HC = O O; C n H 2 n + 1 O C = O; C H 3 O C = O N O R f 1 ; CH 3 O CH 2 OH CH 2 CH kl CH 2 CH m. Rf 1 CH 2 O C=O CH CH 2 CH H 3 CCH 3 C C C O Rf 2 Rf 3 ; ; N; PEGylated oligoperoxides Polymeric surfactants combining PEG and polyelectrolute side chains
15 I. 3. Polymer analogous transformations of peroxide-containing oligoelectrolytes via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups C H 3 O C H 2 O HC H 2 C H k l. C H 2 C H m R f 1 C H 2 O C = O C H 2 C H 2 C H : O H 3 C C H 3 CC C O C H 3 C HN HC = O O; C n H 2 n + 1 O C = O; C H 3 O C = O N O R f 1 ; CH 3 O CH 2 OH CH 2 CH kl CH 2 CH m. Rf 1 CH 2 O C=O CH CH 2 CH H 3 CCH 3 C C C O Rf 2 Rf 3 ; ; N; PEGylated oligoperoxides Polymeric surfactants combining PEG and polyelectrolute side chains
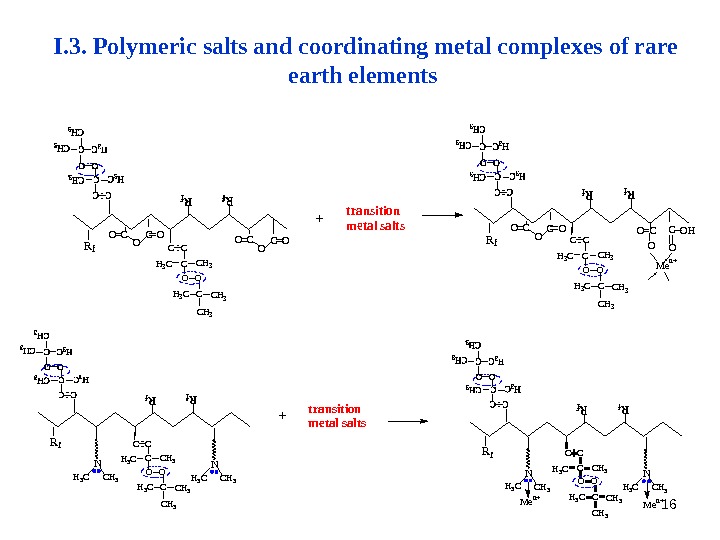
 16 H 3 C C H 3 CO OH 3 C C H 3 CC C M e n ++ t r a n s i t i o n m e t a l s a l t s R f N C H 3 H 3 C C H 3 NR f C C C C H 3 H 3 C O O C C H 3 H 3 C M e n + OC OO CO CC OORf transition metal salts+ Rf O CC OO Men+ OH OO O=CC C C CCH 3 H 3 C O O CCH 3 H 3 C CH 3 C O O H 3 CCH 3 C C CI. 3. Polymeric salts and coordinating metal complexes of rare earth elements
16 H 3 C C H 3 CO OH 3 C C H 3 CC C M e n ++ t r a n s i t i o n m e t a l s a l t s R f N C H 3 H 3 C C H 3 NR f C C C C H 3 H 3 C O O C C H 3 H 3 C M e n + OC OO CO CC OORf transition metal salts+ Rf O CC OO Men+ OH OO O=CC C C CCH 3 H 3 C O O CCH 3 H 3 C CH 3 C O O H 3 CCH 3 C C CI. 3. Polymeric salts and coordinating metal complexes of rare earth elements
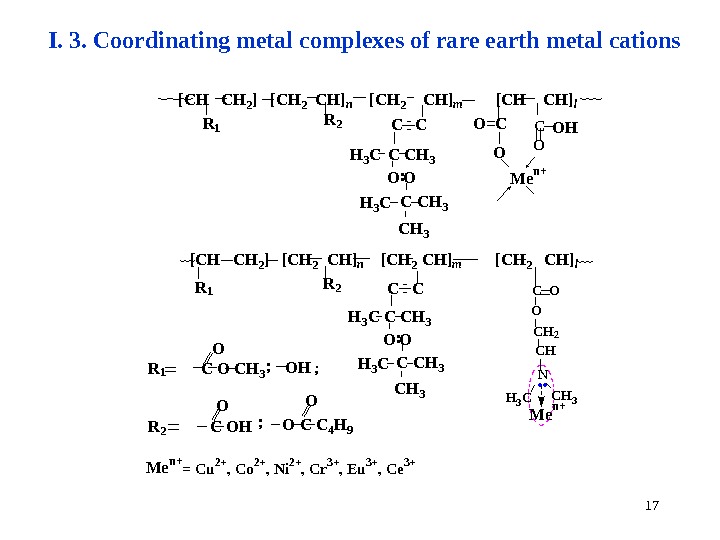
 17 I. 3. Coordinating metal complexes of rare earth metal cations M e n +O O H : O H 3 C CH 3 C O = C C H 3 C C C O[ C H 2 ] [ C H 2 C H ] n [ C H 2 C H ] m [ C H ] l R 1 R 2 C O C H 3 O H C O H O C C 4 H 9 O O O; ; C O M e n + = C u 2 + , C o 2 + , N i 2 + , C r 3 + , E u 3 + , C e 3 +. . C O O C H 2 C H N C H 3 CR 2 R 1[ C H 2 ] [ C H 2 C H ] n [ C H 2 C H ] m [ C H 2 C H ] l OC C C C H 3 C H 3 H 3 C C H 3 C O : M e n +;
17 I. 3. Coordinating metal complexes of rare earth metal cations M e n +O O H : O H 3 C CH 3 C O = C C H 3 C C C O[ C H 2 ] [ C H 2 C H ] n [ C H 2 C H ] m [ C H ] l R 1 R 2 C O C H 3 O H C O H O C C 4 H 9 O O O; ; C O M e n + = C u 2 + , C o 2 + , N i 2 + , C r 3 + , E u 3 + , C e 3 +. . C O O C H 2 C H N C H 3 CR 2 R 1[ C H 2 ] [ C H 2 C H ] n [ C H 2 C H ] m [ C H 2 C H ] l OC C C C H 3 C H 3 H 3 C C H 3 C O : M e n +;
 18 • controlled design of a structure • controlled molecular weight (1, 000 – 30, 000 g/mole) • narrowed molecular weight distribution • controlled macro and microstructure • controlled functionality and reactivity • controlled solubility, surface activity • biocompatibility and non toxicity. Why these oligoperoxide based oligoelectrolytes? • Capability to form free radicals and initiate radical reactions
18 • controlled design of a structure • controlled molecular weight (1, 000 – 30, 000 g/mole) • narrowed molecular weight distribution • controlled macro and microstructure • controlled functionality and reactivity • controlled solubility, surface activity • biocompatibility and non toxicity. Why these oligoperoxide based oligoelectrolytes? • Capability to form free radicals and initiate radical reactions
 19 II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles * Luminescent and scintillation properties of the materials were studied in I. Franko National University under the guidance of Professor A. Voloshinovskii
19 II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles * Luminescent and scintillation properties of the materials were studied in I. Franko National University under the guidance of Professor A. Voloshinovskii
 II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles II. 1. Synthesis of polymeric salts and coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and luminescent polymeric nanoparticles (30 – 150 nm) via water dispersion polymerization initiated and stabilized by OMC. II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent fragments as a result of reactions with reactive phosphors. II. 3. Formation of nanosized micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants containing organic phosphors in hydrophobic core as a result of solubilization in water. II. 4. Synthesis of oligoelectrolyte based nanogels containing coordinated rare earth cations or filled with organic phosphors such as fluorescein in the pores.
II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles II. 1. Synthesis of polymeric salts and coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and luminescent polymeric nanoparticles (30 – 150 nm) via water dispersion polymerization initiated and stabilized by OMC. II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent fragments as a result of reactions with reactive phosphors. II. 3. Formation of nanosized micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants containing organic phosphors in hydrophobic core as a result of solubilization in water. II. 4. Synthesis of oligoelectrolyte based nanogels containing coordinated rare earth cations or filled with organic phosphors such as fluorescein in the pores.
 II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles II. 5. Encapsulation of the phosphors (fluorescein, pyrazolyne and others) in the core of functional polymeric nanoparticles via water dispersion polymerization. II. 6. Template synthesis of functionalized mineral nanoparticles consisting of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 salts doped with cations of Pr +3 , Ce +3 , Eu +2 and Eu +3 core and oligoperoxide shell possessing controlled luminescent and scintillation abilities and capable of grafting functional polymer chains. II. 7. Formation of luminescent nanolayers of controlled thickness and functionality on flat plate surfaces via deposition of functional polymeric and polymer-mineral nanocomposites and futher radical grafting functional polymeric brushes.
II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral nanoparticles II. 5. Encapsulation of the phosphors (fluorescein, pyrazolyne and others) in the core of functional polymeric nanoparticles via water dispersion polymerization. II. 6. Template synthesis of functionalized mineral nanoparticles consisting of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 salts doped with cations of Pr +3 , Ce +3 , Eu +2 and Eu +3 core and oligoperoxide shell possessing controlled luminescent and scintillation abilities and capable of grafting functional polymer chains. II. 7. Formation of luminescent nanolayers of controlled thickness and functionality on flat plate surfaces via deposition of functional polymeric and polymer-mineral nanocomposites and futher radical grafting functional polymeric brushes.
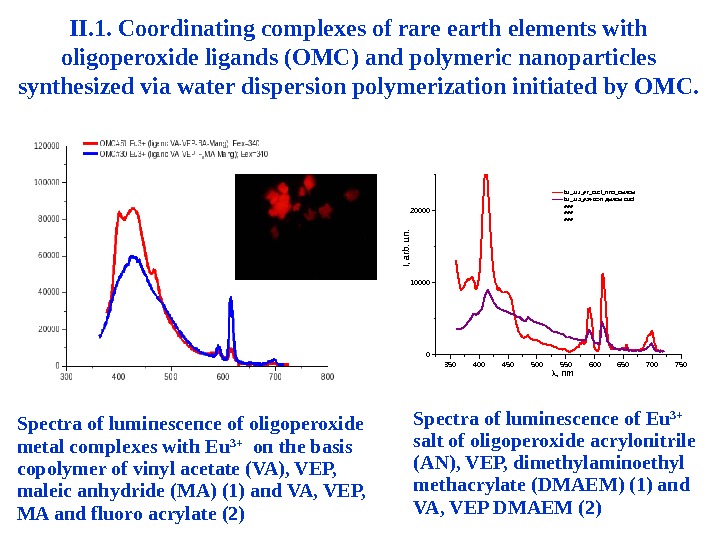
 II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. Spectra of luminescence of oligoperoxide metal complexes with Eu 3+ on the basis copolymer of vinyl acetate (VA), VEP, maleic anhydride (MA) (1) and VA, VEP, MA and fluoro acrylate (2) 350 400 450 500 550 600 650 700 75001000020000 b 1_111_#2_Eu. Cl_H 2 O_DMAEM b 1_113_#34 ВЭП ДМАЕМ Eu. Cl ### ### , nm I, arb. un. Spectra of luminescence of Eu 3+ salt of oligoperoxide acrylonitrile (AN), VEP, dimethylaminoethyl methacrylate (DMAEM) (1) and VA, VEP DMAEM (2)
II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. Spectra of luminescence of oligoperoxide metal complexes with Eu 3+ on the basis copolymer of vinyl acetate (VA), VEP, maleic anhydride (MA) (1) and VA, VEP, MA and fluoro acrylate (2) 350 400 450 500 550 600 650 700 75001000020000 b 1_111_#2_Eu. Cl_H 2 O_DMAEM b 1_113_#34 ВЭП ДМАЕМ Eu. Cl ### ### , nm I, arb. un. Spectra of luminescence of Eu 3+ salt of oligoperoxide acrylonitrile (AN), VEP, dimethylaminoethyl methacrylate (DMAEM) (1) and VA, VEP DMAEM (2)
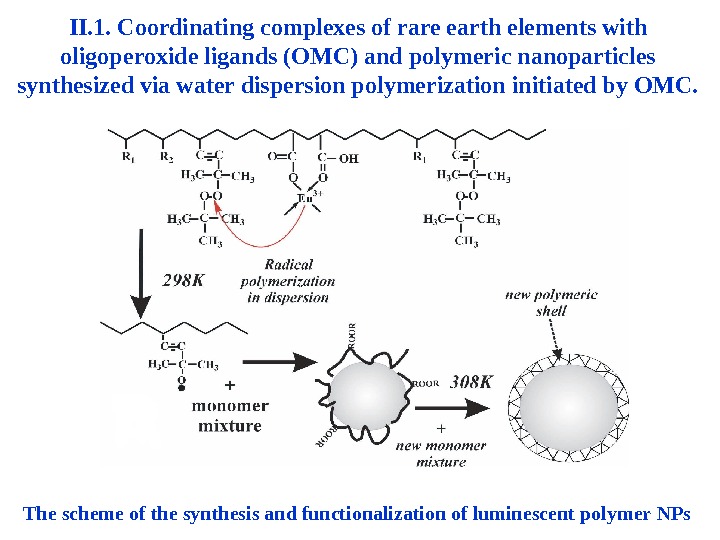
 II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. The scheme of the synthesis and functionalization of luminescent polymer NPs
II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. The scheme of the synthesis and functionalization of luminescent polymer NPs
 II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. Luminescence spectrum (b) of Eu +3 containing OMC (1) and polymer NPs (2) synthesized in the presence of OMC; TEM images of luminescent NPs (c).
