1 of 45 © Boardworks Ltd 2010

How does NMR spectroscopy work? 2 of 45 © Boardworks Ltd 2010
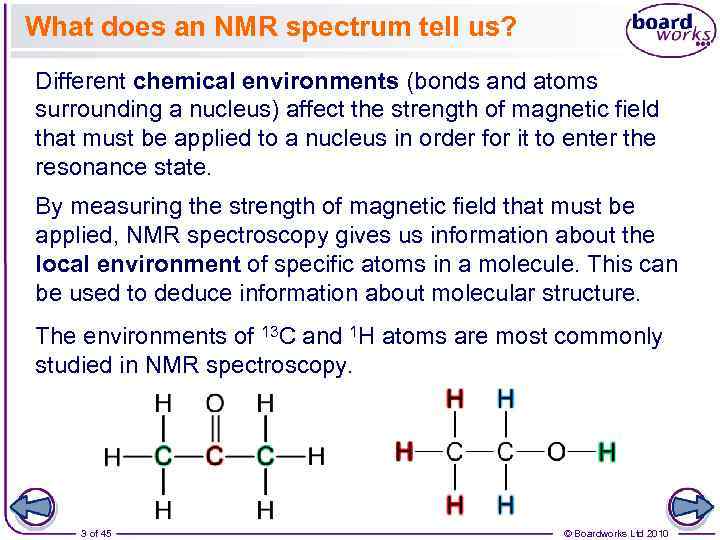
What does an NMR spectrum tell us? Different chemical environments (bonds and atoms surrounding a nucleus) affect the strength of magnetic field that must be applied to a nucleus in order for it to enter the resonance state. By measuring the strength of magnetic field that must be applied, NMR spectroscopy gives us information about the local environment of specific atoms in a molecule. This can be used to deduce information about molecular structure. The environments of 13 C and 1 H atoms are most commonly studied in NMR spectroscopy. 3 of 45 © Boardworks Ltd 2010
Carbon-13 NMR spectroscopy 4 of 45 © Boardworks Ltd 2010
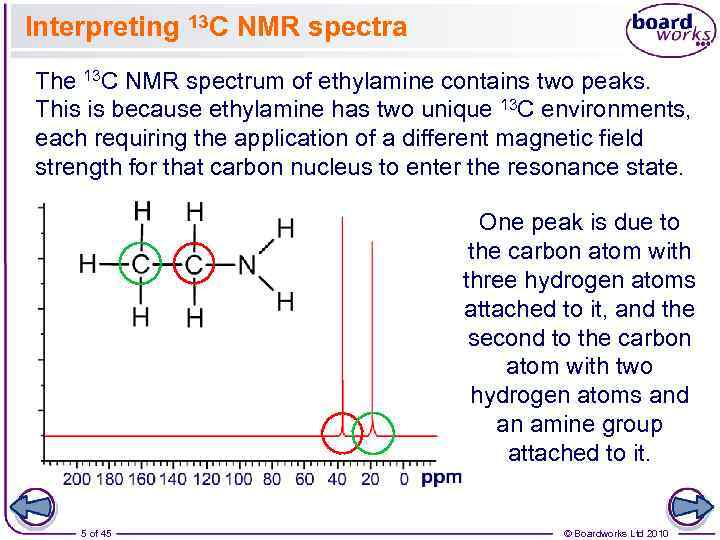
Interpreting 13 C NMR spectra The 13 C NMR spectrum of ethylamine contains two peaks. This is because ethylamine has two unique 13 C environments, each requiring the application of a different magnetic field strength for that carbon nucleus to enter the resonance state. One peak is due to the carbon atom with three hydrogen atoms attached to it, and the second to the carbon atom with two hydrogen atoms and an amine group attached to it. 5 of 45 © Boardworks Ltd 2010
Interpreting 13 C NMR spectra activity 6 of 45 © Boardworks Ltd 2010