II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. Luminescence spectrum (b) of Eu +3 containing OMC (1) and polymer NPs (2) synthesized in the presence of OMC; TEM images of luminescent NPs (c).
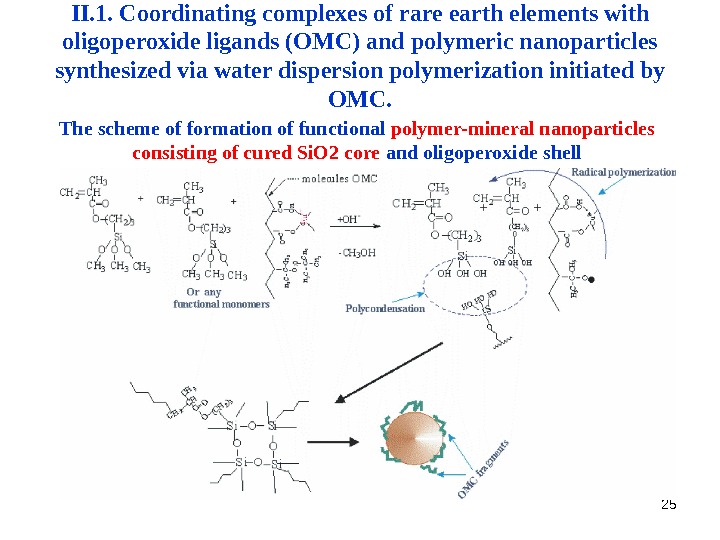
 25 The scheme of formation of functional polymer-mineral nanoparticles consisting of cured Si. O 2 core and oligoperoxide shell. II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC.
25 The scheme of formation of functional polymer-mineral nanoparticles consisting of cured Si. O 2 core and oligoperoxide shell. II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC.
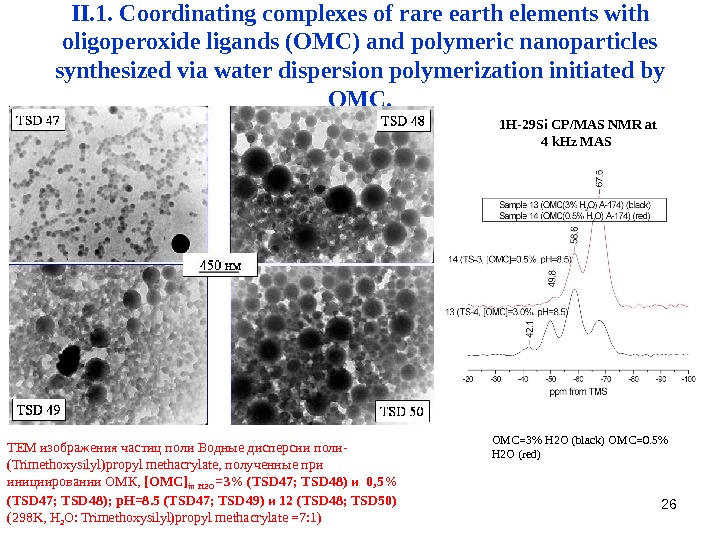
 26 II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. TEM изображения частиц поли Водные дисперсии поли- (Trimethoxysilyl)propyl methacrylate, полученные при инициировании ОМК, [ OMC ] in H 2 O =3% (TSD 47; TSD 48) и 0, 5% (TSD 47; TSD 48); p. H=8. 5 (TSD 4 7 ; TSD 49 ) и 12 (TSD 48; TSD 50) (298 K, Н 2 О: Trimethoxysilyl)propyl methacrylate =7: 1) OMC=3% H 2 O (black) OMC=0. 5% H 2 O (red) 1 H-29 Si CP/MAS NMR at 4 k. Hz MAS
26 II. 1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via water dispersion polymerization initiated by OMC. TEM изображения частиц поли Водные дисперсии поли- (Trimethoxysilyl)propyl methacrylate, полученные при инициировании ОМК, [ OMC ] in H 2 O =3% (TSD 47; TSD 48) и 0, 5% (TSD 47; TSD 48); p. H=8. 5 (TSD 4 7 ; TSD 49 ) и 12 (TSD 48; TSD 50) (298 K, Н 2 О: Trimethoxysilyl)propyl methacrylate =7: 1) OMC=3% H 2 O (black) OMC=0. 5% H 2 O (red) 1 H-29 Si CP/MAS NMR at 4 k. Hz MAS
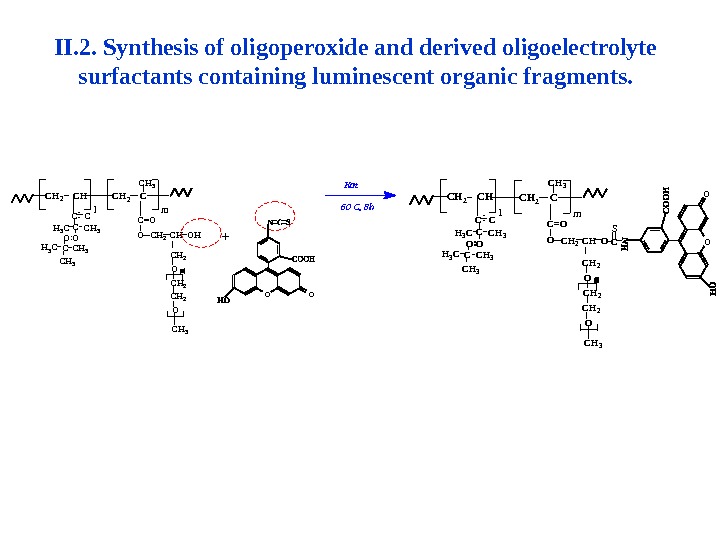
 II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments. C H 3 O C H 2 O Hl. C H 2 C H m C H 2 O C = O C H 2 C H 2 C : O H 3 C C H 3 CC C O C H 3 O OO O+ K a t 6 0 C , 8 h OOC H 3 OC C C C H 3 C H 3 H 3 C CH 3 C O : C H 2 C C H 2 C HC = O O C H 2 m. C H 2 C H l O C H 2 OC H 2 O C H 3 C S
II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments. C H 3 O C H 2 O Hl. C H 2 C H m C H 2 O C = O C H 2 C H 2 C : O H 3 C C H 3 CC C O C H 3 O OO O+ K a t 6 0 C , 8 h OOC H 3 OC C C C H 3 C H 3 H 3 C CH 3 C O : C H 2 C C H 2 C HC = O O C H 2 m. C H 2 C H l O C H 2 OC H 2 O C H 3 C S
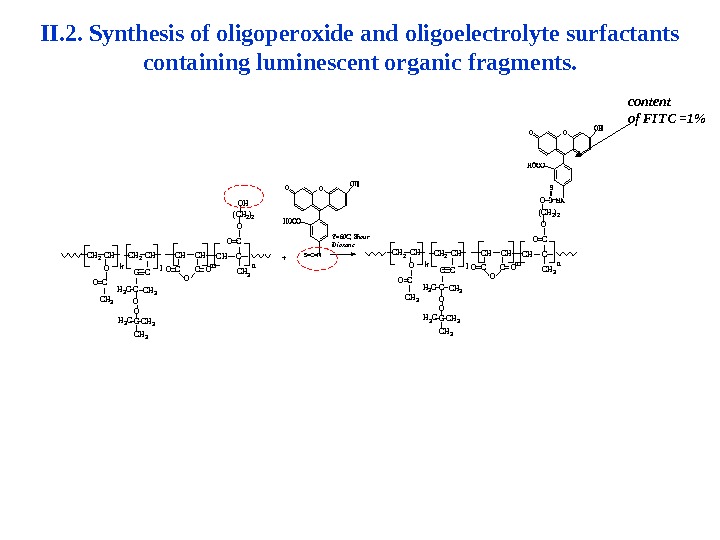
 II. 2. Synthesis of oligoperoxide and oligoelectrolyte surfactants containing luminescent organic fragments. content of FITC =1%mlk O CC O O CH 3 H 3 CC C C CH 3 O CH CHCH 2 CHCH Cn. CH 3 CO O (CH 2)2 OH O OO O O (CH 2)2 O OC CH 3 n. CH CCH 2 CHCH CH O CH 3 C C CH 3 CCH 3 O C OO C klm OO S=C=N T=60 C, 8 hour. Dioxane + H 3 C CH 3 COOCCH 3 H
II. 2. Synthesis of oligoperoxide and oligoelectrolyte surfactants containing luminescent organic fragments. content of FITC =1%mlk O CC O O CH 3 H 3 CC C C CH 3 O CH CHCH 2 CHCH Cn. CH 3 CO O (CH 2)2 OH O OO O O (CH 2)2 O OC CH 3 n. CH CCH 2 CHCH CH O CH 3 C C CH 3 CCH 3 O C OO C klm OO S=C=N T=60 C, 8 hour. Dioxane + H 3 C CH 3 COOCCH 3 H
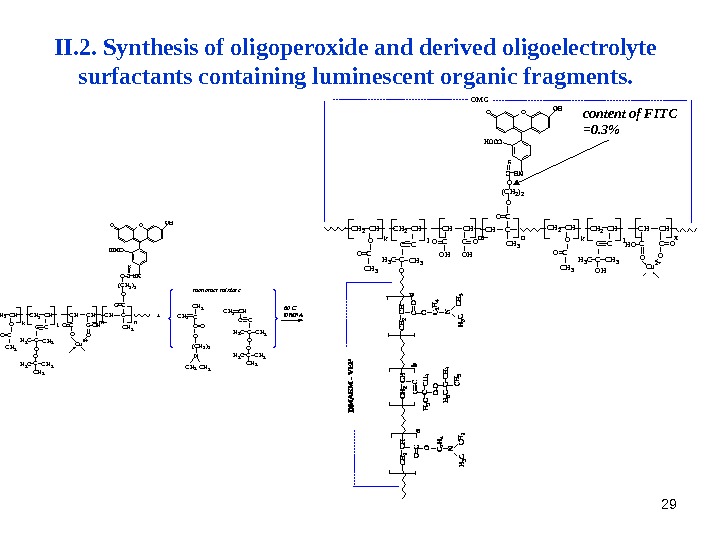
 29 II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments. H 3 C C H 3 CO C u 2 +O O HC O O ( C H 2 ) 2 O O C C H 3 n. C H C C H 2 C H C H O C H 3 C C CH 3 C C H 3 O O C k l m OO + C H 2 CC H 3 C = O O ( C H 2 ) 2 N C H 3 O C H 3 H 3 C C O C C H 3 H 3 CC H 2 C Hm o n o m e r m i x t u r e 6 0 C D M F A O M C C u 2 +H O OCC H 2 C H C H O C H 3 C C CH 3 C C H 3 O H C O O C Ok l x m lk O HO C C O O C H 3 H 3 C C C H 3 O C HC H 2 C H C n C H 3 CO O( C H 2 ) 2 OO O content of FITC =0. 3%
29 II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments. H 3 C C H 3 CO C u 2 +O O HC O O ( C H 2 ) 2 O O C C H 3 n. C H C C H 2 C H C H O C H 3 C C CH 3 C C H 3 O O C k l m OO + C H 2 CC H 3 C = O O ( C H 2 ) 2 N C H 3 O C H 3 H 3 C C O C C H 3 H 3 CC H 2 C Hm o n o m e r m i x t u r e 6 0 C D M F A O M C C u 2 +H O OCC H 2 C H C H O C H 3 C C CH 3 C C H 3 O H C O O C Ok l x m lk O HO C C O O C H 3 H 3 C C C H 3 O C HC H 2 C H C n C H 3 CO O( C H 2 ) 2 OO O content of FITC =0. 3%
 30 The excitation and emission spectra of VA — VEP — MA — HEMA + FITC — graft — VEP — DMAEM branched copolymer II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent fragments as a result of reactions with reactive phosphors. 200 300 400 500 600 700510 Emission. Intensity, arb. un. Wavelength, nm. Excitation
30 The excitation and emission spectra of VA — VEP — MA — HEMA + FITC — graft — VEP — DMAEM branched copolymer II. 2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent fragments as a result of reactions with reactive phosphors. 200 300 400 500 600 700510 Emission. Intensity, arb. un. Wavelength, nm. Excitation
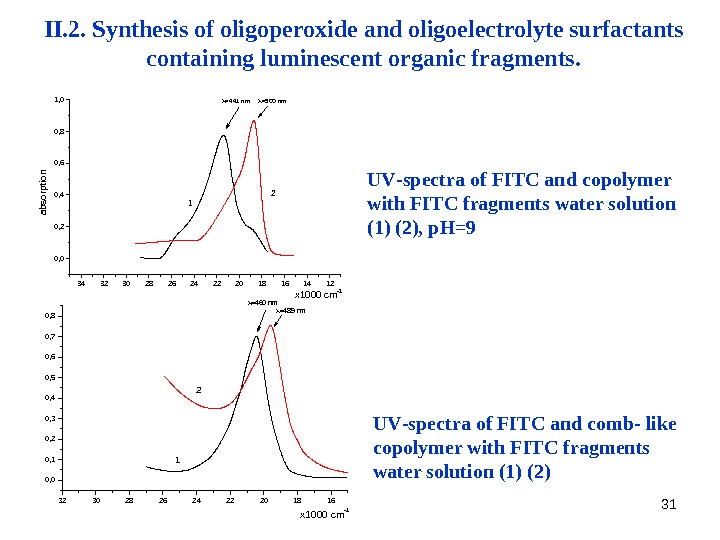
 31343230282624222018161412 0, 0 0, 2 0, 4 0, 6 0, 8 1, 0 2 1 =500 nm =441 nmabsorption x 1000 cm -1 UV — spectra of FITC and copolymer with FITC fragments water solution (1) (2), р. Н=9 32 30 28 26 24 22 20 18 160, 00, 10, 20, 30, 40, 50, 60, 70, 8 2 1 =489 nm =460 nm x 1000 cm -1 UV — spectra of FITC and comb- like copolymer with FITC fragments water solution (1) (2)II. 2. Synthesis of oligoperoxide and oligoelectrolyte surfactants containing luminescent organic fragments.
31343230282624222018161412 0, 0 0, 2 0, 4 0, 6 0, 8 1, 0 2 1 =500 nm =441 nmabsorption x 1000 cm -1 UV — spectra of FITC and copolymer with FITC fragments water solution (1) (2), р. Н=9 32 30 28 26 24 22 20 18 160, 00, 10, 20, 30, 40, 50, 60, 70, 8 2 1 =489 nm =460 nm x 1000 cm -1 UV — spectra of FITC and comb- like copolymer with FITC fragments water solution (1) (2)II. 2. Synthesis of oligoperoxide and oligoelectrolyte surfactants containing luminescent organic fragments.
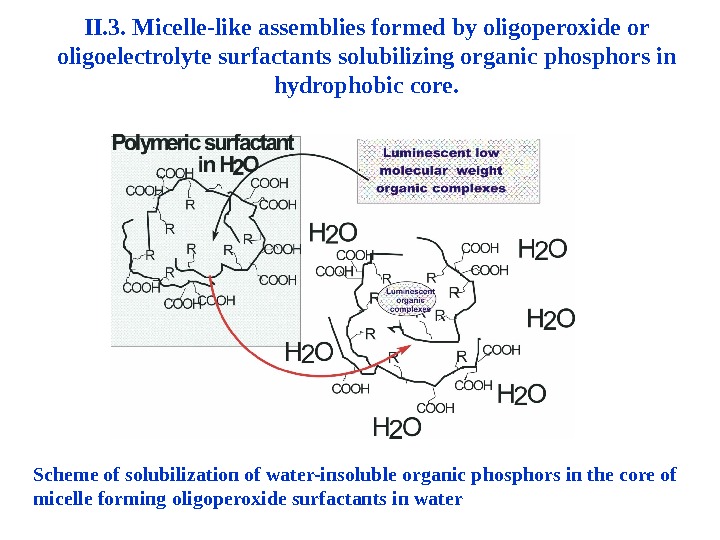
 II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Scheme of solubilization of water-insoluble organic phosphors in the core of micelle forming oligoperoxide surfactants in water
II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Scheme of solubilization of water-insoluble organic phosphors in the core of micelle forming oligoperoxide surfactants in water
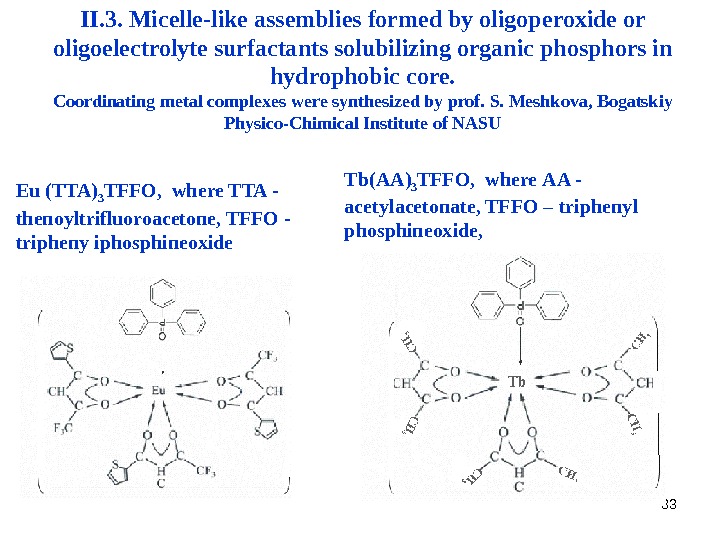
 33 II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Coordinating metal complexes were synthesized by prof. S. Meshkova, Bogatskiy Physico-Chimical Institute of NASU Eu (TTA) 3 TFFO , where TTA — thenoyltrifluoroacetone, TFFO — tripheny iphosphineoxide Tb ( A A) 3 TFFO , where A A — a cetylacetonate, TFFO – tripheny l phosphineoxide ,
33 II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Coordinating metal complexes were synthesized by prof. S. Meshkova, Bogatskiy Physico-Chimical Institute of NASU Eu (TTA) 3 TFFO , where TTA — thenoyltrifluoroacetone, TFFO — tripheny iphosphineoxide Tb ( A A) 3 TFFO , where A A — a cetylacetonate, TFFO – tripheny l phosphineoxide ,
 II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. 200 300 400 500 600 7000, 250, 500, 751, 00 em = 615 nm. Intensity, arb. un. , nm. T = 300 K exc = 390 nm The excitation and e mission spectra of Eu 3+ for Eu (TTA) in the micelle hydrophobic zones of oliogoperoxide surfactants 200 250 300 350 400 450 500 550 600 6500500010000150002000025000300003500040000450000 Intensity, , nm Ex=380 nm Em=540 nm 3 2 1 B B B Spectra of excitation (1, 2) and luminescence (3) of the complex Tb(AА) 3 solubilized in micelles formed by oligo (VA — MAN — MP (Т-3), concentration of the complex is 0. 5% (1, 3) and 0. 3% (2) ;
II. 3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. 200 300 400 500 600 7000, 250, 500, 751, 00 em = 615 nm. Intensity, arb. un. , nm. T = 300 K exc = 390 nm The excitation and e mission spectra of Eu 3+ for Eu (TTA) in the micelle hydrophobic zones of oliogoperoxide surfactants 200 250 300 350 400 450 500 550 600 6500500010000150002000025000300003500040000450000 Intensity, , nm Ex=380 nm Em=540 nm 3 2 1 B B B Spectra of excitation (1, 2) and luminescence (3) of the complex Tb(AА) 3 solubilized in micelles formed by oligo (VA — MAN — MP (Т-3), concentration of the complex is 0. 5% (1, 3) and 0. 3% (2) ;
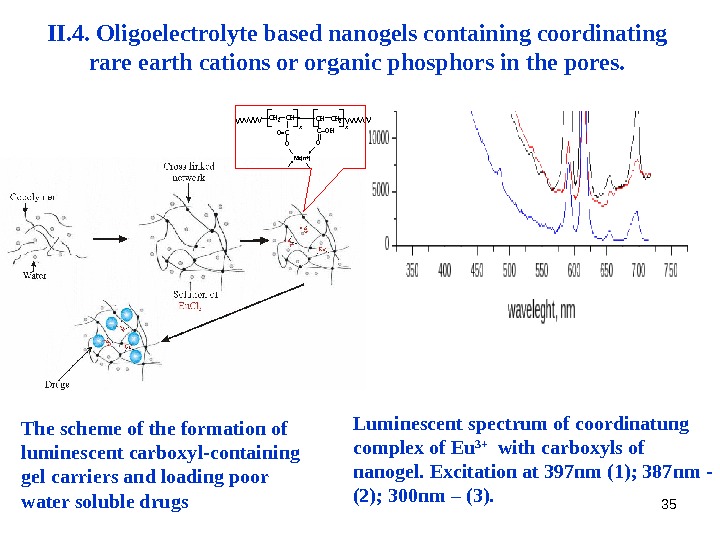
 35 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The scheme of the formation of luminescent carboxyl-containing gel carriers and loading poor water soluble drugs Luminescent spectrum of coordinatung complex of Eu 3+ with carboxyls of nanogel. Excitation at 397 nm ( 1 ) ; 387 nm — ( 2 ) ; 300 nm – ( 3 ). x. CH 2 O CO=COH Me(n+) O CH 2 CHx
35 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The scheme of the formation of luminescent carboxyl-containing gel carriers and loading poor water soluble drugs Luminescent spectrum of coordinatung complex of Eu 3+ with carboxyls of nanogel. Excitation at 397 nm ( 1 ) ; 387 nm — ( 2 ) ; 300 nm – ( 3 ). x. CH 2 O CO=COH Me(n+) O CH 2 CHx
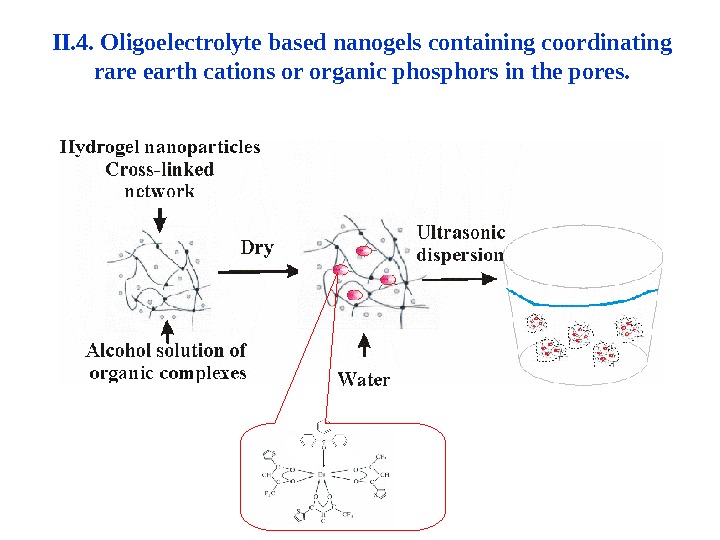
 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores.
II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores.