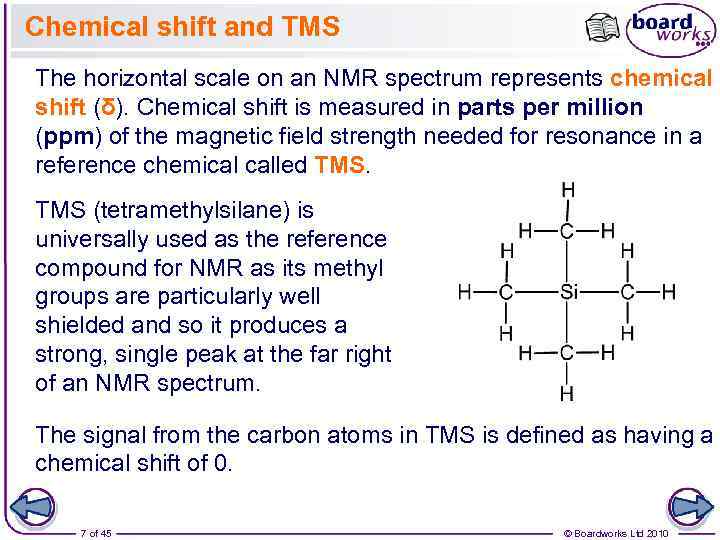
Chemical shift and TMS The horizontal scale on an NMR spectrum represents chemical shift (δ). Chemical shift is measured in parts per million (ppm) of the magnetic field strength needed for resonance in a reference chemical called TMS (tetramethylsilane) is universally used as the reference compound for NMR as its methyl groups are particularly well shielded and so it produces a strong, single peak at the far right of an NMR spectrum. The signal from the carbon atoms in TMS is defined as having a chemical shift of 0. 7 of 45 © Boardworks Ltd 2010
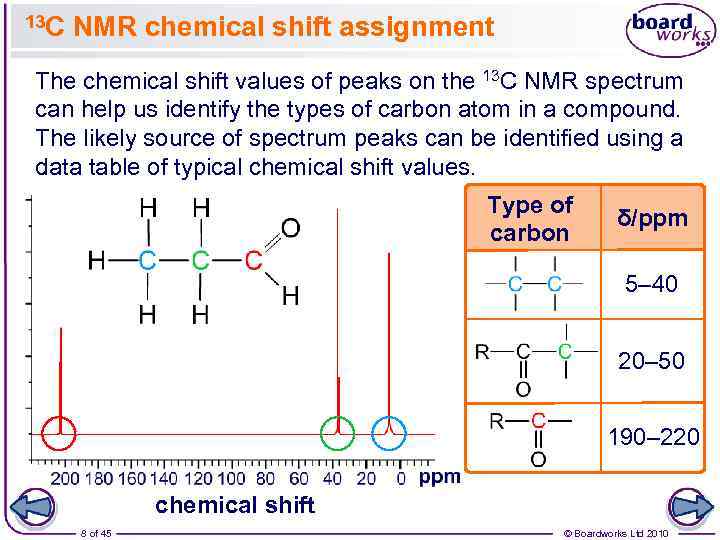
13 C NMR chemical shift assignment The chemical shift values of peaks on the 13 C NMR spectrum can help us identify the types of carbon atom in a compound. The likely source of spectrum peaks can be identified using a data table of typical chemical shift values. Type of carbon δ/ppm 5– 40 20– 50 190– 220 chemical shift 8 of 45 © Boardworks Ltd 2010
13 C NMR chemical shift activity 9 of 45 © Boardworks Ltd 2010

Proton NMR spectroscopy 10 of 45 © Boardworks Ltd 2010
Interpreting 1 H NMR spectra activity 11 of 45 © Boardworks Ltd 2010
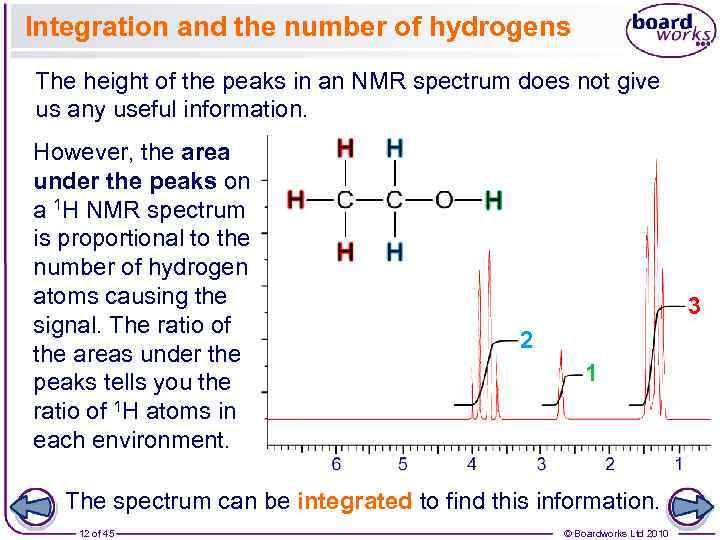