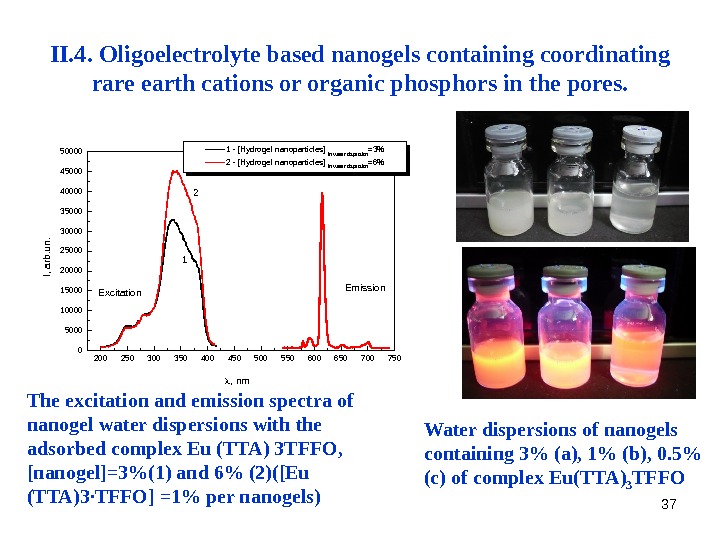
 37 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The excitation and emission spectra of nanogel water dispersions with the adsorbed complex Eu (TTA) 3 TFFO, [nanogel]=3%(1) and 6% (2)([Eu (TTA)3·TFFO] =1% per nanogels) 200 250 300 350 400 450 500 550 600 650 700 7500500010000150002000025000300003500040000450000 Emission 2 1 I, arb. un. , nm 1 — [Hydrogel nanoparticles] in Water dispersion =3% 2 — [Hydrogel nanoparticles] in Water dispersion =6% Excitation Water dispersions of nanogels containing 3% ( a), 1% (b), 0. 5% (c) of complex Eu(TTA) 3 TFFO
37 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The excitation and emission spectra of nanogel water dispersions with the adsorbed complex Eu (TTA) 3 TFFO, [nanogel]=3%(1) and 6% (2)([Eu (TTA)3·TFFO] =1% per nanogels) 200 250 300 350 400 450 500 550 600 650 700 7500500010000150002000025000300003500040000450000 Emission 2 1 I, arb. un. , nm 1 — [Hydrogel nanoparticles] in Water dispersion =3% 2 — [Hydrogel nanoparticles] in Water dispersion =6% Excitation Water dispersions of nanogels containing 3% ( a), 1% (b), 0. 5% (c) of complex Eu(TTA) 3 TFFO
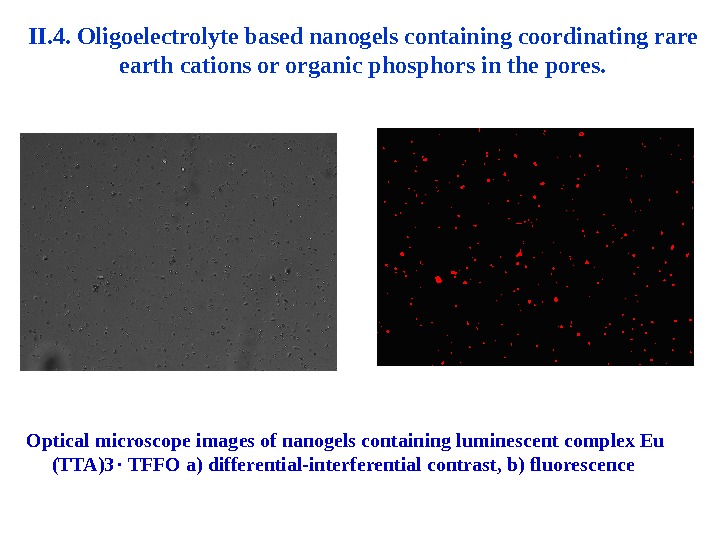
 II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. Optical microscope images of nanogels containing luminescent complex Eu ( TTA )3 TFFO a ) differential-interferential contrast , b ) fluorescence
II. 4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. Optical microscope images of nanogels containing luminescent complex Eu ( TTA )3 TFFO a ) differential-interferential contrast , b ) fluorescence
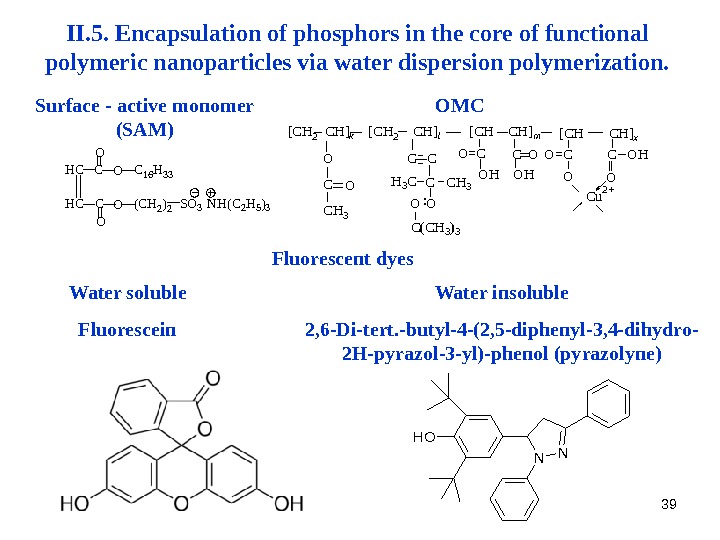
 39 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. HC HC C C O OC 16 H 33 (CH 2)2 SO 3 NH(C 2 H 5)3 Surface — active monomer (SAM) Cu 2+ C(CH 3)3 OO : O H 3 C O=C CH 3 C C C O OC OH [CH 2 CH]k [CH 2 CH]l [CH CH]m CO C O [CH CH]x OHOH O=C OMC Water soluble Fluorescein Water insoluble 2, 6 -Di-tert. -butyl-4 -(2, 5 -diphenyl-3, 4 -dihydro- 2 H-pyrazol-3 -yl)-phenol (pyrazolyne) NN OHFluorescent dyes
39 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. HC HC C C O OC 16 H 33 (CH 2)2 SO 3 NH(C 2 H 5)3 Surface — active monomer (SAM) Cu 2+ C(CH 3)3 OO : O H 3 C O=C CH 3 C C C O OC OH [CH 2 CH]k [CH 2 CH]l [CH CH]m CO C O [CH CH]x OHOH O=C OMC Water soluble Fluorescein Water insoluble 2, 6 -Di-tert. -butyl-4 -(2, 5 -diphenyl-3, 4 -dihydro- 2 H-pyrazol-3 -yl)-phenol (pyrazolyne) NN OHFluorescent dyes
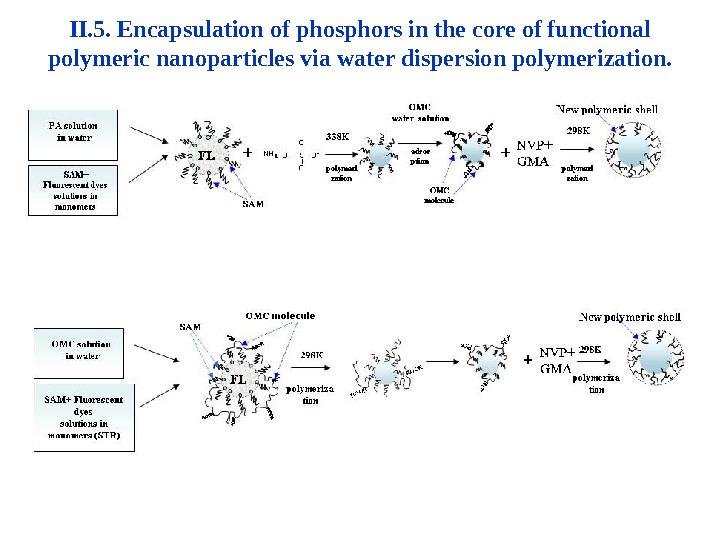
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization.
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization.
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. 0, 0 0, 1 0, 2 0, 3 0, 4 0, 5 0, 6707580859095100 21 Relative content in respect of charged fluorescein (%) particles size, m -5, 0 x 10 -190, 05, 0 x 10 -191, 0 x 10 -181, 5 x 10 -182, 0 x 10 -182, 5 x 10 -183, 0 x 10 -183, 5 x 10 -184, 0 x 10 -18 Total content of fluorescein per particle (mole/particle) The dependences of relative in respect of charged amount (1) and total (2) contents of fluorescein encapsulated per one functional nanoparticle on nanoparticle size. (■, – monomer mixture: STR: SAM, initiator – PA, ●, ♦– monomer mixture: STR: SAM, initiator – OMC, ▲, ▼– monomer mixture: MMA-BA-GMA: SAM, initiator – OMC)
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. 0, 0 0, 1 0, 2 0, 3 0, 4 0, 5 0, 6707580859095100 21 Relative content in respect of charged fluorescein (%) particles size, m -5, 0 x 10 -190, 05, 0 x 10 -191, 0 x 10 -181, 5 x 10 -182, 0 x 10 -182, 5 x 10 -183, 0 x 10 -183, 5 x 10 -184, 0 x 10 -18 Total content of fluorescein per particle (mole/particle) The dependences of relative in respect of charged amount (1) and total (2) contents of fluorescein encapsulated per one functional nanoparticle on nanoparticle size. (■, – monomer mixture: STR: SAM, initiator – PA, ●, ♦– monomer mixture: STR: SAM, initiator – OMC, ▲, ▼– monomer mixture: MMA-BA-GMA: SAM, initiator – OMC)
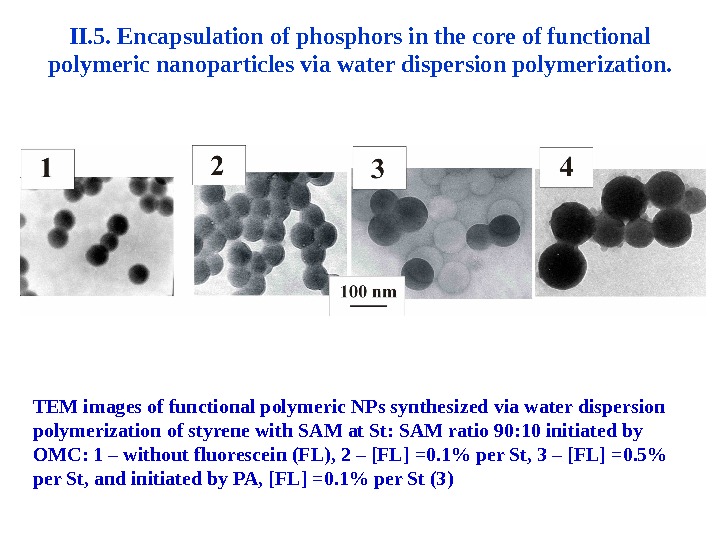
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. TEM images of functional polymeric NPs synthesized via water dispersion polymerization of styrene with SAM at St: SAM ratio 90: 10 initiated by OMC: 1 – without fluorescein (FL), 2 – [FL] =0. 1% per St, 3 – [FL] =0. 5% per St, and initiated by PA, [FL] =0. 1% per St (3)
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. TEM images of functional polymeric NPs synthesized via water dispersion polymerization of styrene with SAM at St: SAM ratio 90: 10 initiated by OMC: 1 – without fluorescein (FL), 2 – [FL] =0. 1% per St, 3 – [FL] =0. 5% per St, and initiated by PA, [FL] =0. 1% per St (3)
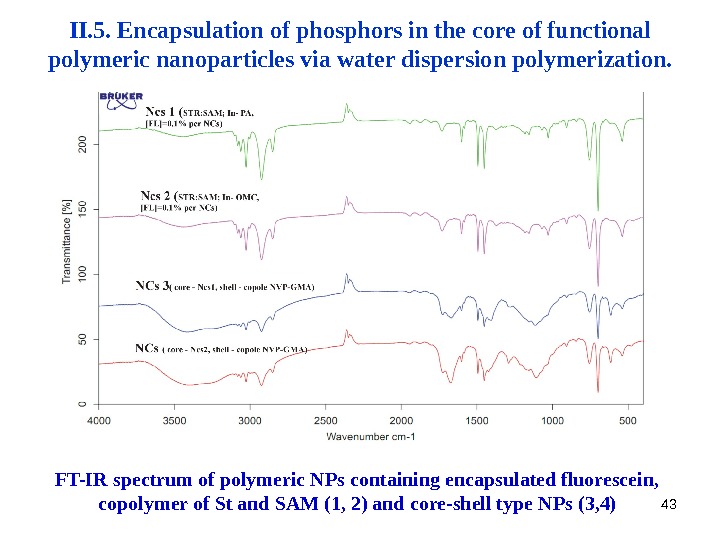
 43 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. FT-IR spectrum of polymeric NPs containing encapsulated fluorescein , copolymer of St and SAM (1, 2) and core-shell type NPs (3, 4)
43 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. FT-IR spectrum of polymeric NPs containing encapsulated fluorescein , copolymer of St and SAM (1, 2) and core-shell type NPs (3, 4)
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. 300400500600 0, 2 0, 4 0, 6 0, 8 1, 02 b Excitation / Emission intensity, a. u. 1 a , nm 1 b Fluorescence (1) and fluorescence excitation (2) spectra of polymeric fluorescein-encapsulated NPs. b – Emission spectra of fluorescein (1) and of fluorescein-encapsulated NPs (2); excitation at 425 nm. F l u o r e s c e i n. N C s F l Green fluorescence of FITS and FITC-encapsulated polystyrene nanoparticles (PSFITS а) in water based systems at distinct dilution (PSFITS а/2, a/4, a/6)
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. 300400500600 0, 2 0, 4 0, 6 0, 8 1, 02 b Excitation / Emission intensity, a. u. 1 a , nm 1 b Fluorescence (1) and fluorescence excitation (2) spectra of polymeric fluorescein-encapsulated NPs. b – Emission spectra of fluorescein (1) and of fluorescein-encapsulated NPs (2); excitation at 425 nm. F l u o r e s c e i n. N C s F l Green fluorescence of FITS and FITC-encapsulated polystyrene nanoparticles (PSFITS а) in water based systems at distinct dilution (PSFITS а/2, a/4, a/6)
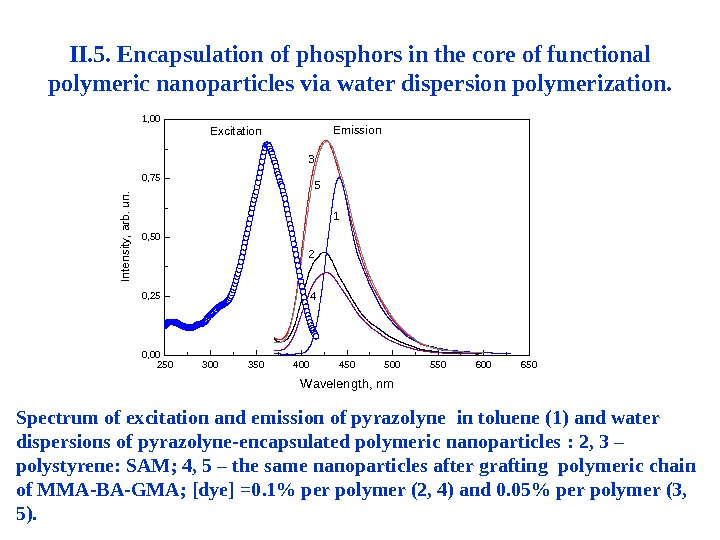
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Spectrum of excitation and emission of pyrazolyne in toluene (1) and water dispersions of pyrazolyne-encapsulated polymeric nanoparticles : 2, 3 – polystyrene: SAM ; 4, 5 – the same nanoparticles after grafting polymeric chain of MMA-BA-GMA ; [ dye ] =0. 1% per polymer (2, 4) and 0. 05% per polymer (3, 5). 250 300 350 400 450 500 550 600 6500, 000, 250, 500, 751, 00 Excitation 5 43 2 1 Wavelength, nm Emission Intensity, arb. un.
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Spectrum of excitation and emission of pyrazolyne in toluene (1) and water dispersions of pyrazolyne-encapsulated polymeric nanoparticles : 2, 3 – polystyrene: SAM ; 4, 5 – the same nanoparticles after grafting polymeric chain of MMA-BA-GMA ; [ dye ] =0. 1% per polymer (2, 4) and 0. 05% per polymer (3, 5). 250 300 350 400 450 500 550 600 6500, 000, 250, 500, 751, 00 Excitation 5 43 2 1 Wavelength, nm Emission Intensity, arb. un.
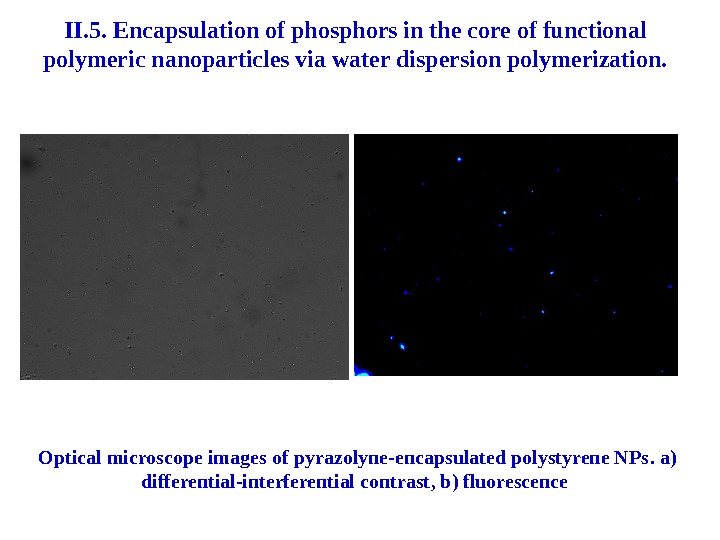
 II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Optical microscope images of pyrazolyne-encapsulated polystyrene NPs. а) differential-interferential contrast , b ) fluorescence
II. 5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Optical microscope images of pyrazolyne-encapsulated polystyrene NPs. а) differential-interferential contrast , b ) fluorescence
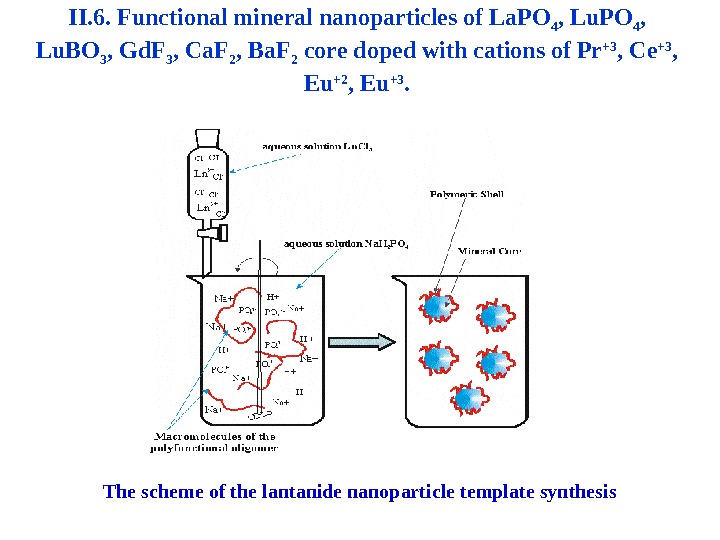
 II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3. The scheme of the lantanide nanoparticle template synthesis
II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3. The scheme of the lantanide nanoparticle template synthesis
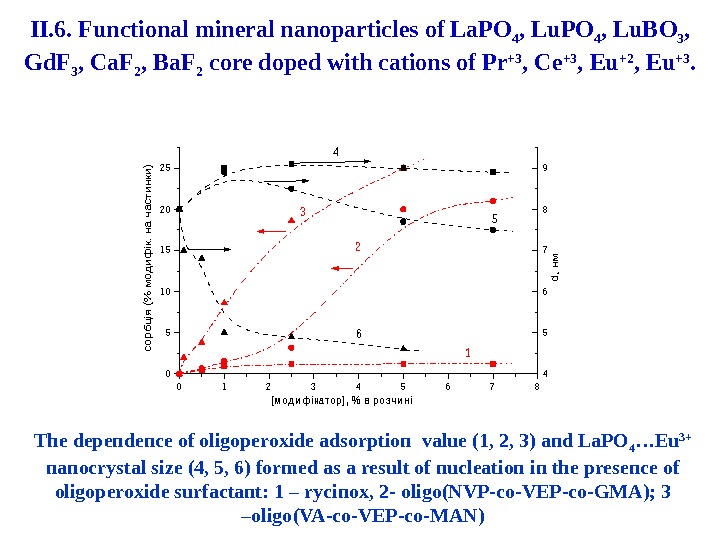
 012345678 0 5 1 0 1 5 2 0 2 5 сорбція (% модифік. на частинки) [м о д и ф ік а то р ] , % в р о з ч и н і 4 5 6 7 8 9 d, нм 6 3 2 5 4 1 The dependence of oligoperoxide adsorption value (1, 2, 3) and La. PO 4 …Eu 3+ nanocrystal size (4, 5, 6 ) formed as a result of nucleation in the presence of oligoperoxide surfactant : 1 – rycinox , 2 — oligo (N VP — co — VEP — co — GMA ); 3 – oligo ( VA — co — VEP — co — MAN )II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3.
012345678 0 5 1 0 1 5 2 0 2 5 сорбція (% модифік. на частинки) [м о д и ф ік а то р ] , % в р о з ч и н і 4 5 6 7 8 9 d, нм 6 3 2 5 4 1 The dependence of oligoperoxide adsorption value (1, 2, 3) and La. PO 4 …Eu 3+ nanocrystal size (4, 5, 6 ) formed as a result of nucleation in the presence of oligoperoxide surfactant : 1 – rycinox , 2 — oligo (N VP — co — VEP — co — GMA ); 3 – oligo ( VA — co — VEP — co — MAN )II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3.
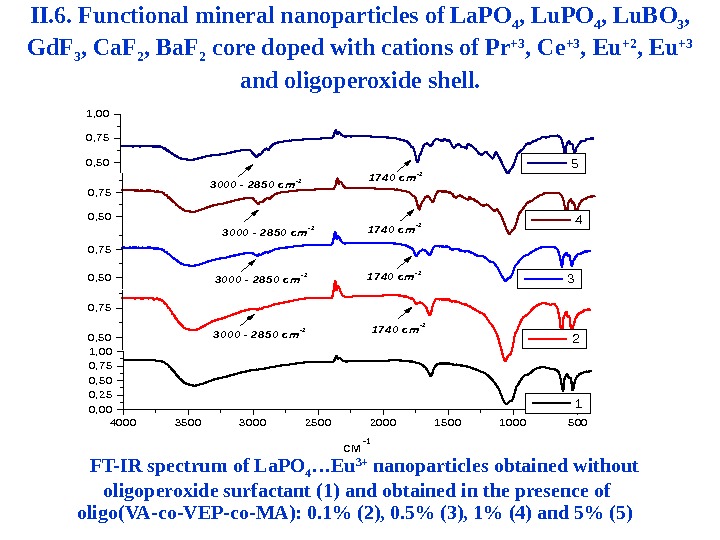
 4 0 0 035 0 03 0 0 02 5 0 02 0 0 01 50 01 0 0 05 0 0 0 , 00 0 , 25 0 , 50 0 , 75 1 , 00 1 c м-1 0 , 5 0 0 , 7 5 2 0 , 5 0 0 , 7 5 3 0 0 0 — 2 8 5 0 c m-1 30 0 0 — 2 8 5 0 c m- 1 3 0 0 0 — 2 8 50 c m-1 1 7 4 0 c m-1 1 7 4 0 cm- 1 1 7 4 0 c m- 1 3 0 , 5 0 0 , 7 5 4 0 , 5 0 0 , 7 5 1 , 0 0 5 FT-IR spectrum of La. PO 4 …Eu 3+ nanoparticles obtained without oligoperoxide surfactant (1) and obtained in the presence of oligo ( VA — co — VEP — co — MA ): 0. 1% (2), 0. 5% (3), 1% (4) and 5% (5) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3 and oligoperoxide shell.
4 0 0 035 0 03 0 0 02 5 0 02 0 0 01 50 01 0 0 05 0 0 0 , 00 0 , 25 0 , 50 0 , 75 1 , 00 1 c м-1 0 , 5 0 0 , 7 5 2 0 , 5 0 0 , 7 5 3 0 0 0 — 2 8 5 0 c m-1 30 0 0 — 2 8 5 0 c m- 1 3 0 0 0 — 2 8 50 c m-1 1 7 4 0 c m-1 1 7 4 0 cm- 1 1 7 4 0 c m- 1 3 0 , 5 0 0 , 7 5 4 0 , 5 0 0 , 7 5 1 , 0 0 5 FT-IR spectrum of La. PO 4 …Eu 3+ nanoparticles obtained without oligoperoxide surfactant (1) and obtained in the presence of oligo ( VA — co — VEP — co — MA ): 0. 1% (2), 0. 5% (3), 1% (4) and 5% (5) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3 and oligoperoxide shell.
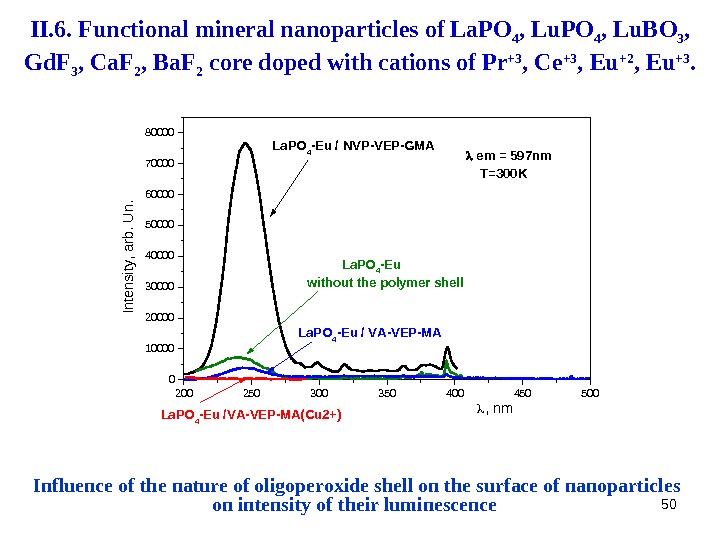
 50 Influence of the nature of oligoperoxide shell on the surface of nanoparticles on intensity of their luminescence 200 250 300 350 400 450 50001000020000300004000050000600007000080000 La. PO 4 -Eu without the polymer shell La. PO 4 -Eu /VA-VEP-MA(Cu 2+) La. PO 4 -Eu / VA-VEP-MALa. PO 4 -Eu / NVP-VEP-GMAIntensity, arb. U n. , nm em = 597 nm T=300 KII. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3.