Integration and the number of hydrogens The height of the peaks in an NMR spectrum does not give us any useful information. However, the area under the peaks on a 1 H NMR spectrum is proportional to the number of hydrogen atoms causing the signal. The ratio of the areas under the peaks tells you the ratio of 1 H atoms in each environment. 3 2 1 The spectrum can be integrated to find this information. 12 of 45 © Boardworks Ltd 2010
Spin coupling 13 of 45 © Boardworks Ltd 2010
Splitting pattern activity 14 of 45 © Boardworks Ltd 2010
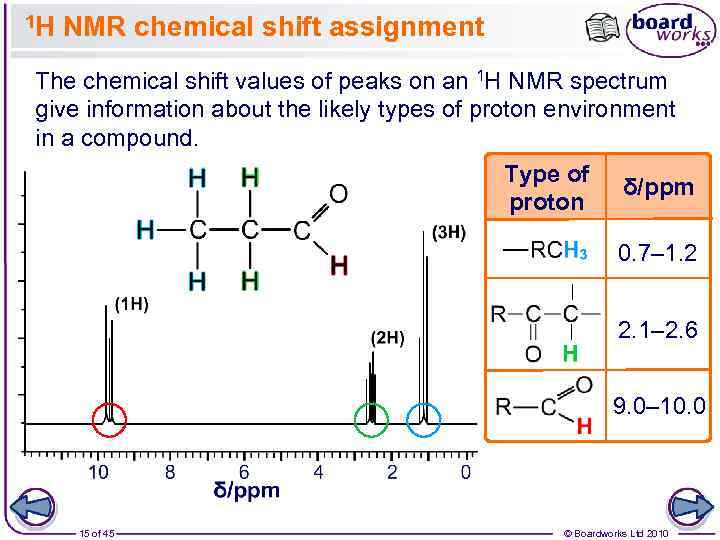
1 H NMR chemical shift assignment The chemical shift values of peaks on an 1 H NMR spectrum give information about the likely types of proton environment in a compound. Type of δ/ppm proton 0. 7– 1. 2 2. 1– 2. 6 9. 0– 10. 0 15 of 45 © Boardworks Ltd 2010
1 H NMR chemical shift assignment activity 16 of 45 © Boardworks Ltd 2010

Uses of NMR spectroscopy uses the same technology as magnetic resonance imaging (MRI). This is an important non-invasive method of gaining information about internal structures in the body used in diagnostic medicine and scientific research. NMR spectroscopy is also used in the pharmaceutical industry to check the purity of compounds. Often, a combination of mass spectrometry, infrared spectroscopy and NMR spectroscopy is used in modern analysis to elucidate the structure of organic molecules. 17 of 45 © Boardworks Ltd 2010