50 Influence of the nature of oligoperoxide shell on the surface of nanoparticles on intensity of their luminescence 200 250 300 350 400 450 50001000020000300004000050000600007000080000 La. PO 4 -Eu without the polymer shell La. PO 4 -Eu /VA-VEP-MA(Cu 2+) La. PO 4 -Eu / VA-VEP-MALa. PO 4 -Eu / NVP-VEP-GMAIntensity, arb. U n. , nm em = 597 nm T=300 KII. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +3.
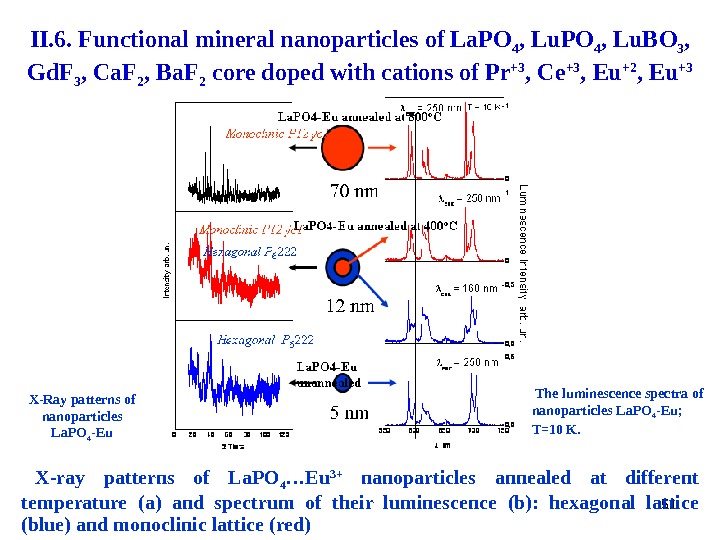
 51 X-Ray patterns of nanoparticles La. PO 4 -Eu The luminescence spectra of nanoparticles La. PO 4 -Eu ; T=10 K. X-ray patterns of La. PO 4 …Eu 3+ nanoparticles annealed at different temperature ( a ) and spectrum of their luminescence ( b ): hexagonal lattice ( blue ) and monoclinic lattice ( red ) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
51 X-Ray patterns of nanoparticles La. PO 4 -Eu The luminescence spectra of nanoparticles La. PO 4 -Eu ; T=10 K. X-ray patterns of La. PO 4 …Eu 3+ nanoparticles annealed at different temperature ( a ) and spectrum of their luminescence ( b ): hexagonal lattice ( blue ) and monoclinic lattice ( red ) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
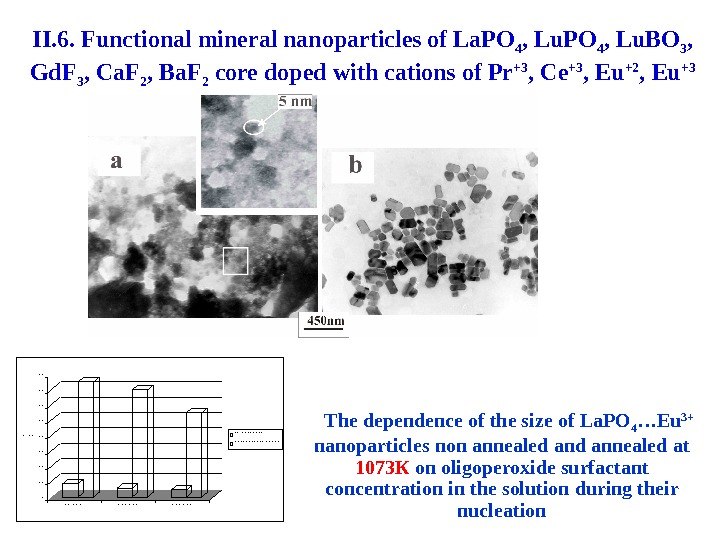
 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 d , н м 0 % П А П 1 , 0 % П А П 2 , 5 % П А П н е в и п а л е н і п р и 1 0 7 3 КThe dependence of the size of La. PO 4 …Eu 3+ nanoparticles non annealed at 1073 К on oligoperoxide surfactant concentration in the solution during their nucleation. II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 d , н м 0 % П А П 1 , 0 % П А П 2 , 5 % П А П н е в и п а л е н і п р и 1 0 7 3 КThe dependence of the size of La. PO 4 …Eu 3+ nanoparticles non annealed at 1073 К on oligoperoxide surfactant concentration in the solution during their nucleation. II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
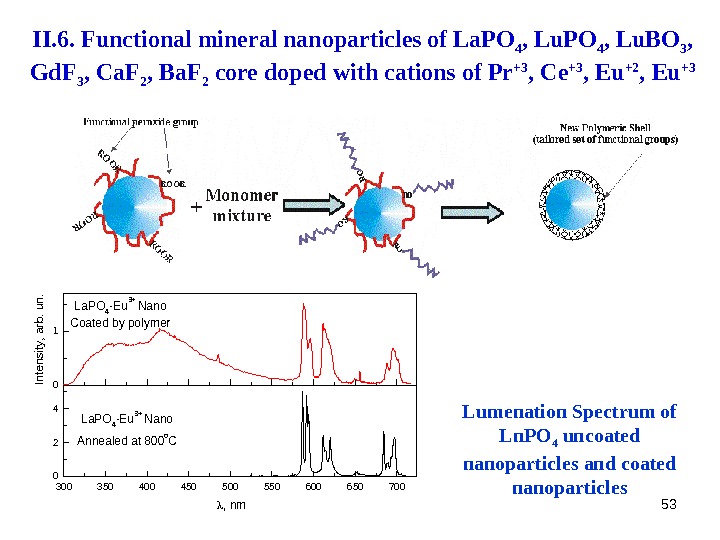
 53300350400450500550600650700 0 2 4 La. PO 4 -Eu 3+ Nano Annealed at 800 o. C Intensity, arb. un. , nm 0 1 La. PO 4 -Eu 3+ Nano Coated by polymer Lumenation Spectrum of Ln. PO 4 uncoated nanoparticles and coated nanoparticles. II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
53300350400450500550600650700 0 2 4 La. PO 4 -Eu 3+ Nano Annealed at 800 o. C Intensity, arb. un. , nm 0 1 La. PO 4 -Eu 3+ Nano Coated by polymer Lumenation Spectrum of Ln. PO 4 uncoated nanoparticles and coated nanoparticles. II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
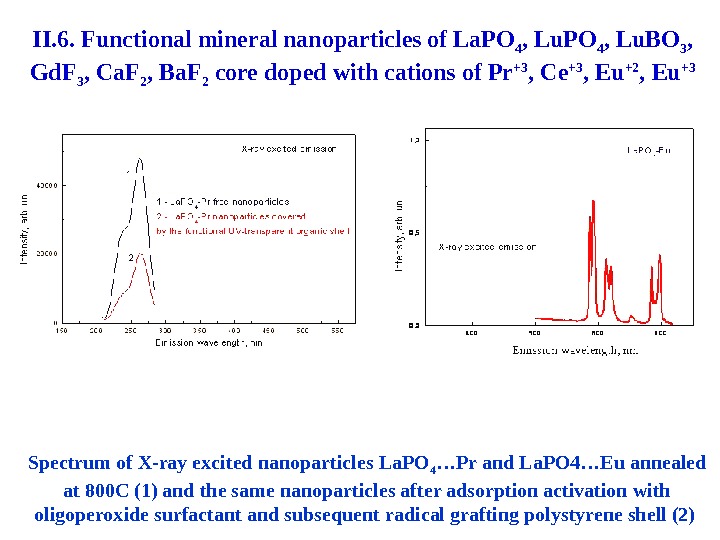
 54 Spectrum of X-ray excited nanoparticles La. PO 4 … Pr and La. PO 4 …Eu annealed at 800 С (1) and the same nanoparticles after adsorption activation with oligoperoxide surfactant and subsequent radical grafting polystyrene shell (2) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
54 Spectrum of X-ray excited nanoparticles La. PO 4 … Pr and La. PO 4 …Eu annealed at 800 С (1) and the same nanoparticles after adsorption activation with oligoperoxide surfactant and subsequent radical grafting polystyrene shell (2) II. 6. Functional mineral nanoparticles of La. PO 4 , Lu. BO 3 , Gd. F 3 , Ca. F 2 , Ba. F 2 core doped with cations of Pr +3 , Ce +3 , Eu +2 , Eu +
 55 II. 7. Luminescent nanolayers on flat plate surfaces deposited from solutions and dispersions of functional polymeric and polymer-mineral nanocomposites. H 3 C C H 3 CO : OH 3 C C H 3 O HO HC O = CC C C O E u 3 +C H 2 C H k x. C H O CO = C O H O L 1 L 2 C H 2 C H l n. C H O O = C C H 3 C H 2 C H O = C m O C 4 H 9 An average thickness (a) and refractive index (b) determined with ellipsometry for PO-Eu adlayers as a function of adsorption time for OP-Eu solutions with concentration: 0. 6 % (triangles), 1. 0 % (circles) and 2. 5 % (squares). Solid lines are a guide to eye. Dashed line in (b) denotes bulk value extrapolated from solution refractometry 050100 0 20 40 60 80 a) Adsorbtion time [min] Adlayer thicknes [nm] 050100 1. 35 1. 50 1. 45 bulk valueb) Adsorbtion time [min] Adlayer refractive indicex 1.
55 II. 7. Luminescent nanolayers on flat plate surfaces deposited from solutions and dispersions of functional polymeric and polymer-mineral nanocomposites. H 3 C C H 3 CO : OH 3 C C H 3 O HO HC O = CC C C O E u 3 +C H 2 C H k x. C H O CO = C O H O L 1 L 2 C H 2 C H l n. C H O O = C C H 3 C H 2 C H O = C m O C 4 H 9 An average thickness (a) and refractive index (b) determined with ellipsometry for PO-Eu adlayers as a function of adsorption time for OP-Eu solutions with concentration: 0. 6 % (triangles), 1. 0 % (circles) and 2. 5 % (squares). Solid lines are a guide to eye. Dashed line in (b) denotes bulk value extrapolated from solution refractometry 050100 0 20 40 60 80 a) Adsorbtion time [min] Adlayer thicknes [nm] 050100 1. 35 1. 50 1. 45 bulk valueb) Adsorbtion time [min] Adlayer refractive indicex 1.
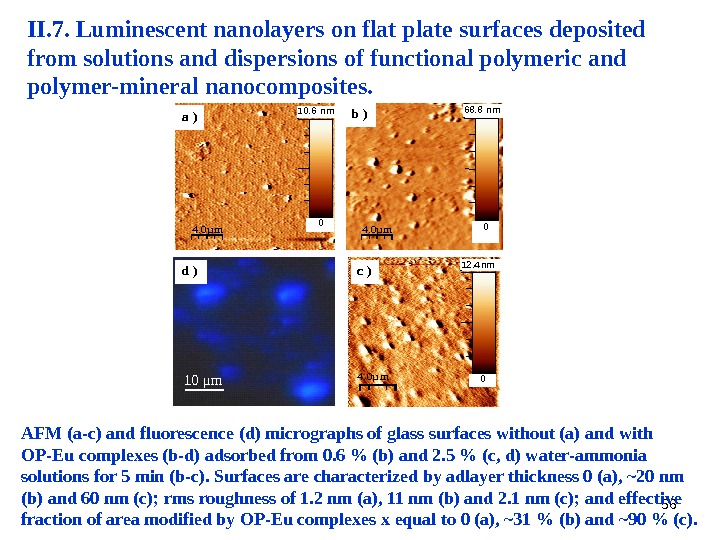
 56 4. 0µm 1 0. 6 nm 0 a ) 4. 0µ m 12. 4 nm 0 4. 0µm 68. 8 nm 0 b ) c ) d ) 10 μ m AFM (a-c) and fluorescence (d) micrographs of glass surfaces without (a) and with OP-Eu complexes (b-d) adsorbed from 0. 6 % (b) and 2. 5 % (c, d) water-ammonia solutions for 5 min (b-c). Surfaces are characterized by adlayer thickness 0 (a), ~20 nm (b) and 60 nm (c); rms roughness of 1. 2 nm (a), 11 nm (b) and 2. 1 nm (c); and effective fraction of area modified by OP-Eu complexes x equal to 0 (a), ~31 % (b) and ~90 % (c). II. 7. Luminescent nanolayers on flat plate surfaces deposited from solutions and dispersions of functional polymeric and polymer-mineral nanocomposites.
56 4. 0µm 1 0. 6 nm 0 a ) 4. 0µ m 12. 4 nm 0 4. 0µm 68. 8 nm 0 b ) c ) d ) 10 μ m AFM (a-c) and fluorescence (d) micrographs of glass surfaces without (a) and with OP-Eu complexes (b-d) adsorbed from 0. 6 % (b) and 2. 5 % (c, d) water-ammonia solutions for 5 min (b-c). Surfaces are characterized by adlayer thickness 0 (a), ~20 nm (b) and 60 nm (c); rms roughness of 1. 2 nm (a), 11 nm (b) and 2. 1 nm (c); and effective fraction of area modified by OP-Eu complexes x equal to 0 (a), ~31 % (b) and ~90 % (c). II. 7. Luminescent nanolayers on flat plate surfaces deposited from solutions and dispersions of functional polymeric and polymer-mineral nanocomposites.
 57 57 • Controlled physically detectable characteristics of nanocomposites and nanoshells • Presence of peroxide links on particle surface provides tailored particle functionalization (epoxide, aldehyde, maleimide etc. ) via graft copolymerization. • Availability of controlled reactive functionality on nanoparticle surface provides attachment of cell recognizing biological vectors (saccharides, lectins, antibodies). Why such oligoperoxide based luminescent nanocomposites and nanolayers? • Controlled particle size and size distribution • Controlled functionality and reactivity
57 57 • Controlled physically detectable characteristics of nanocomposites and nanoshells • Presence of peroxide links on particle surface provides tailored particle functionalization (epoxide, aldehyde, maleimide etc. ) via graft copolymerization. • Availability of controlled reactive functionality on nanoparticle surface provides attachment of cell recognizing biological vectors (saccharides, lectins, antibodies). Why such oligoperoxide based luminescent nanocomposites and nanolayers? • Controlled particle size and size distribution • Controlled functionality and reactivity
 58 III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment. * Cellular study was fulfilled in Lviv Institute of Cell Biology under the guidance of Professor R. Stoika
58 III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment. * Cellular study was fulfilled in Lviv Institute of Cell Biology under the guidance of Professor R. Stoika
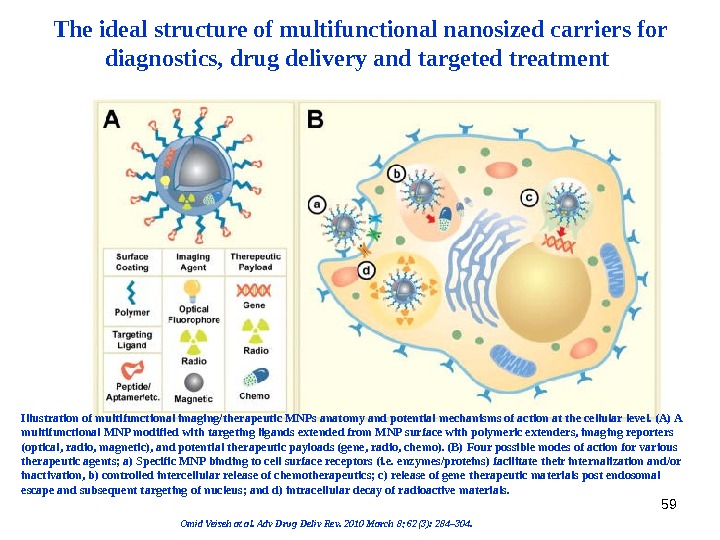
 59 The ideal structure of multifunctional nanosized carriers for diagnostics, drug delivery and targeted treatment Illustration of multifunctional imaging/therapeutic MNPs anatomy and potential mechanisms of action at the cellular level. (A) A multifunctional MNP modified with targeting ligands extended from MNP surface with polymeric extenders, imaging reporters (optical, radio, magnetic), and potential therapeutic payloads (gene, radio, chemo). (B) Four possible modes of action for various therapeutic agents; a) Specific MNP binding to cell surface receptors (i. e. enzymes/proteins) facilitate their internalization and/or inactivation, b) controlled intercellular release of chemotherapeutics; c) release of gene therapeutic materials post endosomal escape and subsequent targeting of nucleus; and d) intracellular decay of radioactive materials. Omid Veiseh at al. Adv Drug Deliv Rev. 2010 March 8; 62(3): 284– 304.
59 The ideal structure of multifunctional nanosized carriers for diagnostics, drug delivery and targeted treatment Illustration of multifunctional imaging/therapeutic MNPs anatomy and potential mechanisms of action at the cellular level. (A) A multifunctional MNP modified with targeting ligands extended from MNP surface with polymeric extenders, imaging reporters (optical, radio, magnetic), and potential therapeutic payloads (gene, radio, chemo). (B) Four possible modes of action for various therapeutic agents; a) Specific MNP binding to cell surface receptors (i. e. enzymes/proteins) facilitate their internalization and/or inactivation, b) controlled intercellular release of chemotherapeutics; c) release of gene therapeutic materials post endosomal escape and subsequent targeting of nucleus; and d) intracellular decay of radioactive materials. Omid Veiseh at al. Adv Drug Deliv Rev. 2010 March 8; 62(3): 284– 304.
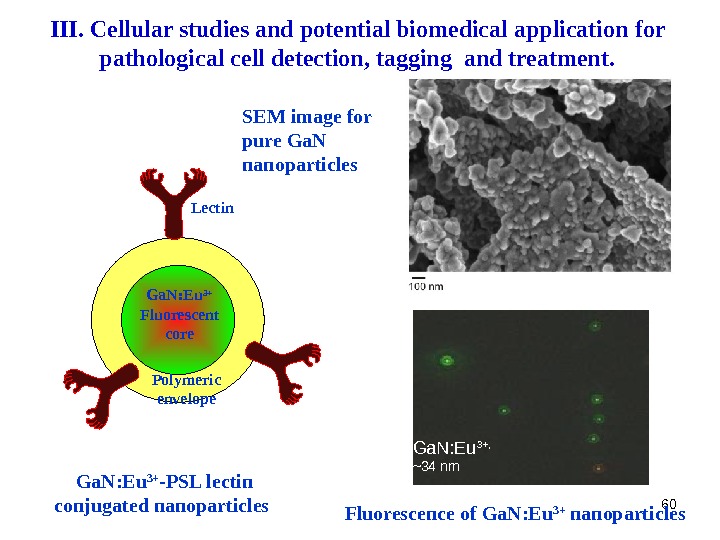
 60 Ga. N: Eu 3+ -PSL lectin conjugated nanoparticles Lectin Ga. N: Eu 3+ Fluorescent core Polymeric envelope SEM image for pure Ga. N nanoparticles Ga. N: Eu 3+, ~34 nm Fluorescence of Ga. N: Eu 3+ nanoparticles III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
60 Ga. N: Eu 3+ -PSL lectin conjugated nanoparticles Lectin Ga. N: Eu 3+ Fluorescent core Polymeric envelope SEM image for pure Ga. N nanoparticles Ga. N: Eu 3+, ~34 nm Fluorescence of Ga. N: Eu 3+ nanoparticles III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
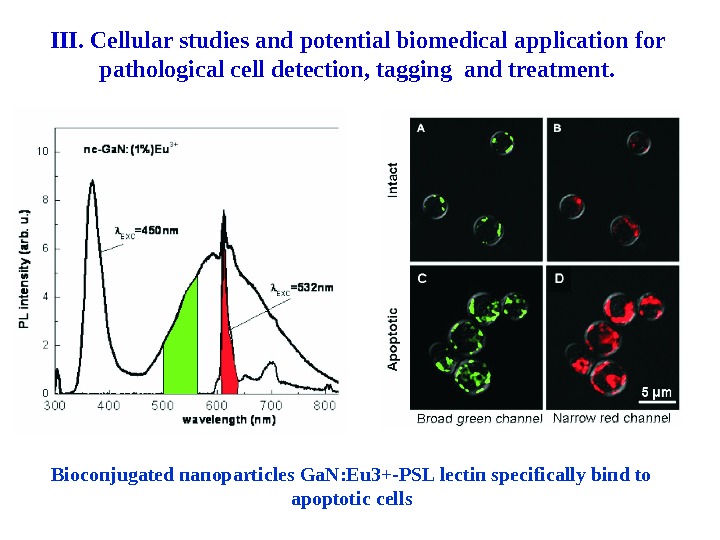
 Bioconjugated nanoparticles Ga. N: Eu 3+-PSL lectin specifically bind to apoptotic cells. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
Bioconjugated nanoparticles Ga. N: Eu 3+-PSL lectin specifically bind to apoptotic cells. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
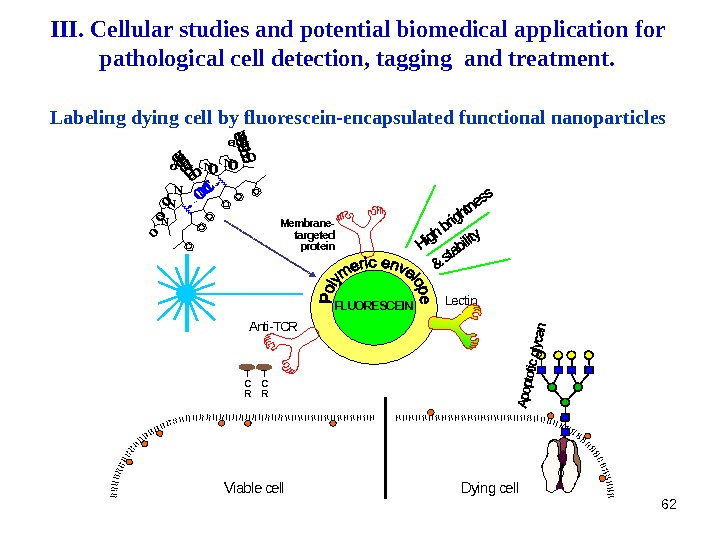
 62 FLUORESCEINT C R Viable cell Dying cell Membrane- targeted protein Lectin Anti-TCR N N NO NN OLabeling dying cell by fluorescein-encapsulated functional nanoparticles. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
62 FLUORESCEINT C R Viable cell Dying cell Membrane- targeted protein Lectin Anti-TCR N N NO NN OLabeling dying cell by fluorescein-encapsulated functional nanoparticles. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
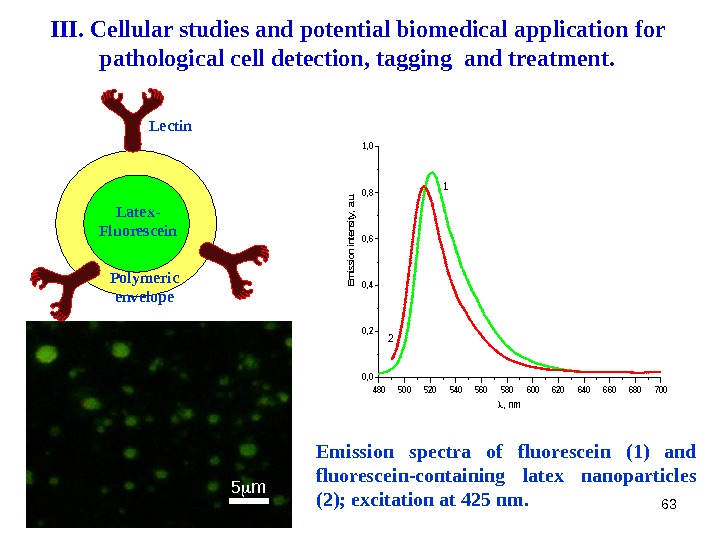
 63 Lectin Latex- Fluorescein Polymeric envelope 5 m Emission spectra of fluorescein (1) and fluorescein-containing latex nanoparticles (2); excitation at 425 nm. 480500520540560580600620640660680700 0, 2 0, 4 0, 6 0, 8 1, 0 2 1 , nm Emission intensity, a. u. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
63 Lectin Latex- Fluorescein Polymeric envelope 5 m Emission spectra of fluorescein (1) and fluorescein-containing latex nanoparticles (2); excitation at 425 nm. 480500520540560580600620640660680700 0, 2 0, 4 0, 6 0, 8 1, 0 2 1 , nm Emission intensity, a. u. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
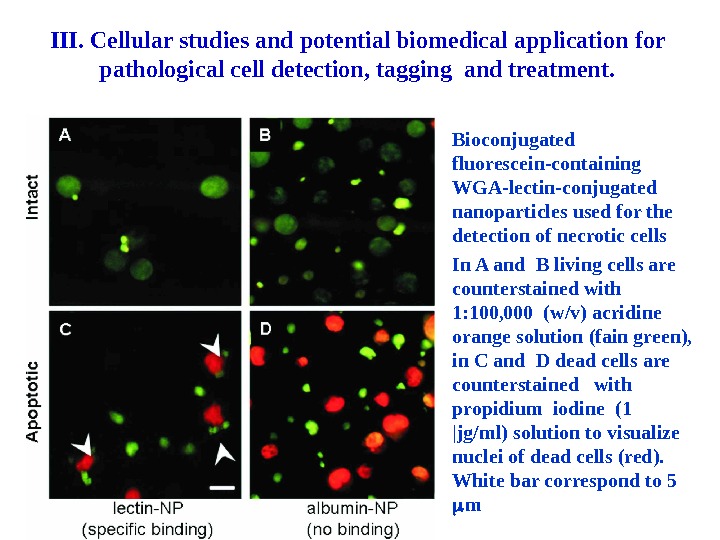
 Bioconjugated fluorescein-containing WGA-lectin-conjugated nanoparticles used for the detection of necrotic cells In A and B living cells are counterstained with 1: 100, 000 (w/v) acridine orange solution (fain green), in C and D dead cells are counterstained with propidium iodine (1 |jg/ml) solution to visualize nuclei of dead cells (red). White bar correspond to 5 m III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
Bioconjugated fluorescein-containing WGA-lectin-conjugated nanoparticles used for the detection of necrotic cells In A and B living cells are counterstained with 1: 100, 000 (w/v) acridine orange solution (fain green), in C and D dead cells are counterstained with propidium iodine (1 |jg/ml) solution to visualize nuclei of dead cells (red). White bar correspond to 5 m III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
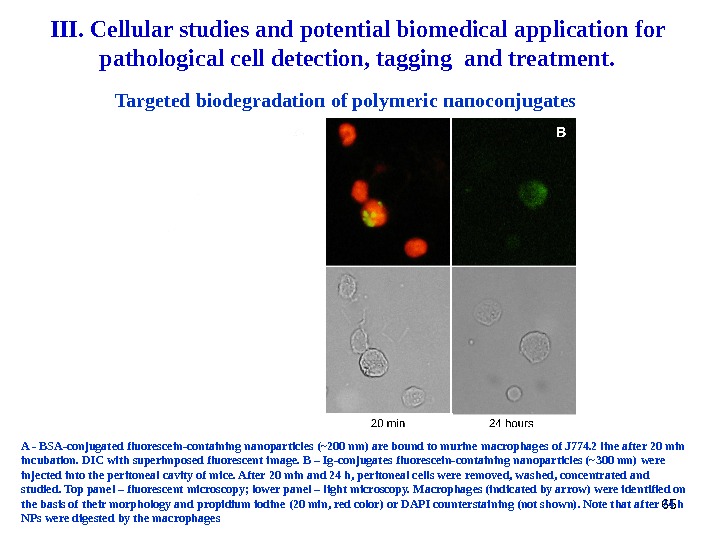
 65 A — BSA-conjugated fluorescein-containing nanoparticles (~200 nm) are bound to murine macrophages of J 774. 2 line after 20 min incubation. DIC with superimposed fluorescent image. B – Ig-conjugates fluorescein-containing nanoparticles (~300 nm) were injected into the peritoneal cavity of mice. After 20 min and 24 h, peritoneal cells were removed, washed, concentrated and studied. Top panel – fluorescent microscopy; lower panel – light microscopy. Macrophages (indicated by arrow) were identified on the basis of their morphology and propidium iodine (20 min, red color) or DAPI counterstaining (not shown). Note that after 24 h NPs were digested by the macrophages Targeted biodegradation of polymeric nanoconjugates. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
65 A — BSA-conjugated fluorescein-containing nanoparticles (~200 nm) are bound to murine macrophages of J 774. 2 line after 20 min incubation. DIC with superimposed fluorescent image. B – Ig-conjugates fluorescein-containing nanoparticles (~300 nm) were injected into the peritoneal cavity of mice. After 20 min and 24 h, peritoneal cells were removed, washed, concentrated and studied. Top panel – fluorescent microscopy; lower panel – light microscopy. Macrophages (indicated by arrow) were identified on the basis of their morphology and propidium iodine (20 min, red color) or DAPI counterstaining (not shown). Note that after 24 h NPs were digested by the macrophages Targeted biodegradation of polymeric nanoconjugates. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
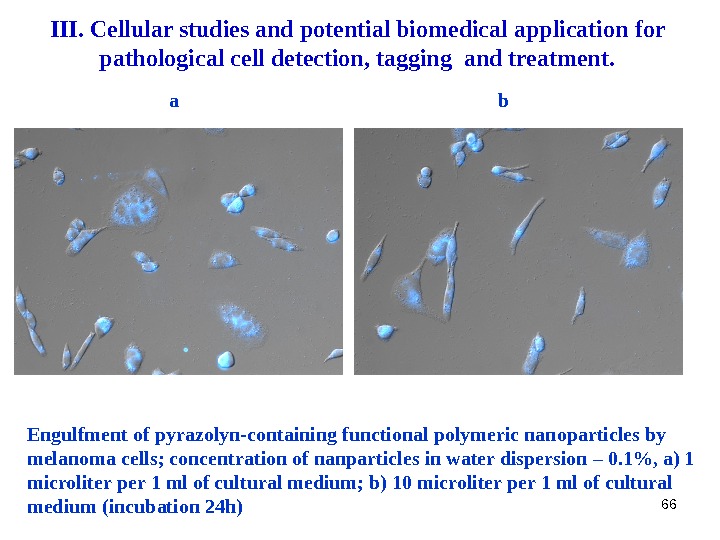
 66 Engulfment of pyrazolyn-containing functional polymeric nanoparticles by melanoma cells ; concentration of nanparticles in water dispersion – 0. 1%, a ) 1 microliter per 1 ml of cultural medium ; b ) 10 microliter per 1 ml of cultural medium ( incubation 24 h ) ba. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
66 Engulfment of pyrazolyn-containing functional polymeric nanoparticles by melanoma cells ; concentration of nanparticles in water dispersion – 0. 1%, a ) 1 microliter per 1 ml of cultural medium ; b ) 10 microliter per 1 ml of cultural medium ( incubation 24 h ) ba. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
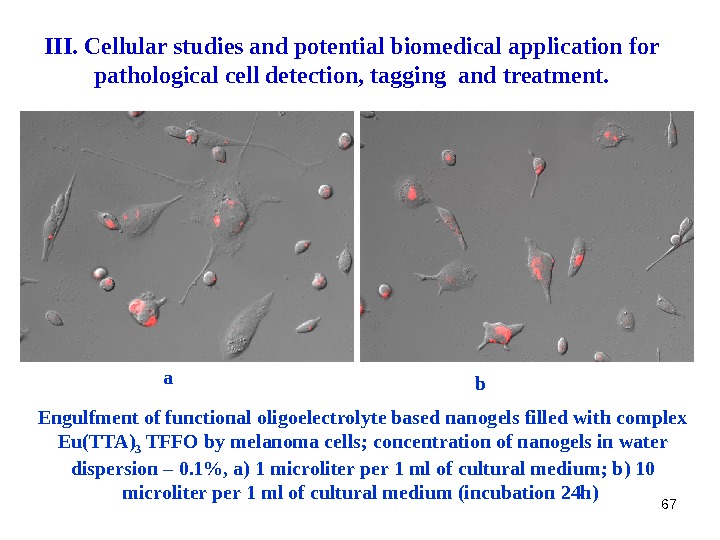
 67 a b. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment. Engulfment of functional oligoelectrolyte based nanogels filled with complex Eu(TTA) 3 TFFO by melanoma cells ; concentration of nanogels in water dispersion – 0. 1%, a ) 1 microliter per 1 ml of cultural medium ; b ) 10 microliter per 1 ml of cultural medium ( incubation 24 h )
67 a b. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment. Engulfment of functional oligoelectrolyte based nanogels filled with complex Eu(TTA) 3 TFFO by melanoma cells ; concentration of nanogels in water dispersion – 0. 1%, a ) 1 microliter per 1 ml of cultural medium ; b ) 10 microliter per 1 ml of cultural medium ( incubation 24 h )
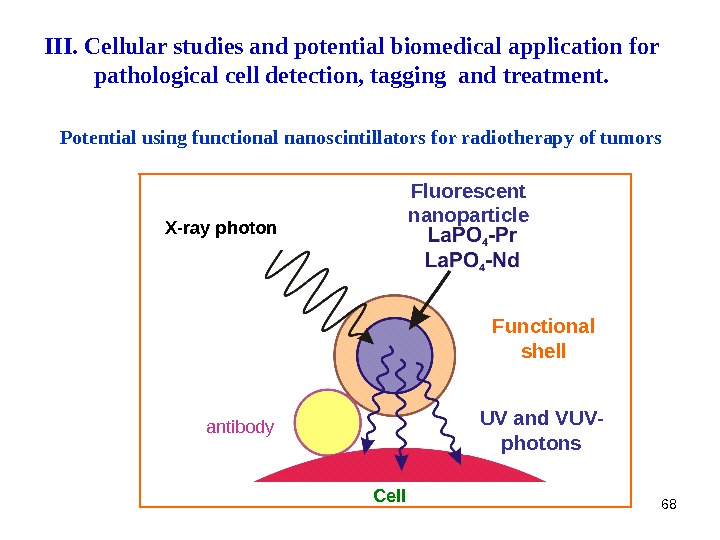
 68 Potential using functional nanoscintillators for r adiotherapy of tumors Fluorescent nanoparticle Functional shell UV and VUV — photons Cellantibody. X — ray photon. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
68 Potential using functional nanoscintillators for r adiotherapy of tumors Fluorescent nanoparticle Functional shell UV and VUV — photons Cellantibody. X — ray photon. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
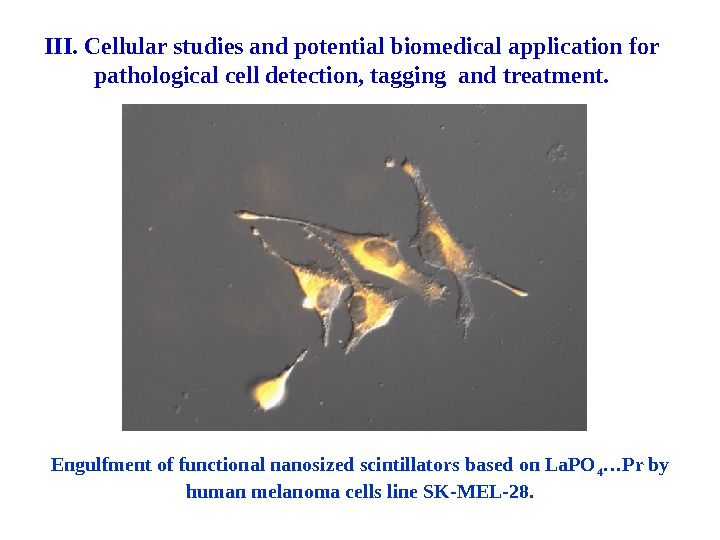
 Engulfment of functional nanosized scintillators based on La. PO 4 …Pr by human melanoma cells line SK-MEL-28. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
Engulfment of functional nanosized scintillators based on La. PO 4 …Pr by human melanoma cells line SK-MEL-28. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
 70 70 Many thanks to my team and partners Dr. N. Mitina, Dr. O. Hevus, Dr. O. Shapoval, Ph. D students O. Myagkota from Lviv Polytechnic National University; Professor A. Voloshinovskii, Professor A. Gektin, Dr. P. Zhmurin, Dr. V. Vistovskiy from I. Franko National University and Kharkiv Institute of Scintillation Materials of NASU • Professor R. Stoika, Dr. R. Bilyy, Dr. R. Panchuk and team from Cell Biology Institute of NASU for the experimental work as well as for the collaboration, ideas and discussion Acknowledgements
70 70 Many thanks to my team and partners Dr. N. Mitina, Dr. O. Hevus, Dr. O. Shapoval, Ph. D students O. Myagkota from Lviv Polytechnic National University; Professor A. Voloshinovskii, Professor A. Gektin, Dr. P. Zhmurin, Dr. V. Vistovskiy from I. Franko National University and Kharkiv Institute of Scintillation Materials of NASU • Professor R. Stoika, Dr. R. Bilyy, Dr. R. Panchuk and team from Cell Biology Institute of NASU for the experimental work as well as for the collaboration, ideas and discussion Acknowledgements

